Mimicking Urinary Tract Infections Caused by Uropathogenic Escherichia coli Using a Human Three-Dimensional Tissue Engineering Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Bacterial Strains and Culture
2.3. Cells
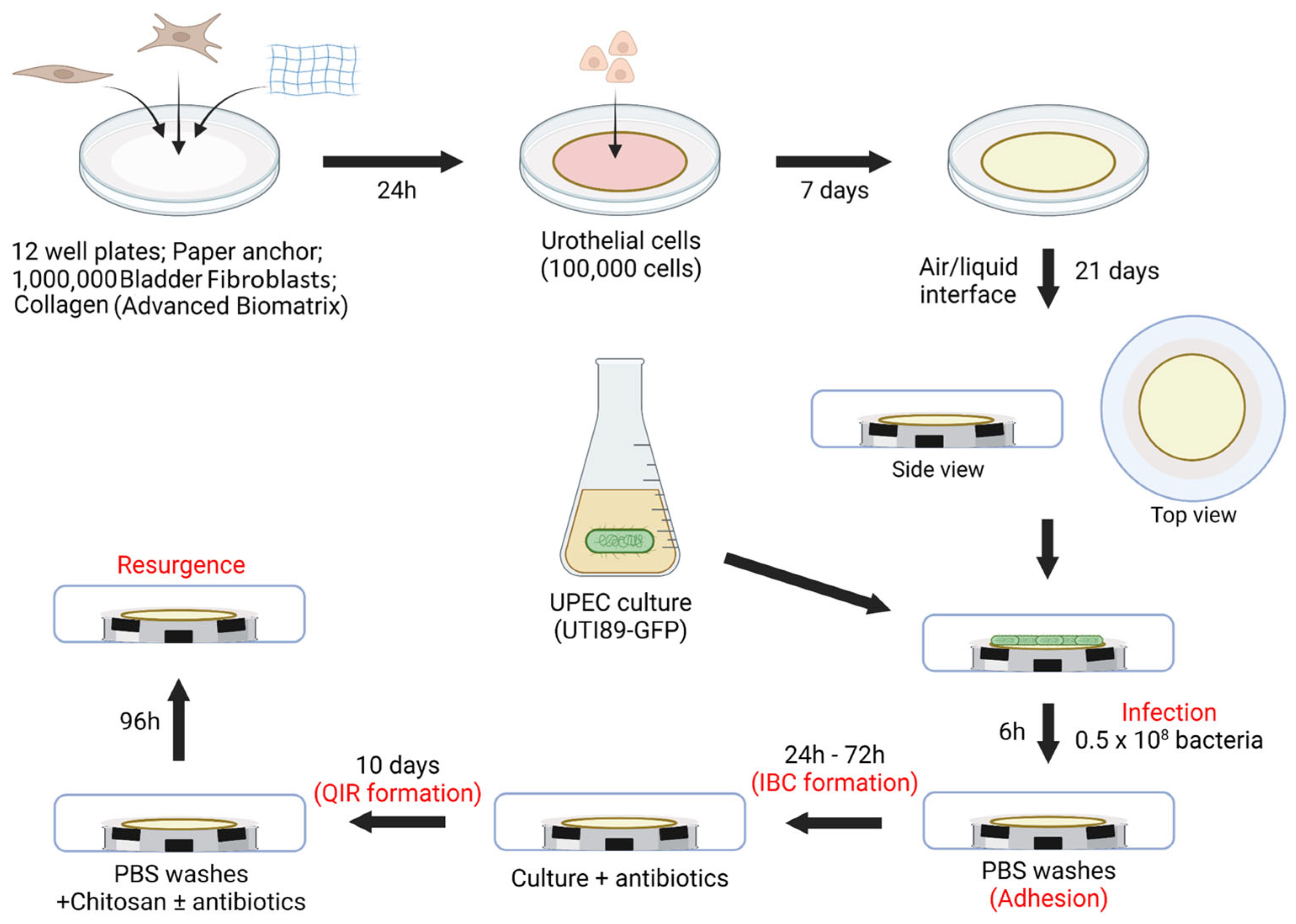
2.4. Three-Dimensional Tissue-Engineered Bladder Mucosa Substitute (Figure 2)
2.5. Histology
2.6. Evaluation of Mannose Coverage
2.7. Infection (Figure 2)
2.8. Scanning Electron Microscopy
2.9. Immunofluorescences
3. Results
3.1. Establishment of the Growth Curve for UPEC UTI-89-GFP and Non-Pathogenic Control BL21-GFP Strains
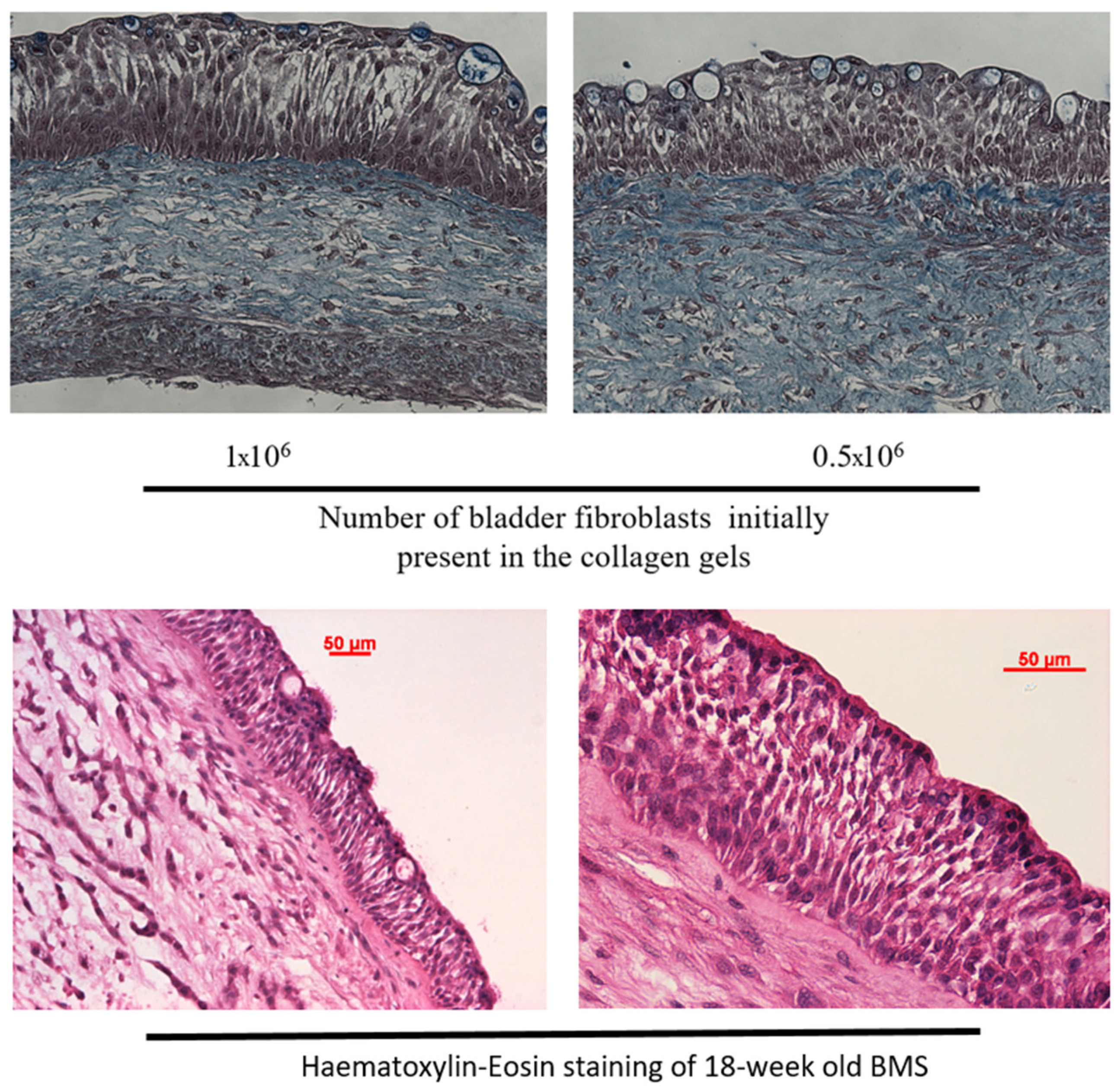
3.2. Histological Characterization of the Bladder Mucosa Substitute (BMS) Reconstructed by Tissue Engineering
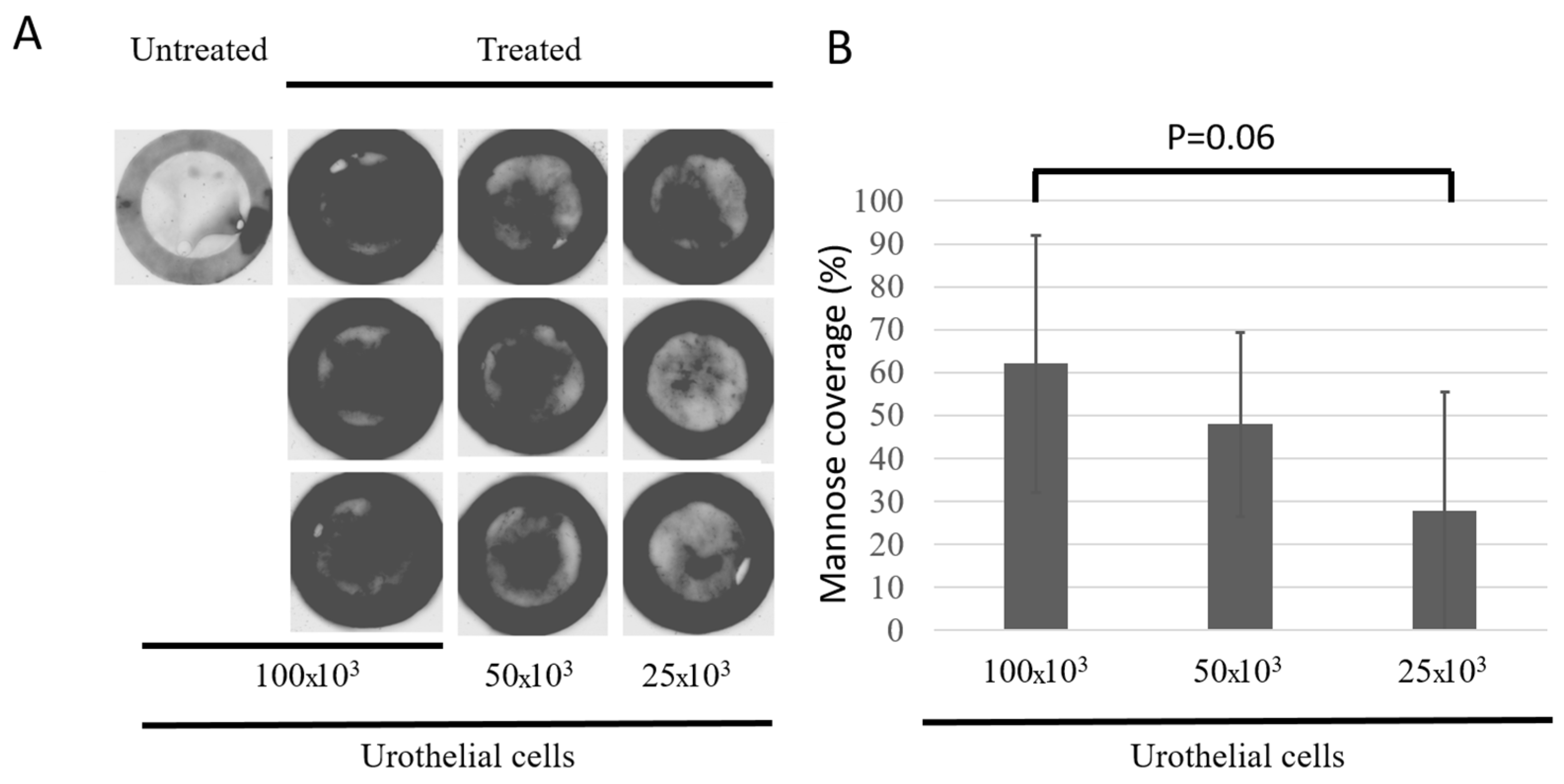
3.3. Characterization of the Urothelium Maturation Using Mannose Presence as a Marker
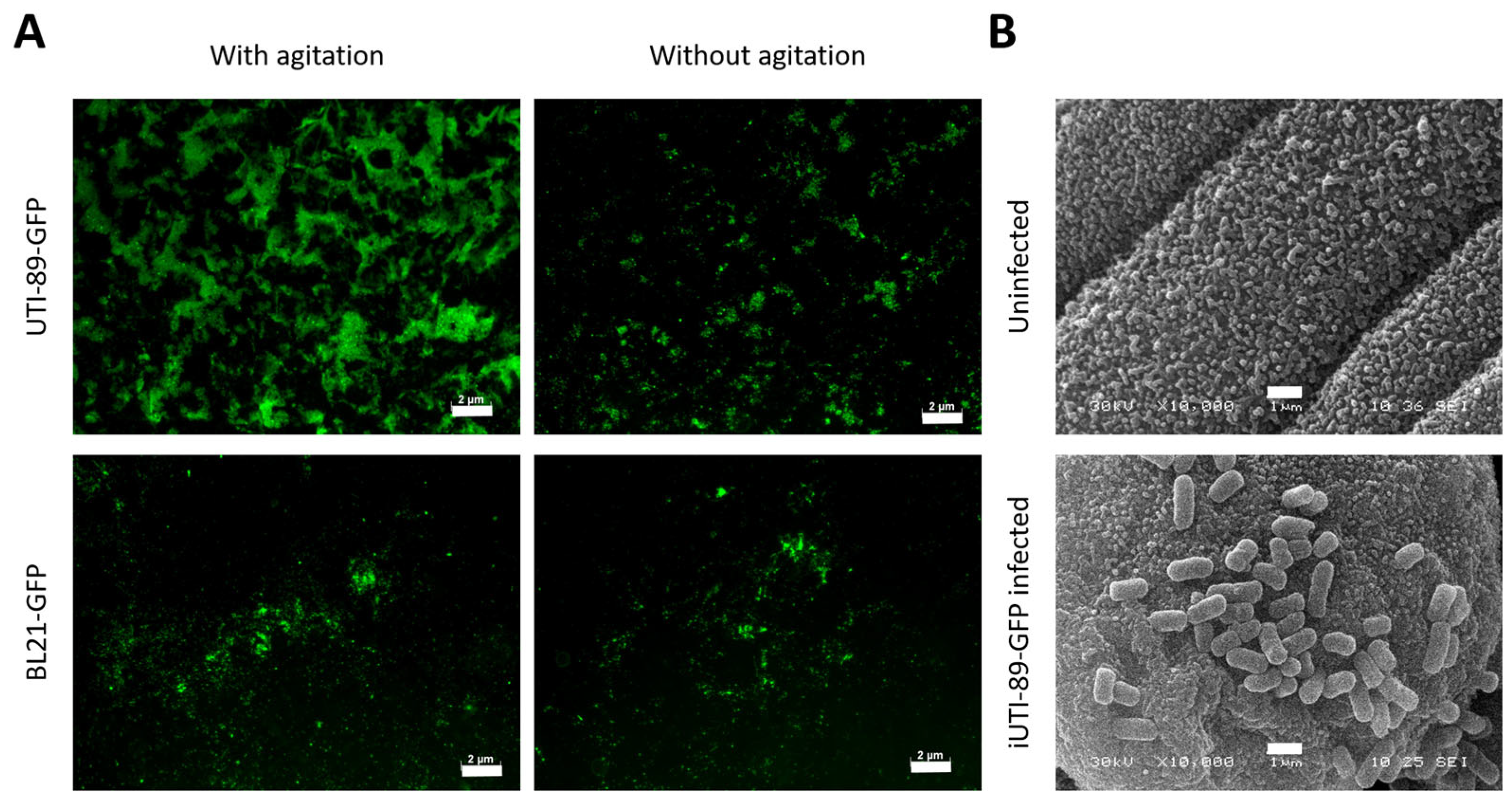
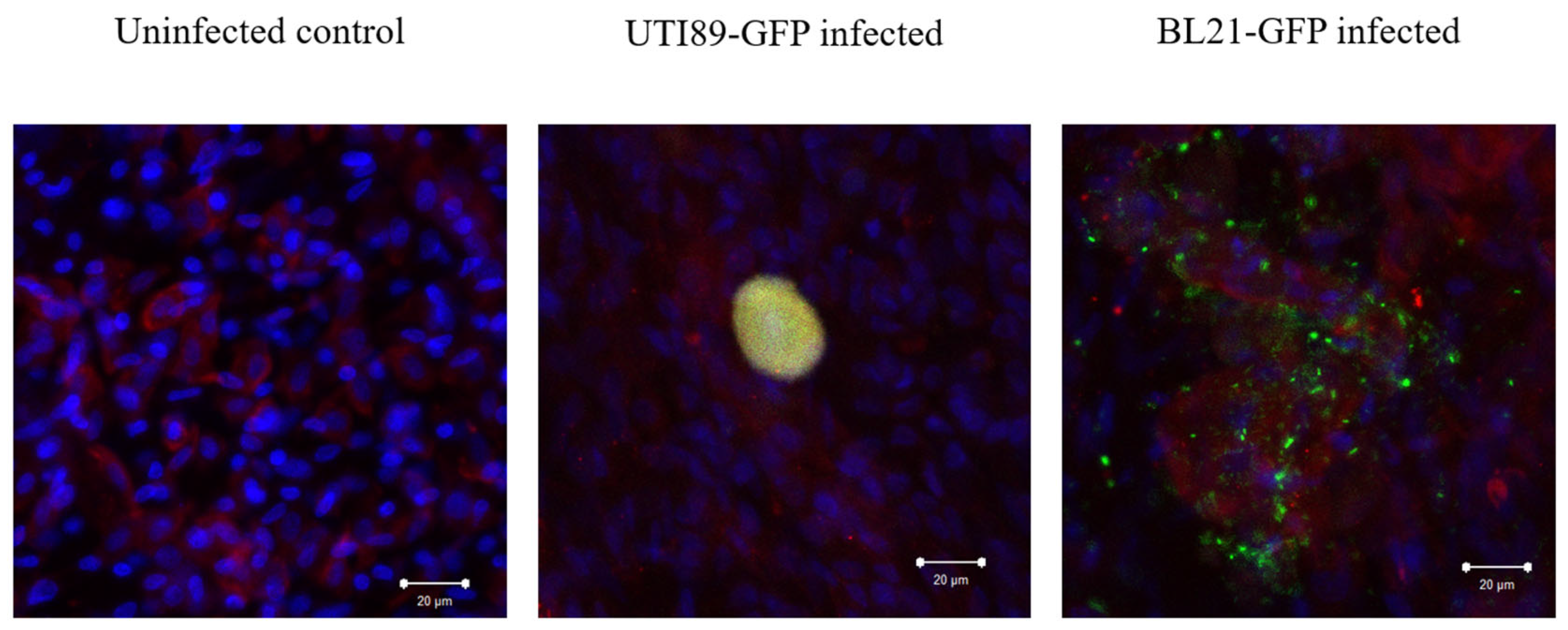
3.4. Adhesion of UPEC on the Urothelial Cells
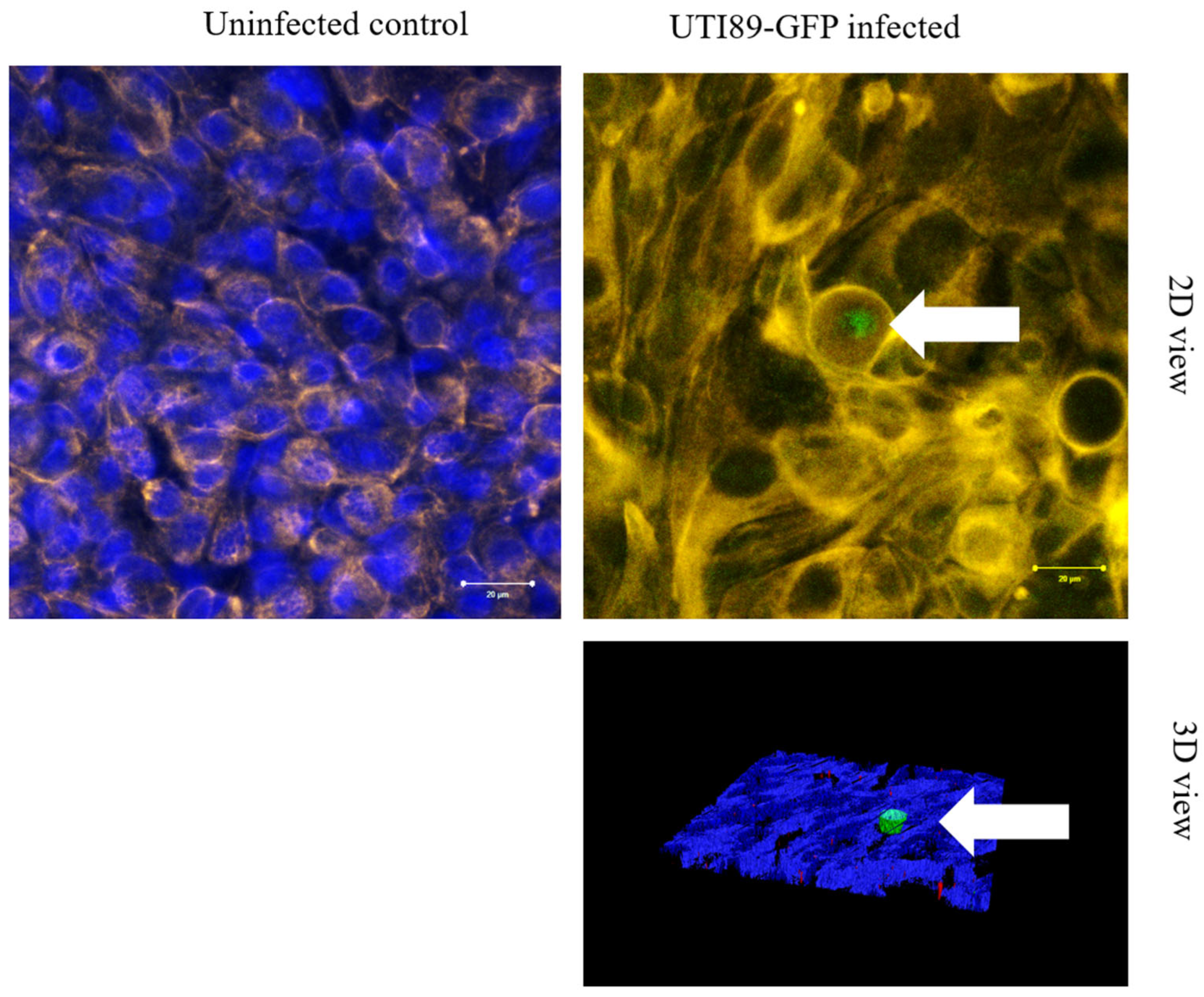
3.5. Invasion of Umbrella Cell Step with the Formation of IBC
3.6. Persistence of UTI89-GFP After Two Weeks of Antibiotic Treatment
3.7. Forced Resurgence of UTI89-GFP from QIR After Chitosan Treatment Can Be Inhibited by Antibiotic Treatment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Gillespie, B.; Koopman, J.; Zhang, L.; Palin, K.; Tallman, P.; Marsh, J.V.; Spear, S.; Sobel, J.D.; Marty, M.J.; et al. Risk factors for second urinary tract infection among college women. Am. J. Epidemiol. 2000, 151, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Mehnert-Kay, S.A. Diagnosis and management of uncomplicated urinary tract infections. Am. Fam. Physician 2005, 72, 451–456. [Google Scholar] [PubMed]
- DeFrances, C.J.; Lucas, C.A.; Buie, V.C.; Golosinskiy, A. 2006 National Hospital Discharge Survey. Natl. Health Stat. Rep. 2008, 5, 1–20. [Google Scholar] [PubMed]
