Abstract
The killer systems of S. cerevisiae are defined by the co-infection of two viral agents, an M virus and a helper virus. Each killer toxin is determined by the type of M virus (ScV-M1, ScV-M2, ScV-M28, and ScV-Mlus), which encodes a specific toxin (K1, K2, K28, and Klus). Since their discovery, interest in their potential use as antimicrobial agents has driven research into the mechanisms of action of these toxins on susceptible cells. This review provides an overview of the key aspects of killer toxins, including their origin and the evolutionary implications surrounding the viruses involved in the killer system, as well as their potential applications in the biomedical field and as a biological control strategy. Special attention is given to the mechanisms of action described to date for the various S. cerevisiae killer toxins.
1. Introduction
The discovery of Saccharomyces cerevisiae strains infected with double-stranded RNA (dsRNA) viruses capable of killing other “sensitive” strains marked the inception of research into killer strains [1,2]. It was then demonstrated that only specific yeast strains could secrete protein-based toxins, resulting in lethal effects on sensitive strains. These secreting strains were termed “killer yeast”, with the secreted protein referred to as “killer toxin”. Subsequent investigations revealed that toxin-secreting strains possess antifungal activity [3,4], and surprisingly, other fungi might also produce similar toxins, such as Zygosaccharomyces bailii, Hanseniaspora uvarum, and Ustilago maydis [5,6,7]. Furthermore, it was discovered that not only do dsRNA viruses confer the coding genes for toxins, but toxins encoded in chromosome and dsDNA plasmids have also been identified [4,8,9,10,11,12].
To date, the primary viruses encoding killer toxins in S. cerevisiae include the M viruses ScV-M1, ScV-M2, ScV-M28, and ScV-Mlus, each encoding a specific toxin (K1, K2, K28, and Klus) and reliant on the coexistence of an L-A helper virus to sustain their replication within the yeast host [13]. These viruses belong to the Totiviridae family, encompassing five viral genera: Giardiavirus, Leishmaniavirus, Trichomonasvirus, Victorivirus, and Totivirus.
ScV-L-A (S. cerevisiae Virus L-A) virions contain a single linear (+) dsRNA molecule ranging from 4.6 to 7 kbp in length. This genome comprises two ORFs: the 5′ ORF encodes the capsid protein Gag, which possesses the ability to bind and remove the 5′-cap of host mRNA via the Gln139-Ser182 region and its interaction with His154 of Gag. This mechanism employs host mRNA as a decoy for the 5′-3′ exoribonuclease, thereby safeguarding the viral genome [14]. The 3′ ORF encodes RdRp (RNA-dependent RNA polymerase) and exhibits ssRNA binding activity, along with capsid structures displaying T = 2* symmetry, predominantly composed of a single coat protein (CP) [15]. The Gag monomer can assume two conformations, A and B, each with significant structural disparities. The capsid consists of 12 AB decamers, wherein each decamer comprises 5 A subunits alternating with 5 B subunits to form a decameric structure [14]. Unlike other viruses, the capsid of ScV-L-A is less densely packed, facilitating enhanced mobility of the template strand and conferring a notable advantage to the virion.
Both L-A and M viruses are regulated by specific factors within the yeast host. The SKI exosome complex, comprising Ski3, Ski8, and the DEVH ATPase Ski2, facilitated by the GTPase Ski7 [16,17,18,19], governs the levels of viruses within the cell [20,21]. Reduced expression levels of these genes have been demonstrated to disrupt viral RNA processes, resulting in the “super killer” phenotype [22,23]. Furthermore, these viruses exhibit high sensitivity to alterations in ribosomal structure [22]. Among the MAK (Maintenance of Killer) genes, mutations in MAK3 (Gag catalytic N-acetyltransferase subunit), MAK10, and MAK31 genes lead to decreased expression of ribosomal subunit 60, thereby disrupting viral RNA replication processes. These genes constitute the NatC acetyltransferase complex, which acetylates the L-A virus Gag capsid protein and contributes to ribosomal maintenance [24,25,26].
2. Evolution of the Family Totiviridae
In recent years, viruses belonging to the Totiviridae family have emerged as a significant model for investigating viral evolution and interactions. It has been revealed that these viruses exhibit a broader host diversity than previously recognized, and the phylogenetic relationships among open reading frames (ORFs) within this family are notably variable. An illustrative example of this variability is UmV-H1, a dsRNA virus hosted by the fungus Ustilago maydis. A study demonstrated that its RdRp shows closer phylogenetic affinity to insect dsRNA viruses compared to other viruses within the same family [27]. Furthermore, two new genera have been proposed within the Totiviridae family: Artivirus, which infects arthropods and some fish species, and Insevirus, which primarily infects insects [28]. Moreover, the Totiviridae family displays distinctive genomic elements, such as type 2A sequences. These sequences consist of oligopeptides containing 8 to 9 amino acid “signature” sequences that regulate the ribosome skipping effect. They are analogous to internal ribosome entry site (IRES) sequences, leading to proteinase-independent polyprotein anchor co-translation. Various viruses containing these type 2A sequences express an anchor site situated within the ORF1 region responsible for encoding Gag. Consequently, this sequence has the capacity to encode 2 to 3 distinct proteins besides Gag. Among them is the RNA-binding protein (RBP) harboring a dsRNA binding domain, alongside a small protein 2 (SP2), which is thought to be associated with the cell entry and extracellular transmission mechanisms of infectious myonecrosis virus (IMNV). Although not ubiquitous among all members of the Totiviridae family, these type 2A sequences seem to represent a significant strategy for enhancing and perpetuating genomic complexity, constituting a pivotal aspect in viral evolution [29].
Another remarkable aspect is the family’s formation of a monophyletic group, in contrast to other dsRNA viruses that exhibit polyphyletic characteristics. Its origins have been postulated to occur just prior to the divergence between Fungi and Protozoa. This proposition finds support in the evidence provided by Victorivirus (a mycovirus), which demonstrates closer phylogenetic relatedness to protozoan viruses (Leishmaniavirus and Trichomonasvirus) than to Totivirus (another mycovirus). Phylogenetic analyses focusing on the amino acid variabilities of RdRp and CP further bolster the hypothesis of substantial divergence, attributed to the viruses’ capacity to infect phylogenetically close yet distinct hosts [28]. Furthermore, the notion of a mono-segmented RNA serving as the common ancestor of dsRNA viruses has been proposed, with descendant genomes later becoming segmented. Consequently, the mono-segmented characteristic observed in Totiviridae positions the family at the foundational level of dsRNA virus evolution [30].
The origin of mycoviruses has been a subject of significant interest since their discovery, primarily due to their lack of an extracellular phase [31,32]. Two complementary hypotheses have emerged to explain their origin and evolution: the Ancestral Coevolution theory, suggesting a long-standing process of coevolution between fungi and viruses, and the Phytovirus Hypothesis, proposing that mycoviruses originated from phytoviruses, possibly through transmission from plants to fungi with which they had a symbiotic relationship, either mutualistic or parasitic [33,34,35]. The evolutionary origins of mycoviruses remain ambiguous, leading to uncertainty regarding the diverse range of hosts they can infect across different viral genera. In the case of the Totiviridae family, the hypothesis posits the existence of an ancestral self-replicating mRNA, yet the mechanism by which they initially infected fungi remains unclear. A study involving the Giardia lamblia virus demonstrated its ability to enter cells through free virions or transfection via electroporation of positive RNA strands transcribed from the original dsRNA virion [36]. Nevertheless, further research is imperative to unveil the initial infective process underlying virus–fungus interactions.
3. The S. cerevisiae Killer Toxins
3.1. The K1 Toxin
The K1 toxin, the original (first) member among killer toxins, was the first to be identified. Its expression is closely associated with the presence of the ScV-M1 virus, characterized by a 1.8 kb genome housing a single ORF responsible for the toxicity and immunity conferred by this toxin [37,38]. This ORF encompasses the pptox sequence, comprising four domains—δ, α, γ, and β—each playing crucial roles in toxin processing, infection, and immunity. The processing of the toxin commences with the import of pptox into the endoplasmic reticulum (ER), where γ undergoes glycosylation and disulfide bridges are formed, linking α and β. Subsequent transportation of the glycosylated pptox to the Golgi involves the action of Kex2p endopeptidases, leading to the removal of δ and γ subunits, thus yielding the mature K1 toxin in the form of a 36 kDa α-β heterodimer. Ultimately, the mature toxin is secreted outside the cell via vesicles [4,39,40,41]. Mutagenesis studies, involving the elimination of disulfide bridges, validate that the toxin’s toxicity hinges on the structural configuration conferred by sulfur bonds, alongside the presence of Cys within the α-subunit, while the β-subunit proves indispensable for binding to the target cell [38,42,43,44]. Although the crystallographic structure of either killer toxin is not currently available, in silico and mutagenesis models of K1 based on Cys indicate that the toxin remains stable due to a disulfide bridge between C92 and C239. The remaining Cys residues of both subunits may exist as free thiol groups or participate in stabilizing intramolecular disulfide arrangements. Notably, C95 and C107 in the α-subunit are implicated in immunity, while C248 and C312 are responsible for binding to intact cells and spheroplasts [42]. These findings complement the proposed immunity and toxicity mechanisms, wherein the toxin enables yeast to self-protect from its lethal effects [45,46]. While the proposed models and data provide insight into the intramolecular structure and interactions of K1, the lack of a three-dimensional model precludes a definitive determination of which residues are free or responsible for maintaining K1 in its final structure.
