Antibacterial, Herbicidal, and Plant Growth-Promoting Properties of Streptomyces sp. STD57 from the Rhizosphere of Adenophora stricta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
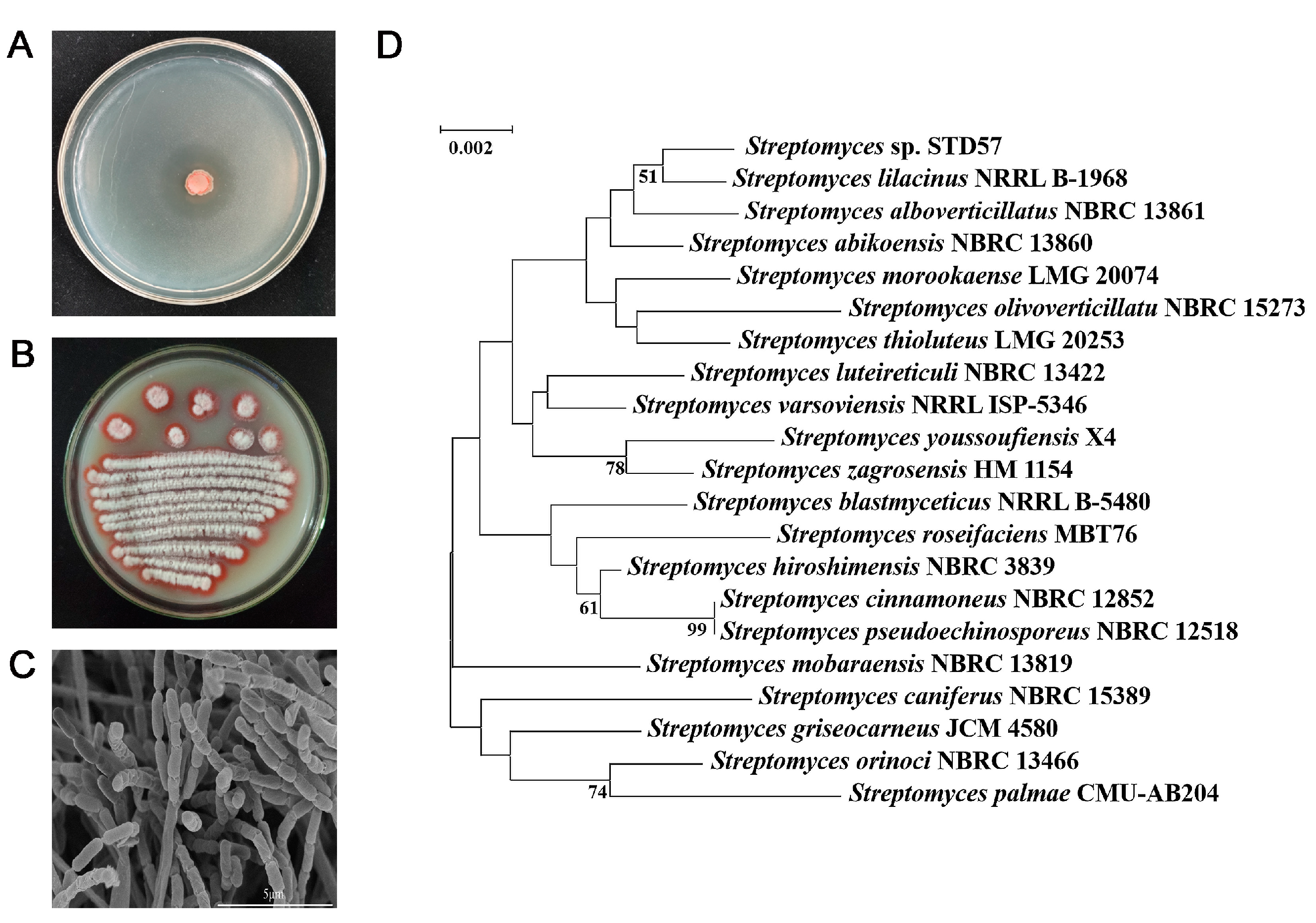
2.2. Isolation and Identification of Strain STD57
2.3. Activity Assessment of Strain STD57 Against R. solanacearum In Vitro
2.4. Biocontrol Efficacy of Strain STD57 Against R. solanacearum
2.5. Activity Evaluation of Strain STD57 on Seed Germination of Weeds and Crops
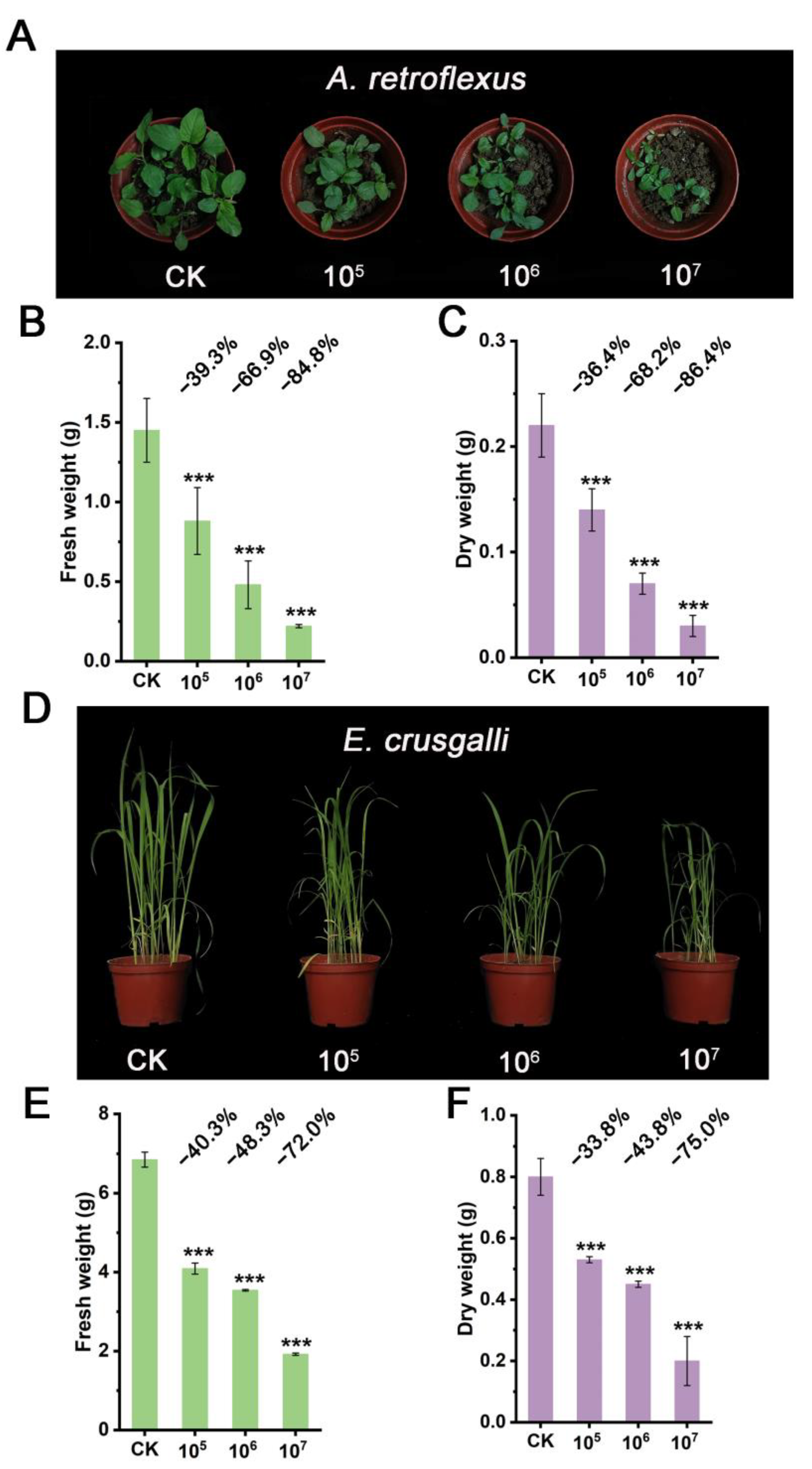
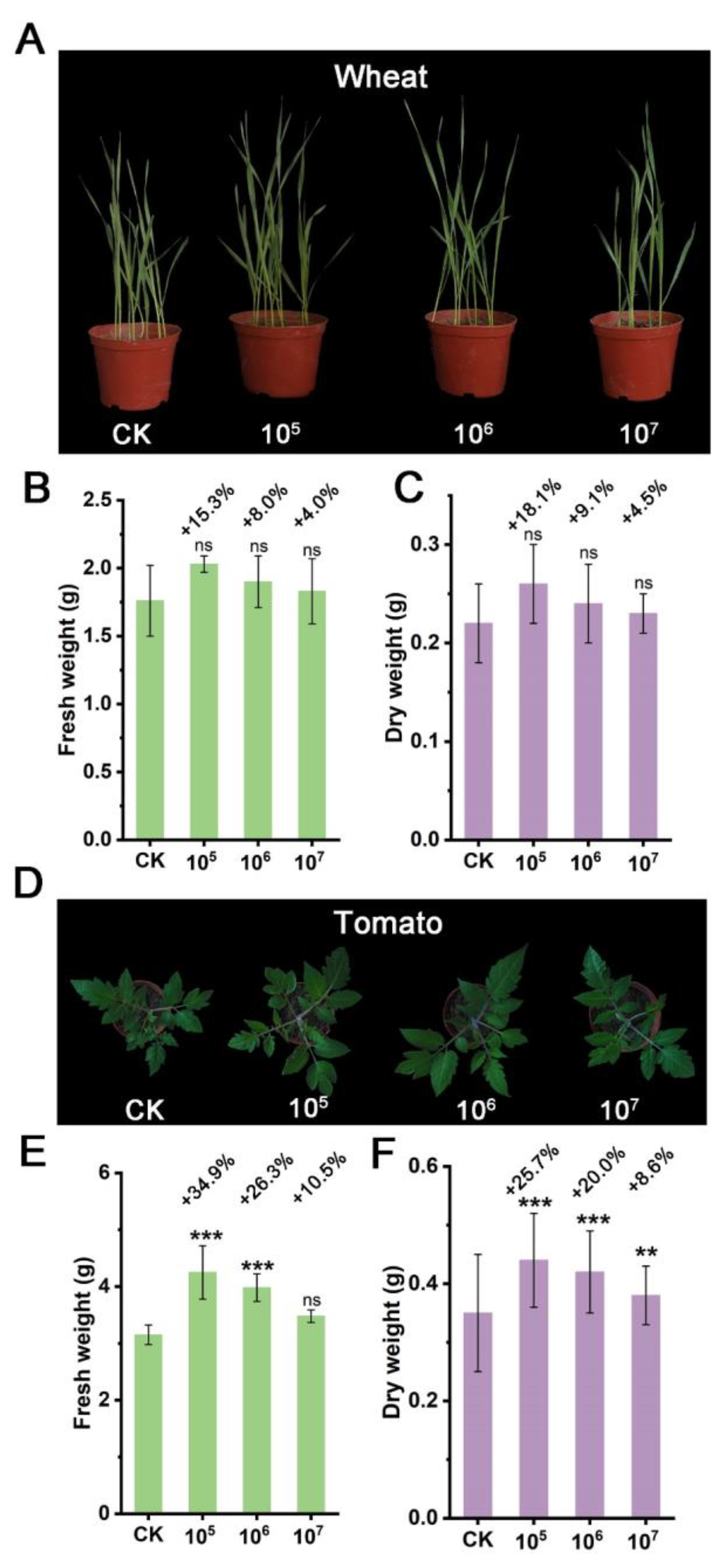
2.6. Effects of Strain STD57 on the Growth of Weeds and Crops in Pot Experiments
2.7. Evaluation of Antibiotic Resistance of Strain STD57
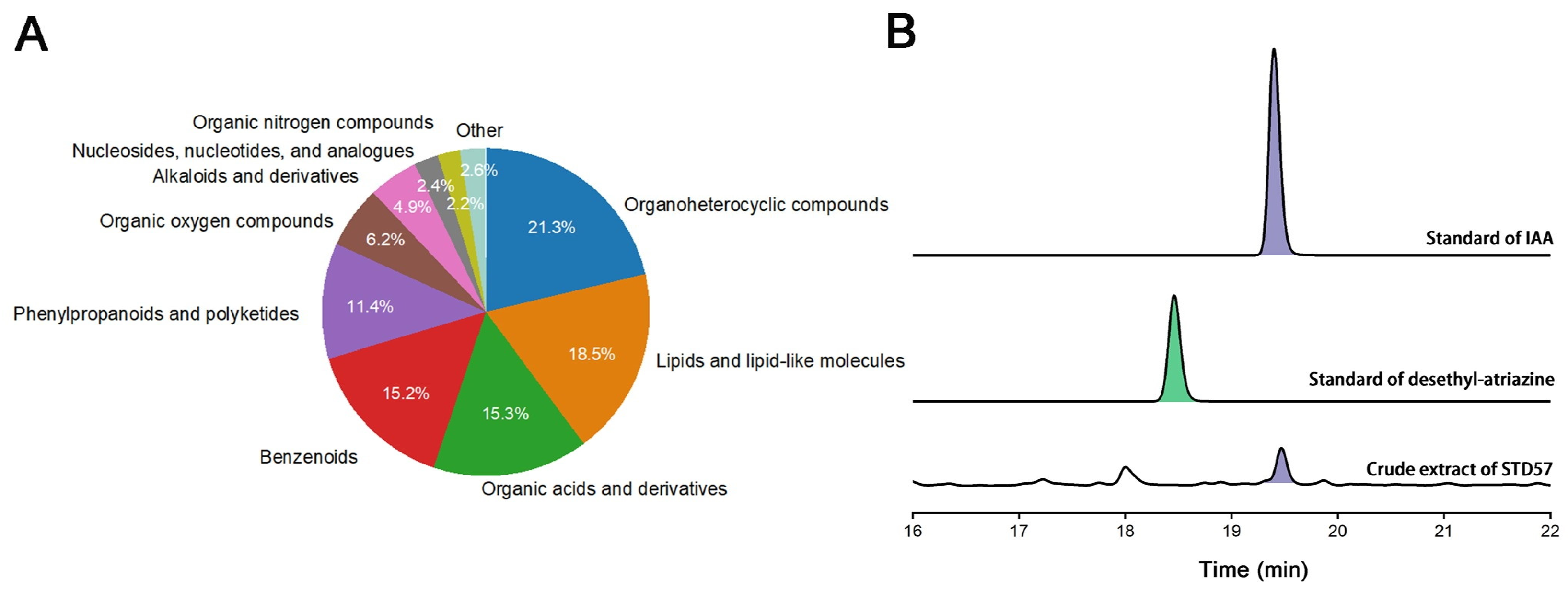
2.8. LC-MS Analysis of Spore Extracts of Strain STD57
2.9. Activity Evaluation of Fermentation Extracts of Strain STD57
2.10. Separation of the Crude Extract of Strain STD57
2.11. Genome Analysis
2.12. Statistical Analysis
3. Results
3.1. Isolation and Identification of Strain STD57 with Antagonistic Activity Against R. solanacearum
3.2. Biocontrol Effect of Strain STD57 on Tomato Bacterial Wilt
3.3. Herbicidal Activity of Strain STD57
3.4. Plant Growth-Promoting Effect of Strain STD57
3.5. Evaluation of Antibiotic Resistance of Strain STD57
3.6. Property of Strain STD57 Active Substances
3.7. Mining the Biosynthetic Potential of Strain STD57