- Litwin, M.S.; Saigal, C.S.; Yano, E.M.; Avila, C.; Geschwind, S.A.; Hanley, J.M.; Joyce, G.F.; Madison, R.; Pace, J.; Polich, S.M.; et al. Urologic Diseases in America Project. Urologic diseases in America Project: Analytical methods and principal findings. J. Urol. 2005, 173, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Ejrnaes, K.; Sandvang, D.; Lundgren, B.; Ferry, S.; Holm, S.; Monsen, T.; Lundholm, R.; Frimodt-Moller, N. Pulsed-field gel electrophoresis typing of Escherichia coli strains from samples collected before and after pivmecillinam or placebo treatment of uncomplicated community-acquired urinary tract infection in women. J. Clin. Microbiol. 2006, 44, 1776–1781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mulvey, M.A.; Schilling, J.D.; Hultgren, S.J. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 2001, 69, 4572–4579. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.E.; Norton, J.P.; Spivak, A.M.; Mulvey, M.A. Urinary tract infections: Current and emerging management strategies. Clin. Infect. Dis. 2013, 57, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shepherd, A.K.; Pottinger, P.S. Management of urinary tract infections in the era of increasing antimicrobial resistance. Med. Clin. North Am. 2013, 97, 737–757. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.O.; Flores, C.; Williams, C.; Flusberg, D.A.; Marr, E.E.; Kwiatkowska, K.M.; Charest, J.L.; Isenberg, B.C.; Rohn, J.L. Recurrent Urinary Tract Infection: A Mystery in Search of Better Model Systems. Front. Cell. Infect. Microbiol. 2021, 11, 691210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veranic, P.; Romih, R.; Jezernik, K. What determines differentiation of urothelial umbrella cells? Eur. J. Cell Biol. 2004, 83, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.R.; Lin, J.H.; Walz, T.; Häner, M.; Yu, J.; Aebi, U.; Sun, T.T. Mammalian uroplakins. A group of highly conserved urothelial differentiation-related membrane proteins. J. Biol. Chem. 1994, 269, 13716–13724. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.A. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am. J. Physiol. Ren. Physiol. 2000, 278, F867–F874. [Google Scholar] [CrossRef] [PubMed]
