Effect of Spirulina on the Rumen Microbiota and Serum Biochemical Parameters of Lambs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals, Experimental Design and Feeding Management
2.3. Serum Parameters Analysis
2.4. Rumen Sample Collection, DNA Extraction, 16S rRNA Gene Amplification and Sequencing
2.5. Statistical Analysis
3. Results
3.1. Analysis of the Diet
3.2. Effects of Spirulina on Serum Biochemical Parameters in Lambs
3.3. Effects of Spirulina on Serum Immunity Statuses in Lambs
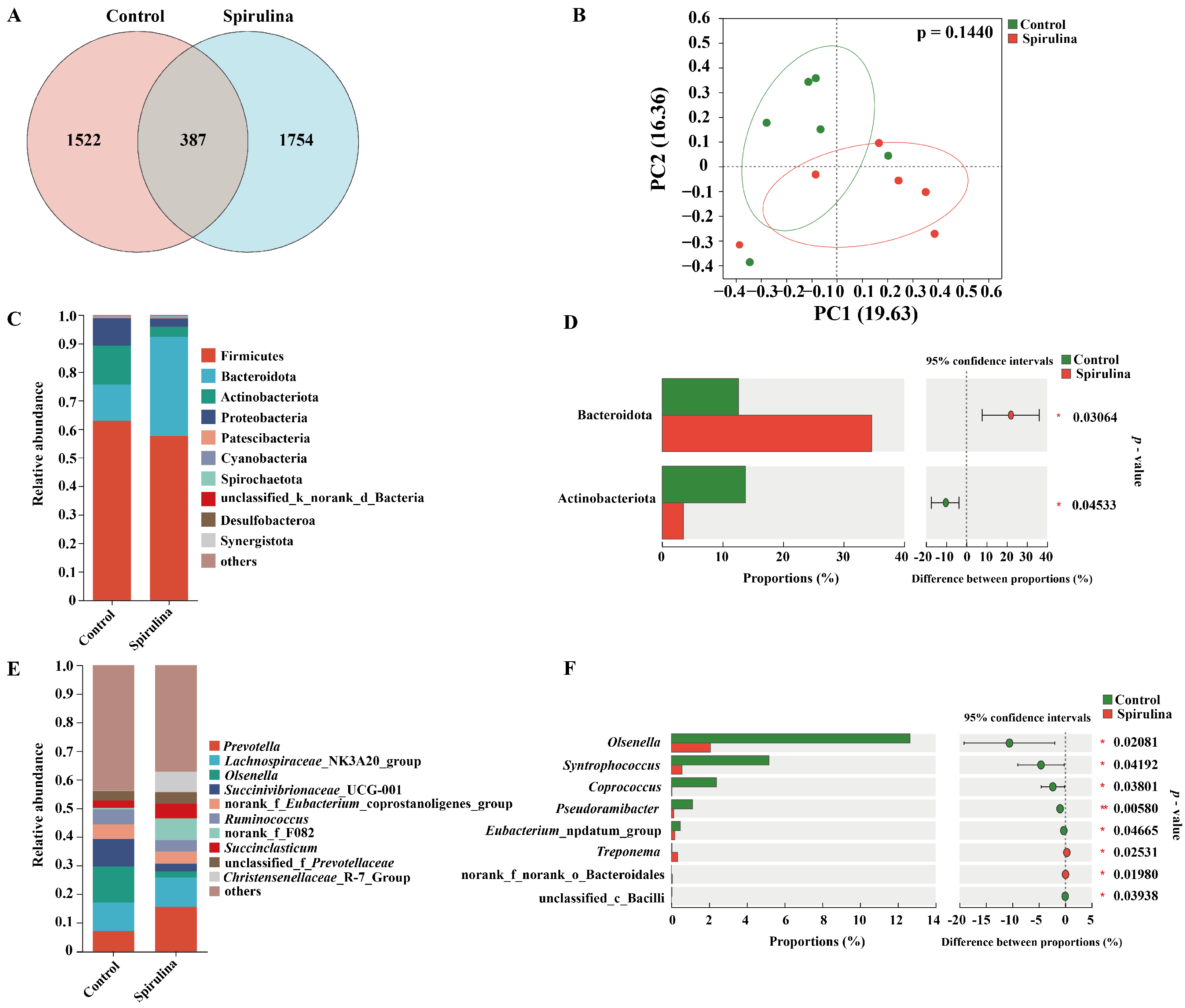
3.4. Rumen Microbiota Diversity Analysis
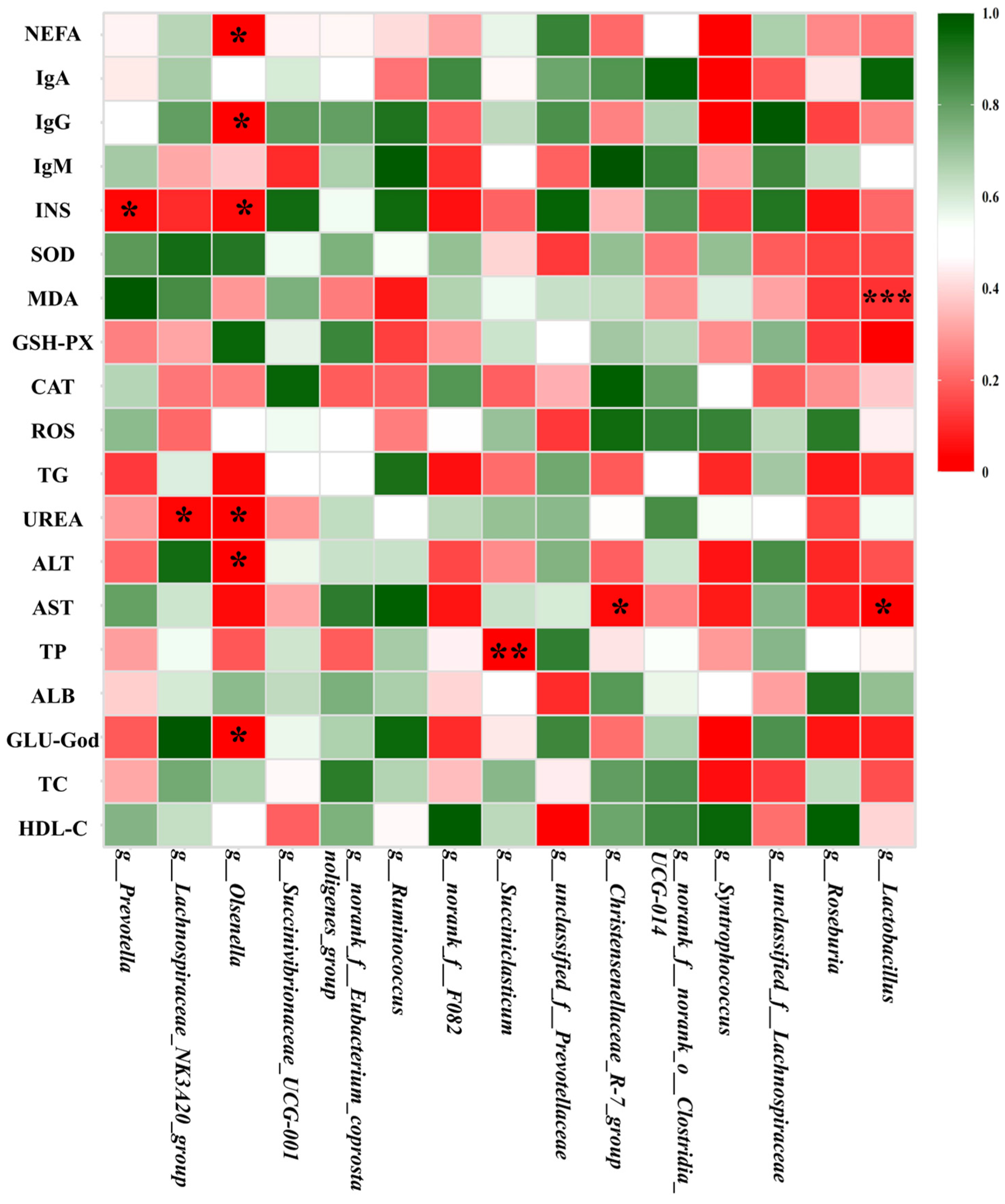
3.5. Mantel Analysis Between the Predominant Rumen Bacteria and Serum Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Holman, B.W.B.; Kashani, A.; Malau-Aduli, A.E.O. Effects of Spirulina (Arthrospira platensis) supplementation level and basal diet on liveweight, body conformation and growth traits in genetically divergent Australian dual-purpose lambs during simulated drought and typical pasture grazing. Small Rumin. Res. 2014, 120, 6–14. [Google Scholar] [CrossRef]
- Beheshtipour, H.; Mortazavian, A.M.; Haratian, P.; Darani, K.K. Effects of Chlorella vulgaris and Arthrospira platensis addition on viability of probiotic bacteria in yogurt and its biochemical properties. Eur. Food Res. Technol. 2012, 235, 719–728. [Google Scholar] [CrossRef]
- Christodoulou, C.; Kotsampasi, B.; Dotas, V.; Simoni, M.; Righi, F.; Tsiplakou, E. The effect of Spirulina supplementation in ewes’ oxidative status and milk quality. Anim. Feed. Sci. Technol. 2022, 295, 115544. [Google Scholar] [CrossRef]
- Alharthi, A.S.; Al-Baadani, H.H.; Alghonaim, A.A.; Al-Garadi, M.A.; Alowaimer, A.N.; Alhidary, I.A. Effects of Spirulina platensis addition on performance, immune response, hematological, selected bacteria activity and rumen morphology of lambs. Ital. J. Anim. Sci. 2024, 23, 1134–1145. [Google Scholar] [CrossRef]
- Christodoulou, C.; Mavrommatis, A.; Loukovitis, D.; Symeon, G.; Dotas, V.; Kotsampasi, B.; Tsiplakou, E. Effect of Spirulina Dietary Supplementation in Modifying the Rumen Microbiota of Ewes. Animals 2023, 13, 740. [Google Scholar] [CrossRef]
- Burgat, V. Residues of drugs of veterinary use in food. LaRevue Du Prat. 1999, 41, 985–990. [Google Scholar]
