Combination of Silicate-Based Soil Conditioners with Plant Growth-Promoting Microorganisms to Improve Drought Stress Resilience in Potato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Conditioners
2.2. Potato Nursery Culture and Transplanting
2.3. Preparation and Application of Microbial Inoculants
2.4. Pot Experiment I
2.5. Pot Experiment II
2.6. Pot Experiment III
2.7. Plant Analysis
2.8. Mycorrhizal Counting
2.9. Statistical Analysis
3. Results
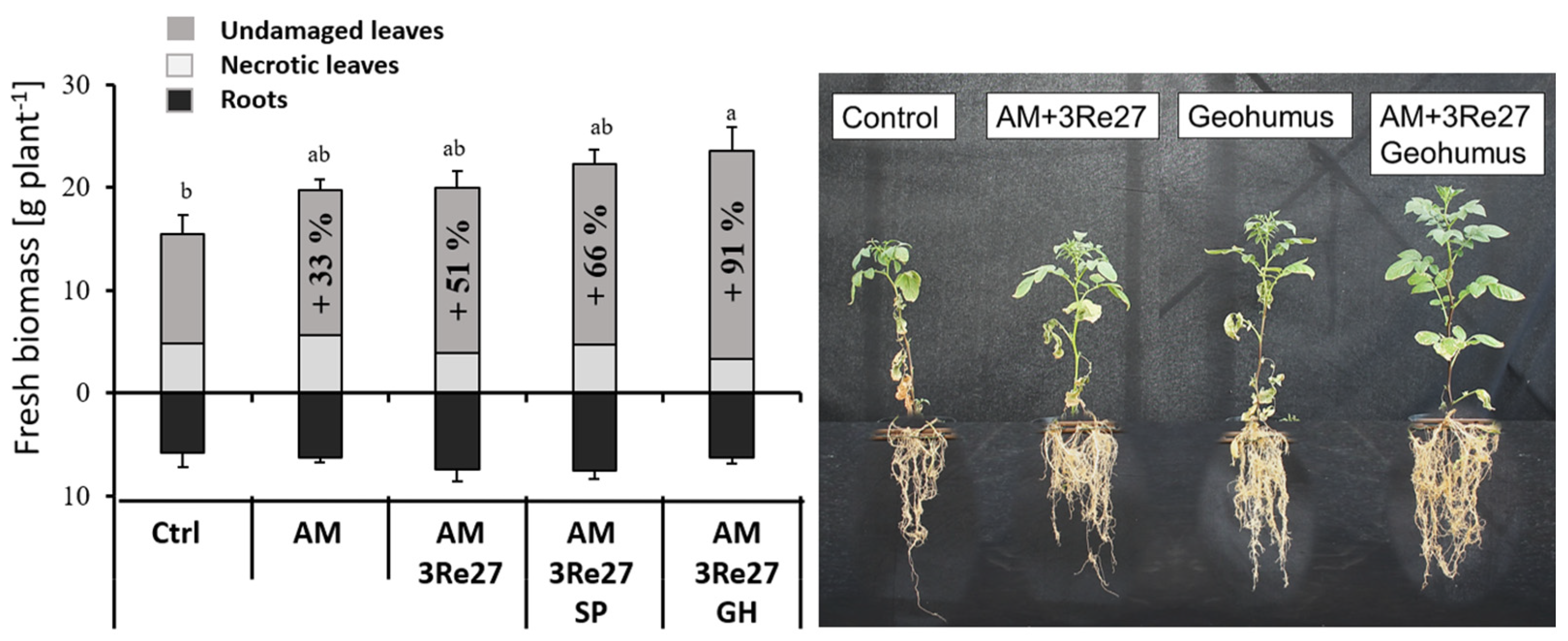
3.1. Drought-Protective Effects on Vegetative Growth
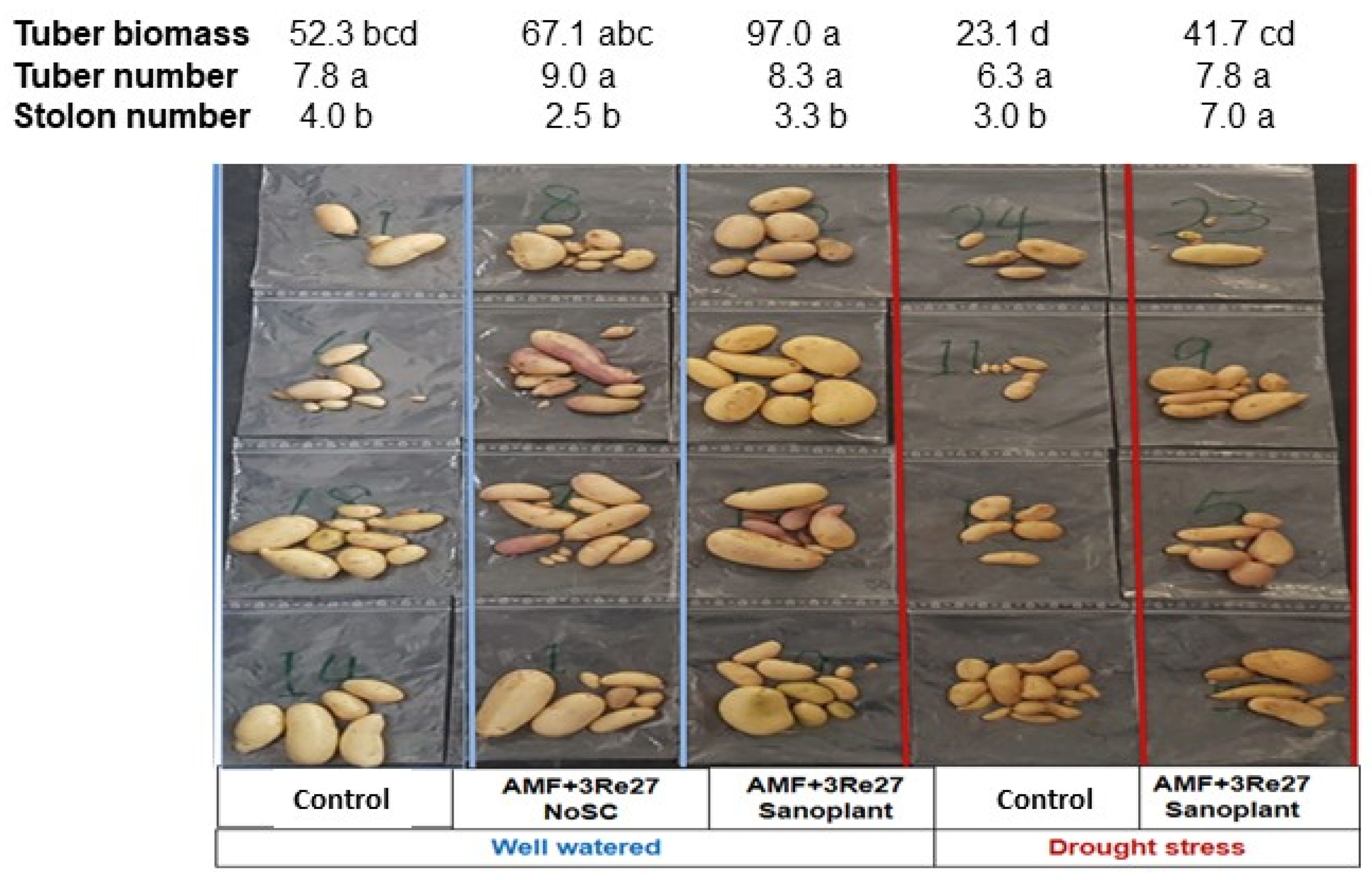
3.2. Drought-Protective Effects on Tuber Formation
3.2.1. Geohumus Experiment
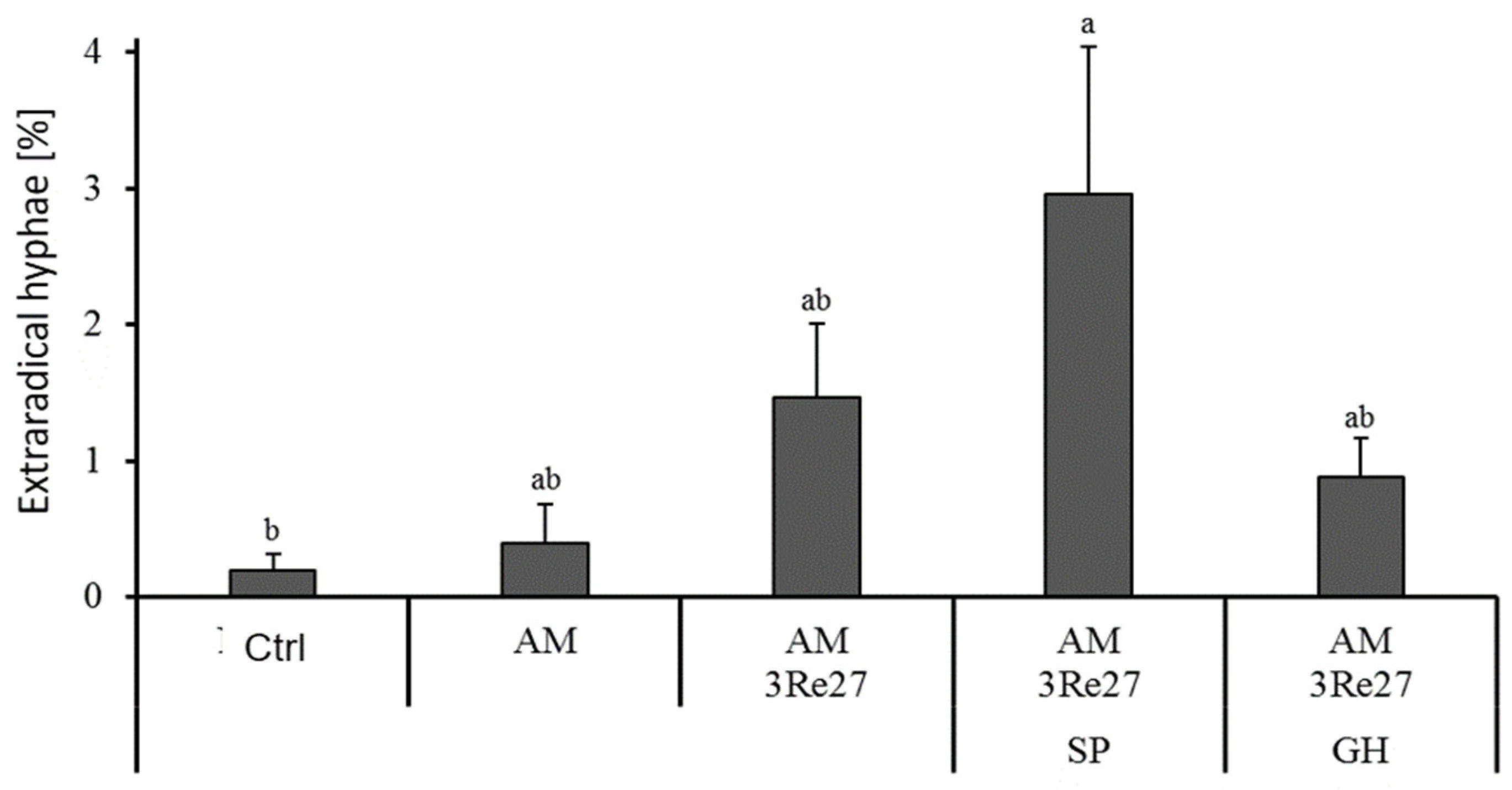
Plant Growth AM Colonization and Nutritional Status
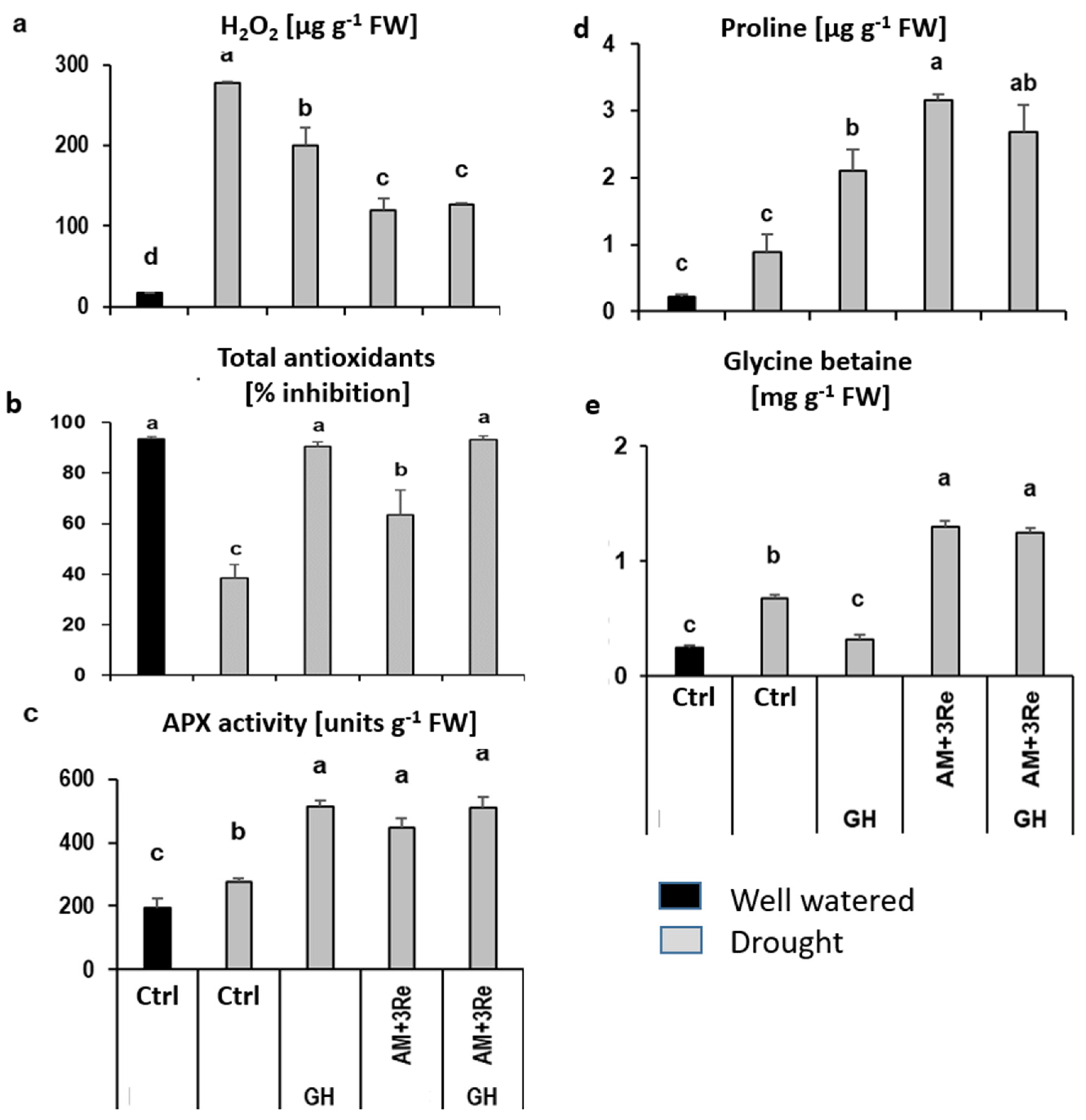
