Effects of Different Dietary Combinations on Blood Biochemical Indicators and Rumen Microbial Ecology in Wenshan Cattle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Experimental Design
2.2. Sequencing and Data Analysis
3. Results
3.1. Growth Performance of Wenshan Cattle
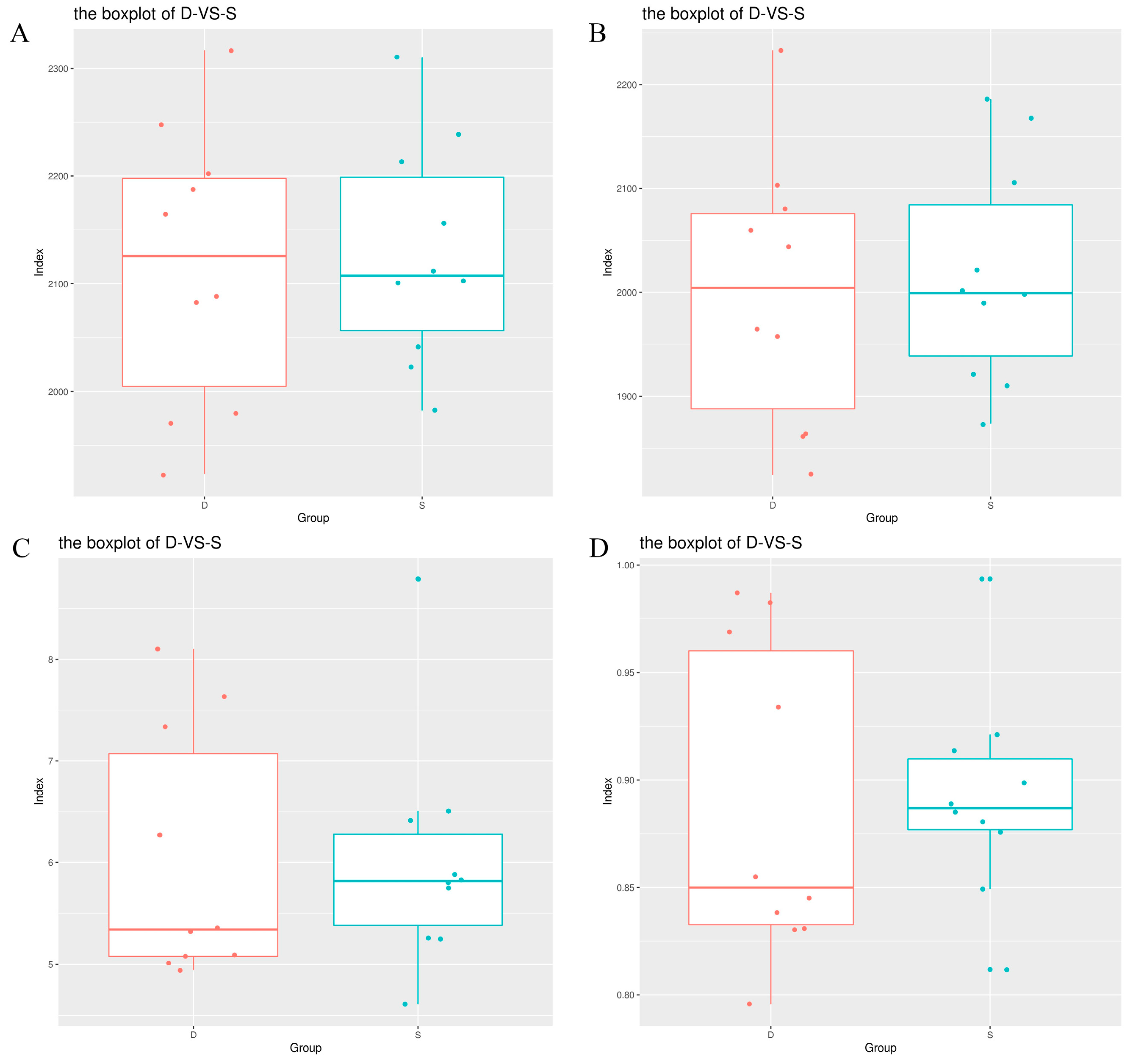
3.2. Blood Index of Wenshan Cattle
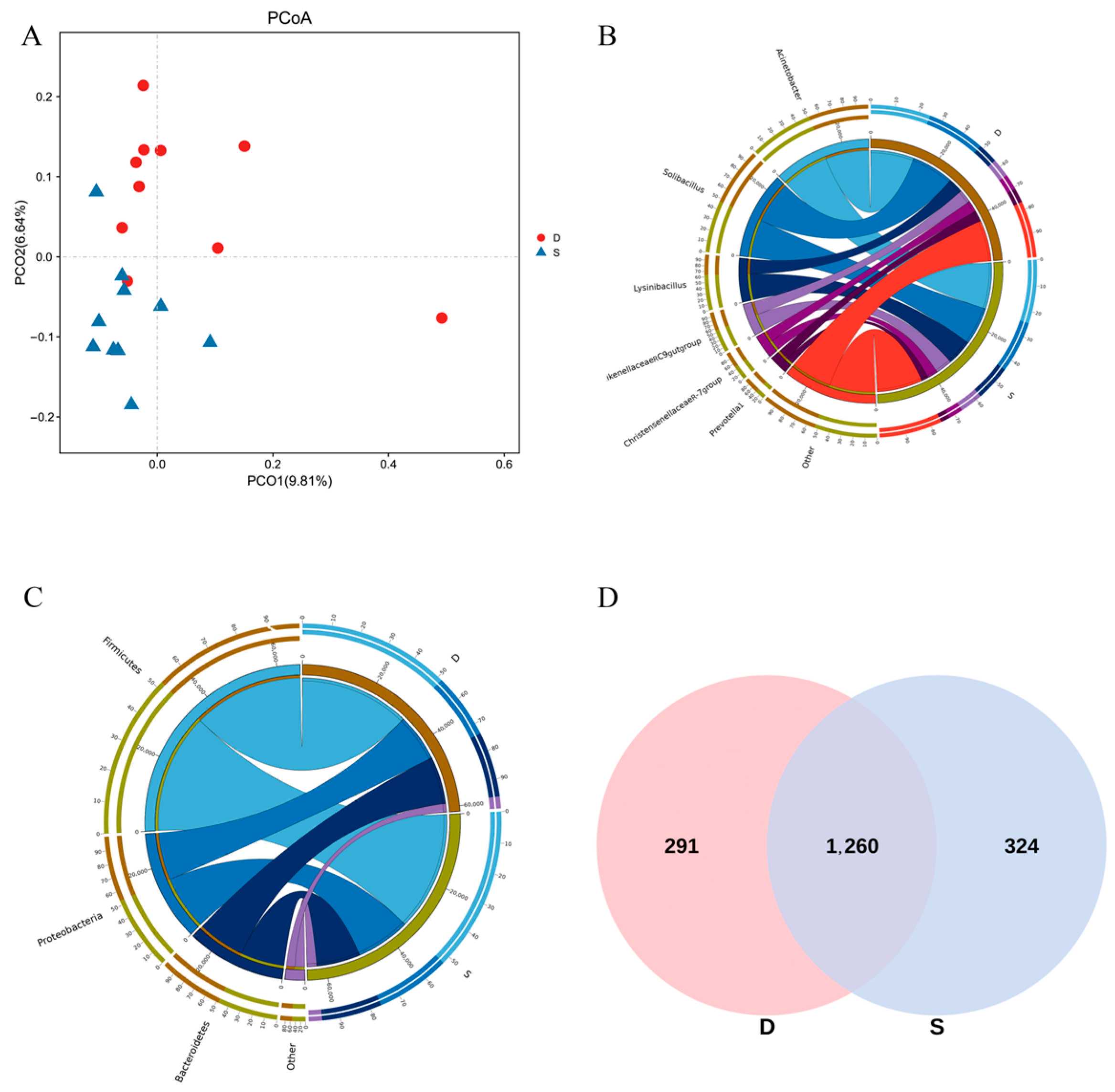
3.3. Rumen Microorganism
3.3.1. Quality Control of Rumen Microbial Original Sequencing Data
3.3.2. Analysis of Species Abundance Composition
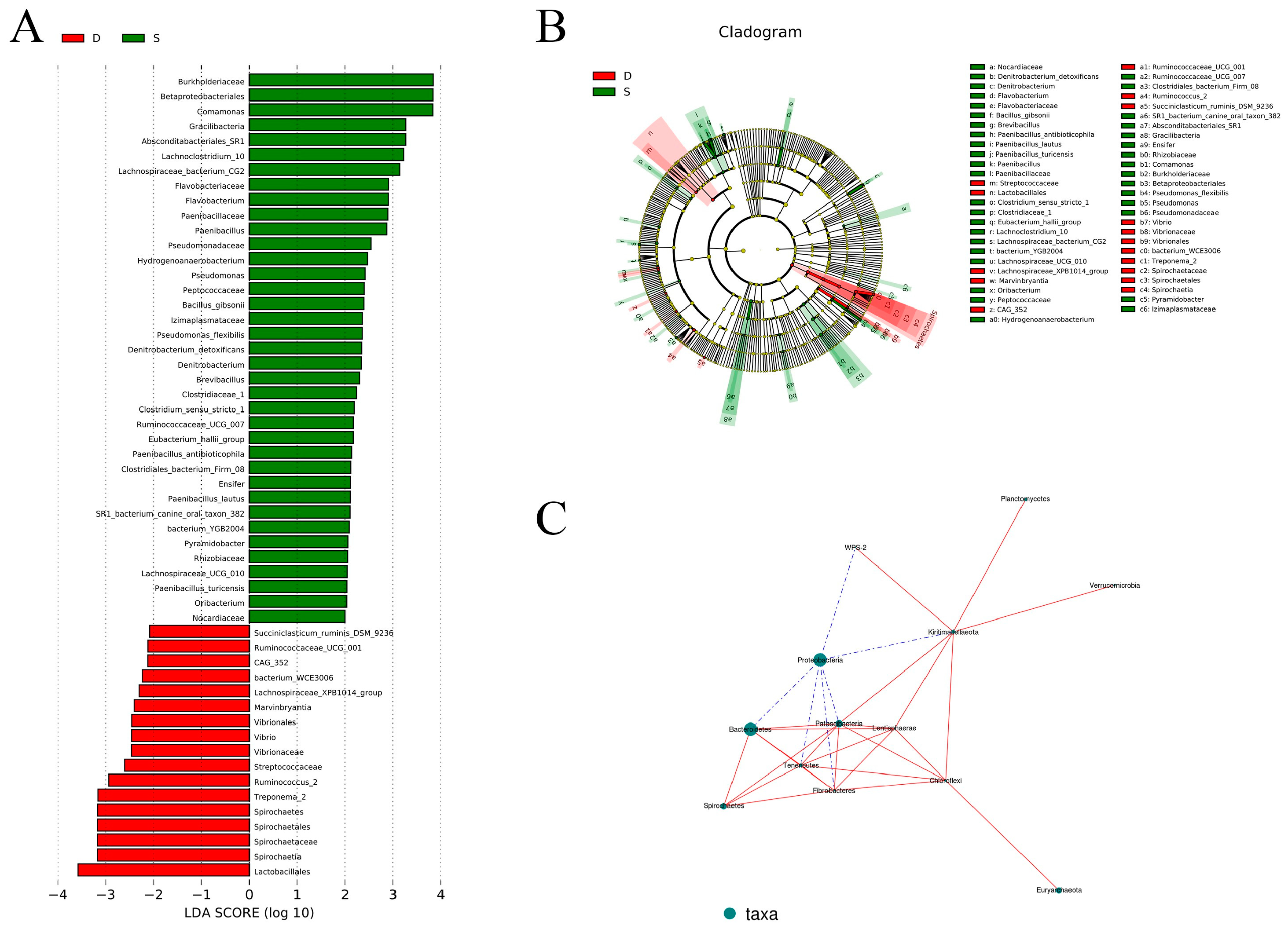
3.3.3. Analysis of Differential Microbial Composition
3.3.4. Analysis of Species Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groot, M.J.; Van’t Hooft, K.E. The Hidden Effects of Dairy Farming on Public and Environmental Health in the Netherlands, India, Ethiopia, and Uganda, Considering the Use of Antibiotics and Other Agro-chemicals. Front. Public Health 2016, 4, 12. [Google Scholar] [CrossRef]
- Goni, I.; Brenes, A.; Centeno, C.; Viveros, A.; Saura-Calixto, F.; Rebole, A.; Arija, I.; Estevez, R. Effect of dietary grape pomace and vitamin E on growth performance, nutrient digestibility, and susceptibility to meat lipid oxidation in chickens. Poult. Sci. 2007, 86, 508–516. [Google Scholar] [CrossRef]
- Alimi, N.; Assani, A.S.; Sanni Worogo, H.; Baco, N.M.; Traore, I.A. Livestock feed resources used as alternatives during feed shortages and their impact on the environment and ruminant performance in West Africa: A systematic review. Front. Vet. Sci. 2024, 11, 1352235. [Google Scholar] [CrossRef] [PubMed]
- Winkler, L.R.; Hasenbeck, A.; Murphy, K.M.; Hermes, J.C. Replacing Corn and Wheat in Layer Diets with Hulless Oats Shows Effects on Sensory Properties and Yolk Quality of Eggs. Front. Nutr. 2017, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jonsson, N.N.; McLaughlin, M.; Burchmore, R.; Johnson, P.C.D.; Jones, R.O.; McGill, S.; Brady, N.; Weidt, S.; Eckersall, P.D. Quantitative TMT-based proteomics revealing host, dietary and microbial proteins in bovine faeces including barley serpin Z4, a prominent component in the head of beer. J. Proteom. 2023, 285, 104941. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, W.; Guo, T.; Li, F.; Li, F.; Ran, T.; Zhang, Z.; Guo, L. Effects of Dietary Barley Starch Contents on the Performance, Nutrient Digestion, Rumen Fermentation, and Bacterial Community of Fattening Hu Sheep. Front. Nutr. 2021, 8, 797801. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Zhao, Q.; Tang, C.; Zhang, J.; Qin, Y. Fermented Soy and Fish Protein Dietary Sources Shape Ileal and Colonic Microbiota, Improving Nutrient Digestibility and Host Health in a Piglet Model. Front. Microbiol. 2022, 13, 911500. [Google Scholar] [CrossRef]
- He, Z.; Liu, S.; Wen, X.; Cao, S.; Zhan, X.; Hou, L.; Li, Y.; Chen, S.; Zheng, H.; Deng, D.; et al. Effect of mixed meal replacement of soybean meal on growth performance, nutrient apparent digestibility, and gut microbiota of finishing pigs. Front. Vet. Sci. 2024, 11, 1321486. [Google Scholar] [CrossRef]
- Maxin, G.; Ouellet, D.R.; Lapierre, H. Effect of substitution of soybean meal by canola meal or distillers grains in dairy rations on amino acid and glucose availability. J. Dairy Sci. 2013, 96, 7806–7817. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, F.; Ma, X.; Li, F.; Wang, Z. Effects of Barley Starch Level in Diet on Fermentation and Microflora in Rumen of Hu Sheep. Animals 2022, 12, 1941. [Google Scholar] [CrossRef]
- Silveira, C.; Oba, M.; Yang, W.Z.; Beauchemin, K.A. Selection of barley grain affects ruminal fermentation, starch digestibility, and productivity of lactating dairy cows. J. Dairy Sci. 2007, 90, 2860–2869. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, S.; Imam, K.; Sun, L.; Wang, Y.; Gu, T.; Wen, B.; Xin, F. The Effect of Xylooligosaccharide, Xylan, and Whole Wheat Bran on the Human Gut Bacteria. Front. Microbiol. 2020, 11, 568457. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xu, H.J.; Ma, G.M.; Sun, Y.K.; Li, Y.; Zhang, Y.G. Digestibility, lactation performance, plasma metabolites, ruminal fermentation, and bacterial communities in Holstein cows fed a fermented corn gluten-wheat bran mixture as a substitute for soybean meal. J. Dairy Sci. 2021, 104, 2866–2880. [Google Scholar] [CrossRef]
- Rehemujiang, H.; Yusuf, H.A.; Ma, T.; Diao, Q.; Kong, L.; Kang, L.; Tu, Y. Fermented cottonseed and rapeseed meals outperform soybean meal in improving performance, rumen fermentation, and bacterial composition in Hu sheep. Front. Microbiol. 2023, 14, 1119887. [Google Scholar] [CrossRef]