- Uyisenga, J.P.; Nzayino, P.; Seneza, R.; Hishamunda, L.; Uwantege, K.; Gasana, N.; Bajyana, E.S. In vitro study of antibacterial and antifungal activity of Spirulina platensis. Int. J. Ecol. Dev. 2010, 16, 80–88. [Google Scholar]
- Abdel-Daim, M.M.; Abuzead, S.M.M.; Halawa, S.M. Protective Role of Spirulina platensis against Acute Deltamethrin-Induced Toxicity in Rats. PLoS ONE 2013, 8, e72991. [Google Scholar] [CrossRef]
- Shokri, H.; Khosravi, A.R.; Taghavi, M. Efficacy of Spirulina platensis on immune functions in cancer mice with systemic candidiasis. J. Mycol. Res. 2014, 1, 7–13. [Google Scholar]
- AbouGabal, A.; Aboul-Ela, H.M.; Ali, E.A.; Khaled, A.E.; Shalaby, O.K. Hepatoprotective, DNA Damage Prevention and Antioxidant Potential of Spirulina platensis on CCl4-Induced Hepatotoxicity in Mice. Am. J. Biomed. Res. 2015, 3, 29–34. [Google Scholar]
- Baghban-Kanani, P.; Azimi-Youvalari, S.; Hosseintabar-Ghasemabad, B.; Slozhenkina, M.; Gorlov, I.; Seidavi, A.; Ayaşan, T.; Laudadio, V. Effects of Horsetail (Equisetum arvense) and Spirulina (Spirulina platensis) Dietary Supplementation on Laying Hens Productivity and Oxidative Status. Animals 2021, 11, 335. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Malau-Aduli, A.E.O. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. 2013, 97, 615–623. [Google Scholar] [CrossRef]
- Zhang, J.G.; Qi, H.B.; Li, M.H.; Wang, Z.H.; Jia, X.F.; Sun, T.; Du, S.; Su, C.; Zhi, M.; Du, W.; et al. Diet Mediate the Impact of Host Habitat on Gut Microbiome and Influence Clinical Indexes by Modulating Gut Microbes and Serum Metabolites. Adv. Sci. 2024, 11, e2310068. [Google Scholar] [CrossRef]
- Altmann, B.A.; Neumann, C.; Velten, S.; Liebert, F.; Morlein, D. Meat quality derived from high inclusion of a micro-alga or insect meal as an alternative protein source in poultry diets: A pilot study. Foods 2018, 7, 34. [Google Scholar] [CrossRef]
- Zeweil, H.S.; Im, A.; Zahran, S.; Ahmed, M.H.; Haiam, M.; As, A. Effect of Spirulina platensis as dietary supplement on some biological traits for chickens under heat stress condition. Asian J. Biomed. Pharm. Sci. 2016, 6, 8–12. [Google Scholar]