3.2. K2 and Klus Toxins
In contrast to the well-studied K1 toxin, the K2 toxin remains poorly understood. Similar to its counterparts (K1, K28, and Klus), this toxin is associated with the presence of a toxin-encoding virus. Specifically, in the case of K2, it is linked to the ScV-M2 virus and the helper virus L-A-2. The processing of the preprotoxin (pptox) is conserved, resulting in the secretion of the mature form composed of a 36 kDa α-β heterodimer [47,48]. Interestingly, its structure differs from K1 and K28 by the absence of a spacer subunit between the α and β subunits, retaining only the signal peptide [49]. Recent studies have demonstrated that the N-terminal sequence of the pptox, in addition to its signaling function, contains a 47 amino acid signal that is both necessary and sufficient to confer immunity to the K2 toxin [50]. However, the mechanism by which this peptide counteracts K2 activity remains unclear.
The Klus toxin remains a poorly studied agent in terms of its structure and mechanism of action following its recent discovery in 2011 from wine-isolated strains [51]. Similar to its counterparts, the ORF at the N-terminal end contains a signal peptide sequence, as well as cleavage sites for the Kex1p and Kex2p peptidases (Lys-Arg- and Arg-Arg- cleavage in the immature protein) and N-glycosylation sites [52]. Interestingly, the ORF of the preprotoxin is related to the chromosomal gene YFR020W (32% identity and 51% similarity), whose function is unknown. However, this relationship opens an intriguing research avenue regarding the evolution of this virus and its co-evolution with the yeast host [53,54,55]. Disulfide bond prediction studies indicate two potential binding sites within the C-terminal domain, predicting a putative 144 aa β-structure. However, the structure of this toxin remains unknown.
3.3. K28 Toxin
The first reports on the K28 toxin were published in the late 1980s and early 1990s, identifying it as a toxic agent of the same family as the K1 and K2 toxins [56], whose expression is determined by the presence of the M28 virus and L28 helper virus [57]. Structurally, both K1 and K28 maintain similarities in their arrangement of domains that make up the preprotoxins (pptox) of both, conserving the prepro region in the N-terminus, which is necessary for the toxin’s secretion system [58], followed by the α (10.5 kDa), γ (containing N-glycosylation sites and recognition sites for Kex1p and Kex2p), and β (11 kDa) subunits, and finally an HDEL retrograde transport sequence. It has been shown experimentally that the mature form of K28 is maintained as a 21 kDa heterodimer at pH less than 6, stabilized by a disulfide bridge between C56 of the α subunit and C333 of β. Interestingly, upon increasing pH, the mature toxin can form agglomerates of trimers and tetramers due to the interactions of the three additional cysteines found in β (C292, C307, and C340), which are involved in the toxicity of the α/β dimer [59,60].
4. The Mechanisms of Action of Killer Toxins
4.1. The Mechanism of Action of K1
Each of the described Killer toxins possesses unique mechanisms of action. To date, the K1 toxin has been the most extensively studied among all Killer toxins. Its toxicity results from the interaction of the mature toxin with the target site of a sensitive cell. However, the exact mechanism of action remains unclear, with multiple scenarios proposed.
The first mechanism described for elucidating K1 activity occurs in two phases. Initially, the mature toxin binds to a 1,6-β-D-glucan receptor on the yeast cell wall [1]. This receptor is ubiquitous in both immune and sensitive yeast strains, and its expression requires the involvement of KRE (Killer Resistance) genes involved in cell wall biosynthesis [44,61]. In the second phase, the mature α/β toxin increases potassium ion flow across the plasma membrane, leading to cell death [62,63]. Most KRE mutants do not interact with the toxin and are thus resistant to K1 action, whereas spheroplasts are sensitive to the toxin. This suggests that K1 action may be independent of the cell wall and primarily associated with the plasma membrane.
The second proposed mechanism indicates that the K1 toxin causes uncontrolled efflux of potassium, protons, ATP, and other small molecules from target cells through pore formation [38,63]. This hypothesis is supported by experimental mitigation of the killer effect, such as the reduction in K1 activity by administering high levels of external potassium, which protects sensitive cells from K1 action [64]. Additionally, spheroplasts and reconstituted liposomes exposed to K1 are depleted entirely of potassium [65].
The third mechanism was discovered incidentally during the sequencing of the S. cerevisiae genome. Genes encoding proteins with structural properties, including the Tok1p channel, were identified. Tok1p is an outward-rectifying potassium channel with two tandem pore domains located in the yeast plasma membrane [66,67,68]. The presence of two tandem pore domains led to various hypotheses about their function, ultimately demonstrating that both pores are crucial for selectivity and work together to form a single potassium-permeation pore [69]. Interestingly, the K1 toxin can activate Tok1p channels in sensitive strains, spheroplasts, and X. laevis oocytes expressing the channel [70,71,72]. Experiments showed that the absence of the channel confers resistance to the toxin, while overexpression increases sensitivity. The action of the K1 toxin on the Tok1p channel of S. cerevisiae occurs in two main phases: (1) In the initial interaction with the Tok1p channel, the mature K1 toxin binds to the Tok1p potassium channel on the plasma membrane of a sensitive cell, activating the channel. This binding leads to increased potassium ion outflow from the cell, creating plasma membrane depolarization. (2) During depolarization and cell death, the potassium loss results in depolarization of the plasma membrane and a disruption in cellular homeostasis. This mechanism is essential for K1 toxicity, as uncontrolled ion flow disrupts normal cellular functions, ultimately causing cell death [70].
Moreover, Sesti et al. [72] proposed the self-immunity mechanism of the K1 toxin in the S. cerevisiae Tok1p potassium channel. According to their study, K1 preprotoxin (pptox) interacts with Tok1p, causing an internal blockade that interferes with the channel’s function, ultimately conferring immunity to the cell against the toxin. The proposed mechanism suggests that K1 pptox acts from within the cell, interacting with the internal side of the Tok1p channel and blocking potassium ion flow through it. This interaction prevents the mature toxin, which acts externally, from exerting its lethal effect on the plasma membrane. To clarify, pptox provides a protective effect by occupying the Tok1p channel from the inside, avoiding pore formation or the potassium flux alteration that the toxin might induce in sensitive cells. This internal blocking mechanism by pptox explains the natural immunity some cells exhibit against the K1 toxin and underscores the significance of Tok1p in the action of this killer toxin. The research further demonstrates that strains lacking Tok1p are resistant to K1, while those overexpressing this channel show increased sensitivity. This model suggests that the action of K1 in sensitive cells heavily relies on the availability and functionality of Tok1p in the plasma membrane [72]. Considering the recognition of the mature toxin by the 1,6-β-D-glucan receptor via the β subunit (Figure 1(B1)), it is hypothesized that the molecular mechanism occurs in two phases: the β subunit initially recognizes this receptor through cysteine 248 [42,43], followed by the α subunit interacting with the Tok1p channel (Figure 1(B2a)). However, the specific molecular interaction between K1 and Tok1p remains uncertain.
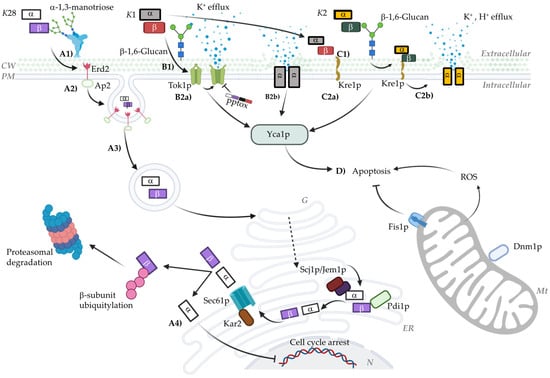
Figure 1.