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ke, J.; Zhu, W.; Yuan, Y.; Du, X.; Xu, A.; Zhang, D.; Cao, S.; Chen, W.; Lin, Y.; Xie, J.; et al. Duality of immune recognition by tomato and virulence activity of the Ralstonia solanacearum exo-polygalacturonase PehC. Plant Cell 2023, 35, 2552–2569. [Google Scholar] [CrossRef] [PubMed]
- Schell, M.A. Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network. Annu. Rev. Phytopathol. 2020, 38, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Meftaul, I.M.; Venkateswarlu, K.; Dharmarajan, R.; Annamalai, P.; Megharaj, M. Pesticides in the urban environment: A potential threat that knocks at the door. Sci. Total Environ. 2020, 711, 134612. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Schmid, M.; Van Tuinen, D.; Berg, G. Plant-driven selection of microbes. Plant Soil 2009, 321, 235–257. [Google Scholar] [CrossRef]
- Kibbey, T.C.; Strevett, K.A. The effect of nanoparticles on soil and rhizosphere bacteria and plant growth in lettuce seedlings. Chemosphere 2019, 221, 703–707. [Google Scholar] [CrossRef]
- Ahmed, W.; Yang, J.; Tan, Y.; Muni, S.; Liu, Q.; Zhang, J.; Ji, G.; Zhao, Z. Ralstonia solanacearum, a deadly pathogen: Revisiting the bacterial wilt biocontrol practices in tobacco and other Solanaceae. Rhizosphere 2022, 21, 100479. [Google Scholar] [CrossRef]
- Lu, Z.; Peng, L.; Dng, H.; Zuo, X.; Peng, J.; Jiang, X. Screening and identifying of antagonistic actinomycetes against Ralstonia solanacearum. Chin. Tob. Sci. 2013, 34, 54–58. [Google Scholar]