- Goiri, I.; Zubiria, I.; Lavin, J.L.; Benhissi, H.; Atxaerandio, R.; Ruiz, R.; Mandaluniz, N.; Garcia-Rodriguez, A. Evaluating the Inclusion of Cold-Pressed Rapeseed Cake in the Concentrate for Dairy Cows upon Ruminal Biohydrogenation Process, Ruminal Microbial Community and Milk Production and Acceptability. Animals 2021, 11, 2553. [Google Scholar] [CrossRef]
- Agwa, H.M.M.; Saleh, H.M.; Ayyat, M.S.; Abdel-Rahman, G.A. Effect of replacing cottonseed meal with canola meal on growth performance, blood metabolites, thyroid function, and ruminal parameters of growing lambs. Trop. Anim. Health Prod. 2023, 55, 122. [Google Scholar] [CrossRef]
- Li, B.; Sun, X.; Huo, Q.; Zhang, G.; Wu, T.; You, P.; He, Y.; Tian, W.; Li, R.; Li, C.; et al. Pelleting of a Total Mixed Ration Affects Growth Performance of Fattening Lambs. Front. Vet. Sci. 2021, 8, 629016. [Google Scholar] [CrossRef]
- Soltani, E.; Naserian, A.A.; Khan, M.A.; Ghaffari, M.H.; Malekkhahi, M. Effects of conditioner retention time during pelleting of starter feed on nutrient digestibility, ruminal fermentation, blood metabolites, and performance of Holstein female dairy calves. J. Dairy Sci. 2020, 103, 8910–8921. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, X.; Li, G.; Lin, M.; Huo, Y.; Li, S.; Zhao, G. Effects of dietary alfalfa flavonoids extraction on growth performance, organ development and blood biochemical indexes of Yangzhou geese aged from 28 to 70 days. Anim. Nutr. 2016, 2, 318–322. [Google Scholar] [CrossRef]
- Hinds, T.D., Jr.; Creeden, J.F.; Gordon, D.M.; Stec, D.F.; Donald, M.C.; Stec, D.E. Bilirubin Nanoparticles Reduce Diet-Induced Hepatic Steatosis, Improve Fat Utilization, and Increase Plasma beta-Hydroxybutyrate. Front. Pharmacol. 2020, 11, 594574. [Google Scholar] [CrossRef]
- Reis, L.G.; Silva, T.H.D.; Salles, M.S.V.; Andrade, A.F.C.; Martins, S.; Takeuchi, P.L.; Vidal, A.M.C.; Netto, A.S. Effect of cow’s milk with different PUFA n-6: N-3 ratios on performance, serum lipid profile, and blood parameters of grower gilts. PLoS ONE 2022, 17, e0258629. [Google Scholar] [CrossRef] [PubMed]
- Noruzi, S.; Torki, M.; Mohammadi, H. Effects of supplementing diet with Thyme (Thymuas vulgaris L.) essential oil and/or selenium yeast on production performance and blood variables of broiler chickens. Vet. Med. Sci. 2022, 8, 1137–1145. [Google Scholar] [CrossRef]