Model of the mechanism of action of Killer toxins. This graphical representation shows the different proposed mechanisms of action for the K1, K2, and K28 toxins. (A1–A4) The transit of the K28 toxin, beginning with the recognition of α1-3 mannose trimers of the cell wall mannoproteins, followed by binding to the Erd2 receptor in an energy-independent process. Once inside the cell, the toxin is transported via retrograde transport to the ER, where it is linearized and transported to the cytosol via the Sec61p complex. In the cytosol, the α subunit enters the nucleus, arresting the cell cycle at G1/S, while the β subunit is polyubiquitinated and degraded in the proteasome. (B1) The recognition of the K1 toxin by the β-1,6-Glucan receptor, followed by recognition of the Tok1p channel (B2a), destabilizing it and causing uncontrolled K+ release. It also shows recognition by the Kre1p receptor (B2b), described as the primary receptor for this toxin. After recognition, the α subunit aggregates in the cell membrane, forming a cationic channel. Similar to K1, the K2 toxin shares the β-1,6-Glucan and Kre1p receptors (C1,C2a). The toxin is subsequently translocated to the membrane (C2b), where its ionophoric action destabilizes the membrane, causing its rupture. (D) The non-canonical pathway of PCD in response to low toxin concentrations. The internalization of the K28 toxin and the action of K1 and K2, in addition to deregulating the cell cycle and membrane, respectively, trigger the activation of the Yca1p caspase, as well as Dnm1p activity and ROS accumulation. Created with Biorender.com.
The fourth and final proposed mechanism presents a viewpoint contrary to the binding of the K1 toxin to the Tok1p potassium channel. It is based on the observation that the tok1-null strain does not show significant changes in sensitivity or immunity to the toxin [61]. Alternatively, the Kre1p receptor located on the yeast cell surface is proposed as the primary binding agent for the toxin [73]. Evidence of its interaction with K1 led to an alternative model where the β subunit of the toxin interacts with its primary receptor, 1,6-β-D-glucan, on the cell wall, followed by translocation and binding to the Kre1p receptor [61]. The importance of this receptor is linked to 1,6-β-D-glucan synthesis, as Δkre1 strains reduce its production by 40% [44,74]. Moreover, these mutants exhibit complete resistance to K1, leading to a new molecular mechanism based on Kre1p’s interaction with K1, subsequently forming cation-selective pores that cause uncontrolled potassium efflux and proton influx (Figure 1(B2b)) [38,40,45,61,63]. However, recent studies suggest that resistance to K1 may not be entirely related to the K1–Kre1p interaction but rather to Kre1p’s role in 1,6-β-D-glucan synthesis [74]. Δkre1 mutants have reduced 1,6-β-D-glucan receptor levels, conferring a K1-resistant phenotype [75]. Thus, the exact molecular mechanism still requires experimental validation.
4.2. The Mechanism of Action of K2 Toxin
Although the K2 toxin has not been extensively studied, key events in its mechanism of action have been determined. It is accepted that K2 operates through a two-phase killing system and that its preprotoxin processing is similar to that of K1 and K28 [76]. However, K2 has distinctive action profiles. One notable similarity is the primary recognition of the target cell. Like K1, K2 uses the 1,6-β-D-glucan receptor and Kre1p [77,78,79] to subsequently induce physiological changes that trigger cell death (Figure 1(C2a,b). It is in this stage that the two toxins differ. This specific difference allows them to kill each other; that is, although strains are immune to their own toxin, K2-producing strains can kill K1-producing strains and vice versa [78,80].
Various studies have focused on determining the particularities that differentiate the mechanisms of these two toxins. It has been discovered that K2 operates optimally at a pH of 4 [81,82], causing effects on the cell surface such as ruptures and undulations of the cell wall due to disruption of the electrochemical gradient [83]. This event may be due to the ionophore activity of the toxin. The uncontrolled influx of sodium, combined with the efflux of potassium and protons, activates a series of stress signals, cell integrity pathways, increased oxygen consumption, and activation of the HOG pathway and phosphoinositide signaling, which have not been detected in response to K1 [48,50,84].
4.3. The Mechanism of Action of K28 Toxin
Unlike the previously described toxins, K28 has a unique mode of action as it targets the cell cycle rather than the cell surface or membrane. Its molecular mechanism is more complex compared to other toxins. However, preprotoxin processing is conserved among killer toxins, resulting in a 21 kDa α/β heterodimer (10.5 kDa and 11 kDa) [59,85]. The recognition and internalization of the toxin are mediated by the β subunit, involving passage through the cell wall by binding to a 185 kDa mannoprotein that recognizes α1-3 and/or α1-2 mannotriose chains, followed by binding to the membrane receptor Erd2p via recognition of the C-terminal HDEL sequence of the β subunit (Figure 1(A1)) [86,87,88,89]. Subsequently, the toxin is transported from the plasma membrane into the cell through a unique process involving mono-ubiquitination by the Uba1p (E1), Ubc4p (E2), and Rsp5p (E3) proteins, supported by the AP2 complex, allowing endocytosis and retrograde transport through the Golgi apparatus and endoplasmic reticulum (ER) (Figure 1(A2,A3)) [45,90,91,92]. This step is crucial as the α/β toxin complex is linearized by Pdi1p to ensure its transport out of the ER [60]. Subsequently, in the cytosol, the β subunit is ubiquitinated and directed to the proteasome, while the α subunit passively diffuses into the nucleus. Once inside the nucleus, the α subunit interacts with nuclear proteins related to cell cycle control, specifically arresting the cell in the G1/S phase (Figure 1(A4)) [91,93,94,95].
4.4. The Mechanism of Action of Klus Toxin and Non-Canonical Pathways
The Klus toxin is the most recently discovered, and its mechanism of action remains unknown. However, this toxin can kill K1, K2, and K28 strains while being immune to its own toxin (a common trait among all). A remarkable characteristic of Klus is its less aggressive effect compared to other toxins, with an optimal temperature range of 28–30 °C and pH 4–4.7 [51]. It is also one of the least prevalent under natural conditions [96]. Despite this, the mechanism by which it causes cell death in sensitive strains is still unknown.
Recent studies have uncovered alternative events to the main proposed mechanisms. These scenarios can be considered as either alternative or simultaneous. Programmed cell death (PCD) in yeast has been shown to be an event triggered by interaction with killer toxins. Apoptosis, a highly regulated mechanism, can be triggered by numerous effectors, most notably reactive oxygen species (ROS) and caspases [45]. Viral killer toxins have been observed to have two pathways of action: (i) a canonical pathway (as described above) and (ii) a secondary pathway (Apoptosis) [97]. High toxin concentrations lead to the canonical pathway, while low concentrations can trigger apoptotic signaling (Figure 1D). For the K1 and K2 toxins, PCD has been hypothesized as a response to their interaction with plasma membrane receptors (Tok1p and Kre1p) and intracellular components such as yeast caspase 1 (Yca1p) and mitochondria [98,99,100]. Increased ROS and the pro-death factor Dnm1p (involved in mitochondrial fission) are the main effectors of PCD, while the mitochondrial pore-forming protein Fis1p inhibits the apoptotic signal [101,102,103,104]. This pathway is significantly slower than the canonical pathway (5.5 times slower). However, for the K28 toxin, the apoptotic pathway is three times faster than its respective canonical pathway [97]. This variability is essentially due to differences in the mechanisms of action of the two toxins; while K1 acts as an ionophore, K28 arrests the cell cycle in G1/S, requiring sensitive cells to be in this phase.
5. The Role of Killer Toxins in Heterologous Expression Systems
Heterologous expression systems have facilitated the manipulation and expression of genes in organisms from different species or cell lines than the original source. These systems enable high production rates of the protein, as well as modifications in the peptide sequence that are not present in its natural source [105]. Eukaryotic organisms, such as filamentous fungi [106], insect cells via baculovirus infection [107,108], plants [109,110], and mammals [111], represent attractive models for studying recombinant proteins in cases where the use of bacteria like E. coli is not entirely viable [112]. The heterologous expression system provides an attractive ease of genetic manipulation and the ability to produce large quantities. In the case of yeasts such as S. cerevisiae and Pichia pastoris, among others, their use as an expression system is suitable for expressing proteins with therapeutic applications and for functional and structural studies [113,114].
In addition to the mentioned species, other killer toxin-producing yeasts like Kluyveromyces wickerhamii, Wickerhamomyces anomalus, and Tetrapisispora phaffii have proven effective for use as antimicrobial agents against fungal infections [115,116], biological control of plant pathogens [117], regulators in food safety and quality [118,119,120], and biofuel production [121]. Their recombinant production is ensured through the heterologous expression of the toxins produced by these yeasts. However, it is necessary to clarify the advantages and disadvantages offered by each expression system according to the application needs. While S. cerevisiae and P. pastoris currently have the most available resources for use as heterologous expression organisms, other yeasts like Kluyveromyces lactis and Yarrowia lipolytica are emerging as promising candidates in this field [122].
