The Bidirectional Relationship Between Cardiovascular Medications and Oral and Gut Microbiome Health: A Comprehensive Review
Abstract
1. Introduction
2. Overview of the Human Microbiome
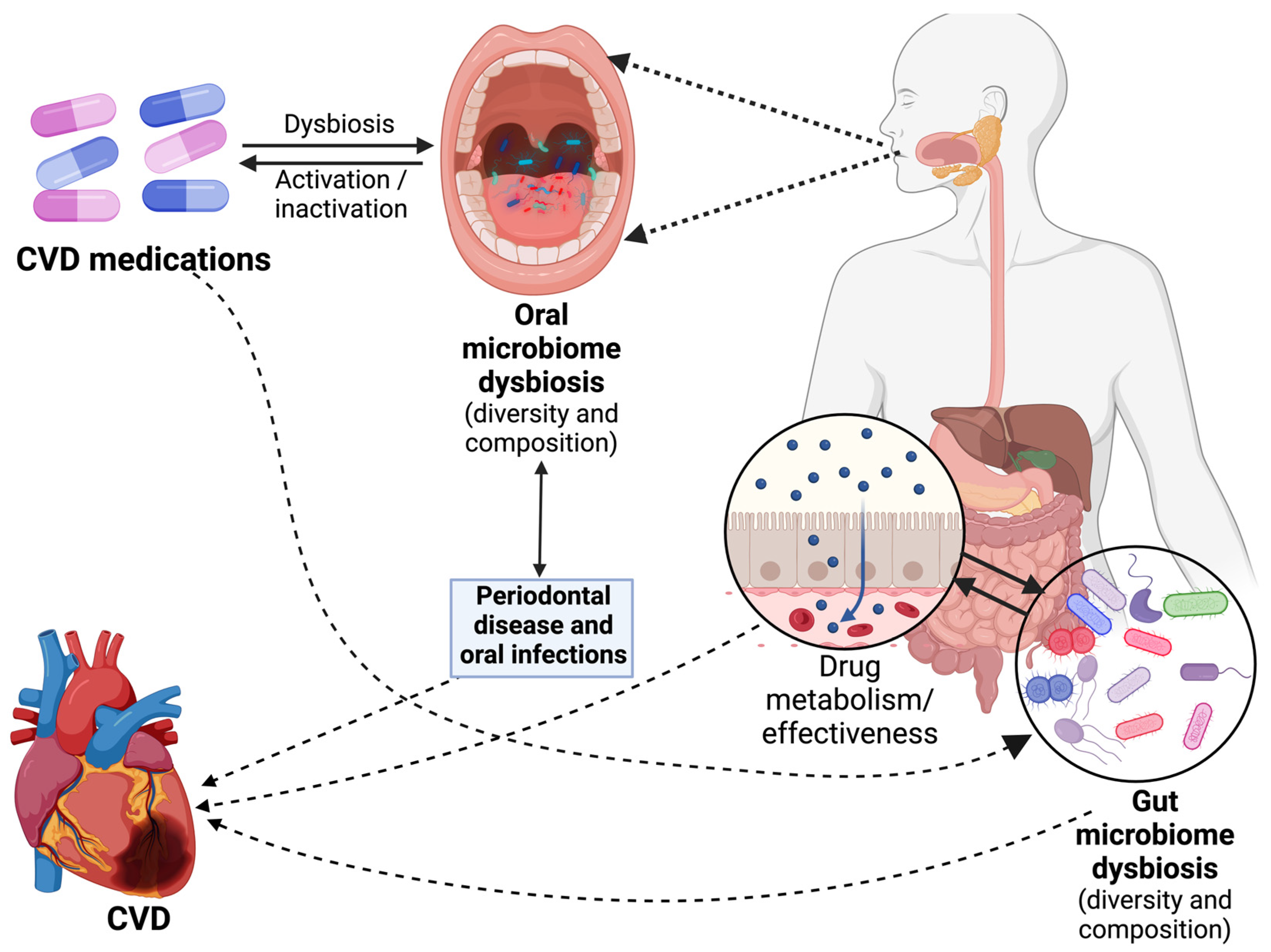
3. The Microbiome and CVD
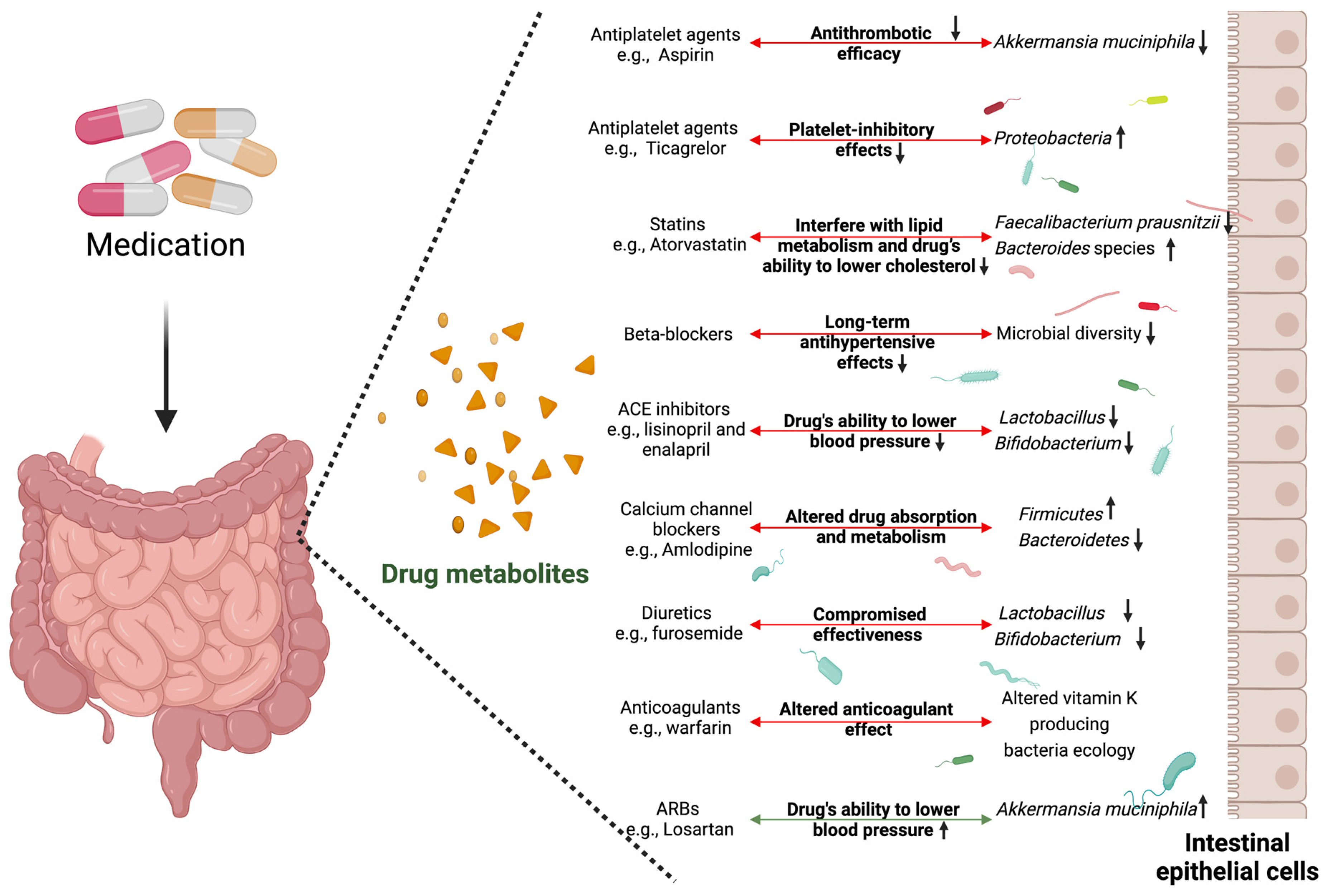
4. Cardiovascular Medications and Microbiome Alterations
| Group | Drug Types | Specific Role | Microbiome Alterations | Reference |
|---|---|---|---|---|
| Antiplatelet agents | Aspirin (Acetylsalicylic Acid), Clopidogrel, Ticagrelor, Prasugrel | Reduce the risk of thrombus formation by preventing the aggregation of platelets and inhibit platelet aggregation. | Gut: Reduce beneficial bacteria like Akkermansia muciniphila and increase Proteobacteria (Ticagrelor). Indirectly affect microbiota through gut immune modulation. | [69,86,87,88] |
| Statins (HMG-CoA reductase inhibitors) | Atorvastatin, Simvastatin, Rosuvastatin, Lovastatin | By lowering LDL-C (improving the lipid profile), it decreases the risk of cardiovascular events such as acute coronary syndromes and stroke. | Gut: Decrease Faecalibacterium prausnitzii and increase Bacteroides. Indirect alterations through bile acid metabolism. Oral: Reduction in periodontal pathogens such as P. gingivalis. | [72,89,90,91] |
| Beta-blockers | Metoprolol, Atenolol, Propranolol, Bisoprolol | Lower heart rate and blood pressure, decrease the force of contraction (negative ionotropic response), and manage conditions such as hypertension, angina, and arrhythmias. | Gut: Decrease gut microbial diversity indirectly. Oral: Alterations in the composition of the oral microbiome in individuals with periodontitis. | [74,92,93,94,95] |
| Angiotensin-converting enzyme (ACE) inhibitors | Lisinopril, Enalapril, Ramipril, Captopril | Lower blood pressure, reduce the strain on the heart, and block the production of angiotensin II. Inhibit the renin angiotensin aldosterone pathway (RAAS), resulting in hypotension and preventing cardiac remodeling. | Gut: Reduce Lactobacillus and Bifidobacterium abundance. | [76,77,96,97] |
| Angiotensin II receptor blockers (ARBs) | Losartan, Valsartan, Olmesartan, Candesartan | Reduce blood pressure by blocking the action of angiotensin II on blood vessels and improve the heart’s pumping ability. Inhibit the RAAS, resulting in hypotension and preventing cardiac remodeling. | Gut: Increase A. muciniphila. | [77,78,98,99,100] |
| Calcium channel blockers | Amlodipine, Diltiazem, Verapamil, Nifedipine | Reduce blood pressure by relaxing and dilating the arterial walls, improve blood supply to the heart, and reduce the myocardial oxygen demand. | Gut: Increase Firmicutes and decrease Bacteroidetes. | [80,101] |
| Diuretics | Hydrochlorothiazide, Furosemide, Chlorthalidone, Spironolactone | By increasing the production of urine, diuretics work on different components of the renal tract to remove excess fluid, reducing the cardiac preload and relieving symptoms of heart failure. | Gut: Reduce Lactobacillus and Bifidobacterium abundance. | [81,102] |
| Nitroglycerin and nitrates | Nitroglycerin, Isosorbide dinitrate, Isosorbide Mononitrate | Provide rapid relief from angina attacks, reduce chest pain, and improve blood flow to the heart during critical situations. | [103,104] | |
| Anticoagulants | Warfarin, Apixaban, Rivaroxaban, Edoxaban | Prevent cardioembolic phenomena including strokes in high-risk conditions such as atrial fibrillation or mitral stenosis. | Gut: Affect vitamin K-producing bacteria. | [84,85,105,106] |
| Antiarrhythmics | Amiodarone, Flecainide, Propafenone, Sotalol | Restore the heart’s normal rhythm in patients with arrhythmias and alleviate symptoms associated with rapid and irregular heart rates. | [107,108] |
5. The Role of the Microbiome in the CVD Medication Response
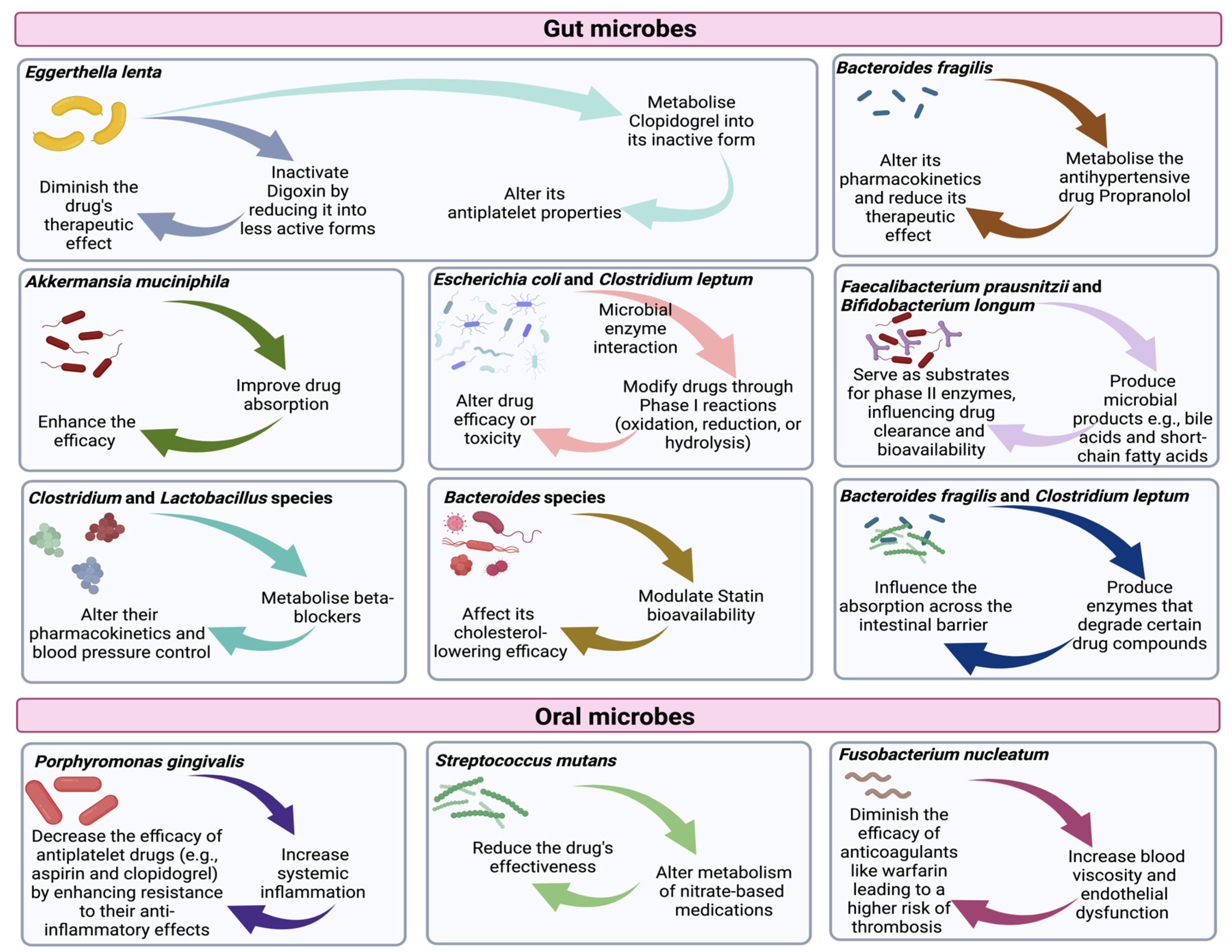
5.1. Drug Metabolism and Bioactivation
5.2. Drug Absorption and Bioavailability
6. Effects of Specific Cardiovascular Medications on the Microbiome
6.1. Effects on Gut Microbiome
6.2. Effects on Oral Microbiome
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization (WHO). Cardiovascular Diseases (CVDs); World Health Organization (WHO): Geneva, Switzerland, 2021. [Google Scholar]
- Wessler, B.S.; Yh, L.L.; Kramer, W.; Cangelosi, M.; Raman, G.; Lutz, J.S.; Kent, D.M. Clinical Prediction Models for Cardiovascular Disease: Tufts Predictive Analytics and Comparative Effectiveness Clinical Prediction Model Database. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.; Wahab, A.; Khan, S.U.; Zaman, U.; ur Rehman, K.; Hamayun, S.; Naeem, M.; Ali, H.; Riaz, T.; Saeed, S.; et al. Stent as a Novel Technology for Coronary Artery Disease and Their Clinical Manifestation. Curr. Probl. Cardiol. 2023, 48, 101415. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A.; Chaitman, B. Angina and Its Management. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.V.; Li, D.L.; Hill, J.A. Heart failure and loss of metabolic control. J. Cardiovasc. Pharmacol. 2014, 63, 302–313. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Miller, M.A. Cardiovascular disease and hypertension in sub-Saharan Africa: Burden, risk and interventions. Intern. Emerg. Med. 2016, 11, 299–305. [Google Scholar] [CrossRef]