- Abdel-Moneim, A.M.E.; Shehata, A.M.; Mohamed, N.G.; Elbaz, A.M.; Ibrahim, N.S. Synergistic effect of Spirulina platensis and selenium nanoparticles on growth performance, serum metabolites, immune responses, and antioxidant capacity of heat-stressed broiler chickens. Biol. Trace Elem. Res. 2021, 200, 768–779. [Google Scholar] [CrossRef]
- Bu, Z.K.; Ge, G.T.; Jia, Y.S.; Du, S. Effect of hay with or without concentrate or pellets on growth performance and meat quality of Ujimqin lambs on the Inner Mongolian Plateau. Anim. Sci. J. 2021, 92, e13553. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, J.P.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Fossati, P.; Prencipe, L. Serum triglycerides determined calorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982, 28, 2007–2080. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Tanja, M.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar]
- SAS/STAT User’s Guide, Release 9.1 ed.; SAS Institute Inc.: Cary, NC, USA, 2007.
- Ismail, F.; Sherif, K.; Rizk, Y.; Hassan, M.; Mekawy, A.; Mahrose, K. Dietary supplementation of spirulina and canthaxanthin boosts laying performance, lipid profile in blood and egg yolk, hatchability, and semen quality of chickens. J. Anim. Physiol. Anim. Nutr. 2023, 107, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Aljumaily, T.K.H.; Taha, A.T. Effects of Spirulina platensis algae extract early feeding on Japanese quail embryos. Adv. Anim. Vet. Sci. 2019, 7, 30–37. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Abd El-Hack, E.M.; Dhama, K. Nutritional and healthical aspects of Spirulina (Arthrospira) for poultry, animals and human. Int. J. Pharmacol. 2015, 12, 36–51. [Google Scholar] [CrossRef]
- Zheng, J.; Inoguchi, T.; Sasaki, S.; Maeda, Y.; McCarty, M.F.; Fujii, M.; Ikeda, N.; Kobayashi, K.; Sonoda, K.; Takayanagi, R. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013, 304, R110–R120. [Google Scholar] [CrossRef]
- Pankaj, P.P.; Varma, M.C. Potential role of Spirulina platensis in maintaining blood parameters in alloxan induced diabetic mice. Int. J. Pharm. Pharm. Sci. 2013, 5, 450–456. [Google Scholar]
- Han, P.; Li Jingjing Zhong, H.; Xie, J.; Zhang, P.; Lu, Q.; Li Jun Xu, P.; Chen, P.; Leng, L.; Zhou, W. Anti-oxidation properties and therapeutic potentials of spirulina. Algal Res. 2021, 55, 102240. [Google Scholar] [CrossRef]