One of the most important aspects of recombinant protein production is ensuring protein secretion. This process depends entirely on its post-translational and/or co-translocation into the lumen of the endoplasmic reticulum (ER), followed by importation into the Golgi. Thus, recombinant protein designs need signal peptides that ensure Golgi importation and subsequent secretion. In the case of S. cerevisiae, one such heterologous expression design involves the use of the K28 toxin prepro sequence, which has proven to be a significant element in ensuring the importation and processing of heterologous proteins into the Golgi, ensuring the secretion of the recombinant peptide into the extracellular medium [58,123]. This strategy allows the use of different varieties of killer toxins as post-translational regulation elements, ensuring the processing of recombinant proteins.
Recently, various killer toxins from S. cerevisiae have been recombinantly expressed for various purposes. One example involves the fusion of the K28 toxin with fluorescent proteins in P. pastoris [124]. This chimeric protein showed proper production and secretion while maintaining its unaltered toxicity. Thus, such toxins fused with fluorescent markers can be utilized for analyzing toxin interactions with different molecular targets. While this strategy can be replicated to study various yeast killer toxins, it is essential to determine that their mechanisms of action, structure, and physicochemical properties differ. Hence, the use of such systems should be evaluated case by case.
6. The Effect of Killer Toxins in the Biomedical Field
Initial evidence of the effect of S. cerevisiae toxins was observed in strains of the same species, which were categorized as “sensitive” because they lacked resistance mechanisms against the producing yeast’s toxins [41,72]. Later studies demonstrated that toxin-producing strains have a mechanism that makes them immune to their own toxin, although this immunity does not extend to other toxins [46], and their effect seemed directed at cell wall components. Consequently, the effect of these toxins was evaluated against various yeast-like fungi such as Pneumocystis carinii, Candida albicans, and Candida glabrata [3,116,125]. A recent study showed that C. albicans contains a homologous gene to the Tok1p potassium channel (one of the possible K1 toxin-binding sites), demonstrating that the presence of Tok1p is related but does not determine sensitivity to the K1 toxin in this yeast [71]. Furthermore, it has been determined that the lethal effects of killer toxins can be enhanced when two toxins with similar mechanisms of action, such as K1 and K2, are used synergistically, showing a greater effect when used together [126]. Additionally, it has been demonstrated that the K28 toxin has a toxic effect on strains of Trichophyton rubrum and Microsporum canis [127], supporting the potential use of killer toxins as potent antifungal agents. The observed effects in these yeasts also enabled the use of killer toxins as an alternative biotyping method for identifying various strains of Candida, Nocardia, Debaryomyces, and Kluyveromyces [128,129,130,131,132].
Interestingly, the effect of killer toxins is not restricted to fungi; they have also been shown to inhibit pathogenic bacterial strains. Various studies have demonstrated that protein fractions and secondary metabolites from different yeasts such as S. cerevisiae, Pichia, Kluyveromyces lactis, Saccharomyces unisporus, Metschnikowia pulcherrima, and Kluyveromyces marxianus can inhibit the growth of Gram-negative and Gram-positive pathogenic bacteria (Escherichia coli, Klepsiella. pneumoniae, Listeria monocytogenes, Proteus mirabilis, Pseudomonas aeruginosa, Staphylococcus aureus, and Salmonella typhimurium) [115,133,134,135,136,137]. However, these studies only demonstrate the effectiveness of protein and metabolic fractions without specifying which toxins are being secreted into the medium or which target sites are susceptible to the killer effect.
The killer toxin produced by Saccharomyces cerevisiae, particularly the K1 toxin, has shown great potential as an alternative to antibiotics due to its ability to inhibit a variety of microbial pathogens. This protein toxin acts, among other mechanisms, by creating pores in the cell walls and plasma membranes of susceptible organisms, leading to cell death. Thanks to its specific mode of action, K1 is particularly effective against certain fungal and bacterial pathogens, positioning it as a potential antifungal and antibacterial tool in biomedical applications.
In addition to inhibiting other strains of S. cerevisiae, the K1 toxin has demonstrated efficacy against pathogenic bacteria. Its mechanism of action involves targeting and binding to specific cell wall components, causing membrane depolarization, and disrupting cellular homeostasis. The use of the K1 toxin and similar Killer toxins could help reduce dependence on conventional antibiotics, particularly for resistant strains. However, additional studies are essential to fully understand the specificity of its action and the immune response it may provoke in different organisms, which is crucial for ensuring its safety and efficacy in therapeutic applications.
Future research will focus on optimizing Killer toxin formulations, identifying specific binding sites on target cells, and evaluating the effects of combining K1 with other antimicrobial agents. Although this research is still in its early stages, it represents a promising advance toward the development of new antimicrobial agents that could complement or even replace traditional antibiotics, especially in treating multidrug-resistant infections.
7. Perspectives
S. cerevisiae killer toxins have formed an intriguing field of research encompassing various areas of analysis, from studying the origin and evolution of the viruses responsible for the killer phenomenon to modeling the molecular interactions of toxins with their molecular targets. Although there is extensive information on killer toxins, many enigmas remain in this model of study. Among the main areas of opportunity in studying killer toxins is the determination of their three-dimensional structural elucidation. While prediction models like AlphaFold and AlphaFold2 have made significant advances with the help of artificial intelligence [138], in silico approaches do not reflect the post-translational modifications experimentally determined [40,46]. Additionally, the possibility of establishing molecular dockings with hypothesized binding sites for each toxin is low. Future studies should focus on resolving the molecular structures and elucidating the specific and shared target sites among different microorganisms. Since the prospect of using killer toxins is of biomedical and biotechnological importance, it is necessary to determine these mechanisms more specifically to ensure the effectiveness and safety of using killer toxins for various purposes.
Author Contributions
C.M.-V., V.M.-T., A.C.-V., J.U.-L., J.T.-B., J.C.-G., J.L.C.-S., T.G.-G. and C.S. wrote the paper and produced the figures. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant A1-S-26966 CONAHCyT; FONDEC and FONFIVE UAQ to V.M.-T. and C.S. C.M.-V. was a CONAHCyT fellow (1003111), and A.C.-V. and J.U.-L. were project fellows (A1-S-26966).
Data Availability Statement
The data presented in this study are available upon request.