- Katoh, M.; Takeda, N.; Arimoto, T.; Abe, H.; Oda, K.; Osuga, Y.; Fujii, T.; Komuro, I. Bevacizumab-related microvascular angina and its management with nicorandil. Int. Heart J. 2017, 58, 803–805. [Google Scholar] [CrossRef]
- Oveissi, F.; Naficy, S.; Lee, A.; Winlaw, D.S.; Dehghani, F. Materials and manufacturing perspectives in engineering heart valves: A review. Mater. Today Bio 2020, 5, 803–805. [Google Scholar] [CrossRef]
- Taghizadeh, B.; Ghavami, L.; Derakhshankhah, H.; Zangene, E.; Razmi, M.; Jaymand, M.; Zarrintaj, P.; Zarghami, N.; Jaafari, M.R.; Moallem Shahri, M.; et al. Biomaterials in Valvular Heart Diseases. Front. Bioeng. Biotechnol. 2020, 8, 529244. [Google Scholar] [CrossRef]
- Maki, K.A.; Kazmi, N.; Barb, J.J.; Ames, N. The Oral and Gut Bacterial Microbiomes: Similarities, Differences, and Connections. Biol. Res. Nurs. 2021, 23, 7–20. [Google Scholar] [CrossRef]
- Kitamoto, S.; Nagao-Kitamoto, H.; Hein, R.; Schmidt, T.M.; Kamada, N. The Bacterial Connection between the Oral Cavity and the Gut Diseases. J. Dent. Res. 2020, 99, 1021–1029. [Google Scholar] [CrossRef]
- Lin, D.; Medeiros, D.M. The microbiome as a major function of the gastrointestinal tract and its implication in micronutrient metabolism and chronic diseases. Nutr. Res. 2023, 112, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, X.; Li, L. Human gut microbiome: The second genome of human body. Protein Cell 2010, 1, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Rupa, P.; Mine, Y. Recent advances in the role of probiotics in human inflammation and gut health. J. Agric. Food Chem. 2012, 60, 8249–8256. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.R.; Gabaldón, T. The human oral microbiome in health and disease: From sequences to ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef]
- Gedif Meseret, A. Oral Biofilm and Its Impact on Oral Health, Psychological and Social Interaction. Int. J. Oral Dent. Health 2021, 7, 127. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef]
- Leviatan, S.; Shoer, S.; Rothschild, D.; Gorodetski, M.; Segal, E. An expanded reference map of the human gut microbiome reveals hundreds of previously unknown species. Nat. Commun. 2022, 13, 3863. [Google Scholar] [CrossRef]
- Carey, H.V.; Assadi-Porter, F.M. The Hibernator Microbiome: Host-Bacterial Interactions in an Extreme Nutritional Symbiosis. Annu. Rev. Nutr. 2017, 37, 477–500. [Google Scholar] [CrossRef]
- Nagpal, R.; Shively, C.A.; Register, T.C.; Craft, S.; Yadav, H. Gut microbiome-Mediterranean diet interactions in improving host health. F1000Research 2019, 8, 699. [Google Scholar] [CrossRef]
- Nagao-Kitamoto, H.; Shreiner, A.B.; Gillilland, M.G.; Kitamoto, S.; Ishii, C.; Hirayama, A.; Kuffa, P.; El-Zaatari, M.; Grasberger, H.; Seekatz, A.M.; et al. Functional Characterization of Inflammatory Bowel Disease-Associated Gut Dysbiosis in Gnotobiotic Mice. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J.; Kogut, M.H. The role of the gut microbiome in shaping the immune system of chickens. Vet. Immunol. Immunopathol. 2018, 204, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Alhajri, N.; Khursheed, R.; Ali, M.T.; Izneid, T.A.; Al-Kabbani, O.; Al-Haidar, M.B.; Al-Hemeiri, F.; Alhashmi, M.; Pot-too, F.H. Cardiovascular health and the intestinal microbial ecosystem: The impact of cardiovascular therapies on the gut microbiota. Microorganisms 2021, 9, 2013. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, E.; Ramadass, B.; Ramprasath, V. Understanding connections and roles of gut microbiome in cardiovascular diseases. Can. J. Microbiol. 2021, 67, 101–111. [Google Scholar] [CrossRef]
- Tonelli, A.; Lumngwena, E.N.; Ntusi, N.A.B. The oral microbiome in the pathophysiology of cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 386–403. [Google Scholar] [CrossRef]
- Chakaroun, R.M.; Olsson, L.M.; Bäckhed, F. The potential of tailoring the gut microbiome to prevent and treat cardiometabolic disease. Nat. Rev. Cardiol. 2023, 20, 217–235. [Google Scholar] [CrossRef]
- Novakovic, M.; Rout, A.; Kingsley, T.; Kirchoff, R.; Singh, A.; Verma, V.; Kant, R.; Chaudhary, R. Role of gut microbiota in cardiovascular diseases. World J. Cardiol. 2020, 12, 110–122. [Google Scholar] [CrossRef]
- Le Bastard, Q.; Berthelot, L.; Soulillou, J.P.; Montassier, E. Impact of non-antibiotic drugs on the human intestinal microbiome. Expert. Rev. Mol. Diagn. 2021, 21, 911–924. [Google Scholar] [CrossRef]
- Dias, A.M.; Cordeiro, G.; Estevinho, M.M.; Veiga, R.; Figueira, L.; Reina-Couto, M.; Magro, F. Gut bacterial microbiome composition and statin intake—A systematic review. Pharmacol. Res. Perspect. 2020, 8, e00601. [Google Scholar] [CrossRef]
