Pharmacodynamic Evaluation of Phage Therapy in Ameliorating ETEC-Induced Diarrhea in Mice Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statement of Ethics
2.2. Identification, Culture, and Drug Resistance Analysis of Bacterial Strains
2.3. Phage Isolation, Purification, and Titer Determination
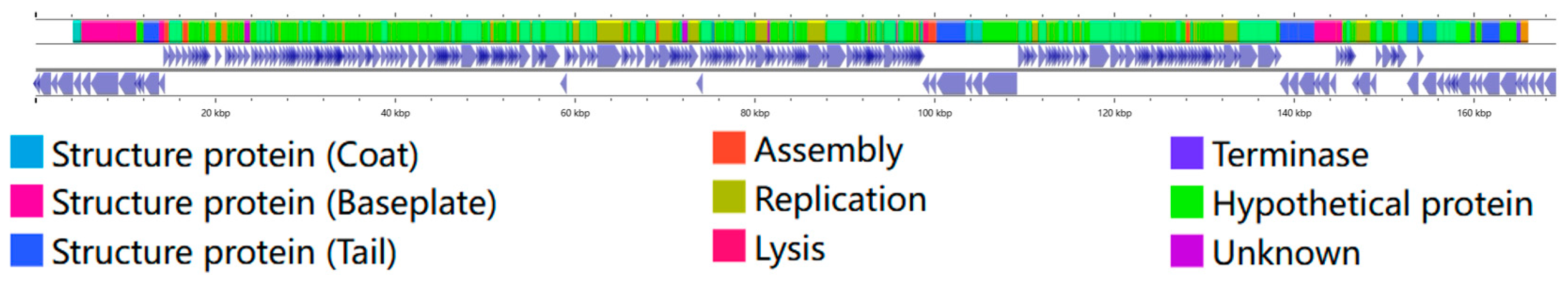
2.4. Phage Genome Sequencing and Annotation
2.5. Phage Morphology Analysis via Transmission Electron Microscopy
2.6. Determination of Host Range of Phage
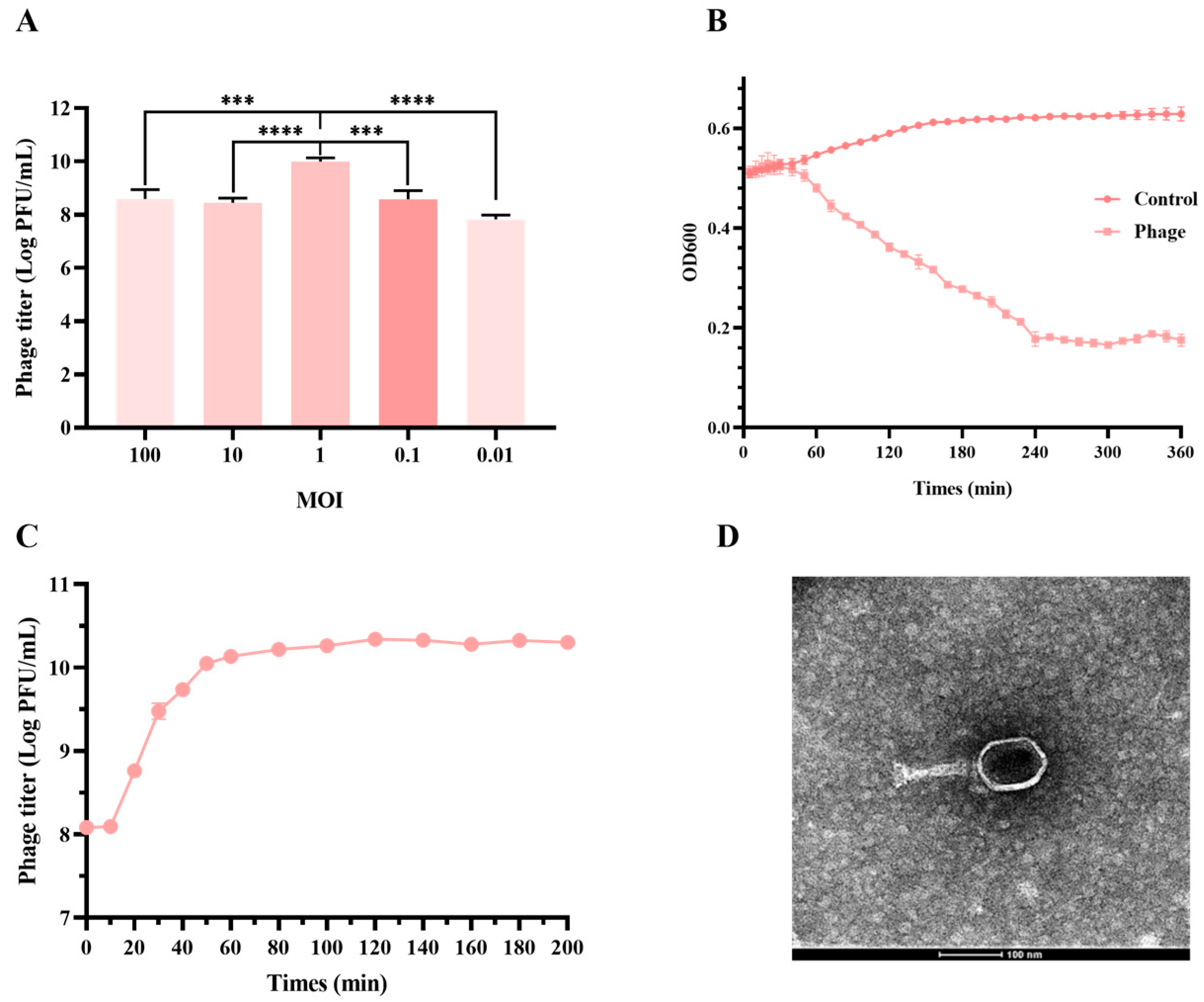
2.7. Assay of Optimal Multiplicity of Infection (MOI) and One-Step Growth
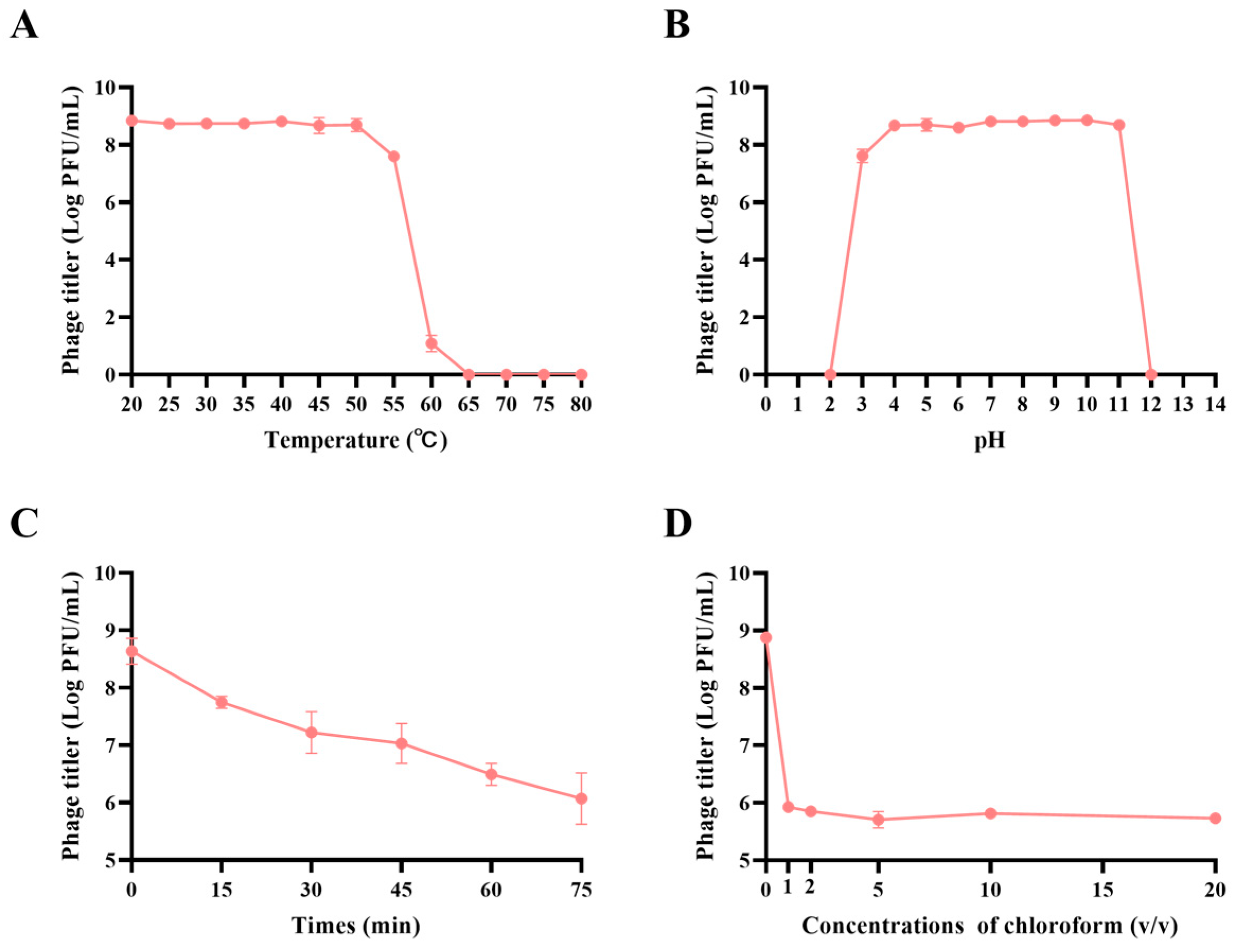
2.8. Assessment of Phage Biological Characteristics
2.9. Assay of Phage’s Inhibition of Bacterial Adhesion
2.10. Mouse Model
2.11. Sample Collection and Processing
2.12. CFU Burden in the Jejunum
2.13. Cytokine Assays
2.14. Construction of Red Fluorescent Protein-Decorated Phage
2.15. In Vivo Imaging of Mice
2.16. Statistical Analysis
3. Results
3.1. Multi-Drug Resistance Profile of ETEC Isolates
3.2. JE01 Phage Effectively Lysed ETEC Strains
3.3. JE01 Phage Exhibited Better Physicochemical Stability
3.4. JE01 Phage Alleviated ETEC-Induced Damage to Porcine Intestinal Epithelial Cells
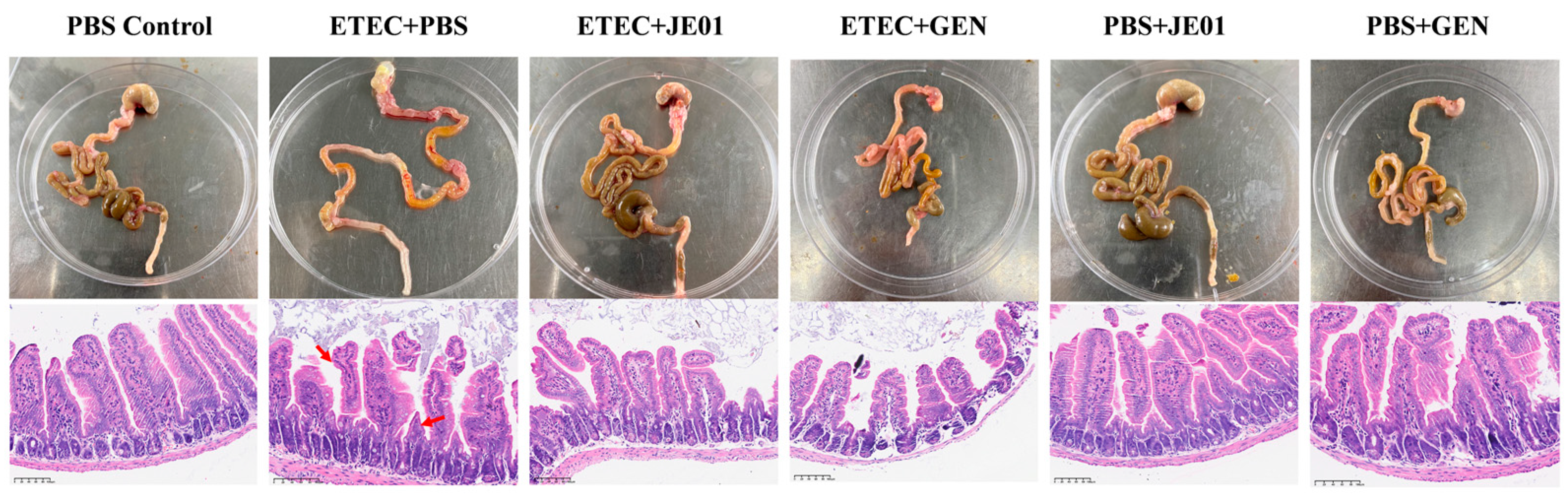
3.5. JE01 Phage Effectively Alleviated ETEC-Induced Diarrhea in Mice
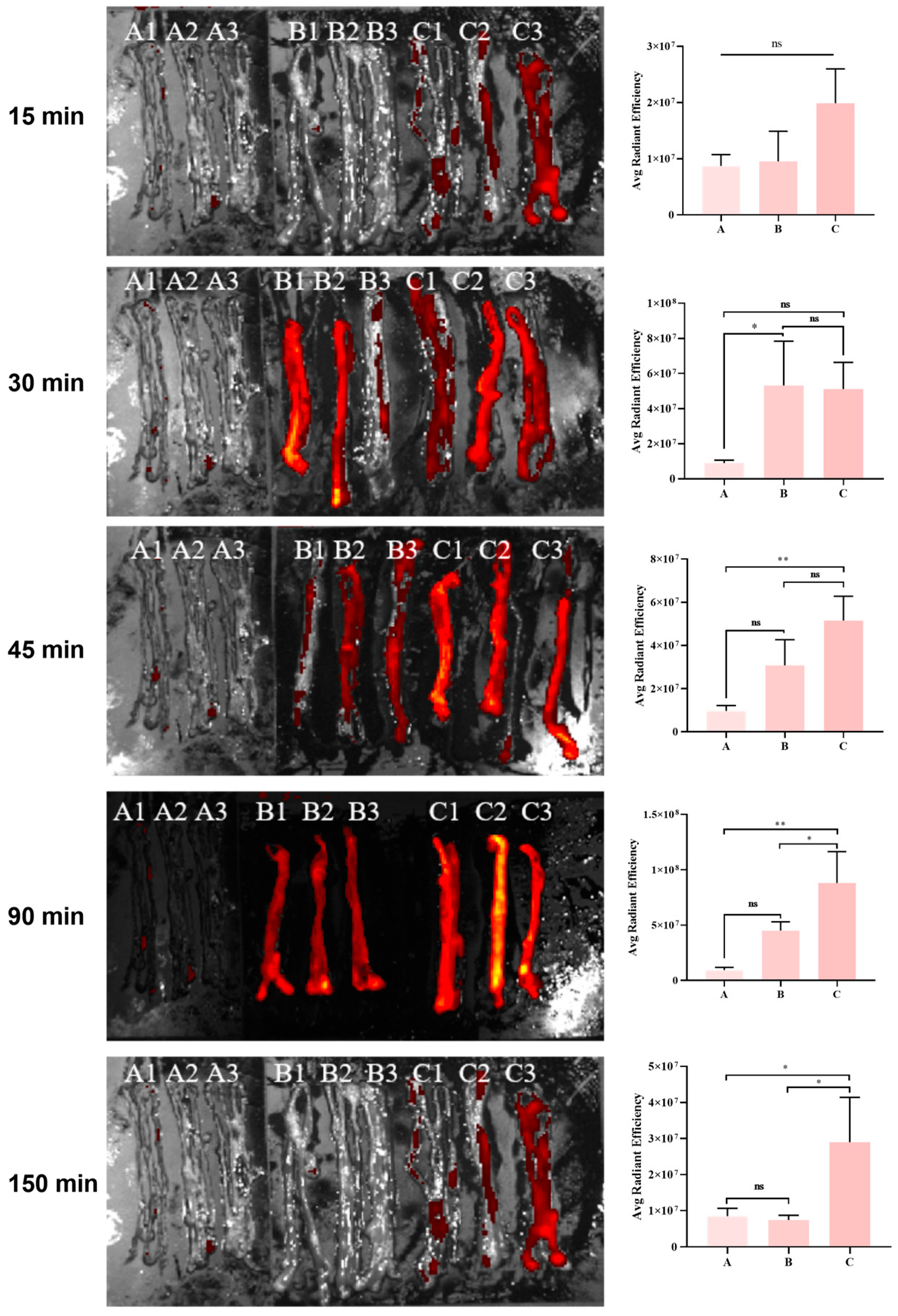
3.6. Metabolic Kinetics of the Phage in the Mouse Intestine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, S.; Xu, H.; Yang, C.; Karmin, O. Regulation of oxidative stress in the intestine of piglets after enterotoxigenic Escherichia coli (ETEC) infection. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119711. [Google Scholar] [CrossRef] [PubMed]