Acknowledgments
We thank Adriana González Gallardo at the Unidad de Proteogenómica at Instituto de Neurobiología de la UNAM; Martha Calahorra and Norma Sánchez at Instituto de Fisiología Celular UNAM; and Rosa Esteban at Salamanca University from Spain for providing the Killer and sensitive strains.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bevan, E.A.; Makower, M. The Physiological Basis of the Killer Character in Yeast. In Genetics Today: Proceedings of the XI International Congress of Genetics, The Hague, The Netherlands, September 1963; Pergamon Press: Oxford, UK, 1963; pp. 202–203. [Google Scholar]
- Herring, A.J.; Bevan, E.A. Virus-like Particles Associated with the Double-Stranded RNA Species Found in Killer and Sensitive Strains of the Yeast Saccharomyces cerevisiae. J. Gen. Virol. 1974, 22, 387–394. [Google Scholar] [CrossRef]
- Magliani, W.; Conti, S.; Gerloni, M.; Bertolotti, D.; Polonelli, L. Yeast Killer Systems. Clin. Microbiol. Rev. 1997, 10, 369–400. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.J.; Breinig, F. The Viral Killer System in Yeast: From Molecular Biology to Application. FEMS Microbiol. Rev. 2002, 26, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Bruenn, J.A.; Ganesa, C.; Flurkey, W.F.; Bozarth, R.F.; Koltin, Y. Structure and Heterologous Expression of the Ustilago maydis Viral Toxin KP4. Mol. Microbiol. 1994, 11, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.J.; Poravou, O.; Trenz, K.; Rehfeldt, K. Unique Double-Stranded RNAs Responsible for the Anti-Candida Activity of the Yeast Hanseniaspora uvarum. J. Virol. 1997, 71, 8852–8855. [Google Scholar] [CrossRef]
- Schmitt, M.J.; Neuhausen, F. Killer Toxin-Secreting Double-Stranded RNA Mycoviruses in the Yeasts Hanseniaspora uvarum and Zygosaccharomyces bailii. J. Virol. 1994, 68, 1765–1772. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Alonso, A.; Marquina, D.; Santos, A. The Biology of Pichia membranifaciens Killer Toxins. Toxins 2017, 9, 112. [Google Scholar] [CrossRef]
- Goto, K.; Iwatuki, Y.; Kitano, K.; Obata, T.; Hara, S. Cloning and Nucleotide Sequence of the KHR Killer Gene of Saccharomyces cerevisiae. Agric. Biol. Chem. 1990, 54, 979–984. [Google Scholar] [CrossRef]
- Goto, K.; Fukuda, H.; Kichise, K.; Kitano, K.; Hara, S. Cloning and Nucleotide Sequence of the KHS Killer Gene of Saccharomyces cerevisiae. Agric. Biol. Chem. 1991, 55, 1953–1958. [Google Scholar] [CrossRef]
- Worsham, P.L.; Bolen, P.L. Killer Toxin Production in Pichia acaciae Is Associated with Linear DNA Plasmids. Curr. Genet. 1990, 18, 77–80. [Google Scholar] [CrossRef]
- Gunge, N.; Tamaru, A.; Ozawa, F.; Sakaguchi, K. Isolation and Characterization of Linear Deoxyribonucleic Acid Plasmids from Kluyveromyces lactis and the Plasmid-Associated Killer Character. J. Bacteriol. 1981, 145, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Icho, T.; Wickner, R.B. The Double-stranded RNA Genome of Yeast Virus L-A Encodes Its Own Putative RNA Polymerase by Fusing Two Open Reading Frames. J. Biol. Chem. 1989, 264, 6716–6723. [Google Scholar] [CrossRef] [PubMed]
- Ghabrial, S.A.; Caston, J.R.; Jiang, D.; Nibert, M.L.; Suzuki, N. 50-Plus Years of Fungal Viruses. Virology 2015, 479–480, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Wickner, R.B.; Ghabrial, S.A.; Nibert, M.L.; Patterson, J.L.; Wang, C.C. Totiviridae. In Virus Taxonomy; Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: Tokyo, Japan, 2012. [Google Scholar]
- Januszyk, K.; Lima, C.D. The eukaryotic RNA exosome. Curr. Opin. Struct. Biol. 2014, 24, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Halbach, F.; Reichelt, P.; Rode, M.; Conti, E. The Yeast Ski Complex: Crystal Structure and RNA Channeling to the Exosome Complex. Cell 2013, 154, 814–826. [Google Scholar] [CrossRef]
- Wang, L.; Lewis, M.S.; Johnson, A.W. Domain interactions within the Ski2/3/8 complex and between the Ski complex and Ski7p. RNA 2005, 11, 1291–1302. [Google Scholar] [CrossRef]
- Benard, L.; Carroll, K.; Valle, R.C.P.; Masison, D.C.; Wickner, R.B. The Ski7 Antiviral Protein Is an EF1-α Homolog That Blocks Expression of Non-Poly(A) mRNA in Saccharomyces cerevisiae. J. Virol. 1999, 73, 2893–2900. [Google Scholar] [CrossRef]
- Johnson, A.W.; Kolodner, R.D. Synthetic Lethality of sep1 (xrn1) ski2 and sep1 (xrn1) ski3 Mutants of Saccharomyces cerevisiae Is Independent of Killer Virus and Suggests a General Role for These Genes in Translation Control. Mol. Cell. Biol. 1995, 15, 2719–2727. [Google Scholar] [CrossRef]
- Weston, S.; Baracco, L.; Keller, C.; Matthews, K.; McGrath, M.E.; Logue, J.; Liang, J.; Dyall, J.; Holbrook, M.R.; Hensley, L.E.; et al. The SKI complex is a broad-spectrum, host-directed antiviral drug target for coronaviruses, influenza, and filoviruses. Proc. Natl. Acad. Sci. USA 2020, 117, 30687–30698. [Google Scholar] [CrossRef]
- Lukša, J.; Celitan, E.; Servienė, E.; Serva, S. Association of ScV-LA Virus with Host Protein Metabolism Determined by Proteomics Analysis and Cognate RNA Sequencing. Viruses 2022, 14, 2345. [Google Scholar] [CrossRef]
- Widner, W.R.; Wickner, R.B. Evidence that the SKI Antiviral System of Saccharomyces cerevisiae Acts by Blocking Expression of Viral mRNA. Mol. Cell. Biol. 1993, 13, 4331–4341. [Google Scholar] [CrossRef] [PubMed]
- Polevoda, B.; Sherman, F. NatC Nα-terminal Acetyltransferase of Yeast Contains Three Subunits, Mak3p, Mak10p, and Mak31p. J. Biol. Chem. 2001, 276, 20154–20159. [Google Scholar] [CrossRef] [PubMed]
- Polevoda, B.; Brown, S.; Cardillo, T.S.; Rigby, S.; Sherman, F. Yeast Nα-terminal acetyltransferases are associated with ribosomes. J. Cell. Biochem. 2008, 103, 492–508. [Google Scholar] [CrossRef]
- Grunwald, S.; Hopf, L.V.M.; Bock-Bierbaum, T.; Lally, C.C.M.; Spahn, C.M.T.; Daumke, O. Divergent architecture of the heterotrimeric NatC complex explains N-terminal acetylation of cognate substrates. Nat. Commun. 2020, 11, 5506. [Google Scholar] [CrossRef]
- Hisano, S.; Zhang, R.; Faruk, M.I.; Kondo, H.; Suzuki, N. A neo-virus lifestyle exhibited by a (+)ssRNA virus hosted in an unrelated dsRNA virus: Taxonomic and evolutionary considerations. Virus Res. 2018, 244, 75–83. [Google Scholar] [CrossRef]
- Liu, J.-J.; Xiang, Y.; Sniezko, R.A.; Schoettle, A.W.; Williams, H.; Zamany, A. Characterization of Cronartium ribicola dsRNAs reveals novel members of the family Totiviridae and viral association with fungal virulence. Virol. J. 2019, 16, 118. [Google Scholar] [CrossRef]
- de Lima, J.G.S.; Teixeira, D.G.; Freitas, T.T.; Lima, J.P.M.S.; Lanza, D.C. Evolutionary origin of 2A-like sequences in Totiviridae genomes. Virus Res. 2019, 259, 1–9. [Google Scholar] [CrossRef]
- Liu, H.; Fu, Y.; Xie, J.; Cheng, J.; Ghabrial, S.A.; Li, G.; Peng, Y.; Yi, X.; Jiang, D. Evolutionary genomics of mycovirus-related dsRNA viruses reveals cross-family horizontal gene transfer and evolution of diverse viral lineages. BMC Evol. Biol. 2012, 12, 91. [Google Scholar] [CrossRef]
- Yu, X.; Li, B.; Fu, Y.; Xie, J.; Cheng, J.; Ghabrial, S.A.; Li, G.; Yi, X.; Jiang, D. Extracellular transmission of a DNA mycovirus and its use as a natural fungicide. Proc. Natl. Acad. Sci. USA 2013, 110, 1452–1457. [Google Scholar] [CrossRef]
- Ayllón, M.A.; Vainio, E.J. Chapter One—Mycoviruses as a Part of the Global Virome: Diversity, Evolutionary Links and Lifestyle. In Advances in Virus Research; Kielian, M., Roossinck, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 115, ISBN 0065-3527. [Google Scholar]
- Sahin, E.; Akata, I. Viruses infecting macrofungi. VirusDisease 2018, 29, 1–18. [Google Scholar] [CrossRef]