- Muller, M.; Canfora, E.E.; Blaak, E.E. Gastrointestinal Transit Time, Glucose Homeostasis and Metabolic Health: Modulation by Dietary Fibers. Nutrients 2018, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Zebeli, Q.; Aschenbach, J.R.; Tafaj, M.; Boguhn, J.; Ametaj, B.N.; Drochner, W. Invited review: Role of physically effective fiber and estimation of dietary fiber adequacy in high-producing dairy cattle. J. Dairy Sci. 2012, 95, 1041–1056. [Google Scholar] [CrossRef]
- Krause, D.O.; Denman, S.E.; Mackie, R.I.; Morrison, M.; Rae, A.L.; Attwood, G.T.; McSweeney, C.S. Opportunities to improve fiber degradation in the rumen: Microbiology, ecology, and genomics. FEMS Microbiol. Rev. 2003, 27, 663–693. [Google Scholar] [CrossRef]
- Xu, Q.; Ungerfeld, E.M.; Morgavi, D.P.; Waters, S.M.; Liu, J.; Du, W.; Zhao, S. Editorial: Rumen microbiome: Interacting with host genetics, dietary nutrients metabolism, animal production, and environment. Front. Microbiol. 2023, 14, 1267149. [Google Scholar] [CrossRef]
- Chen, P.; Li, Y.; Wang, M.; Shen, Y.; Liu, M.; Xu, H.; Ma, N.; Cao, Y.; Li, Q.; Abdelsattar, M.M.; et al. Optimizing dietary rumen-degradable starch to rumen-degradable protein ratio improves lactation performance and nitrogen utilization efficiency in mid-lactating Holstein dairy cows. Front. Vet. Sci. 2024, 11, 1330876. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Y.; Rahman, A.; Chen, M.; Li, N.; Wu, T.; Qi, Y.; Zheng, N.; Zhao, S.; Wang, J. Rumen microbiota succession throughout the perinatal period and its association with postpartum production traits in dairy cows: A review. Anim. Nutr. 2024, 18, 17–26. [Google Scholar] [CrossRef]
- Chuang, S.T.; Ho, S.T.; Tu, P.W.; Li, K.Y.; Kuo, Y.L.; Shiu, J.S.; Wang, S.Y.; Chen, M.J. The Rumen Specific Bacteriome in Dry Dairy Cows and Its Possible Relationship with Phenotypes. Animals 2020, 10, 1791. [Google Scholar] [CrossRef]
- Xu, S.Y.; Feng, X.R.; Zhao, W.; Bi, Y.L.; Diao, Q.Y.; Tu, Y. Rumen and hindgut microbiome regulate average daily gain of preweaning Holstein heifer calves in different ways. Microbiome 2024, 12, 131. [Google Scholar] [CrossRef]
