Optimization of Citrus Pulp Waste-Based Medium for Improved Bacterial Nanocellulose Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Precultivation Conditions
2.3. CPW Processing and Characterization
2.4. BC Processing and Material Characterization
2.5. Preliminary Assessment of BC Production from CPW
2.6. Plackett–Burman Design
2.7. Central Composite Design (CCD)
2.8. Validation Experiment
2.9. Baker’s Yeast Hydrolysate Preparation and Characterization
2.10. HPLC Analysis
3. Results and Discussion
3.1. Preliminary Assessment of BC Production on CPW
3.2. Plackett-Burman Screening
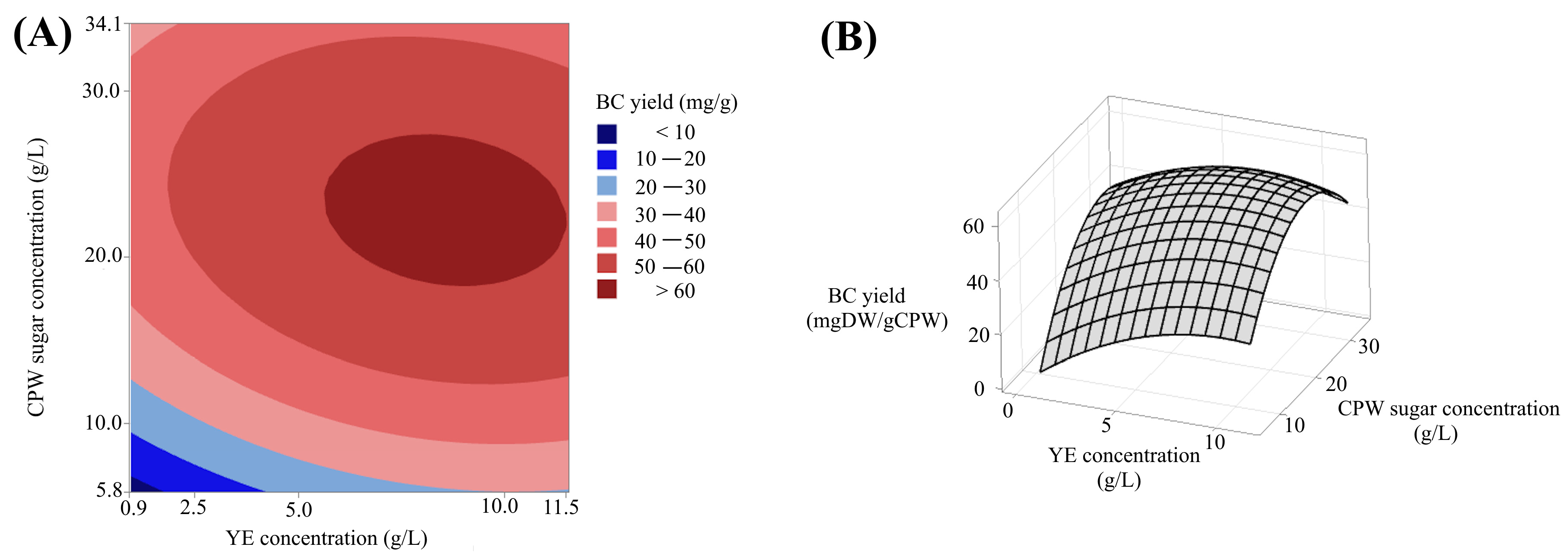
3.3. Central Composite Design
3.4. Model Validation
3.5. Material Characteraization
3.6. Replacing YE with Baker’s Yeast Hydrolysate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Xu, X.; Chen, X.; Yuan, F.; Sun, B.; Xu, Y.; Yang, J.; Sun, D. Complete genome sequence of the cellulose-producing strain Komagataeibacter nataicola RZS01. Sci. Rep. 2017, 7, 4431. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Murakami, S.; Shinke, R.; Aoki, K. Genetic Characteristics of Cellulose-Forming Acetic Acid Bacteria Identified Phenotypically as Gluconacetobacter xylinus. Biosci. Biotechnol. Biochem. 2000, 64, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Ryngajłło, M.; Kubiak, K.; Jędrzejczak-Krzepkowska, M.; Jacek, P.; Bielecki, S. Comparative genomics of the Komagataeibacter strains—Efficient bionanocellulose producers. Microbiologyopen 2018, 8, e00731. [Google Scholar] [CrossRef] [PubMed]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Mangayil, R.; Rajala, S.; Pammo, A.; Sarlin, E.; Luo, J.; Santala, V.; Karp, M.; Tuukkanen, S. Engineering and Characterization of Bacterial Nanocellulose Films as Low Cost and Flexible Sensor Material. ACS Appl. Mater. Interfaces 2017, 9, 19048–19056. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Holwerda, E.K.; Hirst, K.D.; Lynd, L.R. A defined growth medium with very low background carbon for culturing Clostridium thermocellum. J. Ind. Microbiol. Biotechnol. 2012, 39, 943–947. [Google Scholar] [CrossRef]
- El-Gendi, H.; Taha, T.H.; Ray, J.B.; Saleh, A.K. Recent advances in bacterial cellulose: A low-cost effective production media, optimization strategies and applications. Cellulose 2022, 29, 7495–7533. [Google Scholar] [CrossRef]
- Cannazza, P.; Rissanen, A.J.; Guizelini, D.; Losoi, P.; Sarlin, E.; Romano, D.; Santala, V.; Mangayil, R. Characterization of Komagataeibacter Isolate Reveals New Prospects in Waste Stream Valorization for Bacterial Cellulose Production. Microorganisms 2021, 9, 2230. [Google Scholar] [CrossRef]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2015, 23, 57–91. [Google Scholar] [CrossRef]
