The Killer Saccharomyces cerevisiae Toxin: From Origin to Biomedical Research
Abstract
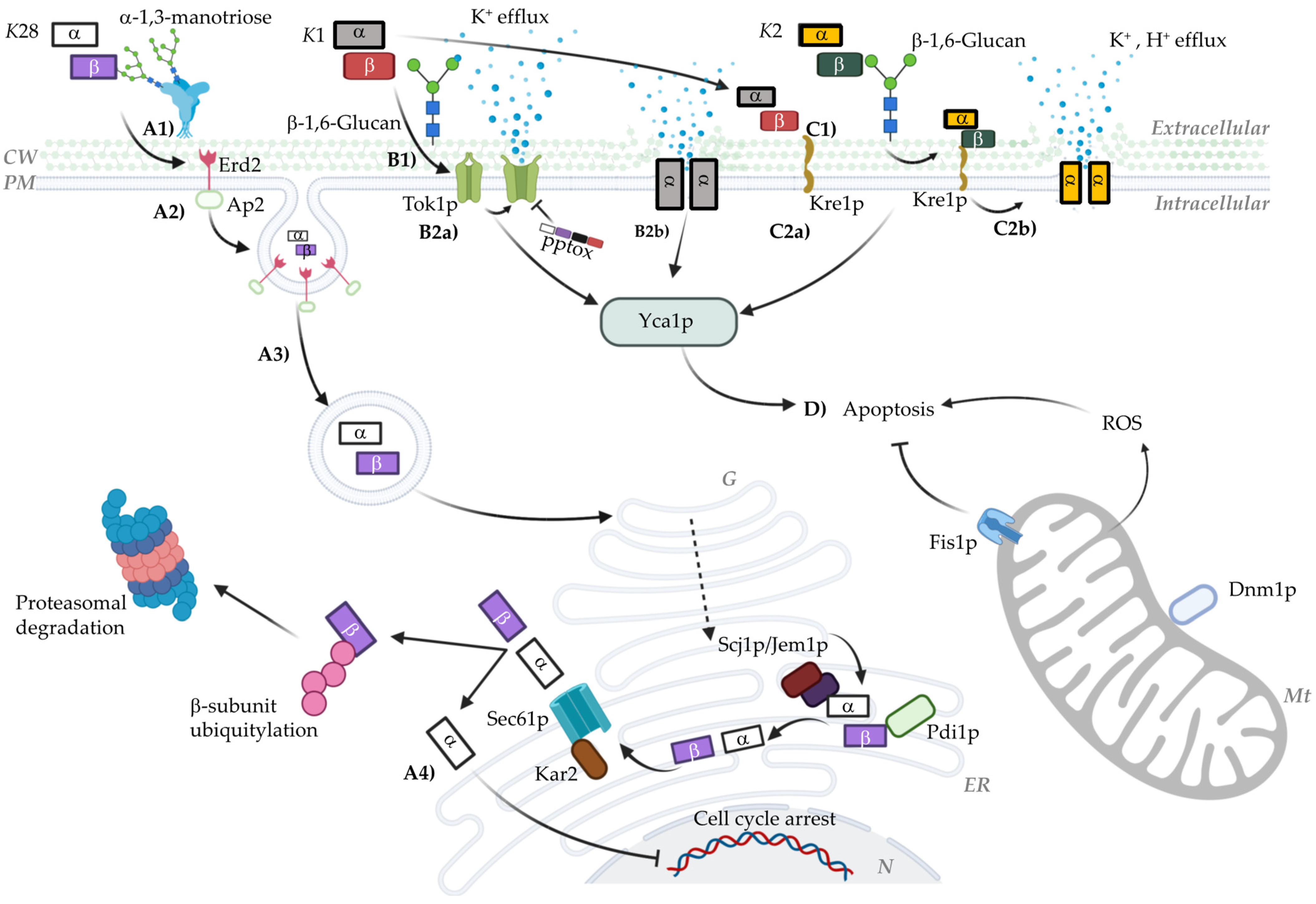
:1. Introduction
2. Evolution of the Family Totiviridae
3. The S. cerevisiae Killer Toxins
3.1. The K1 Toxin
3.2. K2 and Klus Toxins
3.3. K28 Toxin
4. The Mechanisms of Action of Killer Toxins
4.1. The Mechanism of Action of K1
4.2. The Mechanism of Action of K2 Toxin
4.3. The Mechanism of Action of K28 Toxin
4.4. The Mechanism of Action of Klus Toxin and Non-Canonical Pathways
5. The Role of Killer Toxins in Heterologous Expression Systems
6. The Effect of Killer Toxins in the Biomedical Field
7. Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bevan, E.A.; Makower, M. The Physiological Basis of the Killer Character in Yeast. In Genetics Today: Proceedings of the XI International Congress of Genetics, The Hague, The Netherlands, September 1963; Pergamon Press: Oxford, UK, 1963; pp. 202–203. [Google Scholar]
- Herring, A.J.; Bevan, E.A. Virus-like Particles Associated with the Double-Stranded RNA Species Found in Killer and Sensitive Strains of the Yeast Saccharomyces cerevisiae. J. Gen. Virol. 1974, 22, 387–394. [Google Scholar] [CrossRef]
- Magliani, W.; Conti, S.; Gerloni, M.; Bertolotti, D.; Polonelli, L. Yeast Killer Systems. Clin. Microbiol. Rev. 1997, 10, 369–400. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.J.; Breinig, F. The Viral Killer System in Yeast: From Molecular Biology to Application. FEMS Microbiol. Rev. 2002, 26, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Bruenn, J.A.; Ganesa, C.; Flurkey, W.F.; Bozarth, R.F.; Koltin, Y. Structure and Heterologous Expression of the Ustilago maydis Viral Toxin KP4. Mol. Microbiol. 1994, 11, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.J.; Poravou, O.; Trenz, K.; Rehfeldt, K. Unique Double-Stranded RNAs Responsible for the Anti-Candida Activity of the Yeast Hanseniaspora uvarum. J. Virol. 1997, 71, 8852–8855. [Google Scholar] [CrossRef]
- Schmitt, M.J.; Neuhausen, F. Killer Toxin-Secreting Double-Stranded RNA Mycoviruses in the Yeasts Hanseniaspora uvarum and Zygosaccharomyces bailii. J. Virol. 1994, 68, 1765–1772. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Alonso, A.; Marquina, D.; Santos, A. The Biology of Pichia membranifaciens Killer Toxins. Toxins 2017, 9, 112. [Google Scholar] [CrossRef]
- Goto, K.; Iwatuki, Y.; Kitano, K.; Obata, T.; Hara, S. Cloning and Nucleotide Sequence of the KHR Killer Gene of Saccharomyces cerevisiae. Agric. Biol. Chem. 1990, 54, 979–984. [Google Scholar] [CrossRef]
- Goto, K.; Fukuda, H.; Kichise, K.; Kitano, K.; Hara, S. Cloning and Nucleotide Sequence of the KHS Killer Gene of Saccharomyces cerevisiae. Agric. Biol. Chem. 1991, 55, 1953–1958. [Google Scholar] [CrossRef]
- Worsham, P.L.; Bolen, P.L. Killer Toxin Production in Pichia acaciae Is Associated with Linear DNA Plasmids. Curr. Genet. 1990, 18, 77–80. [Google Scholar] [CrossRef]
- Gunge, N.; Tamaru, A.; Ozawa, F.; Sakaguchi, K. Isolation and Characterization of Linear Deoxyribonucleic Acid Plasmids from Kluyveromyces lactis and the Plasmid-Associated Killer Character. J. Bacteriol. 1981, 145, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Icho, T.; Wickner, R.B. The Double-stranded RNA Genome of Yeast Virus L-A Encodes Its Own Putative RNA Polymerase by Fusing Two Open Reading Frames. J. Biol. Chem. 1989, 264, 6716–6723. [Google Scholar] [CrossRef] [PubMed]
- Ghabrial, S.A.; Caston, J.R.; Jiang, D.; Nibert, M.L.; Suzuki, N. 50-Plus Years of Fungal Viruses. Virology 2015, 479–480, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Wickner, R.B.; Ghabrial, S.A.; Nibert, M.L.; Patterson, J.L.; Wang, C.C. Totiviridae. In Virus Taxonomy; Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: Tokyo, Japan, 2012. [Google Scholar]
- Januszyk, K.; Lima, C.D. The eukaryotic RNA exosome. Curr. Opin. Struct. Biol. 2014, 24, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Halbach, F.; Reichelt, P.; Rode, M.; Conti, E. The Yeast Ski Complex: Crystal Structure and RNA Channeling to the Exosome Complex. Cell 2013, 154, 814–826. [Google Scholar] [CrossRef]