- Jiang, F.; Gao, Y.; Peng, Z.; Ma, X.; You, Y.; Hu, Z.; He, A.; Liao, Y. Isoacids supplementation improves growth performance and feed fiber digestibility associated with ruminal bacterial community in yaks. Front. Microbiol. 2023, 14, 1175880. [Google Scholar] [CrossRef] [PubMed]
- Rabee, A.E.; Mohamed, M.G.M.; Sallam, A.; Elwakeel, E.A.; Mohammed, R.S.; Sabra, E.A.; Abdel-Wahed, A.M.; Mourad, D.M.; Hamed, A.A.; Hafez, O.R. Rumen fermentation and microbiota in Shami goats fed on condensed tannins or herbal mixture. BMC Vet. Res. 2024, 20, 35. [Google Scholar] [CrossRef]
- Martinez Boggio, G.; Monteiro, H.F.; Lima, F.S.; Figueiredo, C.C.; Bisinotto, R.S.; Santos, J.E.P.; Mion, B.; Schenkel, F.S.; Ribeiro, E.S.; Weigel, K.A.; et al. Host and rumen microbiome contributions to feed efficiency traits in Holstein cows. J. Dairy Sci. 2024, 107, 3090–3103. [Google Scholar] [CrossRef]
- Russell, J.B.; Rychlik, J.L. Factors that alter rumen microbial ecology. Science 2001, 292, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Plaizier, J.C.; Li, S.; Danscher, A.M.; Derakshani, H.; Andersen, P.H.; Khafipour, E. Changes in Microbiota in Rumen Digesta and Feces Due to a Grain-Based Subacute Ruminal Acidosis (SARA) Challenge. Microb. Ecol. 2017, 74, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Elmhadi, M.E.; Ali, D.K.; Khogali, M.K.; Wang, H. Subacute ruminal acidosis in dairy herds: Microbiological and nutritional causes, consequences, and prevention strategies. Anim. Nutr. 2022, 10, 148–155. [Google Scholar] [CrossRef]
- Gorka, P.; Kowalski, Z.M.; Zabielski, R.; Guilloteau, P. Invited review: Use of butyrate to promote gastrointestinal tract development in calves. J. Dairy Sci. 2018, 101, 4785–4800. [Google Scholar] [CrossRef]
- Ma, L.; Yang, Y.; Liu, W.; Bu, D. Sodium butyrate supplementation impacts the gastrointestinal bacteria of dairy calves before weaning. Appl. Microbiol. Biotechnol. 2023, 107, 3291–3304. [Google Scholar] [CrossRef]
- Liu, H.; Han, X.; Zhao, N.; Hu, L.; Wang, X.; Luo, C.; Chen, Y.; Zhao, X.; Xu, S. The Gut Microbiota Determines the High-Altitude Adaptability of Tibetan Wild Asses (Equus kiang) in Qinghai-Tibet Plateau. Front. Microbiol. 2022, 13, 949002. [Google Scholar] [CrossRef]