- Pearson, M.N.; Beever, R.E.; Boine, B.; Arthur, K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 2009, 10, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Hough, B.; Steenkamp, E.; Wingfield, B.; Read, D. Fungal Viruses Unveiled: A Comprehensive Review of Mycoviruses. Viruses 2023, 15, 1202. [Google Scholar] [CrossRef] [PubMed]
- Ghabrial, S.A. Origin, Adaptation and Evolutionary Pathways of Fungal Viruses. Virus Genes 1998, 16, 119–131. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbeini, M.; Bostian, K.A. Viruses in fungi: Infection of yeast with the K1 and K2 killer viruses. Proc. Natl. Acad. Sci. USA 1987, 84, 4293–4297. [Google Scholar] [CrossRef]
- Skipper, N.; Bussey, H. Mode of action of yeast toxins: Energy requirement for Saccharomyces cerevisiae killer toxin. J. Bacteriol. 1977, 129, 668–677. [Google Scholar] [CrossRef]
- Tipper, D.J.; Bostian, K.A. Double-stranded ribonucleic acid killer systems in yeasts. Microbiol. Rev. 1984, 48, 125–156. [Google Scholar] [CrossRef]
- Gier, S.; Schmitt, M.J.; Breinig, F. Analysis of Yeast Killer Toxin K1 Precursor Processing via Site-Directed Mutagenesis: Implications for Toxicity and Immunity. mSphere 2020, 5, 00979-19. [Google Scholar] [CrossRef]
- Bussey, H.; Boone, C.; Zhu, H.; Vernet, T.; Whiteway, M.; Thomas, D.Y. Genetic and molecular approaches to synthesis and action of the yeast killer toxin. Experientia 1990, 46, 193–200. [Google Scholar] [CrossRef]
- Gier, S.; Lermen, M.; Schmitt, M.J.; Breinig, F. Substitution of cysteines in the yeast viral killer toxin K1 precursor reveals novel insights in heterodimer formation and immunity. Sci. Rep. 2019, 9, 13127. [Google Scholar] [CrossRef]
- Zhu, H.; Bussey, H. Mutational Analysis of the Functional Domains of Yeast K1 Killer Toxin. Mol. Cell. Biol. 1991, 11, 175–181. [Google Scholar] [CrossRef]
- Boone, C.; Sommer, S.S.; Hensel, A.; Bussey, H. Yeast KRE genes provide evidence for a pathway of cell wall beta-glucan assembly. J. Cell Biol. 1990, 110, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.J.; Breinig, F. Yeast viral killer toxins: Lethality and self-protection. Nat. Rev. Microbiol. 2006, 4, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.; Bussey, H.; Greene, D.; Thomas, D.Y.; Vernet, T. Yeast killer toxin: Site-directed mutations implicate the precursor protein as the immunity component. Cell 1986, 46, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, P.; Radler, F. Comparison of the killer toxin of several yeasts and the purification of a toxin of type K2. Arch. Microbiol. 1984, 137, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Orentaite, I.; Poranen, M.M.; Oksanen, H.M.; Daugelavicius, R.; Bamford, D.H. K2 killer toxin-induced physiological changes in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2016, 16, fow003. [Google Scholar] [CrossRef]
- Schmitt, M.; Björn, B. Viral Killer Toxins. In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 428–440. ISBN 978-0-12-809633-8. [Google Scholar]
- Prins, R.C.; Billerbeck, S. The Signal Sequence of Yeast Killer Toxin K2 Confers Producer Self-Protection and Allows Conversion into a Modular Toxin-Antitoxin System. bioRxiv 2023, 43, 114449. [Google Scholar] [CrossRef]
- Rodríguez-Cousiño, N.; Maqueda, M.; Ambrona, J.; Zamora, E.; Esteban, R.; Ramírez, M. A New Wine Saccharomyces cerevisiae Killer Toxin (Klus), Encoded by a Double-Stranded RNA Virus, with Broad Antifungal Activity Is Evolutionarily Related to a Chromosomal Host Gene. Appl. Environ. Microbiol. 2011, 77, 1822–1832. [Google Scholar] [CrossRef]
- Rodríguez-Cousiño, N.; Gómez, P.; Esteban, R. L-A-lus, a New Variant of the L-A Totivirus Found in Wine Yeasts with Klus Killer Toxin-Encoding Mlus Double-Stranded RNA: Possible Role of Killer Toxin-Encoding Satellite RNAs in the Evolution of Their Helper Viruses. Appl. Environ. Microbiol. 2013, 79, 4661–4674. [Google Scholar] [CrossRef]
- Fredericks, L.R.; Lee, M.D.; Crabtree, A.M.; Boyer, J.M.; Kizer, E.A.; Taggart, N.T.; Roslund, C.R.; Hunter, S.S.; Kennedy, C.B.; Willmore, C.G.; et al. The Species-Specific Acquisition and Diversification of a K1-like Family of Killer Toxins in Budding Yeasts of the Saccharomycotina. PLOS Genet. 2021, 17, e1009341. [Google Scholar] [CrossRef]
- Ramírez, M.; Velázquez, R.; Maqueda, M.; Martínez, A. Genome Organization of a New Double-Stranded RNA LA Helper Virus from Wine Torulaspora delbrueckii Killer Yeast as Compared with Its Saccharomyces Counterparts. Front. Microbiol. 2020, 11, 593846. [Google Scholar] [CrossRef]
- Maqueda, M.; Rodriguez-Cousiño, N.; Esteban, R.; Zamora, E.; Ramírez, M. Characterization of a new Saccharomyces cerevisiae dsRNA virus encoding a killer toxin with broad antifungal activity. In Proceedings of the Microbes in Applied Research—Current Advances and Challenges, Malaga, Spain, 14–16 September 2011; pp. 603–607. [Google Scholar]
- Schmittt, M.J.; Tipper, D.J. K28, A Unique Double-Stranded RNA Killer Virus of Saccharomyces cerevisiae. Mol. Cell. Biol. 1990, 10, 4807–4815. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.J.; Tipper, D.J. Genetic analysis of maintenance and expression of L and M double-stranded RNAs from yeast killer virus K28. Yeast 1992, 8, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Eiden-Plach, A.; Zagorc, T.; Heintel, T.; Carius, Y.; Breinig, F.; Schmitt, M.J. Viral Preprotoxin Signal Sequence Allows Efficient Secretion of Green Fluorescent Protein by Candida glabrata, Pichia pastoris, Saccharomyces cerevisiae, and Schizosaccharomyces pombe. Appl. Environ. Microbiol. 2004, 70, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.J.; Tipper, D.J. Sequence of the M28 dsRNA: Preprotoxin Is Processed to an α/β Heterodimeric Protein Toxin. Virology 1995, 213, 341–351. [Google Scholar] [CrossRef]
- Suzuki, Y.; Schwartz, S.L.; Mueller, N.C.; Schmitt, M.J. Cysteine residues in a yeast viral A/B toxin crucially control host cell killing via pH-triggered disulfide rearrangements. Mol. Biol. Cell 2017, 28, 1123–1131. [Google Scholar] [CrossRef]
- Breinig, F.; Tipper, D.J.; Schmitt, M.J. Kre1p, the Plasma Membrane Receptor for the Yeast K1 Viral Toxin. Cell 2002, 108, 395–405. [Google Scholar] [CrossRef]
- Bussey, H.; Sherman, D.; Somers, J.M. Action of Yeast Killer Factor: A Resistant Mutant with Sensitive Spheroplasts. J. Bacteriol. 1973, 113, 1193–1197. [Google Scholar] [CrossRef]
- de la Peña, P.; Barros, F.; Gascón, S.; Lazo, P.S.; Ramos, S. Effect of yeast killer toxin on sensitive cells of Saccharomyces cerevisiae. J. Biol. Chem. 1981, 256, 10420–10425. [Google Scholar] [CrossRef]
- Kurzweilová, H.; Sigler, K. Factors affecting the susceptibility of sensitive yeast cells to killer toxin K1. Folia Microbiol. 1993, 38, 524–526. [Google Scholar] [CrossRef]
- Martinac, B.; Zhu, H.; Kubalski, A.; Zhou, X.L.; Culbertson, M.; Bussey, H.; Kung, C. Yeast K1 killer toxin forms ion channels in sensitive yeast spheroplasts and in artificial liposomes. Proc. Natl. Acad. Sci. USA 1990, 87, 6228–6232. [Google Scholar] [CrossRef]
- Loukin, S.H.; Saimi, Y. K+-Dependent Composite Gating of the Yeast K+ Channel, Tok1. Biophys. J. 1999, 77, 3060–3070. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ketchum, K.A.; Joiner, W.J.; Sellers, A.J.; Kaczmarek, L.K.; Goldstein, S.A.N. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature 1995, 376, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Bertl, A.; Ramos, J.; Ludwig, J.; Lichtenberg-Fraté, H.; Reid, J.; Bihler, H.; Calero, F.; Martínez, P.; Ljungdahl, P.O. Characterization of potassium transport in wild-type and isogenic yeast strains carrying all combinations of trk1, trk2 and tok1 null mutations. Mol. Microbiol. 2003, 47, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, C.; Naranjo, D.; Coria, R.; Peña, A.; Vaca, L. Splitting the Two Pore Domains from TOK1 Results in Two Cationic Channels with Novel Functional Properties. J. Biol. Chem. 2002, 277, 4797–4805. [Google Scholar] [CrossRef]
