The First Description of the Microbial Diversity in the Amarillo River (La Rioja, Argentina), a Natural Extreme Environment Where the Whole Microbial Community Paints the Landscape Yellow
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sample Collection
2.2. Enrichment Cultures
2.3. DNA Extraction and Sequencing
2.4. Bioinformatic Analysis
3. Results
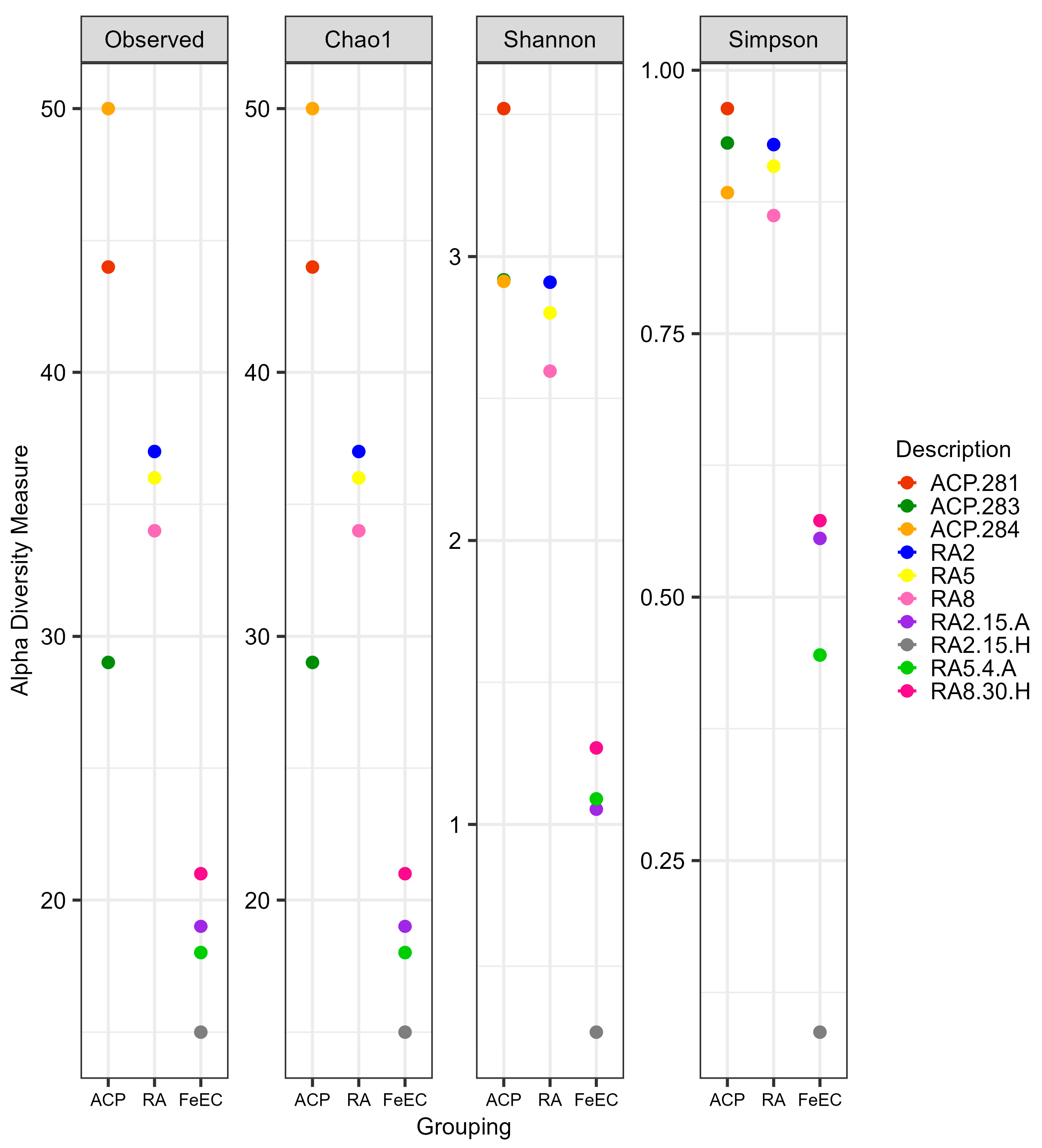