- von Mentzer, A.; Svennerholm, A.-M. Colonization factors of human and animal-specific enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 2023, 32, 448–464. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Pig vaccination strategies based on enterotoxigenic Escherichia coli toxins. Braz. J. Microbiol. 2021, 52, 2499–2509. [Google Scholar] [CrossRef]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal enterotoxigenic Escherichia coli. EcoSal Plus 2016, 47. [Google Scholar] [CrossRef]
- Luppi, A. Swine enteric colibacillosis: Diagnosis, therapy and antimicrobial resistance. Porc. Health Manag. 2017, 3, 16. [Google Scholar] [CrossRef]
- Nadeau, E.; Fairbrother, J.; Zentek, J.; Bélanger, L.; Tremblay, D.; Tremblay, C.-L.; Röhe, I.; Vahjen, W.; Brunelle, M.; Hellmann, K. Efficacy of a single oral dose of a live bivalent E. coli vaccine against post-weaning diarrhea due to F4 and F18-positive enterotoxigenic E. coli. Vet. J. 2017, 226, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M.; Duran, C.O.; Burch, D.G. Antimicrobial resistance in swine production. Anim. Health Res. Rev. 2008, 9, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Laird, T.J.; Abraham, S.; Jordan, D.; Pluske, J.R.; Hampson, D.J.; Trott, D.J.; O’Dea, M. Porcine enterotoxigenic Escherichia coli: Antimicrobial resistance and development of microbial-based alternative control strategies. Vet. Microbiol. 2021, 258, 109117. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Rayamahji, N.; Lee, W.J.; Cha, S.B.; Shin, M.K.; Roh, Y.M.; Sang, H.Y. Genotypes, antibiogram, and pulsed-field gel electrophoresis profiles of Escherichia coli strains from piglets in Korea. J. Vet. Diagn. Investig. 2009, 21, 510–516. [Google Scholar] [CrossRef]
- Smith, M.; Jordan, D.; Chapman, T.; Chin, J.-C.; Barton, M.; Do, T.; Fahy, V.; Fairbrother, J.; Trott, D. Antimicrobial resistance and virulence gene profiles in multi-drug resistant enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea. Vet. Microbiol. 2010, 145, 299–307. [Google Scholar] [CrossRef]