- Wang, L.; Lewis, M.S.; Johnson, A.W. Domain interactions within the Ski2/3/8 complex and between the Ski complex and Ski7p. RNA 2005, 11, 1291–1302. [Google Scholar] [CrossRef]
- Benard, L.; Carroll, K.; Valle, R.C.P.; Masison, D.C.; Wickner, R.B. The Ski7 Antiviral Protein Is an EF1-α Homolog That Blocks Expression of Non-Poly(A) mRNA in Saccharomyces cerevisiae. J. Virol. 1999, 73, 2893–2900. [Google Scholar] [CrossRef]
- Johnson, A.W.; Kolodner, R.D. Synthetic Lethality of sep1 (xrn1) ski2 and sep1 (xrn1) ski3 Mutants of Saccharomyces cerevisiae Is Independent of Killer Virus and Suggests a General Role for These Genes in Translation Control. Mol. Cell. Biol. 1995, 15, 2719–2727. [Google Scholar] [CrossRef]
- Weston, S.; Baracco, L.; Keller, C.; Matthews, K.; McGrath, M.E.; Logue, J.; Liang, J.; Dyall, J.; Holbrook, M.R.; Hensley, L.E.; et al. The SKI complex is a broad-spectrum, host-directed antiviral drug target for coronaviruses, influenza, and filoviruses. Proc. Natl. Acad. Sci. USA 2020, 117, 30687–30698. [Google Scholar] [CrossRef]
- Lukša, J.; Celitan, E.; Servienė, E.; Serva, S. Association of ScV-LA Virus with Host Protein Metabolism Determined by Proteomics Analysis and Cognate RNA Sequencing. Viruses 2022, 14, 2345. [Google Scholar] [CrossRef]
- Widner, W.R.; Wickner, R.B. Evidence that the SKI Antiviral System of Saccharomyces cerevisiae Acts by Blocking Expression of Viral mRNA. Mol. Cell. Biol. 1993, 13, 4331–4341. [Google Scholar] [CrossRef] [PubMed]
- Polevoda, B.; Sherman, F. NatC Nα-terminal Acetyltransferase of Yeast Contains Three Subunits, Mak3p, Mak10p, and Mak31p. J. Biol. Chem. 2001, 276, 20154–20159. [Google Scholar] [CrossRef] [PubMed]
- Polevoda, B.; Brown, S.; Cardillo, T.S.; Rigby, S.; Sherman, F. Yeast Nα-terminal acetyltransferases are associated with ribosomes. J. Cell. Biochem. 2008, 103, 492–508. [Google Scholar] [CrossRef]
- Grunwald, S.; Hopf, L.V.M.; Bock-Bierbaum, T.; Lally, C.C.M.; Spahn, C.M.T.; Daumke, O. Divergent architecture of the heterotrimeric NatC complex explains N-terminal acetylation of cognate substrates. Nat. Commun. 2020, 11, 5506. [Google Scholar] [CrossRef]
- Hisano, S.; Zhang, R.; Faruk, M.I.; Kondo, H.; Suzuki, N. A neo-virus lifestyle exhibited by a (+)ssRNA virus hosted in an unrelated dsRNA virus: Taxonomic and evolutionary considerations. Virus Res. 2018, 244, 75–83. [Google Scholar] [CrossRef]
- Liu, J.-J.; Xiang, Y.; Sniezko, R.A.; Schoettle, A.W.; Williams, H.; Zamany, A. Characterization of Cronartium ribicola dsRNAs reveals novel members of the family Totiviridae and viral association with fungal virulence. Virol. J. 2019, 16, 118. [Google Scholar] [CrossRef]
- de Lima, J.G.S.; Teixeira, D.G.; Freitas, T.T.; Lima, J.P.M.S.; Lanza, D.C. Evolutionary origin of 2A-like sequences in Totiviridae genomes. Virus Res. 2019, 259, 1–9. [Google Scholar] [CrossRef]
- Liu, H.; Fu, Y.; Xie, J.; Cheng, J.; Ghabrial, S.A.; Li, G.; Peng, Y.; Yi, X.; Jiang, D. Evolutionary genomics of mycovirus-related dsRNA viruses reveals cross-family horizontal gene transfer and evolution of diverse viral lineages. BMC Evol. Biol. 2012, 12, 91. [Google Scholar] [CrossRef]
- Yu, X.; Li, B.; Fu, Y.; Xie, J.; Cheng, J.; Ghabrial, S.A.; Li, G.; Yi, X.; Jiang, D. Extracellular transmission of a DNA mycovirus and its use as a natural fungicide. Proc. Natl. Acad. Sci. USA 2013, 110, 1452–1457. [Google Scholar] [CrossRef]
- Ayllón, M.A.; Vainio, E.J. Chapter One—Mycoviruses as a Part of the Global Virome: Diversity, Evolutionary Links and Lifestyle. In Advances in Virus Research; Kielian, M., Roossinck, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 115, ISBN 0065-3527. [Google Scholar]
- Sahin, E.; Akata, I. Viruses infecting macrofungi. VirusDisease 2018, 29, 1–18. [Google Scholar] [CrossRef]
- Pearson, M.N.; Beever, R.E.; Boine, B.; Arthur, K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 2009, 10, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Hough, B.; Steenkamp, E.; Wingfield, B.; Read, D. Fungal Viruses Unveiled: A Comprehensive Review of Mycoviruses. Viruses 2023, 15, 1202. [Google Scholar] [CrossRef] [PubMed]