Physiological Stress Indicators
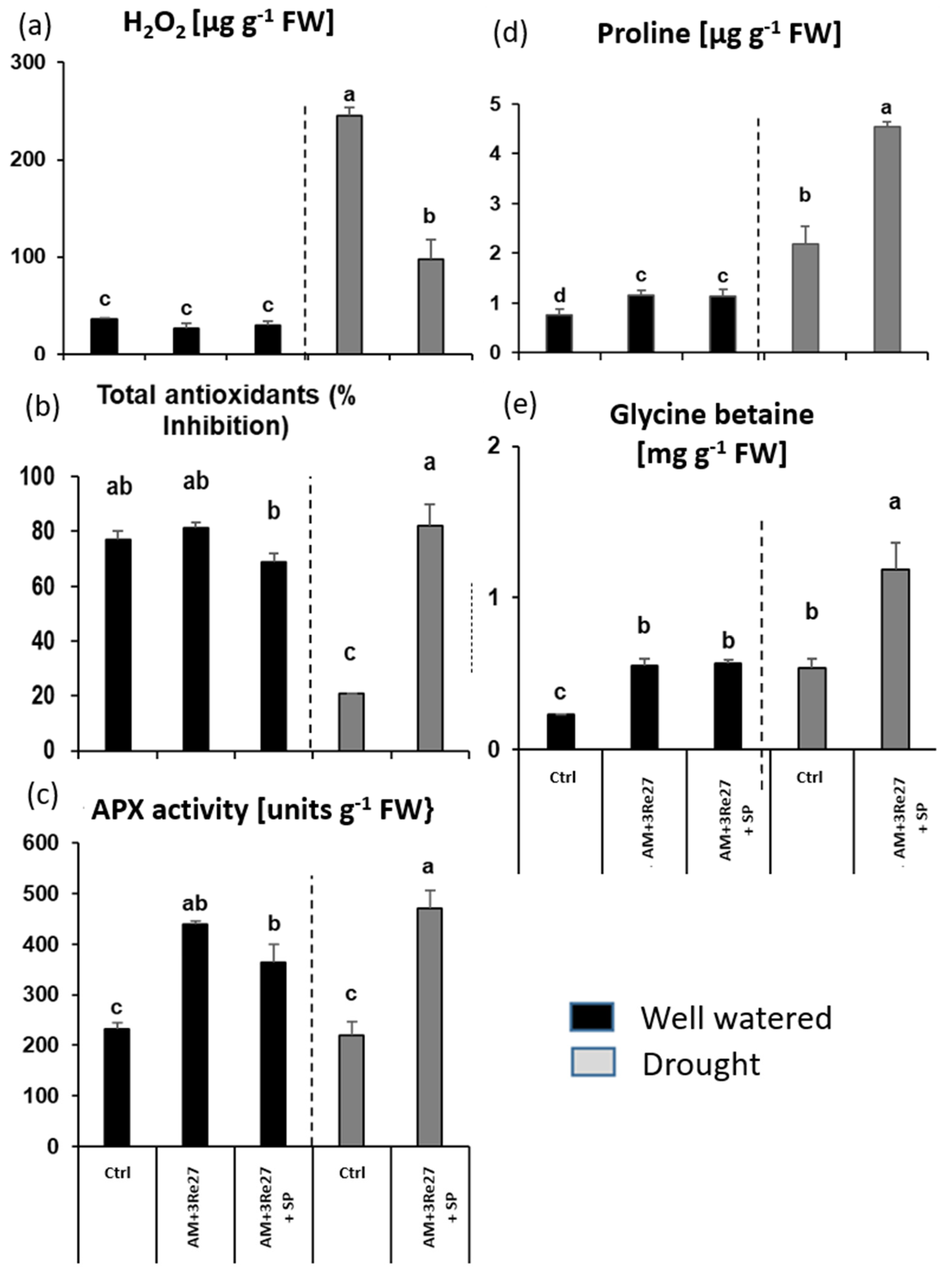
3.2.2. Sanoplant Experiment
Plant Growth, AM Colonization, and Nutritional Status
Physiological Stress Indicators
4. Discussion
4.1. Impact of Soil Conditioners on Soil Water Relationships
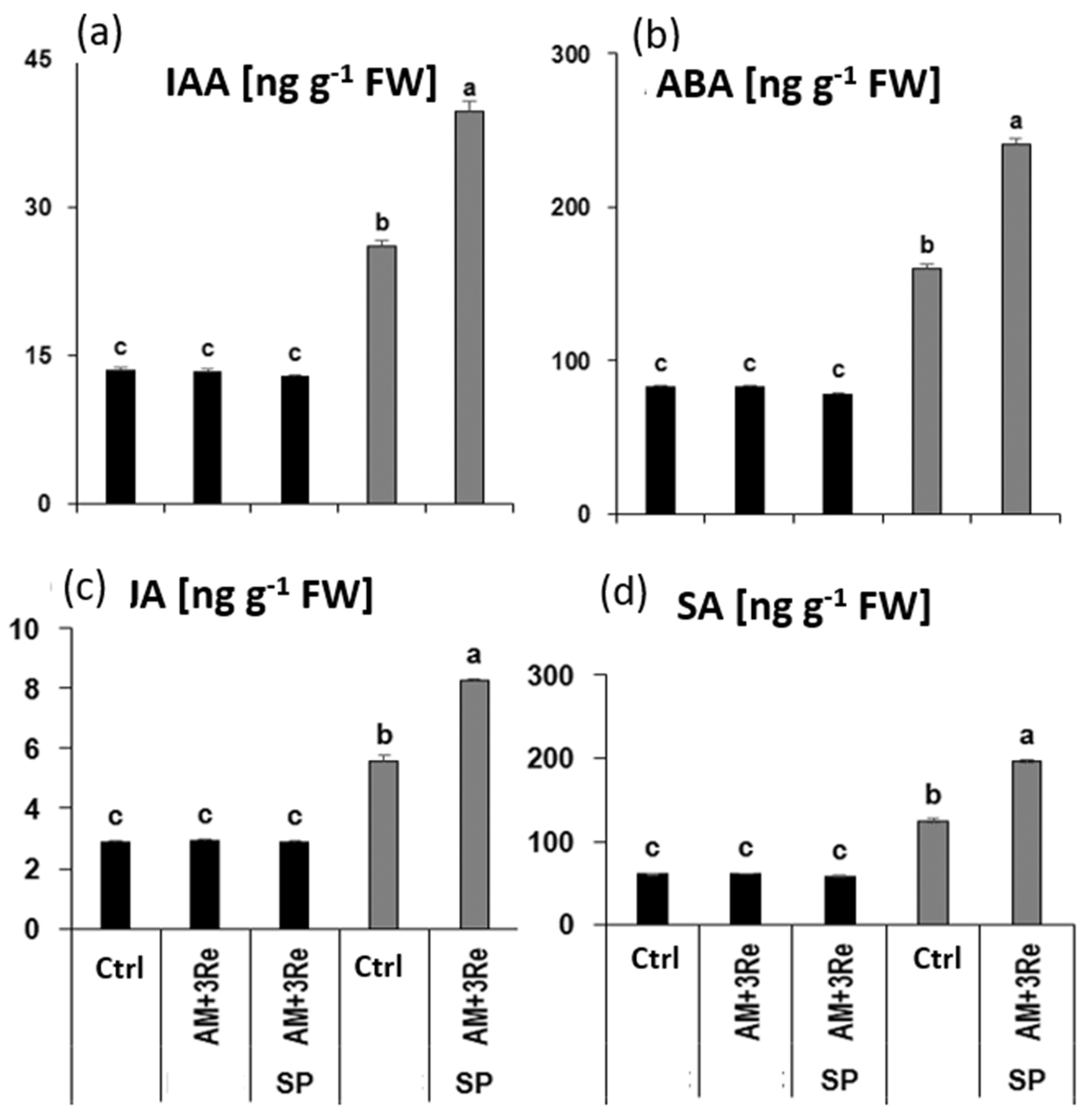
4.2. Oxidative Stress Defense and Hormonal Status
4.3. Biomass Partitioning, Tuber Formation, and Plant-Nutritional Status
4.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Islam, S.M.F.; Karim, Z. World’s demand for food and water: The consequences of climate change. In Desalination-Challenges and Opportunities; IntechOpen: London, UK, 2019; pp. 57–84. [Google Scholar]
- FAO. FAO Statistical Databases FAOSTAT. 2014. Available online: https://www.fao.org/faostat/en/#home (accessed on 20 October 2023).