- Ashelford, K.E.; Day, M.J.; Fry, J.C. Elevated abundance of bacteriophage infecting bacteria in soil. Appl. Environ. Microbiol. 2003, 69, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Loessner, M.J. Beyond antibacterials–exploring bacteriophages as antivirulence agents. Curr. Opin. Biotechnol. 2021, 68, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Kakasis, A.; Panitsa, G. Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. Int. J. Antimicrob. Agents 2019, 53, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yao, J.; He, J.; Liu, H.; Jiang, Y.; Zhao, D.; Shi, Q.; Zhou, J.; Hu, H.; Lan, P. Clinical and laboratory insights into the threat of hypervirulent Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2024, 64, 107275. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-K.; Jean, S.-S.; Lee, Y.-L.; Lu, M.-C.; Ko, W.-C.; Lin, H.-J.; Liu, P.-Y.; Hsueh, P.-R. Bacteriophages and phage-delivered CRISPR-Cas system as antibacterial therapy. Int. J. Antimicrob. Agents 2022, 59, 106475. [Google Scholar] [CrossRef]
- Gao, D.; Ji, H.; Wang, L.; Li, X.; Hu, D.; Zhao, J.; Wang, S.; Tao, P.; Li, X.; Qian, P. Fitness trade-offs in phage cocktail-resistant salmonella enterica serovar enteritidis results in increased antibiotic susceptibility and reduced virulence. Microbiol. Spectr. 2022, 10, e02914-22. [Google Scholar] [CrossRef]
- Titécat, M.; Rousseaux, C.; Dubuquoy, C.; Foligné, B.; Rahmouni, O.; Mahieux, S.; Desreumaux, P.; Woolston, J.; Sulakvelidze, A.; Wannerberger, K. Safety and efficacy of an AIEC-targeted bacteriophage cocktail in a mice colitis model. J. Crohn’s Colitis 2022, 16, 1617–1627. [Google Scholar] [CrossRef]
- Ferreira, A.; Silva, D.; Almeida, C.; Rodrigues, M.E.; Silva, S.; Castro, J.; Mil-Homens, D.; García-Meniño, I.; Mora, A.; Henriques, M. Effect of phage vB_EcoM_FJ1 on the reduction of ETEC O9: H9 infection in a neonatal pig cell line. Vet. Res. 2023, 54, 26. [Google Scholar] [CrossRef]