- Ghabrial, S.A. Origin, Adaptation and Evolutionary Pathways of Fungal Viruses. Virus Genes 1998, 16, 119–131. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbeini, M.; Bostian, K.A. Viruses in fungi: Infection of yeast with the K1 and K2 killer viruses. Proc. Natl. Acad. Sci. USA 1987, 84, 4293–4297. [Google Scholar] [CrossRef]
- Skipper, N.; Bussey, H. Mode of action of yeast toxins: Energy requirement for Saccharomyces cerevisiae killer toxin. J. Bacteriol. 1977, 129, 668–677. [Google Scholar] [CrossRef]
- Tipper, D.J.; Bostian, K.A. Double-stranded ribonucleic acid killer systems in yeasts. Microbiol. Rev. 1984, 48, 125–156. [Google Scholar] [CrossRef]
- Gier, S.; Schmitt, M.J.; Breinig, F. Analysis of Yeast Killer Toxin K1 Precursor Processing via Site-Directed Mutagenesis: Implications for Toxicity and Immunity. mSphere 2020, 5, 00979-19. [Google Scholar] [CrossRef]
- Bussey, H.; Boone, C.; Zhu, H.; Vernet, T.; Whiteway, M.; Thomas, D.Y. Genetic and molecular approaches to synthesis and action of the yeast killer toxin. Experientia 1990, 46, 193–200. [Google Scholar] [CrossRef]
- Gier, S.; Lermen, M.; Schmitt, M.J.; Breinig, F. Substitution of cysteines in the yeast viral killer toxin K1 precursor reveals novel insights in heterodimer formation and immunity. Sci. Rep. 2019, 9, 13127. [Google Scholar] [CrossRef]
- Zhu, H.; Bussey, H. Mutational Analysis of the Functional Domains of Yeast K1 Killer Toxin. Mol. Cell. Biol. 1991, 11, 175–181. [Google Scholar] [CrossRef]
- Boone, C.; Sommer, S.S.; Hensel, A.; Bussey, H. Yeast KRE genes provide evidence for a pathway of cell wall beta-glucan assembly. J. Cell Biol. 1990, 110, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.J.; Breinig, F. Yeast viral killer toxins: Lethality and self-protection. Nat. Rev. Microbiol. 2006, 4, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.; Bussey, H.; Greene, D.; Thomas, D.Y.; Vernet, T. Yeast killer toxin: Site-directed mutations implicate the precursor protein as the immunity component. Cell 1986, 46, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, P.; Radler, F. Comparison of the killer toxin of several yeasts and the purification of a toxin of type K2. Arch. Microbiol. 1984, 137, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Orentaite, I.; Poranen, M.M.; Oksanen, H.M.; Daugelavicius, R.; Bamford, D.H. K2 killer toxin-induced physiological changes in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2016, 16, fow003. [Google Scholar] [CrossRef]
- Schmitt, M.; Björn, B. Viral Killer Toxins. In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 428–440. ISBN 978-0-12-809633-8. [Google Scholar]
- Prins, R.C.; Billerbeck, S. The Signal Sequence of Yeast Killer Toxin K2 Confers Producer Self-Protection and Allows Conversion into a Modular Toxin-Antitoxin System. bioRxiv 2023, 43, 114449. [Google Scholar] [CrossRef]
- Rodríguez-Cousiño, N.; Maqueda, M.; Ambrona, J.; Zamora, E.; Esteban, R.; Ramírez, M. A New Wine Saccharomyces cerevisiae Killer Toxin (Klus), Encoded by a Double-Stranded RNA Virus, with Broad Antifungal Activity Is Evolutionarily Related to a Chromosomal Host Gene. Appl. Environ. Microbiol. 2011, 77, 1822–1832. [Google Scholar] [CrossRef]
- Rodríguez-Cousiño, N.; Gómez, P.; Esteban, R. L-A-lus, a New Variant of the L-A Totivirus Found in Wine Yeasts with Klus Killer Toxin-Encoding Mlus Double-Stranded RNA: Possible Role of Killer Toxin-Encoding Satellite RNAs in the Evolution of Their Helper Viruses. Appl. Environ. Microbiol. 2013, 79, 4661–4674. [Google Scholar] [CrossRef]
- Fredericks, L.R.; Lee, M.D.; Crabtree, A.M.; Boyer, J.M.; Kizer, E.A.; Taggart, N.T.; Roslund, C.R.; Hunter, S.S.; Kennedy, C.B.; Willmore, C.G.; et al. The Species-Specific Acquisition and Diversification of a K1-like Family of Killer Toxins in Budding Yeasts of the Saccharomycotina. PLOS Genet. 2021, 17, e1009341. [Google Scholar] [CrossRef]