- Wang, Z.; Gao, C.; Yang, J.; Du, R.; Zeng, F.; Bing, H.; Xia, B.; Shen, Y.; Liu, C. Endophytic Streptomyces sp. NEAU-ZSY13 from the leaf of Perilla frutescens, as a promising broad-spectrum biocontrol agent against soil-borne diseases. Front. Microbiol. 2023, 14, 1243610. [Google Scholar] [CrossRef]
- Wang, D.; Shen, H.; Ran, L. Biocontrol of bacterial wilt in Eucalyptus urophylla and growth promotion by Bacillus subtilis strain CN181. Hebei J. For. Orchard Res. 2015, 30, 331–334. [Google Scholar]
- Wang, N.; Wang, L.; Zhu, K.; Hou, S.; Chen, L.; Mi, D.; Gui, Y.; Qi, Y.; Jiang, C.; Guo, J.H. Plant root exudates are involved in Bacillus cereus AR156 mediated biocontrol against Ralstonia solanacearum. Front. Microbiol. 2019, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.X.; Liu, C.Y.; Wu, G.J.; van Loon, L.C.; Bakker, P.A.H.M. Suppression of bacterial wilt in Eucalyptus urophylla by fluorescent Pseudomonas spp. in China. Biol. Control 2005, 32, 111–120. [Google Scholar] [CrossRef]
- Clough, S.E.; Jousset, A.; Elphinstone, J.G.; Friman, V.P. Combining in vitro and in vivo screening to identify efficient Pseudomonas biocontrol strains against the phytopathogenic bacterium Ralstonia solanacearum. Microbiologyopen 2022, 11, e1283. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.; Mazumder, A.; Sikdar, S.; Zhao, Y.M.; Hao, J.; Song, C.; Wang, Y.; Sarkar, R.; Islam, S.; Zhang, Y.; et al. Streptomyces: The biofactory of secondary metabolites. Front. Microbiol. 2022, 13, 968053. [Google Scholar] [CrossRef]