- Khieokhajonkhet, A.; Aeksiri, N.; Ratanasut, K.; Kannika, K.; Suwannalers, P.; Tatsapong, P.; Inyawilert, W.; Kaneko, G. Effects of dietary Hericium erinaceus powder on growth, hematology, disease resistance, and expression of genes related immune response against thermal challenge of Nile tilapia (Oreochromis niloticus). Anim. Feed. Sci. Technol. 2022, 290, 115342. [Google Scholar] [CrossRef]
- Watcharachaisoponsiri, T.; Sornchan, P.; Charoenkiatkul, S.; Suttisansanee, U. The α-glucosidase and α-amylase inhibitory activity from different chili pepper extracts. Int. Food Res. J. 2016, 23, 1439–1455. [Google Scholar]
- Khieokhajonkhet, A.; Suwannalers, P.; Aeksiri, N.; Ratanasut, K.; Chitmanat, C.; Inyawilert, W.; Phromkunthong, W.; Kaneko, G. Effects of dietary red pepper extracts on growth, hematology, pigmentation, disease resistance, and growth- and immune-related gene expressions of goldfish (Carassius auratus). Anim. Feed Sci. Technol. 2023, 301, 115658. [Google Scholar] [CrossRef]
- Kolluri, G.; Marappan, G.; Yadav, A.S.; Kumar, A.; Mariappan, A.K.; Tyagi, J.S.; Rokade, J.J.; Govinthasamy, P. Effects of Spirulina (Arthrospira platensis) as a drinking water supplement during cyclical chronic heat stress on broiler chickens: Assessing algal composition, production, stress, health and immune-biochemical indices. J. Therm. Biol. 2022, 103, 103100. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Liu, Y.-Y.; Wan, X.Z.; Huang, Z.R.; Liu, B.; Zhao, C. Regulatory efficacy of the polyunsaturated fatty acids from microalgae Spirulina platensis on lipid metabolism and gut microbiota in high-fat diet rats. Int. J. Mol. Sci. 2018, 19, 3075. [Google Scholar] [CrossRef]
- Long, M.; Zhang, Y.; Li, P.; Yang, S.H.; Zhang, W.K.; Han, J.X.; Wang, Y.; He, J.B. Intervention of grape seed proanthocyanidin extract on the subchronic immune injury in mice induced by aflatoxin B1. Int. J. Mol. Sci. 2016, 17, 516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Zhang, X.T.; Jin, P.T.; Zaho, H.B.; Liu, X.; Sheng, Q.K. Effects of oral administration of Spirulina platensis and probiotics on serum immunity indexes, colonic immune factors, fecal odor, and fecal flora in mice. Anim. Sci. J. 2021, 92, e13593. [Google Scholar] [CrossRef]