- Hannan, T.J.; Totsika, M.; Mansfield, K.J.; Moore, K.H.; Schembri, M.A.; Hultgren, S.J. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol. Rev. 2012, 36, 616–648. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, B.; Zhou, G.; Chan, S.Y.; Shapiro, E.; Kong, X.P.; Wu, X.R.; Sun, T.T.; Costello, C.E. Distinct glycan structures of uroplakins Ia and Ib: Structural basis for the selective binding of FimH adhesin to uroplakin Ia. J. Biol. Chem. 2006, 281, 14644–14653. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.J.; Hultgren, S.J. Requirement of Rho-family GTPases in the invasion of Type 1-piliated uropathogenic Escherichia coli. Cell. Microbiol. 2002, 4, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Justice, S.S.; Hung, C.; Theriot, J.A.; Fletcher, D.A.; Anderson, G.G.; Footer, M.J.; Hultgren, S.J. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 1333–1338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderson, G.G.; Palermo, J.J.; Schilling, J.D.; Roth, R.; Heuser, J.; Hultgren, S.J. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 2003, 301, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Hannan, T.J.; Mysorekar, I.U.; Hung, C.S.; Isaacson-Schmid, M.L.; Hultgren, S.J. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 2010, 6, e1001042. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderson, G.G.; Dodson, K.W.; Hooton, T.M.; Hultgren, S.J. Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol. 2004, 12, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schilling, J.D.; Lorenz, R.G.; Hultgren, S.J. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect. Immun. 2002, 70, 7042–7049. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mysorekar, I.U.; Mulvey, M.A.; Hultgren, S.J.; Gordon, J.I. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J. Biol. Chem. 2002, 277, 7412–7419. [Google Scholar] [CrossRef] [PubMed]