- Ramírez, M.; Velázquez, R.; Maqueda, M.; Martínez, A. Genome Organization of a New Double-Stranded RNA LA Helper Virus from Wine Torulaspora delbrueckii Killer Yeast as Compared with Its Saccharomyces Counterparts. Front. Microbiol. 2020, 11, 593846. [Google Scholar] [CrossRef]
- Maqueda, M.; Rodriguez-Cousiño, N.; Esteban, R.; Zamora, E.; Ramírez, M. Characterization of a new Saccharomyces cerevisiae dsRNA virus encoding a killer toxin with broad antifungal activity. In Proceedings of the Microbes in Applied Research—Current Advances and Challenges, Malaga, Spain, 14–16 September 2011; pp. 603–607. [Google Scholar]
- Schmittt, M.J.; Tipper, D.J. K28, A Unique Double-Stranded RNA Killer Virus of Saccharomyces cerevisiae. Mol. Cell. Biol. 1990, 10, 4807–4815. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.J.; Tipper, D.J. Genetic analysis of maintenance and expression of L and M double-stranded RNAs from yeast killer virus K28. Yeast 1992, 8, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Eiden-Plach, A.; Zagorc, T.; Heintel, T.; Carius, Y.; Breinig, F.; Schmitt, M.J. Viral Preprotoxin Signal Sequence Allows Efficient Secretion of Green Fluorescent Protein by Candida glabrata, Pichia pastoris, Saccharomyces cerevisiae, and Schizosaccharomyces pombe. Appl. Environ. Microbiol. 2004, 70, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.J.; Tipper, D.J. Sequence of the M28 dsRNA: Preprotoxin Is Processed to an α/β Heterodimeric Protein Toxin. Virology 1995, 213, 341–351. [Google Scholar] [CrossRef]
- Suzuki, Y.; Schwartz, S.L.; Mueller, N.C.; Schmitt, M.J. Cysteine residues in a yeast viral A/B toxin crucially control host cell killing via pH-triggered disulfide rearrangements. Mol. Biol. Cell 2017, 28, 1123–1131. [Google Scholar] [CrossRef]
- Breinig, F.; Tipper, D.J.; Schmitt, M.J. Kre1p, the Plasma Membrane Receptor for the Yeast K1 Viral Toxin. Cell 2002, 108, 395–405. [Google Scholar] [CrossRef]
- Bussey, H.; Sherman, D.; Somers, J.M. Action of Yeast Killer Factor: A Resistant Mutant with Sensitive Spheroplasts. J. Bacteriol. 1973, 113, 1193–1197. [Google Scholar] [CrossRef]
- de la Peña, P.; Barros, F.; Gascón, S.; Lazo, P.S.; Ramos, S. Effect of yeast killer toxin on sensitive cells of Saccharomyces cerevisiae. J. Biol. Chem. 1981, 256, 10420–10425. [Google Scholar] [CrossRef]
- Kurzweilová, H.; Sigler, K. Factors affecting the susceptibility of sensitive yeast cells to killer toxin K1. Folia Microbiol. 1993, 38, 524–526. [Google Scholar] [CrossRef]
- Martinac, B.; Zhu, H.; Kubalski, A.; Zhou, X.L.; Culbertson, M.; Bussey, H.; Kung, C. Yeast K1 killer toxin forms ion channels in sensitive yeast spheroplasts and in artificial liposomes. Proc. Natl. Acad. Sci. USA 1990, 87, 6228–6232. [Google Scholar] [CrossRef]
- Loukin, S.H.; Saimi, Y. K+-Dependent Composite Gating of the Yeast K+ Channel, Tok1. Biophys. J. 1999, 77, 3060–3070. [Google Scholar] [CrossRef] [PubMed]
- Ketchum, K.A.; Joiner, W.J.; Sellers, A.J.; Kaczmarek, L.K.; Goldstein, S.A.N. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature 1995, 376, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Bertl, A.; Ramos, J.; Ludwig, J.; Lichtenberg-Fraté, H.; Reid, J.; Bihler, H.; Calero, F.; Martínez, P.; Ljungdahl, P.O. Characterization of potassium transport in wild-type and isogenic yeast strains carrying all combinations of trk1, trk2 and tok1 null mutations. Mol. Microbiol. 2003, 47, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, C.; Naranjo, D.; Coria, R.; Peña, A.; Vaca, L. Splitting the Two Pore Domains from TOK1 Results in Two Cationic Channels with Novel Functional Properties. J. Biol. Chem. 2002, 277, 4797–4805. [Google Scholar] [CrossRef]
- Ahmed, A.; Sesti, F.; Ilan, N.; Shih, T.M.; Sturley, S.L.; Goldstein, S.A. A Molecular Target for Viral Killer Toxin: TOK1 Potassium Channels. Cell 1999, 99, 283–291. [Google Scholar] [CrossRef]
- Baev, D.; Rivetta, A.; Li, X.S.; Vylkova, S.; Bashi, E.; Slayman, C.L.; Edgerton, M. Killing of Candida albicans by Human Salivary Histatin 5 Is Modulated, but Not Determined, by the Potassium Channel TOK1. Infect. Immun. 2003, 71, 3251–3260. [Google Scholar] [CrossRef]