- Kim, N.; Gu, M.J.; Kye, Y.-C.; Ju, Y.-J.; Hong, R.; Ju, D.B.; Pyung, Y.J.; Han, S.H.; Park, B.-C.; Yun, C.-H. Bacteriophage EK99P-1 alleviates enterotoxigenic Escherichia coli K99-induced barrier dysfunction and inflammation. Sci. Rep. 2022, 12, 941. [Google Scholar] [CrossRef]
- Cheng, D.; Sun, H.; Xu, J.; Gao, S. PCR detection of virulence factor genes in Escherichia coli isolates from weaned piglets with edema disease and/or diarrhea in China. Vet. Microbiol. 2006, 115, 320–328. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, P.; Ji, W.; Fu, Q.; Wang, H.; Yan, Y.; Sun, J. SLPW: A virulent bacteriophage targeting methicillin-resistant Staphylococcus aureus in vitro and in vivo. Front. Microbiol. 2016, 7, 934. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Gao, Y.; Xue, Y.; Liu, Y.; Zeng, X.; Cheng, Y.; Ma, J.; Wang, H.; Sun, J.; Wang, Z. Bacteriophage cocktails protect dairy cows against mastitis caused by drug resistant Escherichia coli infection. Front. Cell. Infect. Microbiol. 2021, 11, 690377. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.J.; McArdle, A.H.; Brown, R.; Scott, H.J.; Gurd, F.N. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch. Surg. 1970, 101, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, P.; Chen, C.; Feng, H.; Zhang, W.; Gu, C.; Wen, G.; Rao, V.B.; Tao, P. Bacteriophage T4 Vaccine Platform for Next-Generation Influenza Vaccine Development. Front. Immunol. 2021, 12, 745625. [Google Scholar] [CrossRef]
- LA, L.A.; Waturangi, D.E. Application of BI-EHEC and BI-EPEC bacteriophages to control enterohemorrhagic and enteropathogenic Escherichia coli on various food surfaces. BMC Res. Notes 2023, 16, 102. [Google Scholar]