- Wilmanski, T.; Kornilov, S.A.; Diener, C.; Conomos, M.P.; Lovejoy, J.C.; Sebastiani, P.; Orwoll, E.S.; Hood, L.; Price, N.D.; Rappaport, N.; et al. Heterogeneity in statin responses explained by variation in the human gut microbiome. Med 2022, 3, 388–405.e6. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, S.; Ferguson, J.F. Gut Microbiome and Response to Cardiovascular Drugs. Circ. Genom. Precis. Med. 2019, 12, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, X.; Su, C.; Wang, L.; Jiang, J.; Hong, B. The human gut microbiome–a new and exciting avenue in cardiovascular drug discovery. Expert Opin. Drug Discov. 2019, 14, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Zarco, M.F.; Vess, T.J.; Ginsburg, G.S. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral. Dis. 2012, 18, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Duran-Pinedo, A.E.; Frias-Lopez, J. Beyond microbial community composition: Functional activities of the oral microbiome in health and disease. Microbes Infect. 2015, 17, 505–516. [Google Scholar] [CrossRef]
- Gacesa, R.; Kurilshikov, A.; Vich Vila, A.; Sinha, T.; Klaassen, M.A.Y.; Bolte, L.A.; Andreu-Sánchez, S.; Chen, L.; Collij, V.; Hu, S.; et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature 2022, 604, 732–739. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Zhao, R.; Huang, R.; Long, H.; Li, Y.; Gao, M.; Lai, W. The dynamics of the oral microbiome and oral health among patients receiving clear aligner orthodontic treatment. Oral Dis. 2020, 26, 473–483. [Google Scholar] [CrossRef]
- Nguyen, T.; Brody, H.; Radaic, A.; Kapila, Y. Probiotics for periodontal health—Current molecular findings. Periodontology 2021, 87, 254–267. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Alam, M.T.; Amos, G.C.A.; Murphy, A.R.J.; Murch, S.; Wellington, E.M.H.; Arasaradnam, R.P. Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog. 2020, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, Q.; Park, C.G.; Klena, J.D.; Anisimov, A.P.; Sun, Z.; Wei, X.; Chen, T. Proteus mirabilis Targets Atherosclerosis Plaques in Human Coronary Arteries via DC-SIGN (CD209). Front. Immunol. 2021, 11, 579010. [Google Scholar] [CrossRef] [PubMed]
- Velsko, I.M.; Chukkapalli, S.S.; Rivera, M.F.; Lee, J.Y.; Chen, H.; Zheng, D.; Bhattacharyya, I.; Gangula, P.R.; Lucas, A.R.; Kesavalu, L. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS ONE 2014, 9, e97811. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, S.; Zhang, Q.; He, C.; Fu, C.; Wei, Q. The role of the gut microbiota in health and cardiovascular diseases. Mol. Biomed. 2022, 3, 30. [Google Scholar] [CrossRef]
- Tu, R.; Xia, J. Stroke and Vascular Cognitive Impairment: The Role of Intestinal Microbiota Metabolite TMAO. CNS Neurol. Disord. Drug Targets 2023, 23, 102–121. [Google Scholar] [CrossRef]
- Panyod, S.; Wu, W.K.; Chen, P.C.; Chong, K.V.; Yang, Y.T.; Chuang, H.L.; Chen, C.C.; Chen, R.A.; Liu, P.Y.; Chung, C.H.; et al. Atherosclerosis amelioration by allicin in raw garlic through gut microbiota and trimethylamine-N-oxide modulation. NPJ Biofilms Microbiomes 2022, 8, 4. [Google Scholar] [CrossRef]
- Elliott Miller, P.; Haberlen, S.A.; Brown, T.T.; Margolick, J.B.; DiDonato, J.A.; Hazen, S.L.; Witt, M.D.; Kingsley, L.A.; Palella, F.J.; Budoff, M.; et al. Intestinal microbiota-produced trimethylamine-N-oxide and its association with coronary stenosis and HIV serostatus. J. Acquir. Immune Defic. Syndr. 2016, 72, 114–118. [Google Scholar] [CrossRef]
- Wang, B.Y.; Qiu, J.; Lian, J.F.; Yang, X.; Zhou, J.Q. Gut Metabolite Trimethylamine-N-Oxide in Atherosclerosis: From Mechanism to Therapy. Front. Cardiovasc. Med. 2021, 8, 723886. [Google Scholar] [CrossRef]
- Pieczynska, M.D.; Yang, Y.; Petrykowski, S.; Horbanczuk, O.K.; Atanasov, A.G.; Horbanczuk, J.O. Gut microbiota and its metabolites in atherosclerosis development. Molecules 2020, 25, 594. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Duttaroy, A.K. Role of gut microbiota and their metabolites on atherosclerosis, hypertension and human blood platelet function: A review. Nutrients 2021, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Aleman, R.S.; Moncada, M.; Aryana, K.J. Leaky Gut and the Ingredients That Help Treat It: A Review. Molecules 2023, 28, 619. [Google Scholar] [CrossRef] [PubMed]
- Poto, R.; Fusco, W.; Rinninella, E.; Cintoni, M.; Kaitsas, F.; Raoul, P.; Caruso, C.; Mele, M.C.; Varricchi, G.; Gasbarrini, A.; et al. The Role of Gut Microbiota and Leaky Gut in the Pathogenesis of Food Allergy. Nutrients 2024, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Roca-Millan, E.; González-Navarro, B.; Del Mar Sabater-Recolons, M.; Marí-Roig, A.; Jané-Salas, E.; López-López, J. Periodontal treatment on patients with cardiovascular disease: Systematic review and meta-analysis. Med. Oral Patol. Oral Cir. Bucal 2018, 23, E681–E690. [Google Scholar] [CrossRef]
- Armingohar, Z.; Jørgensen, J.J.; Kristoffersen, A.K.; Abesha-Belay, E.; Olsen, I. Bacteria and bacterial DNA in atherosclerotic plaque and aneurysmal wall biopsies from patients with and without periodontitis. J. Oral Microbiol. 2014, 6, 23408. [Google Scholar] [CrossRef]
- Marcano, R.; Rojo, M.Á.; Cordoba-Diaz, D.; Garrosa, M. Pathological and therapeutic approach to endotoxin-secreting bacteria involved in periodontal disease. Toxins 2021, 13, 533. [Google Scholar] [CrossRef]