- Sesti, F.; Shih, T.M.; Nikolaeva, N.; Goldstein, S.A. Immunity to K1 Killer Toxin: Internal TOK1 Blockade. Cell 2001, 105, 637–644. [Google Scholar] [CrossRef]
- Roemer, T.; Bussey, H. Yeast Kre1p is a cell surface O-glycoprotein. Mol. Genet. Genom. 1995, 249, 209–216. [Google Scholar] [CrossRef]
- Breinig, F.; Schleinkofer, K.; Schmitt, M.J. Yeast Kre1p is GPI-anchored and involved in both cell wall assembly and architecture. Microbiology 2004, 150, 3209–3218. [Google Scholar] [CrossRef]
- Pagé, N.; Gérard-Vincent, M.; Ménard, P.; Beaulieu, M.; Azuma, M.; Dijkgraaf, G.J.P.; Li, H.; Marcoux, J.; Nguyen, T.; Dowse, T.; et al. A Saccharomyces cerevisiae Genome-Wide Mutant Screen for Altered Sensitivity to K1 Killer Toxin. Genetics 2003, 163, 875–894. [Google Scholar] [CrossRef]
- Dignard, D.; Whiteway, M.; Germain, D.; Tessier, D.; Thomas, D.Y. Expression in yeast of a cDNA copy of the K2 killer toxin gene. Mol. Genet. Genom. 1991, 227, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Lukša, J.; Podoliankaitė, M.; Vepštaitė, I.; Strazdaitė-Žielienė, Ž.; Urbonavičius, J.; Servienė, E. Yeast β-1,6-Glucan Is a Primary Target for the Saccharomyces cerevisiae K2 Toxin. Eukaryot. Cell 2015, 14, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Novotná, D.; Flegelová, H.; Janderová, B. Different action of killer toxins K1 and K2 on the plasma membrane and the cell wall of Saccharomyces cerevisiae. FEMS Yeast Res. 2004, 4, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Flegelová, H.; Novotná, D.; Vojtísková, K.; Janderová, B. Isolation and characterization of Saccharomyces cerevisiae mutants with a different degree of resistance to killer toxins K1 and K2. FEMS Yeast Res. 2002, 2, 73–79. [Google Scholar] [CrossRef]
- Bussey, H.; Vernet, T.; Sdicu, A.-M. Mutual antagonism among killer yeasts: Competition between Kl and K2 killers and a novel cDNA-based K1-K2 killer strain of Saccharomyces cerevisiae. Can. J. Microbiol. 1988, 34, 38–44. [Google Scholar] [CrossRef]
- Lebionka, A.; Servienë, E.; Melvydas, V. Isolation and Purification of Yeast Saccharomyces cerevisiae K2 Killer Toxin. Biologija 2002, 4, 7–9. [Google Scholar]
- Lukša, J.; Serva, S.; Servienė, E. Saccharomyces cerevisiae K2 toxin requires acidic environment for unidirectional folding into active state. Mycoscience 2016, 57, 51–57. [Google Scholar] [CrossRef]
- Vadasz, A.; Jagganath, D.; Pretorius, I.; Gupthar, A.S. Electron microscopy of the K2 killer effect of Saccharomyces cerevisiae T206 on a mesophilic wine yeast. Antonie van Leeuwenhoek 2000, 78, 117–122. [Google Scholar] [CrossRef]
- Servienė, E.; Lukša, J.; Orentaitė, I.; Lafontaine, D.L.J.; Urbonavičius, J. Screening the Budding Yeast Genome Reveals Unique Factors Affecting K2 Toxin Susceptibility. PLoS ONE 2012, 7, e50779. [Google Scholar] [CrossRef]
- Schmitt, M.J. Cloning and expression of a cDNA copy of the viral K28 killer toxin gene in yeast. Mol. Genet. Genom. 1995, 246, 236–246. [Google Scholar] [CrossRef]
- Schmitt, M.; Radler, F. Mannoprotein of the Yeast Cell Wall as Primary Receptor for the Killer Toxin of Saccharomyces cerevisiae Strain 28. Microbiology 1987, 133, 3347–3354. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Radler, F. Molecular structure of the cell wall receptor for killer toxin KT28 in Saccharomyces cerevisiae. J. Bacteriol. 1988, 170, 2192–2196. [Google Scholar] [CrossRef]
- Schmitt, M.J.; Radler, F. Blockage of cell wall receptors for yeast killer toxin KT28 with antimannoprotein antibodies. Antimicrob. Agents Chemother. 1990, 34, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.; Blum, A.; Gießelmann, E.; Dausend, J.; Rammo, D.; Müller, N.C.; Tschacksch, E.; Steimer, M.; Spindler, J.; Becherer, U.; et al. H/KDEL receptors mediate host cell intoxication by a viral A/B toxin in yeast. Sci. Rep. 2016, 6, 31105. [Google Scholar] [CrossRef] [PubMed]
- Heiligenstein, S.; Eisfeld, K.; Sendzik, T.; Jimenéz-Becker, N.; Breinig, F.; Schmitt, M.J. Retrotranslocation of a viral A/B toxin from the yeast endoplasmic reticulum is independent of ubiquitination and ERAD. EMBO J. 2006, 25, 4717–4727. [Google Scholar] [CrossRef]