- Nazari, M.T.; Schommer, V.A.; Braun, J.C.A.; dos Santos, L.F.; Lopes, S.T.; Simon, V.; Machado, B.S.; Ferrari, V.; Colla, L.M.; Piccin, J.S. Using Streptomyces spp. as plant growth promoters and biocontrol agents. Rhizosphere 2023, 27, 100741. [Google Scholar] [CrossRef]
- Devi, S.; Sharma, M.; Manhas, R.K. Investigating the plant growth promoting and biocontrol potentiality of endophytic Streptomyces SP. SP5 against early blight in Solanum lycopersicum seedlings. BMC Microbiol. 2022, 22, 285. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Chen, Z.; Zhang, H.; Wang, Z.; Zhu, Y.; Liu, B. A Streptomyces sp. strain: Isolation, identification, and potential biocontrol agent against soilborne diseases of tomato plants. Biol. Control 2019, 136, 104004. [Google Scholar] [CrossRef]
- Qin, S.; Chen, H.H.; Zhao, G.Z.; Li, J.; Zhu, W.Y.; Xu, L.H.; Jiang, J.H.; Li, W.J. Abundant and diverse endophytic actinobacteria associated with medicinal plant Maytenus austroyunnanensis in Xishuangbanna tropical rainforest revealed by culture-dependent and culture-independent methods. Environ. Microbiol. Rep. 2012, 4, 522–531. [Google Scholar] [CrossRef]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Kim, S.B.; Brown, R.; Oldfield, C.; Gilbert, S.C.; Iliarionov, S.; Goodfellow, M. Gordonia amicalis sp. nov., a novel dibenzothiophene-desulphurizing actinomycete. Int. J. Syst. Evol. Microbiol. 