- Blancas-Luciano, B.E.; Zamora-Chimal, J.; da Silva-de Rosenzweig, P.G.; Ramos-Mares, M.; Fernández-Presas, A.M. Macrophages immunomodulation induced by Porphyromonas gingivalis and oral antimicrobial peptides. Odontology 2023, 111, 778–792. [Google Scholar] [CrossRef]
- Lin, J.; Huang, D.; Xu, H.; Zhan, F.; Tan, X.L. Macrophages: A communication network linking Porphyromonas gingivalis infection and associated systemic diseases. Front. Immunol. 2022, 13, 952040. [Google Scholar] [CrossRef]
- Arnao, V.; Tuttolomondo, A.; Daidone, M.; Pinto, A. Lipoproteins in Atherosclerosis Process. Curr. Med. Chem. 2019, 26, 1525–1543. [Google Scholar] [CrossRef] [PubMed]
- Ganther, S.; Radaic, A.; Malone, E.; Kamarajan, P.; Chang, N.Y.N.; Tafolla, C.; Zhan, L.; Fenno, J.C.; Kapila, Y.L. Treponema denticola dentilisin triggered TLR2/ MyD88 activation upregulates a tissue destructive program involving MMPs via Sp1 in human oral cells. PLoS Pathog. 2021, 17, e1009311. [Google Scholar] [CrossRef] [PubMed]
- Saleki, K.; Alijanizade, P.; Moradi, S.; Rahmani, A.; Banazadeh, M.; Mohamadi, M.H.; Shahabi, F.; Nouri, H.R. Engineering a novel immunogenic chimera protein utilizing bacterial infections associated with atherosclerosis to induce a deviation in adaptive immune responses via Immunoinformatics approaches. Infect. Genet. Evol. 2022, 102, 105290. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Han, N.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. Pathogenesis of important virulence factors of Porphyromonas gingivalis via toll-like receptors. Front. Cell. Infect. Microbiol. 2019, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Plemmenos, G.; Evangeliou, E.; Polizogopoulos, N.; Chalazias, A.; Deligianni, M.; Piperi, C. Central Regulatory Role of Cytokines in Periodontitis and Targeting Options. Curr. Med. Chem. 2020, 28, 3032–3058. [Google Scholar] [CrossRef]
- Zeng, X.T.; Leng, W.D.; Lam, Y.Y.; Yan, B.P.; Wei, X.M.; Weng, H.; Kwong, J.S.W. Periodontal disease and carotid atherosclerosis: A meta-analysis of 17,330 participants. Int. J. Cardiol. 2016, 203, 1044–1051. [Google Scholar] [CrossRef]
- Mollace, R.; Maiuolo, J.; Mollace, V. The Role of Endothelial Dysfunction in the Connection Between Gut Microbiota, Vascular Injury, and Arterial Hypertension; Springer International Publishing: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Wu, W.K.; Ivanova, E.A.; Orekhov, A.N. Gut microbiome: A possible common therapeutic target for treatment of atherosclerosis and cancer. Semin. Cancer Biol. 2021, 70, 85–97. [Google Scholar] [CrossRef]
- Brennan, C.A.; Nakatsu, G.; Comeau, C.A.G.; Drew, D.A.; Glickman, J.N.; Schoen, R.E.; Chan, A.T.; Garrett, W.S. Aspirin modulation of the colorectal cancer-associated microbe fusobacterium nucleatum. mBio 2021, 12. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Tong, F.; Cai, Y.; Zhang, Y.; Song, H.; Tian, X.; Yan, C.; Han, Y. Gut microbiota induces high platelet response in patients with ST segment elevation myocardial infarction after ticagrelor treatment. eLife 2022, 11, e70240. [Google Scholar] [CrossRef]
- McCabe, L.R.; Parameswaran, N. Understanding the Gut-Bone Signaling Axis: Mechanisms and Therapeutic Implications. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1033. [Google Scholar]
- Garg, Y.; Kanwar, N.; Chopra, S.; Tambuwala, M.M.; Dodiya, H.; Bhatia, A.; Kanwal, A. Microbiome Medicine: Microbiota in Development and Management of Cardiovascular Diseases. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 1344–1356. [Google Scholar] [CrossRef]
- Jia, Q.; Li, H.; Zhou, H.; Zhang, X.; Zhang, A.; Xie, Y.; Li, Y.; Lv, S.; Zhang, J. Role and Effective Therapeutic Target of Gut Microbiota in Heart Failure. Cardiovasc. Ther. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khmil, N.V.; Kolesnikov, V.G.; Altuhov, O.L. Evaluation of disorders of adaptive mechanisms in heart failure by microwave dielectrometry. Radiotekhnika 2022, 209, 200–205. [Google Scholar] [CrossRef]
- Jaworska, K.; Koper, M.; Ufnal, M. Gut microbiota and renin-angiotensin system: A complex interplay at local and systemic levels. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 321, G355–G366. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Gong, J.Y.; Xing, K.; Liu, M.Z.; Ren, H.; Luo, J.Q. Pharmacomicrobiomics: Exploiting the Drug-Microbiota Interactions in Antihypertensive Treatment. Front. Med. 2022, 8, 742394. [Google Scholar] [CrossRef] [PubMed]
- Avery, E.G.; Bartolomaeus, H.; Maifeld, A.; Marko, L.; Wiig, H.; Wilck, N.; Rosshart, S.P.; Forslund, S.K.; Müller, D.N. The Gut Microbiome in Hypertension: Recent Advances and Future Perspectives. Circ. Res. 2021, 128, 934–950. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Y.; Nan, L.; Zhu, Q.; Wang, S.; Xie, Y.; Dong, X.; Cao, C.; Lin, X.; Lu, Y.; et al. Impact of structurally diverse polysaccharides on colonic mucin O-glycosylation and gut microbiota. NPJ Biofilms Microbiomes 2023, 9, 97. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Huang, W.; Jin, M.; Gao, Z. The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm. Sin. B 2021, 11, 1789–1812. [Google Scholar] [CrossRef]