- Valenti, F.; Porto, S.M.C.; Chinnici, G.; Selvaggi, R.; Cascone, G.; Arcidiacono, C.; Pecorino, B. Use of citrus pulp for biogas production: A GIS analysis of citrus-growing areas and processing industries in South Italy. Land Use Policy 2017, 66, 151–161. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, C.-Y.; Shieh, C.-J.; Wang, H.-M.D.; Tseng, C.-Y. Hydrolysis of Orange Peel with Cellulase and Pectinase to Produce Bacterial Cellulose using Gluconacetobacter xylinus. Waste Biomass Valorization 2019, 10, 85–93. [Google Scholar] [CrossRef]
- Khan, U.M.; Sameen, A.; Aadil, R.M.; Shahid, M.; Sezen, S.; Zarrabi, A.; Ozdemir, B.; Sevindik, M.; Kaplan, D.N.; Selamoglu, Z.; et al. Citrus Genus and Its Waste Utilization: A Review on Health-Promoting Activities and Industrial Application. Evidence-Based Complement. Altern. Med. 2021, 2021, 2488804. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.K.; Lionetto, F.; Nisi, R.; Stoppa, M.; Licciulli, A. Sustainable Production of Stiff and Crystalline Bacterial Cellulose from Orange Peel Extract. Sustainability 2022, 14, 2247. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Production and Characterization of Bacterial Cellulose from Citrus Peels. Waste Biomass Valorization 2019, 10, 2165–2175. [Google Scholar] [CrossRef]
- Andritsou, V.; de Melo, E.M.; Tsouko, E.; Ladakis, D.; Maragkoudaki, S.; Koutinas, A.A.; Matharu, A.S. Synthesis and Characterization of Bacterial Cellulose from Citrus-Based Sustainable Resources. ACS Omega 2018, 3, 10365–10373. [Google Scholar] [CrossRef]
- Zeng, X.; Small, D.P.; Wan, W. Statistical optimization of culture conditions for bacterial cellulose production by Acetobacter xylinum BPR 2001 from maple syrup. Carbohydr. Polym. 2011, 85, 506–513. [Google Scholar] [CrossRef]
- Plackett, R.L.; Burman, J.P. The Design of Optimum Multifactorial Experiments. Biometrika 1946, 33, 305–325. [Google Scholar] [CrossRef]
- Mohite, B.V.; Kamalja, K.K.; Patil, S.V. Statistical optimization of culture conditions for enhanced bacterial cellulose production by Gluconoacetobacter hansenii NCIM 2529. Cellulose 2012, 19, 1655–1666. [Google Scholar] [CrossRef]
- Vazquez, A.; Foresti, M.L.; Cerrutti, P.; Galvagno, M. Bacterial Cellulose from Simple and Low Cost Production Media by Gluconacetobacter xylinus. J. Polym. Environ. 2013, 21, 545–554. [Google Scholar] [CrossRef]
- Bagewadi, Z.K.; Dsouza, V.; Yaraguppi, D.A.; Mulla, S.I.; Deshpande, S.H.; Shettar, S.S. Low cost production of bacterial cellulose through statistical optimization and developing its composites for multipurpose applications. Process. Biochem. 2022, 125, 47–60. [Google Scholar] [CrossRef]
- Bilgi, E.; Bayir, E.; Sendemir-Urkmez, A.; Hames, E.E. Optimization of bacterial cellulose production by Gluconacetobacter xylinus using carob and haricot bean. Int. J. Biol. Macromol. 2016, 90, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Ruby-Figueroa, R. Design of Experiment (DOE). In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 533–535. [Google Scholar]