- Eto, D.S.; Sundsbak, J.L.; Mulvey, M.A. Actin-gated intracellular growth and resurgence of uropathogenic Escherichia coli. Cell. Microbiol. 2006, 8, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Hvidberg, H.; Struve, C.; Krogfelt, K.A.; Christensen, N.; Rasmussen, S.N.; Frimodt-Møller, N. Development of a long-term ascending urinary tract infection mouse model for antibiotic treatment studies. Antimicrob. Agents Chemother. 2000, 44, 156–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kerrn, M.B.; Struve, C.; Blom, J.; Frimodt-Møller, N.; Krogfelt, K.A. Intracellular persistence of Escherichia coli in urinary bladders from mecillinam-treated mice. J. Antimicrob. Chemother. 2005, 55, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Kerec, M.; Bogataj, M.; Veranic, P.; Mrhar, A. Permeability of pig urinary bladder wall: The effect of chitosan and the role of calcium. Eur. J. Pharm. Sci. 2005, 25, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Erman, A.; Kerec Kos, M.; Žakelj, S.; Resnik, N.; Romih, R.; Veranič, P. Correlative study of functional and structural regeneration of urothelium after chitosan-induced injury. Histochem. Cell Biol. 2013, 140, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.E.; Norton, J.P.; Wiles, T.J.; Mulvey, M.A. Strengths and Limitations of Model Systems for the Study of Urinary Tract Infections and Related Pathologies. Microbiol. Mol. Biol. Rev. 2016, 80, 351–367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hannan, T.J.; Hunstad, D.A. A Murine Model for Escherichia coli Urinary Tract Infection. Bact. Persistence Methods Protoc. 2016, 1333, 159–175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carey, A.J.; Tan, C.K.; Ipe, D.S.; Sullivan, M.J.; Cripps, A.W.; Schembri, M.A.; Ulett, G.C. Urinary tract infection of mice to model human disease: Practicalities, implications and limitations. Crit. Rev. Microbiol. 2016, 42, 780–799. [Google Scholar] [CrossRef] [PubMed]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114. [Google Scholar] [PubMed] [PubMed Central]