- Palmu, J.; Lahti, L.; Niiranen, T. Targeting gut microbiota to treat hypertension: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 1248. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Q.; Liu, Y.; Wang, L.; Ge, Z.; Li, Z.; Feng, S.; Wu, C. Gut microbiota and hypertension: Association, mechanisms and treatment. Clin. Exp. Hypertens. 2023, 45, 2195135. [Google Scholar] [CrossRef]
- Stickel, S.; Gin-Sing, W.; Wagenaar, M.; Gibbs, J.S.R. The practical management of fluid retention in adults with right heart failure due to pulmonary arterial hypertension. Eur. Heart J. Suppl. 2019, 21, K46–K53. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Y.; Zhu, H.; Huang, W.H.; Cai, X.H.; Li, D.; Lv, Y.J.; Zhou, H.H.; Luo, F.Y.; Zhang, W.; et al. The Relationship Among Intestinal Bacteria, Vitamin K and Response of Vitamin K Antagonist: A Review of Evidence and Potential Mechanism. Front. Med. 2022, 9, 829304. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Singla, R.K.; Qin, Q.; Ding, Y.; Liu, L.; Ding, X.; Qu, W.; Huang, C.; Shen, Z.; Shen, B.; et al. Exploring the complex relationship between vitamin K, gut microbiota, and warfarin variability in cardiac surgery patients. Int. J. Surg. 2023, 109, 3861–3871. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Meng, X.; Tian, X.; Johnston, S.C.; Li, H.; Bath, P.M.; Zuo, Y.; Xie, X.; Jing, J.; Lin, J.; et al. Effect of Hypertension on Efficacy and Safety of Ticagrelor-Aspirin Versus Clopidogrel-Aspirin in Minor Stroke or Transient Ischemic Attack. Stroke 2022, 53, 2799–2808. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.S.; Tsai, C.F.; Hsu, Y.H.; Ter, O.C. Efficacy of aspirin, clopidogrel, and ticlopidine in stroke prevention: A population-based case-cohort study in Taiwan. PLoS ONE 2021, 15, e0242466. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Park, M.S.; Choi, K.H.; Cho, K.H.; Kim, B.J.; Park, J.M.; Kang, K.; Lee, S.J.; Kim, J.G.; Cha, J.K.; et al. Comparative Effectiveness of Aspirin and Clopidogrel Versus Aspirin in Acute Minor Stroke or Transient Ischemic Attack. Stroke 2019, 50, 101–109. [Google Scholar] [CrossRef]
- Rosen, J.B.; Jimenez, J.G.; Pirags, V.; Vides, H.; Hanson, M.E.; Massaad, R.; McPeters, G.; Brudi, P.; Triscari, J. A comparison of efficacy and safety of an ezetimibe/simvastatin combination compared with other intensified lipid-lowering treatment strategies in diabetic patients with symptomatic cardiovascular disease. Diabetes Vasc. Dis. Res. 2013, 10, 277–286. [Google Scholar] [CrossRef]
- Demoz, G.T.; Wahdey, S.; Kasahun, G.G.; Hagazy, K.; Kinfe, D.G.; Tasew, H.; Bahrey, D.; Niriayo, Y.L. Prescribing pattern of statins for primary prevention of cardiovascular diseases in patients with type 2 diabetes: Insights from Ethiopia. BMC Res. Notes 2019, 12, 386. [Google Scholar] [CrossRef]
- Hakim, S.; Chowdhury, M.A.B.; Haque, M.d.A.; Ahmed, N.U.; Paul, G.K.; Uddin, M.d.J. The availability of essential medicines for cardiovascular diseases at healthcare facilities in low- and middle-income countries: The case of Bangladesh. PLOS Glob. Public Health 2022, 2, e0001154. [Google Scholar] [CrossRef]
- Al-Gobari, M.; El Khatib, C.; Pillon, F.; Gueyffier, F. Beta-blockers for the prevention of sudden cardiac death in heart failure patients: A meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2013, 13, 52. [Google Scholar] [CrossRef]
- Terlecki, M.; Wojciechowska, W.; Klocek, M.; Olszanecka, A.; Stolarz-Skrzypek, K.; Grodzicki, T.; Małecki, M.; Katra, B.; Garlicki, A.; Bociąga-Jasik, M.; et al. Association between cardiovascular disease, cardiovascular drug therapy, and in-hospital outcomes in patients with COVID-19: Data from a large single-center registry in Poland. Kardiol. Pol. 2021, 79, 773–780. [Google Scholar] [CrossRef]
- Jin, S.; Kostka, K.; Posada, J.D.; Kim, Y.; Seo, S.I.; Lee, D.Y.; Shah, N.H.; Roh, S.; Lim, Y.H.; Chae, S.G.; et al. Prediction of major depressive disorder following beta-blocker therapy in patients with cardiovascular diseases. J. Pers. Med. 2020, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.N.; Plante, T.B.; Infeld, M.; Callas, P.W.; Juraschek, S.P.; Dougherty, G.B.; Meyer, M. Association of β-Blocker Use with Heart Failure Hospitalizations and Cardiovascular Disease Mortality Among Patients with Heart Failure with a Preserved Ejection Fraction: A Secondary Analysis of the TOPCAT Trial. JAMA Netw. Open 2019, 2, e1916598. [Google Scholar] [CrossRef] [PubMed]
- Huz, V.S.; Zaliska, O.M. Analysis of dynamics of the drug list in the affordable medicines program for treatment of cardiovascular diseases. Farmatsevtychnyi Zhurnal 2019, 21–30. [Google Scholar] [CrossRef]
- Imaeva, A.E.; Balanova, Y.A.; Kontsevaya, A.V.; Kapustina, A.V.; Duplyakov, D.V.; Malysheva, O.H.; Osipova, I.V.; Petrichko, T.A.; Kropanin, G.I.; Kasimov, R.A.; et al. Availability and affordability of medicines for the treatment of cardiovascular diseases in pharmacies in six regions of the Russian Federation. Ration. Pharmacother. Cardiol. 2018, 14, 804–815. [Google Scholar] [CrossRef]