- Luo, J.; Efimova, E.; Losoi, P.; Santala, V.; Santala, S. Wax ester production in nitrogen-rich conditions by metabolically engineered Acinetobacter baylyi ADP1. Metab. Eng. Commun. 2020, 10, e00128. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Hu, J.; Fu, L.; Zhang, L. Optimization of enzymolysis-ultrasonic assisted extraction of polysaccharides from Momordica charabtia L. by response surface methodology. Carbohydr. Polym. 2015, 115, 701–706. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Zhao, C.; Zhang, Z.; Nie, D.; Tang, W.; Li, Y. The Chemical Composition and Antibacterial and Antioxidant Activities of Five Citrus Essential Oils. Molecules 2022, 27, 7044. [Google Scholar] [CrossRef]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Bailina, Y.; Ge, Z.; Ding, T.; Ye, X.; Liu, D. Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J. Food Eng. 2014, 126, 72–81. [Google Scholar] [CrossRef]
- Kurosumi, A.; Sasaki, C.; Yamashita, Y.; Nakamura, Y. Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr. Polym. 2009, 76, 333–335. [Google Scholar] [CrossRef]
- Lee, S.; Abraham, A.; Lim, A.C.S.; Choi, O.; Gil Seo, J.; Sang, B.-I. Characterisation of bacterial nanocellulose and nanostructured carbon produced from crude glycerol by Komagataeibacter sucrofermentans. Bioresour. Technol. 2021, 342, 125918. [Google Scholar] [CrossRef]
- Efthymiou, M.-N.; Tsouko, E.; Pateraki, C.; Papagiannopoulos, A.; Tzamalis, P.; Pispas, S.; Bethanis, K.; Mantala, I.; Koutinas, A. Property evaluation of bacterial cellulose nanostructures produced from confectionery wastes. Biochem. Eng. J. 2022, 186, 108575. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Matsumoto, K.; Nagata, K.; Sato, T. Extraction of Trehalose from Thermally-treated Bakers’ Yeast. Biosci. Biotechnol. Biochem. 1994, 58, 1226–1230. [Google Scholar] [CrossRef]
- Jules, M.; Guillou, V.; François, J.; Parrou, J.-L. Two Distinct Pathways for Trehalose Assimilation in the Yeast Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2004, 70, 2771–2778. [Google Scholar] [CrossRef] [PubMed]




| CPW Sugar Concentration (A) | Initial pH (B) | Inoculum Concentration (C) | YE Concentration (D) | BC Yield (mgDW/gCPW) a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Runs | g/L | Level | pH | Level | OD600nm | Level | g/L | Level | Final pH | |
| 1 | 40 | +1 | 7 | +1 | 0.05 | −1 | 5 | +1 | 12.0 ± 4.5 | 2.8 |
| 2 | 20 | −1 | 7 | +1 | 0.2 | +1 | 5 | +1 | 34.0 ± 4.6 | 3 |
| 3 | 20 | −1 | 7 | +1 | 0.2 | +1 | 0 | −1 | 13.0 ± 0.9 | 2.8 |
| 4 | 20 | −1 | 5 | −1 | 0.05 | −1 | 5 | +1 | 26.5 ± 2.8 | 3 |
| 5 | 40 | +1 | 5 | −1 | 0.2 | +1 | 5 | +1 | 31.0 ± 10.9 | 2.5 |
| 6 | 20 | −1 | 5 | −1 | 0.05 | −1 | 0 | −1 | 18.0 ± 4.6 | 2.5 |
| 7 | 20 | −1 | 5 | −1 | 0.2 | +1 | 5 | +1 | 31.0 ± 1.5 | 3 |
| 8 | 40 | +1 | 7 | +1 | 0.05 | −1 | 5 | +1 | 23.5 ± 3.2 | 2.5 |
| 9 | 40 | +1 | 7 | +1 | 0.2 | +1 | 0 | −1 | 8.0 ± 2.2 | 3 |
| 10 | 20 | −1 | 7 | +1 | 0.05 | −1 | 0 | −1 | 12.5 ± 2.3 | 3 |
| 11 | 40 | +1 | 5 | −1 | 0.2 | +1 | 0 | −1 | 11.0 ± 0.6 | 2.5 |
| 12 | 40 | +1 | 5 | −1 | 0.05 | −1 | 0 | −1 | 7.0 ± 1.1 | 2.8 |
| YE | CPW Sugars | BC Yield (mgDW/gCPW) a | ||||
|---|---|---|---|---|---|---|
| Runs | A (g/L) | Code A | B (g/L) | Code B | Final pH | |