- Chabaud, S.; Marcoux, T.L.; Deschênes-Rompré, M.P.; Rousseau, A.; Morissette, A.; Bouhout, S.; Bernard, G.; Bolduc, S. Lysophosphatidic acid enhances collagen deposition and matrix thickening in engineered tissue. J. Tissue Eng. Regen. Med. 2015, 9, E65–E75. [Google Scholar] [CrossRef] [PubMed]
- Chabaud, S.; Rousseau, A.; Marcoux, T.L.; Bolduc, S. Inexpensive production of near-native engineered stromas. J. Tissue Eng. Regen. Med. 2017, 11, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Chabaud, S.; Saba, I.; Baratange, C.; Boiroux, B.; Leclerc, M.; Rousseau, A.; Bouhout, S.; Bolduc, S. Urothelial cell expansion and differentiation are improved by exposure to hypoxia. J. Tissue Eng. Regen. Med. 2017, 11, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, S.; Chabaud, S.; Bolduc, S. Organ-specific matrix self-assembled by mesenchymal cells improves the normal urothelial differentiation in vitro. World J. Urol. 2016, 34, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Eshaghi, M.; Mehershahi, K.S.; Chen, S.L. Brighter Fluorescent Derivatives of UTI89 Utilizing a Monomeric vGFP. Pathogens 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bouhout, S.; Goulet, F.; Bolduc, S. A Novel and Faster Method to Obtain a Differentiated 3-Dimensional Tissue Engineered Bladder. J. Urol. 2015, 194, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, S.; Chabaud, S.; Bolduc, S. Collagen hollow structure for bladder tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Hultgren, S.J.; Schwan, W.R.; Schaeffer, A.J.; Duncan, J.L. Regulation of production of type 1 pili among urinary tract isolates of Escherichia coli. Infect. Immun. 