- Devaux, A.; Kromann, P.; Ortiz, O. Potatoes for sustainable global food security. Potato Res. 2014, 57, 185–199. [Google Scholar] [CrossRef]
- Hijmans, R.J. The effect of climate change on global potato production. Am. J. Potato Res. 2003, 80, 271–279. [Google Scholar] [CrossRef]
- Raymundo, R.; Asseng, S.; Robertson, R.; Petsakos, A.; Hoogenboom, G.; Quiroz, R.; Wolf, J. Climate change impact on global potato production. Eur. J. Agron. 2018, 100, 87–98. [Google Scholar] [CrossRef]
- Mohamed, H.I.; El-Beltagi, H.E.D.S.; Abd-Elsalam, K.A. Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management; Springer International Publishing: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Steinberg, P.D.; Rillig, M.C. Differential decomposition of arbuscular mycorrhizal fungal hyphae and glomalin. Soil Biol. Biochem. 2003, 35, 191–194. [Google Scholar] [CrossRef]
- Tyagi, J.; Sultan, E.; Mishra, A.; Kumari, M.; Pudake, R.N. The impact of AMF symbiosis in alleviating drought tolerance in field crops. In Mycorrhiza-Nutrient Uptake, Biocontrol, Ecorestoration; Springer: Cham, Switzerland, 2017; pp. 211–234. [Google Scholar]
- Kim, Y.C.; Glick, B.R.; Bashan, Y.; Ryu, C.M. Enhancement of plant drought tolerance by microbes. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 383–413. [Google Scholar]
- Ruiz-Lozano, J.M.; Porcel, R.; Bárzana, G.; Azcón, R.; Aroca, R. Contribution of arbuscular mycorrhizal symbiosis to plant drought tolerance: State of the art. In Plant Responses to Drought Stress; Gabler: Wiesbaden, Germany, 2012; pp. 335–362. [Google Scholar]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef]
- Duo, L.A.; Liu, C.X.; Zhao, S.L. Alleviation of drought stress in turfgrass by the combined application of nano-compost and microbes from compost. Russ. J. Plant Physiol. 2018, 65, 419–426. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Garbaye, J.; Tarkka, M. The mycorrhiza helper bacteria revisited. New Phytol. 2007, 176, 22–36. [Google Scholar] [CrossRef]
- Yusran, Y.; Roemheld, V.; Mueller, T. Effects of Pseudomonas sp. “Proradix” and Bacillus amyloliquefaciens FZB42 on the establishment of AMF infection, nutrient acquisition and growth of tomato affected by Fusarium oxysporum Schlecht f.sp. radicis-lycopersici Jarvis and shoemaker. In Proceedings of the International Plant Nutrition Colloquium XVI, Sacramento, CA, USA, 26–30 August 2009; p. 1106. [Google Scholar]
- Thonar, C.; Lekfeldt, J.D.S.; Cozzolino, V.; Kundel, D.; Kulhánek, M.; Mosimann, C.; Mäder, P. Potential of three microbial bio-effectors to promote maize growth and nutrient acquisition from alternative phosphorous fertilizers in contrasting soils. Chem. Biol. Technol. Agric. 2017, 4, 7. [Google Scholar] [CrossRef]
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef]
- Bradáčová, K.; Florea, A.S.; Bar-Tal, A.; Minz, D.; Yermiyahu, U.; Shawahna, R.; Weinmann, M. Microbial consortia versus single-strain inoculants: An advantage in PGPM-assisted tomato production. Agronomy 2019, 9, 105. [Google Scholar] [CrossRef]
- Nkebiwe, P.M.; Stevens Lekfeldt, J.D.; Symanczik, S.; Thonar, C.; Mäder, P.; Bar-Tal, A.; Halpern, M.; Biro, B.; Bradacova, K.; Caniullan, P.C.; et al. Effectiveness of bio-effectors on maize, wheat and tomato performance and phosphorus acquisition from greenhouse to field scales in Europe and Israel: A meta-analysis. Front. Plant Sci. 2024, 15, 1333249. [Google Scholar] [CrossRef] [PubMed]
- Abobatta, W. Impact of hydrogel polymer in agricultural sector. Adv. Agric. Environ. Sci. Open Access 2018, 1, 59–64. [Google Scholar] [CrossRef]
- Saha, A.; Sekharan, S.; Manna, U. Superabsorbent hydrogel (SAH) as a soil amendment for drought management: A review. Soil Tillage Researc 2020, 204, 104736. [Google Scholar] [CrossRef]
- Rydlova, J.; Püschel, D. Arbuscular mycorrhiza, but not hydrogel, alleviates drought stress of ornamental plants in peat-based substrate. Appl. Soil Ecol. 2020, 146, 103394. [Google Scholar] [CrossRef]
- Abdel-Nasser, G.; Al-Omran, A.M.; Falatah, A.M.; Sheta, A.S.; Al-Harbi, A.R. Impact of natural conditioners on water retention, infiltration and evaporation characteristics of sandy soil. J. Appl. Sci. 2007, 7, 1699–1708. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Yang, F.; Wang, J.; Song, S.; Rao, P.; Wang, R.; Liu, S.; Zhang, F. Novel controlled release microspheric soil conditioner based on the temperature and pH dual-stimuli response. J. Agric. Food Chem. 2020, 68, 7819–7829. [Google Scholar] [CrossRef]
- Lertsarawut, P.; Rattanawongwiboon, T.; Tangthong, T.; Laksee, S.; Kwamman, T.; Phuttharak, B.; Hemvichian, K. Starch-Based Super Water Absorbent: A Promising and Sustainable Way to Increase Survival Rate of Trees Planted in Arid Areas. Polymers 2021, 13, 1314. [Google Scholar] [CrossRef]
- Li, Y.; Shi, H.; Zhang, H.; Chen, S. Amelioration of drought effects in wheat and cucumber by the combined application of super absorbent polymer and potential biofertilizer. PeerJ 2019, 7, e6073. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Zhu, M.; Wan, H.; Chen, Z.; Yang, N.; Duan, J.; Wei, Z.; Hu, T.; Liu, F. Effects of Plant Growth Promoting Rhizobacteria (PGPR) Strain Bacillus licheniformis with Biochar Amendment on Potato Growth and Water Use Efficiency under Reduced Irrigation Regime. Agronomy 2022, 12, 1031. [Google Scholar] [CrossRef]