- Song, Y.; Sun, L.; Zhang, S.; Fan, K.; Wang, H.; Shi, Y.; Shen, Y.; Wang, W.; Zhang, J.; Han, X.; et al. Enzymes and microorganisms jointly promote the fermentation of rapeseed cake. Front. Nutr. 2022, 9, 989410. [Google Scholar] [CrossRef]
- Jami, E.; White, B.A.; Mizrahi, I. Potential role of the bovine rumen microbiome in modulating milk composition and feed efficiency. PLoS ONE 2014, 9, e85423. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.S.; Soundharrajan, I.; Kim, D.; Baik, M.; Ha, S.; Choi, K.C. Microbiota and Serum Metabolic Profile Changes in Korean Native Hanwoo Steer in Response to Diet Feeding Systems. Int. J. Mol. Sci. 2022, 23, 2391. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.R. Supplementation Strategies for Growing and Finishing Beef Cattle on Tall Fescue Pastures in the Southeast; Virginia Tech: Blacksburg, VA, USA, 2024. [Google Scholar]
| Ingredients | Component Ratio (%) | |
|---|---|---|
| Control Group | Experimental Group | |
| Corn | 60 | 30 |
| Soybean meal | 12 | 5 |
| Rapeseed meal | 0 | 6 |
| barley | 0 | 40 |
| White sugar | 0 | 2 |
| Wheat bran | 0 | 10 |
| Calcium hydrogen phosphate | 1 | 0.75 |
| salt | 1 | 0.5 |
| Sodium carbonate | 1 | 0.75 |
| additive | 0 | 2 |
| Vegetable oil | 0 | 3 |
| Concentrate feed | 15 | 0 |
| molasses | 10 | 0 |
| total | 100 | 100 |
| Digestible energy DE (MJ/Kg) | 13.73 | 13.55 |
| Crude protein (CP) | 16.22 | 13.12 |
| Ether extract (EE) | 2.4 | 2.33 |
| Neutral detergent fibre (NDF) | 7.21 | 15.77 |
| Acid detergent fibre (ADF) | 2.77 | 6.32 |
| Calcium (Ca) | 0.21 | 0.29 |
| phosphorus (P) | 0.31 | 0.4 |
| Index | Item | Control Group | Experimental Group |
|---|---|---|---|
| Body weight (Kg) | Before the experiment | 188.86 ± 56.69 | 203.64 ± 12.95 |
| After the experiment | 293.71 ± 52.03 | 311.57 ± 30.44 | |
| Gain weight | 104.86 ± 14.31 | 107.92 ± 29.96 | |
| Average daily gain | 0.8729 ± 0.12 | 0.90 ± 0.25 | |
| Body height (cm) | Before the experiment | 116 ± 3.05 | 117.86 ± 3.13 |
| After the experiment | 118.43 ± 3.55 | 121.00 ± 2.77 | |
| Gain | 2.43 ± 1.62 | 3.14 ± 1.95 | |
| Average growth | 0.02 ± 0.01 | 0.03 ± 0.02 | |
| Body length (cm) | Before the experiment | 117.86 ± 9.79 | 123.14 ± 4.63 |
| After the experiment | 125.86 ± 8.93 | 133.85 ± 11.28 | |
| Gain | 8.00 ± 7.90 | 10.71 ± 9.27 | |