- Liang, Y.X.; Bao, Y.J.; Gao, X.X.; Deng, K.P.; An, S.Y.; Wang, Z.B.; Huang, X.N.; Liu, D.; Liu, Z.N.; Wang, F.; et al. Effects of spirulina supplementation on lipid metabolism disorder, oxidative stress caused by high-energy dietary in Hu sheep. Meat Sci. 2020, 164, 108094. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímova, B.; Wan, D.; Kuca, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Movahhedkhah, S.; Rasouli, B.; Seidavi, A.; Mazzei, D.; Laudadio, V.; Tufarelli, V. Summer Savory (Satureja hortensis L.) Extract as Natural Feed Additive in Broilers: Effects on Growth, Plasma Constituents, Immune Response, and Ileal Microflora. Animals 2019, 9, 87. [Google Scholar] [CrossRef]
- Du, S.; Bu, Z.; You, S.; Bao, J.; Jia, Y. Diversity of growth performance and rumen microbiota vary with feed types. Front. Sustain. Food Syst. 2022, 6, 1004373. [Google Scholar] [CrossRef]
- Abd El-Hady, A.M.; Elghalid, O.A.; Elnaggar, A.S.; Abd El-khalek, E. Growth performance and physiological status evaluation of Spirulina platensis algae supplementation in broiler chicken diet. Livest. Sci. 2022, 263, 105009. [Google Scholar] [CrossRef]
- Xiao, Y.Z.; Sun, L.; Xin, X.P.; Xu, L.J.; Du, S. Physicochemical characteristics and microbial community succession during oat silage prepared without or with Lactiplantibacillus plantarum or Lentilactobacillus buchneri. Microbiol. Spectr. 2023, 11, e0222823. [Google Scholar] [CrossRef]
- Li, L.; Guo, W.L.; Zhang, W.; Xu, J.X.; Qian, M.; Bai, W.D.; Zhang, Y.Y.; Rao, P.F.; Ni, L.; Lv, X.C. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Huws, S.A.; Edwards, J.E.; Creevey, C.J.; Stevens, P.R.; Lin, W.C.; Girdwood, S.E.; Pachebat, J.A.; Kingston-Smith, A.H. Temporal dynamics of the metabolaically active rumen bacteria colonizing fresh perennial ryegrass. FEMS Microbiol. Ecol. 2016, 92, 137. [Google Scholar] [CrossRef] [PubMed]