- Waqar, A.; Bano, A.; Ajmal, M. Effects of PGPR bioinoculants, hydrogel and biochar on growth and physiology of soybean under drought stress. Commun. Soil Sci. Plant Anal. 2022, 53, 826–847. [Google Scholar] [CrossRef]
- Truong, V.K.; Mainwaring, D.E.; Murugaraj, P.; Nguyen, D.H.; Ivanova, E.P. Impact of confining 3-D polymer networks on dynamics of bacterial ingress and self-organisation. J. Mater. Chem. B 2015, 3, 8704–8710. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Murugaraj, P.; Mathes, F.; Tan, B.K.; Truong, V.K.; Murphy, D.V.; Mainwaring, D.E. Copolymers enhance selective bacterial community colonization for potential root zone applications. Sci. Rep. 2017, 7, 15902. [Google Scholar] [CrossRef] [PubMed]
- Mathes, F.; Murugaraj, P.; Bougoure, J.; Pham, V.T.; Truong, V.K.; Seufert, M.; Murphy, D.V. Engineering rhizobacterial community resilience with mannose nanofibril hydrogels towards maintaining grain production under drying climate stress. Soil Biol. Biochem. 2020, 142, 107715. [Google Scholar] [CrossRef]
- Su, A.Y.; Niu, S.Q.; Liu, Y.Z.; He, A.L.; Zhao, Q.; Paré, P.W.; Zhang, J.L. Synergistic effects of Bacillus amyloliquefaciens (GB03) and water retaining agent on drought tolerance of perennial ryegrass. Int. J. Mol. Sci. 2017, 18, 2651. [Google Scholar] [CrossRef]
- Holliman, P.J.; Clark, J.A.; Williamson, J.C.; Jones, D.L. Model and field studies of the degradation of cross-linked polyacrylamide gels used during the revegetation of slate waste. Sci. Total Environ. 2005, 336, 13–24. [Google Scholar] [CrossRef]
- Dell’Ambrogio, G.; Wong, J.W.Y.; Ferrari, B.J.D. Ecotoxicological Effects of Polyacrylate, Acrylic Acid, Polyacrylamide and Acrylamide on Soil and Water Organisms; Swiss Centre for Applied Ecotoxicology: Lausanne, Switzerland, 2019. [Google Scholar]
- Mamun, A.A.; Neumann, G.; Moradtalab, N.; Ahmed, A.; Dupuis, B.; Darbon, G.; Weinmann, M. Microbial consortia versus single-strain inoculants as drought stress protectants in potato affected by the form of N supply. Horticulturae 2024, 10, 102. [Google Scholar] [CrossRef]
- Boguszewska-Mańkowska, D.; Zarzyńska, K.; Nosalewicz, A. Drought differentially affects root system size and architecture of potato cultivars with differing drought tolerance. Am. J. Potato Res. 2020, 97, 54–62. [Google Scholar] [CrossRef]
- VDLUFA (Verband Deutscher Landwirtschaftlicher Untersuchungs-und Forschungsanstalten e.V. Speyer, Germany). Phosphordü ngung nach Bodenuntersuchung und Pflanzenbedarf. VDLUFA: Darmsatdt, Germany, 2018. [Google Scholar]
- Öhlinger, R. Maximum Water-Holding Capacity. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 385–386. [Google Scholar]
- Duong, V.N. Environmental Effects on Physical Properties of Geohumus and Effects of Its Application on Drought Responses in Maize. Ph.D. Thesis, University of Hohenheim, Hohenheim, Germany, 2013. [Google Scholar]
- Buchmann, C. The Swelling of Interparticulate Hydrogels in Soil and Their Contribution to Soil Structural Stability and Soil-Water Interactions. Ph.D. Thesis, University of Koblenz, Koblenz, Germany, 2018. [Google Scholar]
- Barrientos-Sanhueza, C.; Mondaca, P.; Tamayo, M.; Álvaro, J.E.; Díaz-Barrera, A.; Cuneo, I.F. Enhancing the mechanical and hydraulic properties of coarse quartz sand using a water-soluble hydrogel based on bacterial alginate for novel application in agricultural contexts. Soil Sci. Soc. Am. J. 2021, 85, 1880–1893. [Google Scholar] [CrossRef]
- Zhang, S.; He, F.; Fang, X.; Zhao, X.; Liu, Y.; Yu, G.; Li, J. Enhancing soil aggregation and acetamiprid adsorption by ecofriendly polysaccharides hydrogel based on Ca2+-amphiphilic sodium alginate. J. Environ. Sci. 2022, 113, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Loján, P.; Demortier, M.; Velivelli, S.L.; Pfeiffer, S.; Suárez, J.P.; De Vos, P.; Declerck, S. Impact of plant growth-promoting rhizobacteria on root colonization potential and life cycle of Rhizophagus irregularis following co-entrapment into alginate beads. J. Appl. Microbiol. 2017, 122, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.R.S.; Piché, Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef] [PubMed]
- Vierheilig, H.; Schweiger, P.; Brundrett, M. An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots. Physiol. Plant. 2005, 125, 393–404. [Google Scholar] [CrossRef]
- Mudra, A. Statitische Methoden für Landwirtschaftliche Versuche; Paul Parey Verlag: Berlin, Germany, 1958. [Google Scholar]
- Wolf, B. Diagnostic Techniques for Improving Crop Production; Food Product Press an Imprint of the Haworth Press: New York, NY, USA, 1996. [Google Scholar]
- Coskun, D.; Deshmukh, R.; Shivaraj, S.M.; Isenring, P.; Bélanger, R.R. Lsi2: A black box in plant silicon transport. Plant Soil 2021, 466, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.S. Effect of soluble salts on water absorption by gel-forming soil conditioners. J. Sci. Food Agric. 1984, 35, 1063–1066. [Google Scholar] [CrossRef]