- Becker, B.; Schmitt, M.J. Yeast Killer Toxin K28: Biology and Unique Strategy of Host Cell Intoxication and Killing. Toxins 2017, 9, 333. [Google Scholar] [CrossRef]
- Eisfeld, K.; Riffer, F.; Mentges, J.; Schmitt, M.J. Endocytotic uptake and retrograde transport of a virally encoded killer toxin in yeast. Mol. Microbiol. 2000, 37, 926–940. [Google Scholar] [CrossRef]
- Schmitt, M.J.; Klavehn, P.; Wang, J.; Schönig, I.; Tipper, D.J. Cell cycle studies on the mode of action of yeast K28 killer toxin. Microbiology 1996, 142, 2655–2662. [Google Scholar] [CrossRef]
- Schmitt, M.; Brendel, M.; Schwarz, R.; Radler, F. Inhibition of DNA Synthesis in Saccharomyces cerevisiae by Yeast Killer Toxin KT28. Microbiol.-Sgm 1989, 135, 1529–1535. [Google Scholar] [CrossRef]
- Liu, G.-L.; Chi, Z.; Wang, G.-Y.; Wang, Z.-P.; Li, Y.; Chi, Z.-M. Yeast killer toxins, molecular mechanisms of their action and their applications. Crit. Rev. Biotechnol. 2015, 35, 222–234. [Google Scholar] [CrossRef]
- Maqueda, M.; Zamora, E.; Álvarez, M.L.; Ramírez, M. Characterization, Ecological Distribution, and Population Dynamics of Saccharomyces Sensu Stricto Killer Yeasts in the Spontaneous Grape Must Fermentations of Southwestern Spain. Appl. Environ. Microbiol. 2012, 78, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.; Dikicioglu, D. Dynamic modelling of the killing mechanism of action by virus-infected yeasts. J. R. Soc. Interface 2019, 16, 20190064. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Herker, E.; Madeo, F.; Schmitt, M.J. Viral killer toxins induce caspase-mediated apoptosis in yeast. J. Cell Biol. 2005, 168, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, C.; Falcone, C. Caspase-dependent apoptosis in yeast. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2008, 1783, 1320–1327. [Google Scholar] [CrossRef]
- Hamann, A.; Brust, D.; Osiewacz, H.D. Apoptosis pathways in fungal growth, development and ageing. Trends Microbiol. 2008, 16, 276–283. [Google Scholar] [CrossRef]
- Schmitt, M.J.; Reiter, J. Viral induced yeast apoptosis. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2008, 1783, 1413–1417. [Google Scholar] [CrossRef]
- Ivanovska, I.; Hardwick, J.M. Viruses activate a genetically conserved cell death pathway in a unicellular organism. J. Cell Biol. 2005, 170, 391–399. [Google Scholar] [CrossRef]
- Falcone, C.; Mazzoni, C. External and internal triggers of cell death in yeast. Cell. Mol. Life Sci. 2016, 73, 2237–2250. [Google Scholar] [CrossRef]
- Fannjiang, Y.; Cheng, W.-C.; Lee, S.J.; Qi, B.; Pevsner, J.; McCaffery, J.M.; Hill, R.B.; Basañez, G.; Hardwick, J.M. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev. 2004, 18, 2785–2797. [Google Scholar] [CrossRef]
- Fisher, D.I.; Mayr, L.M.; Roth, R.G. Expression Systems. In Encyclopedia of Cell Biology; Bradshaw, R.A., Stahl, P.D., Eds.; Academic Press: Waltham, MA, USA, 2016; pp. 54–65. ISBN 978-0-12-394796-3. [Google Scholar]
- Ward, O.P. Production of recombinant proteins by filamentous fungi. Biotechnol. Adv. 2012, 30, 1119–1139. [Google Scholar] [CrossRef]
- Kost, T.A.; Condreay, J.P.; Jarvis, D.L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol. 2005, 23, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gennerich, A. Insect Cell-Based Expression of Cytoskeletal Motor Proteins for Single-Molecule Studies. In Single Molecular Analysis. Methods in Molecular Biology; Humana: New York, NY, USA, 2024; Volume 2694, pp. 69–90. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.-K.; Park, J.-S.; Lee, K.-R. Plant-made pharmaceuticals: Exploring studies for the production of recombinant protein in plants and assessing challenges ahead. Plant Biotechnol. Rep. 2023, 17, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Bulaon, C.J.I.; Malla, A.; Phoolcharoen, W. Biotechnological Insights on the Expression and Production of Antimicrobial Peptides in Plants. Molecules 2021, 26, 4032. [Google Scholar] [CrossRef] [PubMed]