- Wata, D.; Ogwu, J.; Dunford, L.; Lawson, G.; Tanna, S. Utilizing quantitative dried blood spot analysis to objectively assess adherence to cardiovascular pharmacotherapy among patients at Kenyatta National Hospital, Nairobi, Kenya. PLoS ONE 2023, 18, e0280137. [Google Scholar] [CrossRef]
- Jatic, Z.; Skopljak, A.; Hebibovic, S.; Sukalo, A.; Rustempasic, E.; Valjevac, A. Effects of Different Antihypertensive Drug Combinations on Blood Pressure and Arterial Stiffness. Med Arch. 2019, 73, 157–162. [Google Scholar] [CrossRef]
- Lee, W.; Kang, J.; Park, J.B.; Seo, W.W.; Lee, S.Y.; Lim, W.H.; Jeon, K.H.; Hwang, I.C.; Kim, H.L. Long-term mortality and cardiovascular events of seven angiotensin receptor blockers in hypertensive patients: Analysis of a national real-world database: A retrospective cohort study. Health Sci. Rep. 2023, 6, e1056. [Google Scholar] [CrossRef]
- Netiazhenko, V.Z. Infusion therapy for cardiovascular diseases: The allowed limits. Infus. Chemother. 2020, 227–230. [Google Scholar] [CrossRef]
- Filippova, A.V.; Ostroumova, O.D. Drug-induced pancreatitis: Focus on drugs used to treat cardiovascular disease. Med. Alph. 2021, 37–42. [Google Scholar] [CrossRef]
- Musa, M. Supply of medicines by humanitarian organizations in war conditions. Tech. BioChemMed 2022, 4, 45–53. [Google Scholar] [CrossRef]
- Farpour-Lambert, N.J.; Martin, X.E.; Bucher Della Torre, S.; von Haller, L.; Ells, L.J.; Herrmann, F.R.; Aggoun, Y. Effectiveness of individual and group programmes to treat obesity and reduce cardiovascular disease risk factors in pre-pubertal children. Clin. Obes. 2019, 9, e12335. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Tang, Y.; Li, B.; Tang, J.; Xu, H.; Zhao, K.; Zhang, X. Stachydrine, a potential drug for the treatment of cardiovascular system and central nervous system diseases. Biomed. Pharmacother. 2023, 161, 114489. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhao, Z.; Simmons, D. Cardiovascular risks and bleeding with non-vitamin K antagonist oral anticoagulant versus warfarin in patients with type 2 diabetes: A tapered matching cohort study. Cardiovasc. Diabetol. 2020, 19, 174. [Google Scholar] [CrossRef] [PubMed]
- Freemantle, N.; Lafuente-Lafuente, C.; Mitchell, S.; Eckert, L.; Reynolds, M. Mixed treatment comparison of dronedarone, amiodarone, sotalol, flecainide, and propafenone, for the management of atrial fibrillation. Europace 2011, 13, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.C.; Silka, M.J.; Bar-Cohen, Y. A comparison of oral flecainide and amiodarone for the treatment of recurrent supraventricular tachycardia in children. PACE-Pacing Clin. Electrophysiol. 2019, 42, 670–677. [Google Scholar] [CrossRef]
- Mavrides, N.; Nemeroff, C.B. Treatment of affective disorders in cardiac disease. Dialogues Clin. Neurosci. 2015, 17, 127–140. [Google Scholar] [CrossRef]
- Franconi, F.; Brunelleschi, S.; Steardo, L.; Cuomo, V. Gender differences in drug responses. Pharmacol. Res. 2007, 55, 81–95. [Google Scholar] [CrossRef]
- Sadee, W.; Wang, D.; Hartmann, K.; Toland, A.E. Pharmacogenomics: Driving Personalized Medicine. Pharmacol. Rev. 2023, 75, 789–814. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Y. Gut microbiome and its meta-omics perspectives: Profound implications for cardiovascular diseases. Gut Microbes 2021, 13, 1936379. [Google Scholar] [CrossRef]
- Kyaw, T.S.; Sandy, M.; Trepka, K.; Goh, J.J.; Yu, K.; Dimassa, V.; Bess, E.N.; Bisanz, J.E.; Turnbaugh, P. Human Gut Actinobacteria Boost Drug Absorption by Secreting P-Glycoprotein ATPase Inhibitors. SSRN Electron. J. 2022, 27, 110122. [Google Scholar] [CrossRef]
- Kumar, K.; Jaiswal, S.K.; Dhoke, G.V.; Srivastava, G.N.; Sharma, A.K.; Sharma, V.K. Mechanistic and structural insight into promiscuity based metabolism of cardiac drug digoxin by gut microbial enzyme. J. Cell Biochem. 2018, 119, 5287–5296. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Q.; Liu, Y.; Wang, L.; Ge, Z.; Li, Z.; Feng, S.; Wu, C. The Novel Interplay between Commensal Gut Bacteria and Metabolites in Diet-Induced Hyperlipidemic Rats Treated with Simvastatin. J. Proteome Res. 2022, 21, 808–821. [Google Scholar] [CrossRef]
- Vourakis, M.; Mayer, G.; Rousseau, G. The role of gut microbiota on cholesterol metabolism in atherosclerosis. Int. J. Mol. Sci. 2021, 22, 8074. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, A.P.; Murugesan, S.; Al Khodor, S.; Terranegra, A. The potential impact of a probiotic: Akkermansia muciniphila in the regulation of blood pressure—The current facts and evidence. J. Transl. Med. 2022, 20, 430. [Google Scholar] [CrossRef] [PubMed]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef]
- Sanz, M.; Marco del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Noites, R.; Teixeira, M.; Cavero-Redondo, I.; Alvarez-Bueno, C.; Ribeiro, F. Apical Periodontitis and Cardiovascular Disease in Adults: A Systematic Review with Meta-Analysis. Rev. Cardiovasc. Med. 2022, 23, 0100. [Google Scholar] [CrossRef]