2000, 50, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. Mega7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Waksman, S.A. “The Actinomycetes” in Classifification, Identification and Descriptions of Genera and Species; Williams and Wilkins: Baltimore, MD, USA, 1961; Volume 2. [Google Scholar]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Zhang, L.; Mu, J.; Jiang, Y.; Fu, H.; Zhang, Y.; Cui, H.; Yu, X.; Ye, Z. Biosynthetic pathways and functions of indole-3-acetic acid in microorganisms. Microorganisms 2023, 11, 2077. [Google Scholar] [CrossRef]
- Villarino, M.; Larena, I.; Melgarejo, P.; De Cal, A. Effect of chemical alternatives to methyl bromide on soil-borne disease incidence and fungal populations in Spanish strawberry nurseries: A long-term study. Pest Manag. Sci 2021, 77, 766–774. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Adegboye, M.F.; Babalola, O.O. Taxonomy and ecology of antibiotic producing actinomycetes. Afr. J. Agric. Res. 2012, 7, 2255–2261. [Google Scholar]
- Tyc, O.; Song, C.; Dickschat, J.S.; Vos, M.; Garbeva, P. The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends Microbiol. 2017, 25, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Aallam, Y.; El Maliki, B.; Dhiba, D.; Lemriss, S.; Souiri, A.; Haddioui, A.; Tarkka, M.; Hamdali, H. Multiple potential plant growth promotion activities of endemic Streptomyces spp. from Moroccan sugar beet fields with their inhibitory activities against Fusarium spp. Microorganisms 2021, 9, 1429. [Google Scholar] [CrossRef] [PubMed]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Estoppey, D.; Roggo, S.; Pistorius, D.; Fuchs, F.; Studer, C.; Ibrahim, A.S.; Aust, T.; Grandjean, F.; Mihalic, M.; et al. Jawsamycin exhibits in vivo antifungal properties by inhibiting Spt14/Gpi3-mediated biosynthesis of glycosylphosphatidylinositol. Nat. Commun. 