- Zhang, J.-M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Alustiza, F.E.; Picco, N.Y.; Bellingeri, R.V.; Terzolo, H.R.; Vivas, A.B. Frequency of virulence genes of Escherichia coli among newborn piglets from an intensive pig farm in Argentina. Rev. Argent Microbiol. 2012, 44, 250–254. [Google Scholar]
- Tsekouras, N.; Meletis, E.; Kostoulas, P.; Labronikou, G.; Athanasakopoulou, Z.; Christodoulopoulos, G.; Billinis, C.; Papatsiros, V.G. Detection of enterotoxigenic Escherichia coli and clostridia in the aetiology of neonatal piglet diarrhoea: Important factors for their prevention. Life 2023, 13, 1092. [Google Scholar] [CrossRef]
- Vu-Khac, H.; Holoda, E.; Pilipcinec, E.; Blanco, M.; Blanco, J.; Dahbi, G.; Mora, A.; López, C.; González, E.; Blanco, J. Serotypes, virulence genes, intimin types and PFGE profiles of Escherichia coli isolated from piglets with diarrhoea in Slovakia. Vet. J. 2007, 174, 176–187. [Google Scholar] [CrossRef]
- Jenkins, T.P.; Ács, N.; Arendrup, E.W.; Swift, A.; Duzs, Á.; Chatzigiannidou, I.; Pichler, M.; Kittilä, T.; Peachey, L.; Gram, L. Protecting the piglet gut microbiota against ETEC-mediated post-weaning diarrhoea using specific binding proteins. npj Biofilms Microbiomes 2024, 10, 42. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Hill, C. Bacteriophages of the human gut: The “known unknown” of the microbiome. Cell Host Microbe 2019, 25, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Acta Kravsi 1961, 186, 1241–1243. [Google Scholar] [CrossRef]
- Lee, C.; Kim, S.; Park, B.; Han, J. Effects of dietary supplementation of bacteriophages against enterotoxigenic Escherichia coli (ETEC) K88 on clinical symptoms of post-weaning pigs challenged with the ETEC pathogen. J. Anim. Physiol. Anim. Nutr. 2017, 101, 88–95. [Google Scholar] [CrossRef]