- James, K.L.; Mogen, A.B.; Brandwein, J.N.; Orsini, S.S.; Ridder, M.J.; Markiewicz, M.A.; Bose, J.L.; Rice, K.C. Interplay of Nitric Oxide Synthase (NOS) and SrrAB in modulation of staphylococcus aureus metabolism and virulence. Infect. Immun. 2019, 87, e00570-18. [Google Scholar] [CrossRef]
- Abbas, M.; Constantin, M.I.; Narendra, A. Pylephlebitis Caused by Fusobacterium nucleatum in a Septuagenarian Healthy Caucasian Male: Atypical Presentation of Lemierre’s Syndrome. Case Rep. Infect. Dis. 2022, 2022, 5160408. [Google Scholar] [CrossRef]
- Da Silveira, T.M.; Silva, C.F.E.; Voucher, R.D.A.; Melchiors Angst, P.D.; Casarin, M.; Pola, N.M. Higher frequency of specific periodontopathogens in hypertensive patients. A pilot study. Braz. Dent. J. 2022, 33, 64–73. [Google Scholar] [CrossRef]
- Lai, Y.; Chu, X.; Di, L.; Gao, W.; Guo, Y.; Liu, X.; Lu, C.; Mao, J.; Shen, H.; Tang, H.; et al. Recent advances in the translation of drug metabolism and pharmacokinetics science for drug discovery and development. Acta Pharm. Sin. B 2022, 12, 2751–2777. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.N.; Surapaneni, S. Role of drug metabolism in drug discovery and development. Med. Res. Rev. 2001, 21, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Kramlinger, V.M.; Dalvie, D.; Heck, C.J.S.; Kalgutkar, A.S.; O’Neill, J.; Su, D.; Teitelbaum, A.M.; Totah, R.A. Future of Biotransformation Science in the Pharmaceutical Industry. Drug Metab. Dispos. 2022, 50, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Kebamo, S.; Tesema, S. The Role of Biotransformation in Drug Discovery and Development. J. Drug Metab. Toxicol. 2015, 6, 2. [Google Scholar] [CrossRef]
- Dhurjad, P.; Dhavaliker, C.; Gupta, K.; Sonti, R. Exploring Drug Metabolism by the Gut Microbiota: Modes of Metabolism and Experimental Approaches. Drug Metab. Dispos. 2022, 50, 224–234. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Q.; Yang, M.; Liu, D.; Hou, X.; Tang, L.; Wang, X.; Lyu, Y.; Chen, X.; Liu, K.; et al. Current trends in drug metabolism and pharmacokinetics. Acta Pharm. Sin. B 2019, 9, 1113–1144. [Google Scholar] [CrossRef]
- Li, H.; He, J.; Jia, W. The influence of gut microbiota on drug metabolism and toxicity. Expert Opin. Drug Metab. Toxicol. 2016, 12, 31–40. [Google Scholar] [CrossRef]
- Koppel, N.; Rekdal, V.M.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356, 6344. [Google Scholar] [CrossRef]
- Pant, A.; Maiti, T.K.; Mahajan, D.; Das, B. Human Gut Microbiota and Drug Metabolism. Microb. Ecol. 2023, 86, 97–111. [Google Scholar] [CrossRef]
- Spanogiannopoulos, P.; Bess, E.N.; Carmody, R.N.; Turnbaugh, P.J. The microbial pharmacists within us: A metagenomic view of xenobiotic metabolism. Nat. Rev. Microbiol. 2016, 14, 273–287. [Google Scholar] [CrossRef]
- Najjar, A.; Najjar, A.; Karaman, R. Newly developed prodrugs and prodrugs in development; an insight of the recent years. Molecules 2020, 25, 884. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Mayers, J.R.; Zhang, Y.; Bhosle, A.; Glasser, N.R.; Nguyen, L.H.; Ma, W.; Bae, S.; Branck, T.; Song, K.; et al. Gut microbial metabolism of 5-ASA diminishes its clinical efficacy in inflammatory bowel disease. Nat. Med. 2023, 29, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Gupta, S.K.; Singh, P.; Ali, V.; Kumar, V.; Verma, M. Drug-metabolizing enzymes: Role in drug resistance in cancer. Clin. Transl. Oncol. 2020, 22, 1667–1680. [Google Scholar] [CrossRef]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug Metabolism in the Liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef]
- Forslund, S.K. Fasting intervention and its clinical effects on the human host and microbiome. J. Intern. Med. 2023, 293, 166–183. [Google Scholar] [CrossRef]
- Selwyn, F.P.; Cheng, S.L.; Bammler, T.K.; Prasad, B.; Vrana, M.; Klaassen, C.; Cui, J.Y. Developmental regulation of drug-processing genes in livers of germ-free mice. Toxicol. Sci. 2015, 147, 84–103. [Google Scholar] [CrossRef]
- Wilson, I.D.; Nicholson, J.K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 2017, 179, 204–222. [Google Scholar] [CrossRef]
- Zimmermann, M.; Patil, K.R.; Typas, A.; Maier, L. Towards a mechanistic understanding of reciprocal drug–microbiome interactions. Mol. Syst. Biol. 2021, 17, e10116. [Google Scholar] [CrossRef]
- Eusuf, D.V.; Thomas, E. Pharmacokinetic variation. Anaesth. Intensive Care Med. 2022, 23, 50–53. [Google Scholar] [CrossRef]
- Zimmermann-Kogadeeva, M.; Zimmermann, M.; Goodman, A.L. Insights from pharmacokinetic models of host-microbiome drug metabolism. Gut Microbes 2020, 11, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, S.M.; Gonzales, C.; Jarmusch, A.K.; Momper, J.D.; Ma, J.D. Contribution of the Gut Microbiome to Drug Disposition, Pharmacokinetic and Pharmacodynamic Variability. Clin. Pharmacokinet. 2021, 60, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.; Sarfraz, M.; Mousa, W.K. The Interplay between Gut Microbiota and Oral Medications and Its Impact on Advancing Precision Medicine. Metabolites 2023, 13, 674. [Google Scholar] [CrossRef] [PubMed]