- Abedi-Koupai, J.; Sohrab, F.; Swarbrick, G. Evaluation of hydrogel application on soil water retention characteristics. J. Plant Nutr. 2008, 31, 317–331. [Google Scholar] [CrossRef]
- Dorraji, S.S.; Golchin, A.; Ahmadi, S. The effects of hydrophilic polymer and soil salinity on corn growth in sandy and loamy soils. Clean Soil Air Water 2010, 38, 584–591. [Google Scholar] [CrossRef]
- Smagin, A.V.; Budnikov, V.I.; Sadovnikova, N.B.; Smagina, M.V.; Sidorova, M.A. Water retention in different types of protective gel compositions for plant rhizosphere. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 368, p. 012049. [Google Scholar]
- Hosseini, S.H.; Niyungeko, C.; Khan, S.; Liang, X. Effects of superabsorbent polyacrylamide hydrogel and gypsum applications on colloidal phosphorus release from agricultural soils. J. Soils Sediments 2021, 21, 925–935. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Ashraf, M.F.M.R.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Berta, G.; Copetta, A.; Gamalero, E.; Bona, E.; Cesaro, P.; Scarafoni, A.; D′Agostino, G. Maize development and grain quality are differentially affected by mycorrhizal fungi and a growth-promoting pseudomonad in the field. Mycorrhiza 2014, 24, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Aalipour, H.; Nikbakht, A.; Etemadi, N. Co-inoculation of Arizona cypress with arbuscular mycorrhiza fungi and Pseudomonas fluorescens under fuel pollution. Mycorrhiza 2019, 29, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Rajasheker, G.; Jawahar, G.; Jalaja, N.; Kumar, S.A.; Kumari, P.H.; Punita, D.L.; Kishor, P.B.K. Role and regulation of osmolytes and ABA interaction in salt and drought stress tolerance. In Plant signaling molecules; Woodhead Publishing: Shaston, UK, 2019; pp. 417–436. [Google Scholar]
- Khan, N.; Bano, A.; Ali, S.; Babar, M. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.; Qiu, J.; Wang, H.; Wang, S.; Tang, L.; Zhang, J. Abscisic acid promotes jasmonic acid biosynthesis via a ‘SAPK10-bZIP72-AOC’pathway to synergistically inhibit seed germination in rice (Oryza sativa). New Phytol. 2020, 228, 1336–1353. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gopal, R.; Dube, B.K. Impact of iron stress on biomass, yield, metabolism and quality of potato (Solanum tuberosum L.). Sci. Hortic. 2006, 108, 1–6. [Google Scholar] [CrossRef]
- Berger, K.C.; Gerloff, G.C. Manganese toxicity of potatoes in relation to strong soil acidity. Soil Sci. Soc. Am. Proc. 1947, 12, 310–314. [Google Scholar] [CrossRef]
- Pathak, D.; Lone, R.; Khan, S.; Koul, K.K. Isolation, screening and molecular characterization of free-living bacteria of potato (Solanum tuberosum L.) and their interplay impact on growth and production of potato plant under Mycorrhizal association. Sci. Hortic. 2019, 252, 388–397. [Google Scholar] [CrossRef]
- Chifetete, V.W.; Dames, J.F. Mycorrhizal interventions for sustainable potato production in Africa. Front. Sustain. Food Syst. 2020, 4, 264. [Google Scholar] [CrossRef]
- Mir, R.A.; Bhat, B.A.; Yousuf, H.; Islam, S.T.; Raza, A.; Rizvi, M.A.; Zargar, S.M. Multidimensional Role of Silicon to Activate Resilient Plant Growth and to Mitigate Abiotic Stress. Front. Plant Sci. 2022, 13, 819658. [Google Scholar] [CrossRef]
- Dutt, S.; Manjul, A.S.; Raigond, P.; Singh, B.; Siddappa, S.; Bhardwaj, V.; Kardile, H.B. Key players associated with tuberization in potato: Potential candidates for genetic engineering. Crit. Rev. Biotechnol. 2017, 37, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Kolachevskaya, O.O.; Lomin, S.N.; Arkhipov, D.V.; Romanov, G.A. Auxins in potato: Molecular aspects and emerging roles in tuber formation and stress resistance. Plant Cell Rep. 2019, 38, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Abelenda, J.A.; Bergonzi, S.; Oortwijn, M.; Sonnewald, S.; Du, M.; Visser, R.G.; Bachem, C.W. Source-sink regulation is mediated by interaction of an FT homolog with a SWEET protein in potato. Curr. Biol. 2019, 29, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Manck-Götzenberger, J.; Requena, N. Arbuscular mycorrhiza symbiosis induces a major transcriptional reprogramming of the potato SWEET sugar transporter family. Front. Plant Sci. 2016, 7, 487. [Google Scholar] [CrossRef] [PubMed]
- Tejada, M.; Hernandez, M.T.; Garcia, C. Application of two organic amendments on soil restoration: Effects on the soil biological properties. J. Environ. Qual. 2006, 35, 1010–1017. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; McLaughlin, N.B.; Mi, J.; Chen, Q.; Liu, J. Effect of synthetic and natural water absorbing soil amendment soil physical properties under potato production in a semi-arid region. Soil Tillage Res. 2015, 148, 31–39. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Zhou, L.; Mi, J.; McLaughlin, N.B.; Liu, J. Effect of synthetic and natural water absorbing soil amendments on soil microbiological parameters under potato production in a semi-arid region. Eur. J. Soil Biol. 2016, 75, 8–14. [Google Scholar] [CrossRef]