| Average growth | 0.07 ± 0.07 | 0.09 ± 0.08 | |
| Chest circumference (cm) | Before the experiment | 141.00 ± 13.51 | 149.71 ± 5.40 |
| After the experiment | 168.57 ± 7.63 | 170.00 ± 4.16 | |
| Gain | 22.57 ± 8.94 | 20.29 ± 4.42 | |
| Average growth | 0.02 ± 0.008 | 0.02 ± 0.01 | |
| Canno round (cm) | Before the experiment | 15.29 ± 1.80 | 14.57 ± 0.98 |
| After the experiment | 14.43 ± 0.53 | 15.14 ± 0.38 * | |
| Gain | 0.86 ± 1.57 | 0.57 ± 0.98 | |
| Average growth | 0.009 ± 0.01 | 0.006 ± 0.01 | |
| Dry matter(Kg) | Intake | 5.13 ± 0.15 | 5.27 ± 0.18 |
| Index | Control Group | Experimental Group | Normal Range |
|---|---|---|---|
| Glutamyl transpeptidase (GGT) (U/L) | 25.36 ± 21.17 | 16.51 ± 3.84 | / |
| Alanine aminotransferase (ALT) (U/L) | 28.08 ± 6.02 | 35.98 ± 5.20 ** | 15–70 |
| Alkaline phosphatase (ALP) (U/L) | 6.98 ± 6.33 | 4.06 ± 2.96 | 18–153 |
| Aspartate transaminase (AST) (U/L) | 78.22 ± 19.52 | 76.81 ± 14.01 | 45–110 |
| Aspartate transaminase/Alanine aminotransferase(S/L) (Ratio) | 2.82 ± 0.68 | 2.16 ± 0.39 * | 0.8–1.2 |
| Serum albumin (ALB) (g/L) | 36.58 ± 1.51 | 37.78 ± 3.13 | 21–36 |
| Globulin (GLOB) (g/L) | 40.79 ± 5.25 | 43.09 ± 8.56 | 34–48.5 |
| Total protein (TP) (g/L) | 77.37 ± 5.53 | 80.87 ± 6.40 | 57–81 |
| Indirect bilirubin (IBIL) (μmol) | 1.56 ± 0.64 | 2.58 ± 0.57 ** | 3–17 |
| Direct bilirubin (DBIL) (μmol/L) | 1.35 ± 0.41 | 1.99 ± 0.43 ** | 0.7–6.8 |
| total bilirubin (TBIL) (μmol/L) | 2.92 ± 0.85 | 4.57 ± 0.95 ** | 0.17–17.1 |
| Triglyceride (TG) (mmol/L) | 0.37 ± 0.32 | 0.29 ± 0.10 | 0.23–0.57 |
| Low-density lipoprotein cholesterol (LDL-CH) (mmol/L) | 1.64 ± 0.64 | 0.97 ± 0.20 ** | 0.65–1.81 |
| High-density lipoprotein cholesterol (HDL-CH) (mmol/L) | 2.43 ± 0.37 | 2.02 ± 0.36 * | 0.4–2.6 |
| CholesterolCHOL) (mmol/L) | 4.91 ± 1.51 | 3.33 ± 0.47 ** | 3.1–5.69 |
| Urea (UREA) (mmol/L) | 3.95 ± 0.63 | 5.38 ± 0.73 ** | 2.8–7.5 |
| Glucose (GLU) (mmol/L) | 7.30 ± 4.14 | 6.76 ± 2.28 | 3.9–5.5 |
| Item | Group | p | |
|---|---|---|---|
| Control | Experimental | ||
| Firmicutes | 50.913 ± 10.753 | 52.560 ± 8.941 | 0.703 |
| Proteobacteria | 20.893 ± 12.835 | 23.101 ± 13.152 | 0.709 |
| Bacteroidetes | 23.207 ± 12.541 | 19.337 ± 7.692 | 0.416 |
| Patescibacteria | 1.201 ± 0.556 | 1.345 ± 1.246 | 0.743 |