1986, 54, 613–620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, J.K.; Gunther, N.W.; Zhao, H.; Johnson, D.E.; Keay, S.K.; Mobley, H.L. In vivo phase variation of Escherichia coli type 1 fimbrial genes in women with urinary tract infection. Infect. Immun. 1998, 66, 3303–3310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dalghi, M.G.; Montalbetti, N.; Carattino, M.D.; Apodaca, G. The Urothelium: Life in a Liquid Environment. Physiol. Rev. 2020, 100, 1621–1705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lavoie, A.; Fugère, C.; Beauparlant, A.; Goyer, B.; Larouche, D.; Paquet, C.; Desgagné, M.; Sauvé, S.; Robitaille, H.; Dunnwald, M.; et al. Human epithelial stem cells persist within tissue-engineered skin produced by the self-assembly approach. Tissue Eng. Part A 2013, 19, 1023–1038. [Google Scholar] [CrossRef] [PubMed]
- Blango, M.G.; Mulvey, M.A. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob. Agents Chemother. 2010, 54, 1855–1863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blango, M.G.; Ott, E.M.; Erman, A.; Veranic, P.; Mulvey, M.A. Forced resurgence and targeting of intracellular uropathogenic Escherichia coli reservoirs. PLoS ONE 2014, 9, e93327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erman, A.; Hergouth, V.K.; Blango, M.G.; Kos, M.K.; Mulvey, M.A.; Veranic, P. Repeated Treatments with Chitosan in Combination with Antibiotics Completely Eradicate Uropathogenic Escherichia coli from Infected Mouse Urinary Bladders. J. Infect. Dis. 2017, 216, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, D.; Zakrajšek, M.; Zhou, G.; Romih, R. Expression and localization of four uroplakins in urothelial preneoplastic lesions. Histochem. Cell Biol. 2011, 136, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, D.; Ovčak, Z.; Vidmar, G.; Romih, R. Altered expression of UPIa, UPIb, UPII, and UPIIIa during urothelial carcinogenesis induced by N-butyl-N-(4-hydroxybutyl)nitrosamine in rats. Virchows Arch. 2011, 458, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Lobban, E.D.; Smith, B.A.; Hall, G.D.; Harnden, P.; Roberts, P.; Selby, P.J.; Trejdosiewicz, L.K.; Southgate, J. Uroplakin gene expression by normal and neoplastic human urothelium. Am. J. Pathol. 