- Cha, S.B.; Yoo, A.N.; Lee, W.J.; Shin, M.K.; Jung, M.H.; Shin, S.W.; Cho, Y.W.; Yoo, H.S. Effect of bacteriophage in enterotoxigenic Escherichia coli (ETEC) infected pigs. J. Vet. Med. Sci. 2012, 74, 1037–1039. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Abdelrahman, F.; Dawoud, A.; Connerton, I.F.; El-Shibiny, A. Encapsulation of E. coli phage ZCEC5 in chitosan–alginate beads as a delivery system in phage therapy. Amb. Express 2019, 9, 87. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Zeng, X.; Cai, S.; Wang, G.; Liu, L.; Huang, S.; Li, N.; Liu, H.; Ding, X. Therapeutic administration of the recombinant antimicrobial peptide microcin J25 effectively enhances host defenses against gut inflammation and epithelial barrier injury induced by enterotoxigenic Escherichia coli infection. FASEB J. 2020, 34, 1018–1037. [Google Scholar] [CrossRef] [PubMed]
- Maura, D.; Galtier, M.; Le Bouguénec, C.; Debarbieux, L. Virulent bacteriophages can target O104: H4 enteroaggregative Escherichia coli in the mouse intestine. Antimicrob. Agents Chemother. 2012, 56, 6235–6242. [Google Scholar] [CrossRef] [PubMed]
- Danis-Wlodarczyk, K.; Dąbrowska, K.; Abedon, S.T. Phage therapy: The pharmacology of antibacterial viruses. Curr. Issues Mol. Biol. 2021, 40, 81–164. [Google Scholar] [CrossRef]
- Kaźmierczak, Z.; Majewska, J.; Milczarek, M.; Owczarek, B.; Dąbrowska, K. Circulation of fluorescently labelled phage in a murine model. Viruses 2021, 13, 297. [Google Scholar] [CrossRef]
- Kaźmierczak, Z.; Piotrowicz, A.; Owczarek, B.; Hodyra, K.; Miernikiewicz, P.; Lecion, D.; Harhala, M.; Górski, A.; Dąbrowska, K. Molecular imaging of T4 phage in mammalian tissues and cells. Bacteriophage 2014, 4, e28364. [Google Scholar] [CrossRef]


| ETEC Strains | Enterotoxin | Drug Resistance | Phage | ||||||
|---|---|---|---|---|---|---|---|---|---|
| STa | STb | LT1 | AMP (R: 88% a) | CIP (R: 64%) | GM (R: 32%) | MEM (R: 0%) | FEP (R: 0%) | JE01 | |
| U74 | + b | − | − | R c | S | S | S | S | + |