| 1 | 2.5 | −1 | 10 | −1 | 25.0 ± 1.8 | 5 |
| 2 | 10 | +1 | 10 | −1 | 43.0 ± 2.0 | 6.5 |
| 3 | 2.5 | −α | 30 | +1 | 50.0 ± 2.1 | 3.5 |
| 4 | 10 | +1 | 30 | +1 | 58.0 ± 6.4 | 4 |
| 5 | 0.9 | 0.9 | 20 | 0 | 46.0 ± 4.4 | 3.5 |
| 6 | 11.6 | +α | 20 | 0 | 58.0 ± 1.5 | 6 |
| 7 | 5 | 0 | 5.9 | −α | 26.0 ± 2.0 | 5.5 |
| 8 | 5 | 0 | 34.1 | +α | 43.0 ± 2.8 | 4 |
| 9 | 5 | 0 | 20 | 0 | 54.0 ± 3.6 | 4 |
| 10 | 5 | 0 | 20 | 0 | 57.0 ± 6.1 | 4 |
| 11 | 5 | 0 | 20 | 0 | 59.0 ± 3.1 | 4.5 |
| 12 | 5 | 0 | 20 | 0 | 56.0 ± 6.4 | 4 |
| 13 | 5 | 0 | 20 | 0 | 61.0 ± 7.3 | 4 |
| Source | df | Adj SS | Adj MS | F Value | p Value | Coefficient (Coded) | SEcoefficient (Coded) |
|---|---|---|---|---|---|---|---|
| Model | 4 | 906.53 | 226.63 | 11.71 | 0.003 | ||
| Linear | 4 | 906.53 | 226.63 | 11.71 | 0.003 | ||
| CPW sugars | 1 | 144.91 | 144.91 | 7.48 | 0.029 | −3.48 | 1.27 |
| Initial medium pH | 1 | 39.24 | 39.24 | 2.03 | 0.198 | −1.81 | 1.27 |
| Inoculum concentration | 1 | 66.74 | 66.74 | 3.45 | 0.106 | 2.36 | 1.27 |
| YE | 1 | 655.64 | 655.64 | 33.86 | 0.001 | 7.39 | 1.27 |
| Error | 7 | 135.53 | 19.36 | ||||
| Lack-of-fit | 6 | 69.41 | 11.57 | 0.17 | 0.946 | ||
| Pure error | 1 | 66.13 | 66.13 | ||||
| Total | 11 | 1042.06 |
| Source | DF | Adj SS | Adj MS | F Value | p Value |
|---|---|---|---|---|---|
| Model | 5 | 1666.43 | 333.287 | 27.67 | 0.000 |
| Linear | 2 | 696.88 | 348.442 | 28.93 | 0.000 |
| YE | 1 | 238.18 | 238.177 | 19.77 | 0.003 |
| CPW (sugars) | 1 | 458.71 | 458.708 | 38.08 | 0.000 |
| Square | 2 | 940.83 | 470.415 | 39.06 | 0.000 |
| YE*YE | 1 | 89.89 | 89.891 | 7.46 | 0.029 |
| CPW sugars*CPW sugars | 1 | 915.85 | 915.846 | 76.04 | 0.000 |
| Two-way interaction | 1 | 17.28 | 17.279 | 1.43 | 0.270 |
| YE*CPW sugars | 1 | 17.28 | 17.279 | 1.43 | 0.270 |
| Error | 7 | 84.31 | 12.044 | ||
| Lack-of-Fit | 3 | 52.46 | 17.486 | 2.20 | 0.231 |
| Pure error | 4 | 31.85 | 7.963 | ||
| Total | 12 | 1750.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minardi, C.; Bersanetti, D.; Sarlin, E.; Santala, V.; Mangayil, R. Optimization of Citrus Pulp Waste-Based Medium for Improved Bacterial Nanocellulose Production. Microorganisms 2024, 12, 2095. https://doi.org/10.3390/microorganisms12102095
Minardi C, Bersanetti D, Sarlin E, Santala V, Mangayil R. Optimization of Citrus Pulp Waste-Based Medium for Improved Bacterial Nanocellulose Production. Microorganisms. 2024; 12(10):2095. https://doi.org/10.3390/microorganisms12102095
Chicago/Turabian StyleMinardi, Carlotta, Davide Bersanetti, Essi Sarlin, Ville Santala, and Rahul Mangayil. 2024. "Optimization of Citrus Pulp Waste-Based Medium for Improved Bacterial Nanocellulose Production" Microorganisms 12, no. 10: 2095. https://doi.org/10.3390/microorganisms12102095
APA StyleMinardi, C., Bersanetti, D., Sarlin, E., Santala, V., & Mangayil, R. (2024). Optimization of Citrus Pulp Waste-Based Medium for Improved Bacterial Nanocellulose Production. Microorganisms, 12(10), 2095. https://doi.org/10.3390/microorganisms12102095