2020, 11, 3387. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, D.; Gao, C.; Zhao, S.; Chen, H.; Li, P.; Yang, X.; Li, D.; Zhao, T.; Jiang, H.; Liu, C. Antibacterial, Herbicidal, and Plant Growth-Promoting Properties of Streptomyces sp. STD57 from the Rhizosphere of Adenophora stricta. Microorganisms 2024, 12, 2245. https://doi.org/10.3390/microorganisms12112245
He D, Gao C, Zhao S, Chen H, Li P, Yang X, Li D, Zhao T, Jiang H, Liu C. Antibacterial, Herbicidal, and Plant Growth-Promoting Properties of Streptomyces sp. STD57 from the Rhizosphere of Adenophora stricta. Microorganisms. 2024; 12(11):2245. https://doi.org/10.3390/microorganisms12112245
Chicago/Turabian StyleHe, Dan, Congting Gao, Shen Zhao, Hongmin Chen, Peng Li, Xishan Yang, Deping Li, Tingting Zhao, Hong Jiang, and Chongxi Liu. 2024. "Antibacterial, Herbicidal, and Plant Growth-Promoting Properties of Streptomyces sp. STD57 from the Rhizosphere of Adenophora stricta" Microorganisms 12, no. 11: 2245. https://doi.org/10.3390/microorganisms12112245
APA StyleHe, D., Gao, C., Zhao, S., Chen, H., Li, P., Yang, X., Li, D., Zhao, T., Jiang, H., & Liu, C. (2024). Antibacterial, Herbicidal, and Plant Growth-Promoting Properties of Streptomyces sp. STD57 from the Rhizosphere of Adenophora stricta. Microorganisms, 12(11), 2245. https://doi.org/10.3390/microorganisms12112245