| U86 | − | + | + | R | S | R | S | S | − |
| XH12 | + | + | − | R | I | S | S | S | + |
| XH18 | − | − | + | R | S | R | S | S | − |
| XH22 | − | + | + | R | R | R | S | S | + |
| K88-4 | + | + | + | I | S | S | S | S | − |
| K99-1 | + | − | − | S | S | S | S | S | + |
| NJ-08 | + | + | + | I | I | S | S | S | + |
| NJ-09 | + | + | − | R | S | S | S | S | − |
| NJ-14 | − | + | + | R | R | R | S | S | + |
| NJ-18 | − | + | + | R | R | I | S | S | − |
| NJ-24 | + | + | − | R | R | R | S | S | − |
| NJ-26 | + | + | + | R | R | S | S | S | − |
| NJ-28 | + | + | − | R | I | S | S | S | + |
| NJ-30 | + | + | + | R | R | I | S | S | + |
| MS-04 | + | + | − | S | S | S | S | S | − |
| MS-12 | + | + | − | I | I | S | S | S | − |
| MS-14 | + | + | − | R | R | R | S | S | − |
| MS-27 | − | − | + | R | S | S | S | S | − |
| MS-31 | + | + | − | R | R | S | S | S | − |
| JD-12 | + | + | − | S | S | S | S | S | − |
| JD-23 | + | − | − | R | R | S | S | S | − |
| JD-27 | − | − | + | R | I | S | S | S | − |
| JD-32 | + | − | − | R | R | S | S | S | + |
| JD-38 | − | − | + | I | I | S | S | S | − |
| Primer | Primer Sequences | |
|---|---|---|
| STa-F | GGGTTGGCAATTTTTATTTCTGT | |
| STa-R | ATTACAACAAAGTTCACAGCAGTA | |
| STb-F | ATGTAAATACCTACAACGGGTGAT | |
| STb-R | TATTTGGGCGCCAAAGCATGCTCC | |
| LT1-F | TAGAGACCGGTATTACAGAAATCTGA | |
| LT1-R | TCATCCCGAATTCTGTTATATATGTC | |
| Porcine IL-8-F | TAGGACCAGAGCCAGGAAGA | |
| Porcine IL-8-R | AGCAGGAAAACTGCCAAGAA | |
| Porcine IL-6-F | CCTCTCCGGACAAAACTGAA | |
| Porcine IL-6-R | TCTGCCAGTACCTCCTTGCT | |
| Porcine β-Actin-F | GGACTTCGAGCAGGAGATGG | |
| Porcine β-Actin-R | GCACCGTGTTGGCGTAGAGG | |
| Murine IL-8-F | TCGAGACCATTTACTGCAACAG | |
| Murine IL-8-R | CATTGCCGGTGGAAATTCCTT | |
| Murine IL-6-F | CTGCAAGAGACTTCCATCCAG | |
| Murine IL-6-R | AGTGGTATAGACAGGTCTGTTGG | |
| Murine TNF-α-F | CAGGCGGTGCCTATGTCTC | |