- Vibart, R.E.; Ganesh, S.; Kirk, M.R.; Kittelmann, S.; Leahy, S.C.; Janssen, P.H.; Pacheco, D. Temporal fermentation and microbial community dynamics in rumens of sheep grazing a ryegrass-based pasture offered either in the morning or in the afternoon. Animal 2019, 2, 2251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, C.; Niu, X.; Zhang, Z.; Li, F. The effects of milk replacer allowance and weaning age on the performance, nutrients digestibility, and ruminal microbiota communities of lambs. Anim. Feed Sci. Technol. 2019, 257, 114263. [Google Scholar] [CrossRef]
- Li, T.T.; Huang, Z.R.; Jia, R.B.; Lv, X.C.; Zhao, C.; Liu, B. Spirulina platensis polysaccharides attenuate lipid and carbohydrate metabolism disorder in high-sucrose and high-fat diet-fed rats in association with intestinal microbiota. Food Res. Int. 2021, 147, 110530. [Google Scholar] [CrossRef]
- Wang, G.; Jiao, T.; Xu, Y.; Li, D.; Si, Q.; Hao, J.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium adolescentis and Lactobacillus rhamnosus alleviate non-alcoholic fatty liver disease induced by a high-fat, high-cholesterol diet through modulation of different gut microbiota-dependent pathways. Food Funct. 2020, 11, 6115–6127. [Google Scholar] [CrossRef] [PubMed]
| Item | CK | Spirulina |
|---|---|---|
| Ingredients (%) | ||
| Maize | 42.63 | 42.63 |
| Soybean meal | 19.60 | 19.60 |
| Flax | 7.84 | 7.84 |
| Alfalfa | 16.10 | 15.80 |
| Wheat bran | 5.88 | 5.88 |
| Premix | 5.00 | 5.00 |
| Salt | 0.98 | 0.98 |
| Soda | 1.17 | 1.17 |
| Hawthorn | 0.10 | 0.10 |
| Malt | 0.10 | 0.10 |
| Dried tangerine peel | 0.10 | 0.10 |
| Medicated Leaven | 0.10 | 0.10 |
| Astragalus membranaceus | 0.10 | 0.10 |
| Atractylodes macrocephala | 0.10 | 0.10 |
| Licorice | 0.10 | 0.10 |
| Epimedium | 0.10 | 0.10 |
| Spirulina | 0.00 | 0.30 |
| Chemical compositions | ||
| Dry matter (%) | 66.69 | 67.03 |
| Crude protein (%DM) | 19.56 | 19.11 |
| Acid detergent fiber (%DM) | 14.21 | 14.34 |
| Neutral detergent fiber (%DM) | 24.42 | 24.33 |
| Item | CK | Spirulina | p-Value |
|---|---|---|---|
| Triacylglycerol (mmol/L) | 0.16 ± 0.01 b | 0.41 ± 0.02 a | <0.0001 |
| Urea (mmol/L) | 3.02 ± 0.12 | 2.97 ± 0.14 | 0.8516 |
| ALT (U/L) | 11.35 ± 1.16 b | 40.98 ± 1.50 a | <0.0001 |
| AST (U/L) | 74.63 ± 2.07 b | 191.73 ± 17.33 a | <0.0001 |
| Total protein (g/L) | 65.88 ± 2.30 | 66.58 ± 1.26 | 0.7950 |
| Albumin (g/L) | 22.60 ± 0.75 | 21.00 ± 0.43 | 0.0928 |