| Spirochaetes | 1.004 ± 0.399 | 0.671 ± 0.201 * | 0.030 |
| Euryarchaeota | 0.705 ± 1.007 | 0.824 ± 0.823 | 0.776 |
| Actinobacteria | 0.445 ± 0.583 | 0.455 ± 0.338 | 0.963 |
| Tenericutes | 0.167 ± 0.084 | 0.230 ± 0.080 | 0.106 |
| Kiritimatiellaeota | 0.273 ± 0.158 | 0.331 ± 0.227 | 0.511 |
| Planctomycetes | 0.758 ± 0.290 | 0.692 ± 0.165 | 0.538 |
| Item | Group | p | |
|---|---|---|---|
| Control | Experimental | ||
| Acinetobacter | 20.393 ± 12.953 | 20.940 ± 13.636 | 0.928 |
| Solibacillus | 19.993 ± 17.095 | 17.505 ± 13.675 | 0.724 |
| Lysinibacillus | 7.031 ± 6.575 | 12.135 ± 9.245 | 0.172 |
| Rikenellaceae_RC9_gut_group | 6.690 ± 1.963 | 7.794 ± 2.696 | 0.309 |
| Christensenellaceae_R-7_group | 5.719 ± 1.778 | 4.943 ± 1.025 | 0.248 |
| Prevotella_1 | 5.859 ± 4.826 | 3.163 ± 1.310 | 0.105 |
| Succiniclasticum | 2.602 ± 1.960 | 1.731 ± 0.964 | 0.223 |
| Saccharofermentans | 1.660 ± 0.688 | 1.957 ± 0.472 | 0.275 |
| Ruminococcaceae_NK4A214_group | 1.497 ± 0.678 | 1.658 ± 1.069 | 0.693 |
| Candidatus_Saccharimonas | 1.014 ± 0.559 | 0.837 ± 0.672 | 0.530 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; He, X.; Lu, Y.; Gao, Z.; Chong, Y.; Hong, J.; Wu, J.; Deng, W.; Xi, D. Effects of Different Dietary Combinations on Blood Biochemical Indicators and Rumen Microbial Ecology in Wenshan Cattle. Microorganisms 2024, 12, 2154. https://doi.org/10.3390/microorganisms12112154
Wu D, He X, Lu Y, Gao Z, Chong Y, Hong J, Wu J, Deng W, Xi D. Effects of Different Dietary Combinations on Blood Biochemical Indicators and Rumen Microbial Ecology in Wenshan Cattle. Microorganisms. 2024; 12(11):2154. https://doi.org/10.3390/microorganisms12112154
Chicago/Turabian StyleWu, Dongwang, Xiaoming He, Ying Lu, Zhendong Gao, Yuqing Chong, Jieyun Hong, Jiao Wu, Weidong Deng, and Dongmei Xi. 2024. "Effects of Different Dietary Combinations on Blood Biochemical Indicators and Rumen Microbial Ecology in Wenshan Cattle" Microorganisms 12, no. 11: 2154. https://doi.org/10.3390/microorganisms12112154
APA StyleWu, D., He, X., Lu, Y., Gao, Z., Chong, Y., Hong, J., Wu, J., Deng, W., & Xi, D. (2024). Effects of Different Dietary Combinations on Blood Biochemical Indicators and Rumen Microbial Ecology in Wenshan Cattle. Microorganisms, 12(11), 2154. https://doi.org/10.3390/microorganisms12112154