| (A) SC Treatment | 100% WHC | 60% WHC | 20% WHC |
| Control | 35.2 n.s. | 21.1 n.s. | 7.0 n.s |
| SP | 37.5 n.s | 22.5 n.s | 7.5 n.s |
| GH | 37.5 n.s. | 22.5 n.s | 7.5 n.s |
| (B) SC Treatment | 100% WHC | 60% WHC | 20% WHC |
| Control | 39.3 n.s | 23.6 n.s | 7.9 n.s |
| SP | 39.9 n.s | 23.9 n.s | 8.0 n.s |
| AM+3Re27+SP | 38.7 n.s | 23.2 n.s | 7.7 n.s |
| GH | 38.9 n.s | 23.3 n.s | 7.8 n.s |
| AM+3Re27+GH | 38.7 n.s | 23.2 n.s | 7.7 n.s |
| Treatments | Tuber Biomass [g Plant−1] | Tuber Number [Plant−1] |
|---|---|---|
| Well watered | 216.2 ± 60.1 a | 25.5 ± 13.5 a |
| Drought | 121.6 ± 34.1 ab | 15.8 ± 4.3 a |
| Drought + GH | 79.9 ± 23.5 b | 10.5 ± 3.5 a |
| Drought + AM + 3Re2-7 | 213.8 ± 30.3 a | 9.5 ± 1.6 a |
| Drought + AM + 3Re2-7 + GH | 67.5 ± 24.4 b | 7.8 ± 0.9 a |
| WW | Drought | GH Drought | AM + 3Re2-7 Drought | AM +3 Re2-7 +GH Drought | |
|---|---|---|---|---|---|
| Total FW [g plant−1] | 490.8 a | 388.0 ab | 368.9 b | 522.0 a | 481.2 ab |
| Relative biomass distribution [%] | |||||
| Shoot | 49.1 | 50.8 | 61.3 | 48.3 | 70.4 |
| Tubers | 44.1 | 31.3 | 21.6 | 41.0 | 14.0 |
| Roots | 6.8 | 17.7 | 17.1 | 10.7 | 9.2 |
| Treatments | P | K | N | Mg | Mn | Zn | Cu |
|---|---|---|---|---|---|---|---|
| Deficiency Threshold | 2.5 mg·g−1 | 60 mg·g−1 | 30 mg·g−1 | 7.0 mg·g−1 | 30 mg·kg−1 | 30 mg·kg−1 | 3 mg·kg−1 |
| Well watered | 1.74 ± 0.18 a | 30.0 ± 3.0 c | 24.2 ± 4.1 c | 6.5 ± 0.4 ab | 27.96 ± 3.3 b | 55.8 ± 5.3 b | 14.8 ± 2.7 a |
| Drought | 1.86 ± 0.10 a | 37.3 ± 1.1 b | 35.5 ± 1.8 b | 6.6 ± 0.5 ab | 45.08 ± 2.6 b | 57.8 ± 5.1 b | 14.2 ± 0.4 a |
| Drought + GH | 2.00 ± 0.28 a | 49.3 ± 3.1 a | 43.2 ± 2.5 a | 7.4 ± 0.8 a | 419.42 ± 90.4 a | 86.8 ± 7.0 a | 16.1 ± 1.8 a |
| Drought + AM + 3Re2-7 | 1.66 ± 0.14 a | 32.5 ± 1.8 bc | 27.3 ± 1.0 c | 5.1 ± 0.4 b | 42.3 ± 8.3 b | 51.9 ± 2.6 b | 13.2 ± 0.5 a |
| Drought + AM + 3Re2-7+GH | 2.14 ± 0.14 a | 52.5 ± 2.0 a | 41.9 ± 1.6 ab | 6.4 ± 0.3 ab | 413.27 ± 50.1 a | 92.7 ± 11.4 a | 16.8 ± 1.5 a |
| WW Control | WW AM+3Re27 | WW SP+AM+3Re27 | Drought Control | AM+3Re27+SP Drought | |
|---|---|---|---|---|---|
| Total FW [g] | 187.1 bc | 244.6a | 212.9 ab | 132.3 c | 132.1 c |
| Relative biomass distribution [%] | |||||
| Shoot | 62.8 | 60.5 | 45.0 | 63.9 | 56.8 |
| Tubers | 28.0 | 25.2 | 45.6 | 17.5 | 31.6 |
| Roots | 9.2 | 14.3 | 9.4 | 18.6 | 11.5 |
| Irrigation | Variants | Root Biomass [g plant−1] | Root Length [m plant−1] | AM Colonization [%] | Extraradical Hyphae [%] | Vesicles [%] |
|---|---|---|---|---|---|---|
| Well watered | Control | 17.2 bc | 51.9 d | 16.7 c | 14.5 b | 8.5 b |
| AM+3Re27 | 29.5 a | 87.2 a | 27.3 ab | 17.5 ab | 12.5 ab | |
| AM+3Re27+SP | 20.1 abc | 59.6 cd | 33.8 a | 28.5 a | 18.5 a | |
| Drought | Control | 24.6 abc | 76.3 abc | 13.7 c | 11.5 b | 5.5 b |
| AM+3Re27+SP | 15.3 c | 64.0 bcd | 17.0 c | 14.0 b | 10.0 b |
| Treatments | P | K | N | Mg | Ca | Si | Mn | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| Deficiency Thresholds | 2.5 mg·g−1 | 60 mg·g−1 | 39 mg·g−1 | 7.0 mg·g−1 | 6 mg·g−1 | 30 mg·g−1 | 30 μg g−1 | 30 μg g−1 | |
| Well watered | Control | 2.81 ab | 45.8 ab | 20.6 bc | 3.4 ab | 11.1 b | 0.49 a | 15.0 c | 50.5 bc |
| AM+3Re27 | 2.75 b | 45.8 ab | 17.8 c | 3.1 abc | 12.8 ab | 0.46 a | 14.0 cd | 44.4 c | |
| AM+3Re27+SP | 2.76 b | 45.0 b | 13.7 d | 2.6 c | 14.7 ab | 0.63 a | 10.0 d | 52.9 abc | |
| Drought | Control | 3.36 a | 50.0 ab | 34.2 a | 3.6 a | 16.2 a | 0.58 a | 38.2 a | 56.2 ab |
| AM+3Re27+SP | 3.12 ab | 54.3 a | 22.0 b | 2.8 bc | 14.3 ab | 0.92 a | 30.9 b | 61.4 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamun, A.A.; Neumann, G.; Moradtalab, N.; Ahmed, A.; Nawaz, F.; Tenbohlen, T.; Feng, J.; Zhang, Y.; Xie, X.; Zhifang, L.; et al. Combination of Silicate-Based Soil Conditioners with Plant Growth-Promoting Microorganisms to Improve Drought Stress Resilience in Potato. Microorganisms 2024, 12, 2128. https://doi.org/10.3390/microorganisms12112128
Mamun AA, Neumann G, Moradtalab N, Ahmed A, Nawaz F, Tenbohlen T, Feng J, Zhang Y, Xie X, Zhifang L, et al. Combination of Silicate-Based Soil Conditioners with Plant Growth-Promoting Microorganisms to Improve Drought Stress Resilience in Potato. Microorganisms. 2024; 12(11):2128. https://doi.org/10.3390/microorganisms12112128
Chicago/Turabian StyleMamun, Abdullah Al, Günter Neumann, Narges Moradtalab, Aneesh Ahmed, Fahim Nawaz, Timotheus Tenbohlen, Jingyu Feng, Yongbin Zhang, Xiaochan Xie, Li Zhifang, and et al. 2024. "Combination of Silicate-Based Soil Conditioners with Plant Growth-Promoting Microorganisms to Improve Drought Stress Resilience in Potato" Microorganisms 12, no. 11: 2128. https://doi.org/10.3390/microorganisms12112128
APA StyleMamun, A. A., Neumann, G., Moradtalab, N., Ahmed, A., Nawaz, F., Tenbohlen, T., Feng, J., Zhang, Y., Xie, X., Zhifang, L., Ludewig, U., Bradáčová, K., & Weinmann, M. (2024). Combination of Silicate-Based Soil Conditioners with Plant Growth-Promoting Microorganisms to Improve Drought Stress Resilience in Potato. Microorganisms, 12(11), 2128. https://doi.org/10.3390/microorganisms12112128