- Capin, J.; Harrison, A.; Raele, R.A.; Yadav, S.K.N.; Baiwir, D.; Mazzucchelli, G.; Quinton, L.; Satchwell, T.J.; Toye, A.M.; Schaffitzel, C.; et al. An engineered baculoviral protein and DNA co-delivery system for CRISPR-based mammalian genome editing. Nucleic Acids Res. 2024, 52, 3450–3468. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.M.V.; Carmo, T.S.; Carvalho, L.S.; Bahia, F.M.; Parachin, N.S. Comparison of Yeasts as Hosts for Recombinant Protein Production. Microorganisms 2018, 6, 38. [Google Scholar] [CrossRef]
- Popova, L.G.; Khramov, D.E.; Nedelyaeva, O.I.; Volkov, V.S. Yeast Heterologous Expression Systems for the Study of Plant Membrane Proteins. Int. J. Mol. Sci. 2023, 24, 10768. [Google Scholar] [CrossRef]
- Tran, A.-M.; Nguyen, T.-T.; Nguyen, C.-T.; Huynh-Thi, X.-M.; Nguyen, C.-T.; Trinh, M.-T.; Tran, L.-T.; Cartwright, S.P.; Bill, R.M.; Tran-Van, H. Pichia pastoris versus Saccharomyces cerevisiae: A case study on the recombinant production of human granulocyte-macrophage colony-stimulating factor. BMC Res. Notes 2017, 10, 148. [Google Scholar] [CrossRef]
- Büyüksırıt-Bedir, T.; Kuleaşan, H. Purification and characterization of a Metschnikowia pulcherrima killer toxin with antagonistic activity against pathogenic microorganisms. Arch. Microbiol. 2022, 204, 337. [Google Scholar] [CrossRef]
- Rosseto, L.R.B.; Martelli, E.C.; da Silva, J.C.; Nascimento, B.L.; Junges, D.S.B.; Delabeneta, M.F.; Paris, A.P.; Auler, M.E.; Menolli, R.A.; Simão, R.C.G.; et al. Susceptibility of Candida albicans Strains Isolated from Vaginal Secretion in Front of the Mycocins of Wickerhamomyces anomalus. Probiotics Antimicrob. Proteins 2022, 14, 595–601. [Google Scholar] [CrossRef]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microbiol. Biotechnol. 2019, 35, 154. [Google Scholar] [CrossRef]
- Carboni, G.; Marova, I.; Zara, G.; Zara, S.; Budroni, M.; Mannazzu, I. Evaluation of Recombinant Kpkt Cytotoxicity on HaCaT Cells: Further Steps towards the Biotechnological Exploitation Yeast Killer Toxins. Foods 2021, 10, 556. [Google Scholar] [CrossRef] [PubMed]
- Agarbati, A.; Ciani, M.; Esin, S.; Agnolucci, M.; Marcheggiani, F.; Tiano, L.; Comitini, F. Comparative Zymocidial Effect of Three Different Killer Toxins against Brettanomyces bruxellensis Spoilage Yeasts. Int. J. Mol. Sci. 2023, 24, 1309. [Google Scholar] [CrossRef] [PubMed]
- Çorbacı, C.; Uçar, F.B. Purification, characterization and in vivo biocontrol efficiency of killer toxins from Debaryomyces hansenii strains. Int. J. Biol. Macromol. 2018, 119, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Chessa, R.; Landolfo, S.; Ciani, M.; Budroni, M.; Zara, S.; Ustun, M.; Cakar, Z.P.; Mannazzu, I. Biotechnological exploitation of Tetrapisispora phaffii killer toxin: Heterologous production in Komagataella phaffii (Pichia pastoris). Appl. Microbiol. Biotechnol. 2017, 101, 2931–2942. [Google Scholar] [CrossRef]
- Wagner, J.M.; Alper, H.S. Synthetic biology and molecular genetics in non-conventional yeasts: Current tools and future advances. Fungal Genet. Biol. 2016, 89, 126–136. [Google Scholar] [CrossRef]
- Eskandari, A.; Nezhad, N.G.; Leow, T.C.; Rahman, M.B.A.; Oslan, S.N. Current achievements, strategies, obstacles, and overcoming the challenges of the protein engineering in Pichia pastoris expression system. World J. Microbiol. Biotechnol. 2023, 40, 39. [Google Scholar] [CrossRef]
- Giesselmann, E.; Becker, B.; Schmitt, M.J. Production of fluorescent and cytotoxic K28 killer toxin variants through high cell density fermentation of recombinant Pichia pastoris. Microb. Cell Factories 2017, 16, 228. [Google Scholar] [CrossRef]
- Sangorrín, M.P.; A Lopes, C.; Rivero, A.; Caballero, A.C. Rapid identification and susceptibility to killer toxins of yeasts isolated from non-systemic mycoses. Rev. Argent. Microbiol. 2007, 39, 230–236. [Google Scholar]
- Bracesco, N.; Salvo, V.A.; Carrau, F.M.; Nunes, E. Physicochemical modification of the excretion product of Saccharomyces cerevisiae killer strains results in fungicidal activity against Candida albicans and Tricophyton mentagrophytes. FEMS Microbiol. Lett. 2006, 256, 132–136. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Radler, F.; Caspritz, G.; Hänel, H. Effect of a Killer Toxin of Yeast on Eucaryotic Systems. Appl. Environ. Microbiol. 1988, 54, 1068–1069. [Google Scholar] [CrossRef]