- Ahmed, A.; Sesti, F.; Ilan, N.; Shih, T.M.; Sturley, S.L.; Goldstein, S.A. A Molecular Target for Viral Killer Toxin: TOK1 Potassium Channels. Cell 1999, 99, 283–291. [Google Scholar] [CrossRef]
- Baev, D.; Rivetta, A.; Li, X.S.; Vylkova, S.; Bashi, E.; Slayman, C.L.; Edgerton, M. Killing of Candida albicans by Human Salivary Histatin 5 Is Modulated, but Not Determined, by the Potassium Channel TOK1. Infect. Immun. 2003, 71, 3251–3260. [Google Scholar] [CrossRef]
- Sesti, F.; Shih, T.M.; Nikolaeva, N.; Goldstein, S.A. Immunity to K1 Killer Toxin: Internal TOK1 Blockade. Cell 2001, 105, 637–644. [Google Scholar] [CrossRef]
- Roemer, T.; Bussey, H. Yeast Kre1p is a cell surface O-glycoprotein. Mol. Genet. Genom. 1995, 249, 209–216. [Google Scholar] [CrossRef]
- Breinig, F.; Schleinkofer, K.; Schmitt, M.J. Yeast Kre1p is GPI-anchored and involved in both cell wall assembly and architecture. Microbiology 2004, 150, 3209–3218. [Google Scholar] [CrossRef]
- Pagé, N.; Gérard-Vincent, M.; Ménard, P.; Beaulieu, M.; Azuma, M.; Dijkgraaf, G.J.P.; Li, H.; Marcoux, J.; Nguyen, T.; Dowse, T.; et al. A Saccharomyces cerevisiae Genome-Wide Mutant Screen for Altered Sensitivity to K1 Killer Toxin. Genetics 2003, 163, 875–894. [Google Scholar] [CrossRef]
- Dignard, D.; Whiteway, M.; Germain, D.; Tessier, D.; Thomas, D.Y. Expression in yeast of a cDNA copy of the K2 killer toxin gene. Mol. Genet. Genom. 1991, 227, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Lukša, J.; Podoliankaitė, M.; Vepštaitė, I.; Strazdaitė-Žielienė, Ž.; Urbonavičius, J.; Servienė, E. Yeast β-1,6-Glucan Is a Primary Target for the Saccharomyces cerevisiae K2 Toxin. Eukaryot. Cell 2015, 14, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Novotná, D.; Flegelová, H.; Janderová, B. Different action of killer toxins K1 and K2 on the plasma membrane and the cell wall of Saccharomyces cerevisiae. FEMS Yeast Res. 2004, 4, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Flegelová, H.; Novotná, D.; Vojtísková, K.; Janderová, B. Isolation and characterization of Saccharomyces cerevisiae mutants with a different degree of resistance to killer toxins K1 and K2. FEMS Yeast Res. 2002, 2, 73–79. [Google Scholar] [CrossRef][Green Version]
- Bussey, H.; Vernet, T.; Sdicu, A.-M. Mutual antagonism among killer yeasts: Competition between Kl and K2 killers and a novel cDNA-based K1-K2 killer strain of Saccharomyces cerevisiae. Can. J. Microbiol. 1988, 34, 38–44. [Google Scholar] [CrossRef]
- Lebionka, A.; Servienë, E.; Melvydas, V. Isolation and Purification of Yeast Saccharomyces cerevisiae K2 Killer Toxin. Biologija 2002, 4, 7–9. [Google Scholar]
- Lukša, J.; Serva, S.; Servienė, E. Saccharomyces cerevisiae K2 toxin requires acidic environment for unidirectional folding into active state. Mycoscience 2016, 57, 51–57. [Google Scholar] [CrossRef]
- Vadasz, A.; Jagganath, D.; Pretorius, I.; Gupthar, A.S. Electron microscopy of the K2 killer effect of Saccharomyces cerevisiae T206 on a mesophilic wine yeast. Antonie van Leeuwenhoek 2000, 78, 117–122. [Google Scholar] [CrossRef]
- Servienė, E.; Lukša, J.; Orentaitė, I.; Lafontaine, D.L.J.; Urbonavičius, J. Screening the Budding Yeast Genome Reveals Unique Factors Affecting K2 Toxin Susceptibility. PLoS ONE 2012, 7, e50779. [Google Scholar] [CrossRef]
- Schmitt, M.J. Cloning and expression of a cDNA copy of the viral K28 killer toxin gene in yeast. Mol. Genet. Genom. 1995, 246, 236–246. [Google Scholar] [CrossRef]
- Schmitt, M.; Radler, F. Mannoprotein of the Yeast Cell Wall as Primary Receptor for the Killer Toxin of Saccharomyces cerevisiae Strain 28. Microbiology 1987, 133, 3347–3354. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Radler, F. Molecular structure of the cell wall receptor for killer toxin KT28 in Saccharomyces cerevisiae. J. Bacteriol. 1988, 170, 2192–2196. [Google Scholar] [CrossRef]
- Schmitt, M.J.; Radler, F. Blockage of cell wall receptors for yeast killer toxin KT28 with antimannoprotein antibodies. Antimicrob. Agents Chemother. 1990, 34, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.; Blum, A.; Gießelmann, E.; Dausend, J.; Rammo, D.; Müller, N.C.; Tschacksch, E.; Steimer, M.; Spindler, J.; Becherer, U.; et al. H/KDEL receptors mediate host cell intoxication by a viral A/B toxin in yeast. Sci. Rep. 2016, 6, 31105. [Google Scholar] [CrossRef] [PubMed]
- Heiligenstein, S.; Eisfeld, K.; Sendzik, T.; Jimenéz-Becker, N.; Breinig, F.; Schmitt, M.J. Retrotranslocation of a viral A/B toxin from the yeast endoplasmic reticulum is independent of ubiquitination and ERAD. EMBO J. 2006, 25, 4717–4727. [Google Scholar] [CrossRef]
- Becker, B.; Schmitt, M.J. Yeast Killer Toxin K28: Biology and Unique Strategy of Host Cell Intoxication and Killing. Toxins 2017, 9, 333. [Google Scholar] [CrossRef]
- Eisfeld, K.; Riffer, F.; Mentges, J.; Schmitt, M.J. Endocytotic uptake and retrograde transport of a virally encoded killer toxin in yeast. Mol. Microbiol. 2000, 37, 926–940. [Google Scholar] [CrossRef]
- Schmitt, M.J.; Klavehn, P.; Wang, J.; Schönig, I.; Tipper, D.J. Cell cycle studies on the mode of action of yeast K28 killer toxin. Microbiology 1996, 142, 2655–2662. [Google Scholar] [CrossRef]
- Schmitt, M.; Brendel, M.; Schwarz, R.; Radler, F. Inhibition of DNA Synthesis in Saccharomyces cerevisiae by Yeast Killer Toxin KT28. Microbiol.-Sgm 1989, 135, 1529–1535. [Google Scholar] [CrossRef]
- Liu, G.-L.; Chi, Z.; Wang, G.-Y.; Wang, Z.-P.; Li, Y.; Chi, Z.-M. Yeast killer toxins, molecular mechanisms of their action and their applications. Crit. Rev. Biotechnol. 2015, 35, 222–234. [Google Scholar] [CrossRef]
- Maqueda, M.; Zamora, E.; Álvarez, M.L.; Ramírez, M. Characterization, Ecological Distribution, and Population Dynamics of Saccharomyces Sensu Stricto Killer Yeasts in the Spontaneous Grape Must Fermentations of Southwestern Spain. Appl. Environ. Microbiol. 2012, 78, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.; Dikicioglu, D. Dynamic modelling of the killing mechanism of action by virus-infected yeasts. J. R. Soc. Interface 2019, 16, 20190064. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Herker, E.; Madeo, F.; Schmitt, M.J. Viral killer toxins induce caspase-mediated apoptosis in yeast. J. Cell Biol. 2005, 168, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, C.; Falcone, C. Caspase-dependent apoptosis in yeast. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2008, 1783, 1320–1327. [Google Scholar] [CrossRef]
- Hamann, A.; Brust, D.; Osiewacz, H.D. Apoptosis pathways in fungal growth, development and ageing. Trends Microbiol. 2008, 16, 276–283. [Google Scholar] [CrossRef]
- Schmitt, M.J.; Reiter, J. Viral induced yeast apoptosis. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2008, 1783, 1413–1417. [Google Scholar] [CrossRef]
- Ivanovska, I.; Hardwick, J.M. Viruses activate a genetically conserved cell death pathway in a unicellular organism. J. Cell Biol. 2005, 170, 391–399. [Google Scholar] [CrossRef]
- Falcone, C.; Mazzoni, C. External and internal triggers of cell death in yeast. Cell. Mol. Life Sci. 2016, 73, 2237–2250. [Google Scholar] [CrossRef]
- Fannjiang, Y.; Cheng, W.-C.; Lee, S.J.; Qi, B.; Pevsner, J.; McCaffery, J.M.; Hill, R.B.; Basañez, G.; Hardwick, J.M. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev. 2004, 18, 2785–2797. [Google Scholar] [CrossRef]
- Fisher, D.I.; Mayr, L.M.; Roth, R.G. Expression Systems. In Encyclopedia of Cell Biology; Bradshaw, R.A., Stahl, P.D., Eds.; Academic Press: Waltham, MA, USA, 2016; pp. 54–65. ISBN 978-0-12-394796-3. [Google Scholar]
- Ward, O.P. Production of recombinant proteins by filamentous fungi. Biotechnol. Adv. 2012, 30, 1119–1139. [Google Scholar] [CrossRef]