- Grosheva, I.; Zheng, D.; Levy, M.; Polansky, O.; Lichtenstein, A.; Golani, O.; Dori-Bachash, M.; Moresi, C.; Shapiro, H.; Del Mare-Roumani, S.; et al. High-Throughput Screen Identifies Host and Microbiota Regulators of Intestinal Barrier Function. Gastroenterology 2020, 159, 1807–1823. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Chang, S.H.; Ko, Y.F.; Hwang, T.L.; Young, J.D.; Ojcius, D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. 2022, 33, 247–265. [Google Scholar] [CrossRef]
- Maher, S.; Leonard, T.W.; Jacobsen, J.; Brayden, D.J. Safety and efficacy of sodium caprate in promoting oral drug absorption: From in vitro to the clinic. Adv. Drug Deliv. Rev. 2009, 61, 1427–1449. [Google Scholar] [CrossRef]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Cao, Z.; Li, W.; Li, H.; Lu, C.; Yang, X.; Liu, Y. Gut microbiota as an “invisible organ” that modulates the function of drugs. Biomed. Pharmacother. 2020, 121, 109653. [Google Scholar] [CrossRef]
- Jia, B.; Zou, Y.; Han, X.; Bae, J.W.; Jeon, C.O. Gut microbiome-mediated mechanisms for reducing cholesterol levels: Implications for ameliorating cardiovascular disease. Trends Microbiol. 2023, 31, 76–91. [Google Scholar] [CrossRef]
- Raju, S.C.; Viljakainen, H.; Figueiredo, R.A.O.; Neuvonen, P.J.; Eriksson, J.G.; Weiderpass, E.; Rounge, T.B. Antimicrobial drug use in the first decade of life influences saliva microbiota diversity and composition. Microbiome 2020, 8, 121. [Google Scholar] [CrossRef]
- Sun, B.; Li, L.; Zhou, X. Comparative analysis of the gut microbiota in distinct statin response patients in East China. J. Microbiol. 2018, 56, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Saberianpour, S.; Abolbashari, S.; Modaghegh, M.H.S.; Karimian, M.S.; Eid, A.H.; Sathyapalan, T.; Sahebkar, A. Therapeutic effects of statins on osteoarthritis: A review. J. Cell. Biochem. 2022, 123, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Caparrós-Martín, J.A.; Lareu, R.R.; Ramsay, J.P.; Peplies, J.; Reen, F.J.; Headlam, H.A.; Ward, N.C.; Croft, K.D.; News-holme, P.; Hughes, J.D.; et al. Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M.; Radjabzadeh, D.; Kiefte-de Jong, J.C.; Uitterlinden, A.G.; Kraaij, R.; Stricker, B.H.; Verbon, A. Long-term effects of antimicrobial drugs on the composition of the human gut microbiota. Gut Microbes 2020, 12, 1795492. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Lin, T.Y.; Hung, S.C.; Liu, P.Y.; Hung, W.C.; Tsai, W.C.; Tsai, Y.C.; Delicano, R.A.; Chuang, Y.S.; Kuo, M.C.; et al. Differences in the microbial composition of hemodialysis patients treated with and without β-blockers. J. Pers. Med. 2021, 11, 198. [Google Scholar] [CrossRef]
- Talathi, S.; Wilkinson, L.; Meloni, K.; Shroyer, M.; Zhang, L.; Ding, Z.; Eipers, P.; Van Der Pol, W.; Martin, C.; Dimmitt, R.; et al. Factors Affecting the Gut Microbiome in Pediatric Intestinal Failure. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 426–432. [Google Scholar] [CrossRef]
- Tabata, T.; Yamashita, T.; Hosomi, K.; Park, J.; Hayashi, T.; Yoshida, N.; Saito, Y.; Fukuzawa, K.; Konishi, K.; Murakami, H.; et al. Gut microbial composition in patients with atrial fibrillation: Effects of diet and drugs. Heart Vessel. 2021, 36, 105–114. [Google Scholar] [CrossRef]
- Kamińska, M.; Aliko, A.; Hellvard, A.; Bielecka, E.; Binder, V.; Marczyk, A.; Potempa, J.; Delaleu, N.; Kantyka, T.; Mydel, P. Effects of statins on multispecies oral biofilm identify simvastatin as a drug candidate targeting Porphyromonas gingivalis. J. Periodontol. 2019, 90, 637–646. [Google Scholar] [CrossRef]
- DeClercq, V.; Nearing, J.T.; Langille, M.G.I. Investigation of the impact of commonly used medications on the oral microbiome of individuals living without major chronic conditions. PLoS ONE 2021, 16, e0261032. [Google Scholar] [CrossRef]
- Kato-Kogoe, N.; Sakaguchi, S.; Kamiya, K.; Omori, M.; Gu, Y.H.; Ito, Y.; Nakamura, S.; Nakano, T.; Tamaki, J.; Ueno, T.; et al. Characterization of Salivary Microbiota in Patients with Atherosclerotic Cardiovascular Disease: A Case-Control Study. J. Atheroscler. Thromb. 2022, 29, 403–421. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmarathne, G.; Kazi, S.; King, S.; Jayasinghe, T.N. The Bidirectional Relationship Between Cardiovascular Medications and Oral and Gut Microbiome Health: A Comprehensive Review. Microorganisms 2024, 12, 2246. https://doi.org/10.3390/microorganisms12112246
Dharmarathne G, Kazi S, King S, Jayasinghe TN. The Bidirectional Relationship Between Cardiovascular Medications and Oral and Gut Microbiome Health: A Comprehensive Review. Microorganisms. 2024; 12(11):2246. https://doi.org/10.3390/microorganisms12112246
Chicago/Turabian StyleDharmarathne, Gangani, Samia Kazi, Shalinie King, and Thilini N. Jayasinghe. 2024. "The Bidirectional Relationship Between Cardiovascular Medications and Oral and Gut Microbiome Health: A Comprehensive Review" Microorganisms 12, no. 11: 2246. https://doi.org/10.3390/microorganisms12112246
APA StyleDharmarathne, G., Kazi, S., King, S., & Jayasinghe, T. N. (2024). The Bidirectional Relationship Between Cardiovascular Medications and Oral and Gut Microbiome Health: A Comprehensive Review. Microorganisms, 12(11), 2246. https://doi.org/10.3390/microorganisms12112246