- Provost, F.; Polonelli, L.; Conti, S.; Fisicaro, P.; Gerloni, M.; Boiron, P. Use of yeast killer system to identify species of the Nocardia asteroides complex. J. Clin. Microbiol. 1995, 33, 8–10. [Google Scholar] [CrossRef]
- Polonelli, L.; Conti, S. Biotyping of Candida Albicans and Other Fungi by Yeast Killer Toxins Sensitivity. Methods Mol. Biol. 2009, 499, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Buzzini, P.; Martini, A. Utilisation of Differential Killer Toxin Sensitivity Patterns for Fingerprinting and Clustering Yeast Strains Belonging to Different Genera. Syst. Appl. Microbiol. 2000, 23, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Polonelli, L.; Archibusacci, C.; Sestito, M.; Morace, G. Killer system: A simple method for differentiating Candida albicans strains. J. Clin. Microbiol. 1983, 17, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Polonelli, L.; Conti, S.; Magliani, W.; Horace, G. Biotyping of pathogenic fungi by the killer system and with monoclonal antibodies. Mycopathologia 1989, 107, 17–23. [Google Scholar] [CrossRef]
- Al-Obaydi, A.H.; Mahmood, N.N.; Alwan, B. Effect of purified killer toxin extracted from Saccharomyces cerevisiae on some pathological microorganisms cause urinary tract infections. Bionatura 2023, 8, 1–10. [Google Scholar] [CrossRef]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Anti-salmonella properties of kefir yeast isolates: An in vitro screening for potential infection control. Saudi J. Biol. Sci. 2022, 29, 550–563. [Google Scholar] [CrossRef]
- Acuña-Fontecilla, A.; Silva-Moreno, E.; Ganga, M.A.; Godoy, L. Evaluation of antimicrobial activity from native wine yeast against food industry pathogenic microorganisms. CyTA-J. Food 2017, 15, 457–465. [Google Scholar] [CrossRef]
- Younis, G.; Awad, A.; Dawod, R.E.; Yousef, N.E. Antimicrobial activity of yeasts against some pathogenic bacteria. Veter. World 2017, 10, 979–983. [Google Scholar] [CrossRef]
- Chen, Y.J.; Du, C.G.; Guo, Y.Q.; Zhao, Y.F.; Aorigele, C.; Wang, C.J.; Simujide, H.; Agima, W.; Zhang, X.Y. Antibacterial spectrum of four compounds from yeasts in koumiss. Pol. J. Veter. Sci. 2021, 24, 167–173. [Google Scholar] [CrossRef]
- Goel, D.; Kumar, R.; Kumar, S. AI-Assisted Methods for Protein Structure Prediction and Analysis. In Microbial Data Intelligence and Computational Techniques for Sustainable Computing; Khamparia, A., Pandey, B., Pandey, D.K., Gupta, D., Eds.; Springer Nature: Singapore, 2024; pp. 365–391. ISBN 978-981-9996-21-6. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Vera, C.; Morales-Tlalpan, V.; Chavez-Vega, A.; Uribe-López, J.; Trujillo-Barrientos, J.; Campos-Guillén, J.; Chávez-Servín, J.L.; García-Gasca, T.; Saldaña, C. The Killer Saccharomyces cerevisiae Toxin: From Origin to Biomedical Research. Microorganisms 2024, 12, 2481. https://doi.org/10.3390/microorganisms12122481
Molina-Vera C, Morales-Tlalpan V, Chavez-Vega A, Uribe-López J, Trujillo-Barrientos J, Campos-Guillén J, Chávez-Servín JL, García-Gasca T, Saldaña C. The Killer Saccharomyces cerevisiae Toxin: From Origin to Biomedical Research. Microorganisms. 2024; 12(12):2481. https://doi.org/10.3390/microorganisms12122481
Chicago/Turabian StyleMolina-Vera, Carlos, Verónica Morales-Tlalpan, Amairani Chavez-Vega, Jennifer Uribe-López, Jessica Trujillo-Barrientos, Juan Campos-Guillén, Jorge Luis Chávez-Servín, Teresa García-Gasca, and Carlos Saldaña. 2024. "The Killer Saccharomyces cerevisiae Toxin: From Origin to Biomedical Research" Microorganisms 12, no. 12: 2481. https://doi.org/10.3390/microorganisms12122481
APA StyleMolina-Vera, C., Morales-Tlalpan, V., Chavez-Vega, A., Uribe-López, J., Trujillo-Barrientos, J., Campos-Guillén, J., Chávez-Servín, J. L., García-Gasca, T., & Saldaña, C. (2024). The Killer Saccharomyces cerevisiae Toxin: From Origin to Biomedical Research. Microorganisms, 12(12), 2481. https://doi.org/10.3390/microorganisms12122481