- Kost, T.A.; Condreay, J.P.; Jarvis, D.L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol. 2005, 23, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gennerich, A. Insect Cell-Based Expression of Cytoskeletal Motor Proteins for Single-Molecule Studies. In Single Molecular Analysis. Methods in Molecular Biology; Humana: New York, NY, USA, 2024; Volume 2694, pp. 69–90. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.-K.; Park, J.-S.; Lee, K.-R. Plant-made pharmaceuticals: Exploring studies for the production of recombinant protein in plants and assessing challenges ahead. Plant Biotechnol. Rep. 2023, 17, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Bulaon, C.J.I.; Malla, A.; Phoolcharoen, W. Biotechnological Insights on the Expression and Production of Antimicrobial Peptides in Plants. Molecules 2021, 26, 4032. [Google Scholar] [CrossRef] [PubMed]
- Capin, J.; Harrison, A.; Raele, R.A.; Yadav, S.K.N.; Baiwir, D.; Mazzucchelli, G.; Quinton, L.; Satchwell, T.J.; Toye, A.M.; Schaffitzel, C.; et al. An engineered baculoviral protein and DNA co-delivery system for CRISPR-based mammalian genome editing. Nucleic Acids Res. 2024, 52, 3450–3468. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.M.V.; Carmo, T.S.; Carvalho, L.S.; Bahia, F.M.; Parachin, N.S. Comparison of Yeasts as Hosts for Recombinant Protein Production. Microorganisms 2018, 6, 38. [Google Scholar] [CrossRef]
- Popova, L.G.; Khramov, D.E.; Nedelyaeva, O.I.; Volkov, V.S. Yeast Heterologous Expression Systems for the Study of Plant Membrane Proteins. Int. J. Mol. Sci. 2023, 24, 10768. [Google Scholar] [CrossRef]
- Tran, A.-M.; Nguyen, T.-T.; Nguyen, C.-T.; Huynh-Thi, X.-M.; Nguyen, C.-T.; Trinh, M.-T.; Tran, L.-T.; Cartwright, S.P.; Bill, R.M.; Tran-Van, H. Pichia pastoris versus Saccharomyces cerevisiae: A case study on the recombinant production of human granulocyte-macrophage colony-stimulating factor. BMC Res. Notes 2017, 10, 148. [Google Scholar] [CrossRef]
- Büyüksırıt-Bedir, T.; Kuleaşan, H. Purification and characterization of a Metschnikowia pulcherrima killer toxin with antagonistic activity against pathogenic microorganisms. Arch. Microbiol. 2022, 204, 337. [Google Scholar] [CrossRef]
- Rosseto, L.R.B.; Martelli, E.C.; da Silva, J.C.; Nascimento, B.L.; Junges, D.S.B.; Delabeneta, M.F.; Paris, A.P.; Auler, M.E.; Menolli, R.A.; Simão, R.C.G.; et al. Susceptibility of Candida albicans Strains Isolated from Vaginal Secretion in Front of the Mycocins of Wickerhamomyces anomalus. Probiotics Antimicrob. Proteins 2022, 14, 595–601. [Google Scholar] [CrossRef]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef]
- Carboni, G.; Marova, I.; Zara, G.; Zara, S.; Budroni, M.; Mannazzu, I. Evaluation of Recombinant Kpkt Cytotoxicity on HaCaT Cells: Further Steps towards the Biotechnological Exploitation Yeast Killer Toxins. Foods 2021, 10, 556. [Google Scholar] [CrossRef] [PubMed]
- Agarbati, A.; Ciani, M.; Esin, S.; Agnolucci, M.; Marcheggiani, F.; Tiano, L.; Comitini, F. Comparative Zymocidial Effect of Three Different Killer Toxins against Brettanomyces bruxellensis Spoilage Yeasts. Int. J. Mol. Sci. 2023, 24, 1309. [Google Scholar] [CrossRef] [PubMed]
- Çorbacı, C.; Uçar, F.B. Purification, characterization and in vivo biocontrol efficiency of killer toxins from Debaryomyces hansenii strains. Int. J. Biol. Macromol. 2018, 119, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Chessa, R.; Landolfo, S.; Ciani, M.; Budroni, M.; Zara, S.; Ustun, M.; Cakar, Z.P.; Mannazzu, I. Biotechnological exploitation of Tetrapisispora phaffii killer toxin: Heterologous production in Komagataella phaffii (Pichia pastoris). Appl. Microbiol. Biotechnol. 2017, 101, 2931–2942. [Google Scholar] [CrossRef]
- Wagner, J.M.; Alper, H.S. Synthetic biology and molecular genetics in non-conventional yeasts: Current tools and future advances. Fungal Genet. Biol. 2016, 89, 126–136. [Google Scholar] [CrossRef]
- Eskandari, A.; Nezhad, N.G.; Leow, T.C.; Rahman, M.B.A.; Oslan, S.N. Current achievements, strategies, obstacles, and overcoming the challenges of the protein engineering in Pichia pastoris expression system. World J. Microbiol. Biotechnol. 2023, 40, 39. [Google Scholar] [CrossRef]
- Giesselmann, E.; Becker, B.; Schmitt, M.J. Production of fluorescent and cytotoxic K28 killer toxin variants through high cell density fermentation of recombinant Pichia pastoris. Microb. Cell Factories 2017, 16, 228. [Google Scholar] [CrossRef]
- Sangorrín, M.P.; A Lopes, C.; Rivero, A.; Caballero, A.C. Rapid identification and susceptibility to killer toxins of yeasts isolated from non-systemic mycoses. Rev. Argent. Microbiol. 2007, 39, 230–236. [Google Scholar]
- Bracesco, N.; Salvo, V.A.; Carrau, F.M.; Nunes, E. Physicochemical modification of the excretion product of Saccharomyces cerevisiae killer strains results in fungicidal activity against Candida albicans and Tricophyton mentagrophytes. FEMS Microbiol. Lett. 2006, 256, 132–136. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Radler, F.; Caspritz, G.; Hänel, H. Effect of a Killer Toxin of Yeast on Eucaryotic Systems. Appl. Environ. Microbiol. 1988, 54, 1068–1069. [Google Scholar] [CrossRef]
- Provost, F.; Polonelli, L.; Conti, S.; Fisicaro, P.; Gerloni, M.; Boiron, P. Use of yeast killer system to identify species of the Nocardia asteroides complex. J. Clin. Microbiol. 1995, 33, 8–10. [Google Scholar] [CrossRef]
- Polonelli, L.; Conti, S. Biotyping of Candida Albicans and Other Fungi by Yeast Killer Toxins Sensitivity. Methods Mol. Biol. 2009, 499, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Buzzini, P.; Martini, A. Utilisation of Differential Killer Toxin Sensitivity Patterns for Fingerprinting and Clustering Yeast Strains Belonging to Different Genera. Syst. Appl. Microbiol. 2000, 23, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Polonelli, L.; Archibusacci, C.; Sestito, M.; Morace, G. Killer system: A simple method for differentiating Candida albicans strains. J. Clin. Microbiol. 1983, 17, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Polonelli, L.; Conti, S.; Magliani, W.; Horace, G. Biotyping of pathogenic fungi by the killer system and with monoclonal antibodies. Mycopathologia 1989, 107, 17–23. [Google Scholar] [CrossRef]
- Al-Obaydi, A.H.; Mahmood, N.N.; Alwan, B. Effect of purified killer toxin extracted from Saccharomyces cerevisiae on some pathological microorganisms cause urinary tract infections. Bionatura 2023, 8, 1–10. [Google Scholar] [CrossRef]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Anti-salmonella properties of kefir yeast isolates: An in vitro screening for potential infection control. Saudi J. Biol. Sci. 2022, 29, 550–563. [Google Scholar] [CrossRef]
- Acuña-Fontecilla, A.; Silva-Moreno, E.; Ganga, M.A.; Godoy, L. Evaluation of antimicrobial activity from native wine yeast against food industry pathogenic microorganisms. CyTA-J. Food 2017, 15, 457–465. [Google Scholar] [CrossRef]
- Younis, G.; Awad, A.; Dawod, R.E.; Yousef, N.E. Antimicrobial activity of yeasts against some pathogenic bacteria. Veter. World 2017, 10, 979–983. [Google Scholar] [CrossRef]
- Chen, Y.J.; Du, C.G.; Guo, Y.Q.; Zhao, Y.F.; Aorigele, C.; Wang, C.J.; Simujide, H.; Agima, W.; Zhang, X.Y. Antibacterial spectrum of four compounds from yeasts in koumiss. Pol. J. Veter. Sci. 2021, 24, 167–173. [Google Scholar] [CrossRef]
- Goel, D.; Kumar, R.; Kumar, S. AI-Assisted Methods for Protein Structure Prediction and Analysis. In Microbial Data Intelligence and Computational Techniques for Sustainable Computing; Khamparia, A., Pandey, B., Pandey, D.K., Gupta, D., Eds.; Springer Nature: Singapore, 2024; pp. 365–391. ISBN 978-981-9996-21-6. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).