1998, 153, 1957–1967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saba, I.; Barat, C.; Chabaud, S.; Reyjon, N.; Leclerc, M.; Jakubowska, W.; Orabi, H.; Lachhab, A.; Pelletier, M.; Tremblay, M.J.; et al. Immunocompetent Human 3D Organ-Specific Hormone-Responding Vaginal Mucosa Model of HIV-1 Infection. Tissue Eng. Part C Methods 2021, 27, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.; Bélanger, S.; Cortez Ghio, S.; Pouliot, R. Eicosapentaenoic acid reduces the proportion of IL-17A-producing T cells in a 3D psoriatic skin model. J. Lipid Res. 2023, 64, 100428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Attiogbe, E.; Larochelle, S.; Chaib, Y.; Mainzer, C.; Mauroux, A.; Bordes, S.; Closs, B.; Gilbert, C.; Moulin, V.J. An in vitro autologous, vascularized, and immunocompetent Tissue Engineered Skin model obtained by the self-assembled approach. Acta Biomater. 2023, 168, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Bouhout, S.; Gauvin, R.; Gibot, L.; Aubé, D.; Bolduc, S. Bladder substitute reconstructed in a physiological pressure environment. J. Pediatr. Urol. 2011, 7, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Caneparo, C.; Chabaud, S.; Fradette, J.; Bolduc, S. Evaluation of a Serum-Free Medium for Human Epithelial and Stromal Cell Culture. Int. J. Mol. Sci. 2022, 23, 10035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hassuna, N.A.; Rabie, E.M.; Mahd, W.K.M.; Refaie, M.M.; Yousef, R.K.M.; Abdelraheem, W.M. Antibacterial effect of vitamin C against uropathogenic E. coli in vitro and in vivo. BMC Microbiol. 