| Glucose (mmol/L) | 5.77 ± 0.20 b | 13.40 ± 0.33 a | <0.0001 |
| Total cholesterol (mmol/L) | 1.34 ± 0.08 | 1.14 ± 0.05 | 0.0657 |
| HDL-C (mmol/L) | 0.44 ± 0.09 | 0.42 ± 0.02 | 0.8487 |
| LDL-C (mmol/L) | 0.40 ± 0.04 | 0.33 ± 0.02 | 0.1217 |
| Item | CK | Spirulina | p-Value |
|---|---|---|---|
| NEFAs (mmol/L) | 0.65 ± 0.05 a | 0.45 ± 0.03 b | 0.0055 |
| IgA (g/L) | 0.35 ± 0.02 | 0.34 ± 0.01 | 0.5625 |
| IgG (g/L) | 8.30 ± 0.34 b | 9.73 ± 0.24 a | 0.0066 |
| IgM (g/L) | 0.57 ± 0.02 | 0.59 ± 0.02 | 0.5219 |
| Insulin (uIU/mL) | 11.13 ± 0.26 b | 13.28 ± 0.47 a | 0.0025 |
| SOD (U/mL) | 79.22 ± 01.50 | 79.43 ± 1.14 | 0.9136 |
| MDA (nmol/mL) | 2.66 ± 0.21 | 3.23 ± 0.27 | 0.1308 |
| GSH-Px (U/mL) | 176.28 ± 5.70 | 178.92 ± 5.10 | 0.8621 |
| CAT (U/mL) | 40.17 ± 1.32 | 43.87 ± 0.84 | 0.1731 |
| Item | CK | Spirulina | p-Value | Total No. |
|---|---|---|---|---|
| No. of sequences | 73,420 ± 3169 | 76,627 ± 4896 | 0.5939 | 900,284 |
| No. of valid sequences | 40,392 ± 2385 | 40,323 ± 1596 | 0.9813 | 484,294 |
| Observed ASV number | 468 ± 70 | 428 ± 69 | 0.6925 | |
| Good’s coverage | >0.99 | >0.99 | 0.9221 | |
| Chao1 value | 470.98 ± 71.12 | 429.81 ± 70.61 | 0.6899 | |
| Shannon index | 4.03 ± 0.22 | 3.08 ± 0.29 | 0.6838 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liu, X.; Zhao, M.; Ma, W.; Wang, Y.; Jia, Y.; Ge, G. Effect of Spirulina on the Rumen Microbiota and Serum Biochemical Parameters of Lambs. Microorganisms 2024, 12, 2473. https://doi.org/10.3390/microorganisms12122473
Wang Z, Liu X, Zhao M, Ma W, Wang Y, Jia Y, Ge G. Effect of Spirulina on the Rumen Microbiota and Serum Biochemical Parameters of Lambs. Microorganisms. 2024; 12(12):2473. https://doi.org/10.3390/microorganisms12122473
Chicago/Turabian StyleWang, Zhijun, Xiangdong Liu, Muqier Zhao, Weiqin Ma, Yuxuan Wang, Yushan Jia, and Gentu Ge. 2024. "Effect of Spirulina on the Rumen Microbiota and Serum Biochemical Parameters of Lambs" Microorganisms 12, no. 12: 2473. https://doi.org/10.3390/microorganisms12122473
APA StyleWang, Z., Liu, X., Zhao, M., Ma, W., Wang, Y., Jia, Y., & Ge, G. (2024). Effect of Spirulina on the Rumen Microbiota and Serum Biochemical Parameters of Lambs. Microorganisms, 12(12), 2473. https://doi.org/10.3390/microorganisms12122473