| Murine TNF-α-R | CGATCACCCCGAAGTTCAGTAG | |
| Murine β-Actin-F | AAGAGCTATGAGCTGCCTGA | |
| Murine β-Actin-R | TACGGATGTCAACGTCACAC | |
| Group (n = 24) | Bacterial Challenge | Treatment |
|---|---|---|
| PBS Control | 50 μL PBS | 50 μL PBS |
| ETEC + PBS | 50 μL ETEC | 50 μL PBS |
| ETEC + JE01 | 50 μL ETEC | 50 μL JE01 |
| ETEC + GEN | 50 μL ETEC | 50 μL GEN |
| PBS + JE01 | 50 μL PBS | 50 μL JE01 |
| PBS + GEN | 50 μL PBS | 50 μL GEN |
| Group (n = 15) | Bacterial Challenge | Treatment |
|---|---|---|
| PBS control | PBS | PBS |
| PBS + PhageRFP | PBS | PhageRFP |
| ETEC + PhageRFP | ETEC | PhageRFP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Xia, L.; Zhang, Y.; Zhao, G.; Zhang, S.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J.; Yan, Y.; et al. Pharmacodynamic Evaluation of Phage Therapy in Ameliorating ETEC-Induced Diarrhea in Mice Models. Microorganisms 2024, 12, 2532. https://doi.org/10.3390/microorganisms12122532
Xiong Y, Xia L, Zhang Y, Zhao G, Zhang S, Ma J, Cheng Y, Wang H, Sun J, Yan Y, et al. Pharmacodynamic Evaluation of Phage Therapy in Ameliorating ETEC-Induced Diarrhea in Mice Models. Microorganisms. 2024; 12(12):2532. https://doi.org/10.3390/microorganisms12122532
Chicago/Turabian StyleXiong, Yangjing, Lu Xia, Yumin Zhang, Guoqing Zhao, Shidan Zhang, Jingjiao Ma, Yuqiang Cheng, Hengan Wang, Jianhe Sun, Yaxian Yan, and et al. 2024. "Pharmacodynamic Evaluation of Phage Therapy in Ameliorating ETEC-Induced Diarrhea in Mice Models" Microorganisms 12, no. 12: 2532. https://doi.org/10.3390/microorganisms12122532
APA StyleXiong, Y., Xia, L., Zhang, Y., Zhao, G., Zhang, S., Ma, J., Cheng, Y., Wang, H., Sun, J., Yan, Y., & Wang, Z. (2024). Pharmacodynamic Evaluation of Phage Therapy in Ameliorating ETEC-Induced Diarrhea in Mice Models. Microorganisms, 12(12), 2532. https://doi.org/10.3390/microorganisms12122532