2023, 23, 112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matuszewski, M.A.; Tupikowski, K.; Dołowy, Ł.; Szymańska, B.; Dembowski, J.; Zdrojowy, R. Uroplakins and their potential applications in urology. Cent. Eur. J. Urol. 2016, 69, 252–257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taganna, J.; de Boer, A.R.; Wuhrer, M.; Bouckaert, J. Glycosylation changes as important factors for the susceptibility to urinary tract infection. Biochem. Soc. Trans. 2011, 39, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Roussel, C.; Chabaud, S.; Lessard-Lord, J.; Cattero, V.; Pellerin, F.A.; Feutry, P.; Bochard, V.; Bolduc, S.; Desjardins, Y. UPEC Colonic-Virulence and Urovirulence Are Blunted by Proanthocyanidins-Rich Cranberry Extract Microbial Metabolites in a Gut Model and a 3D Tissue-Engineered Urothelium. Microbiol. Spectr. 2022, 10, e0243221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellerin, F.-A.; Dufresne, É.; Chabaud, S.; Orabi, H.; Bolduc, S. Mimicking Urinary Tract Infections Caused by Uropathogenic Escherichia coli Using a Human Three-Dimensional Tissue Engineering Model. Microorganisms 2024, 12, 2155. https://doi.org/10.3390/microorganisms12112155
Pellerin F-A, Dufresne É, Chabaud S, Orabi H, Bolduc S. Mimicking Urinary Tract Infections Caused by Uropathogenic Escherichia coli Using a Human Three-Dimensional Tissue Engineering Model. Microorganisms. 2024; 12(11):2155. https://doi.org/10.3390/microorganisms12112155
Chicago/Turabian StylePellerin, Félix-Antoine, Élodie Dufresne, Stéphane Chabaud, Hazem Orabi, and Stéphane Bolduc. 2024. "Mimicking Urinary Tract Infections Caused by Uropathogenic Escherichia coli Using a Human Three-Dimensional Tissue Engineering Model" Microorganisms 12, no. 11: 2155. https://doi.org/10.3390/microorganisms12112155
APA StylePellerin, F.-A., Dufresne, É., Chabaud, S., Orabi, H., & Bolduc, S. (2024). Mimicking Urinary Tract Infections Caused by Uropathogenic Escherichia coli Using a Human Three-Dimensional Tissue Engineering Model. Microorganisms, 12(11), 2155. https://doi.org/10.3390/microorganisms12112155


_Di_Marco.png)