3.1. High-Throughput Sequencing Metrics and Biodiversity Analysis
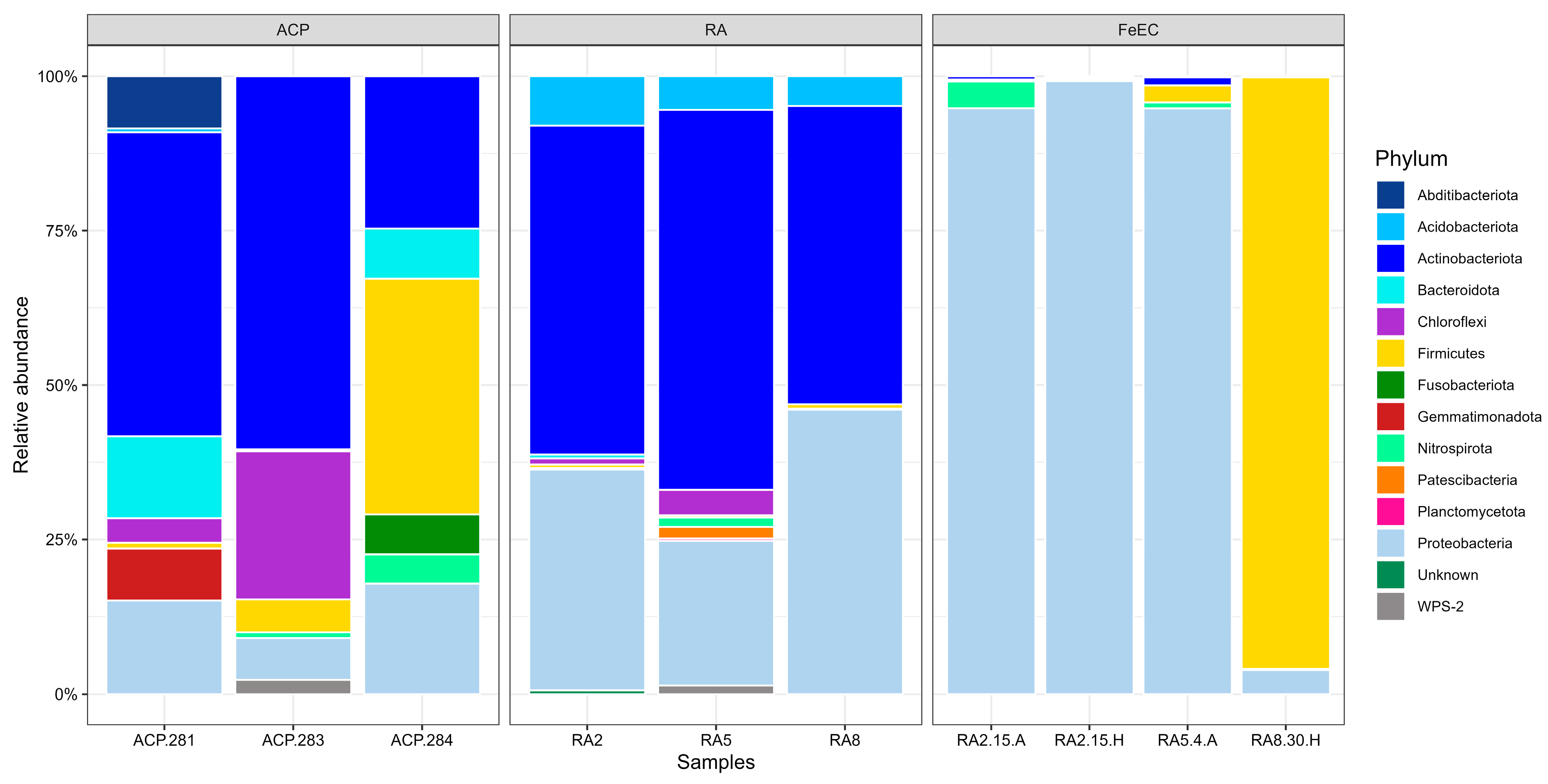
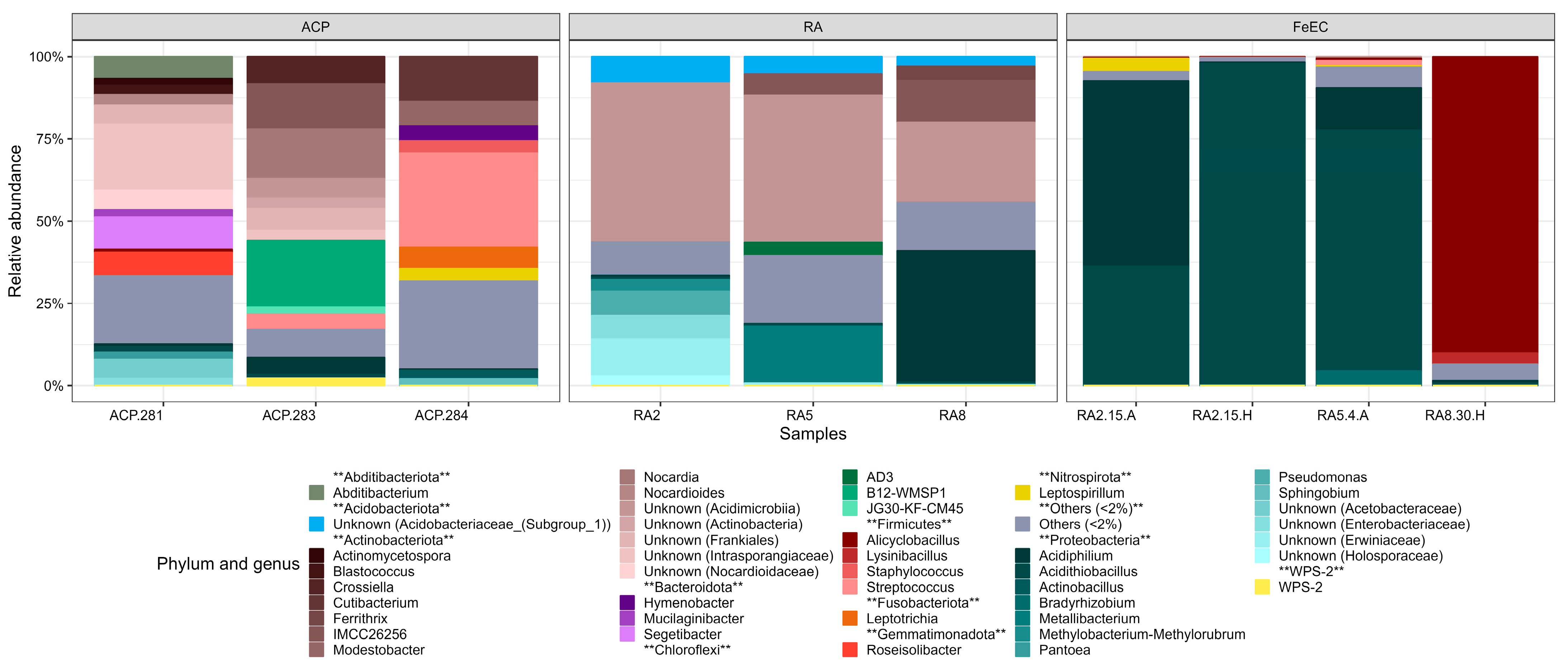
3.1.1. Bacterial Diversity in the Amarillo River System and the Fe(II) Enrichment Cultures
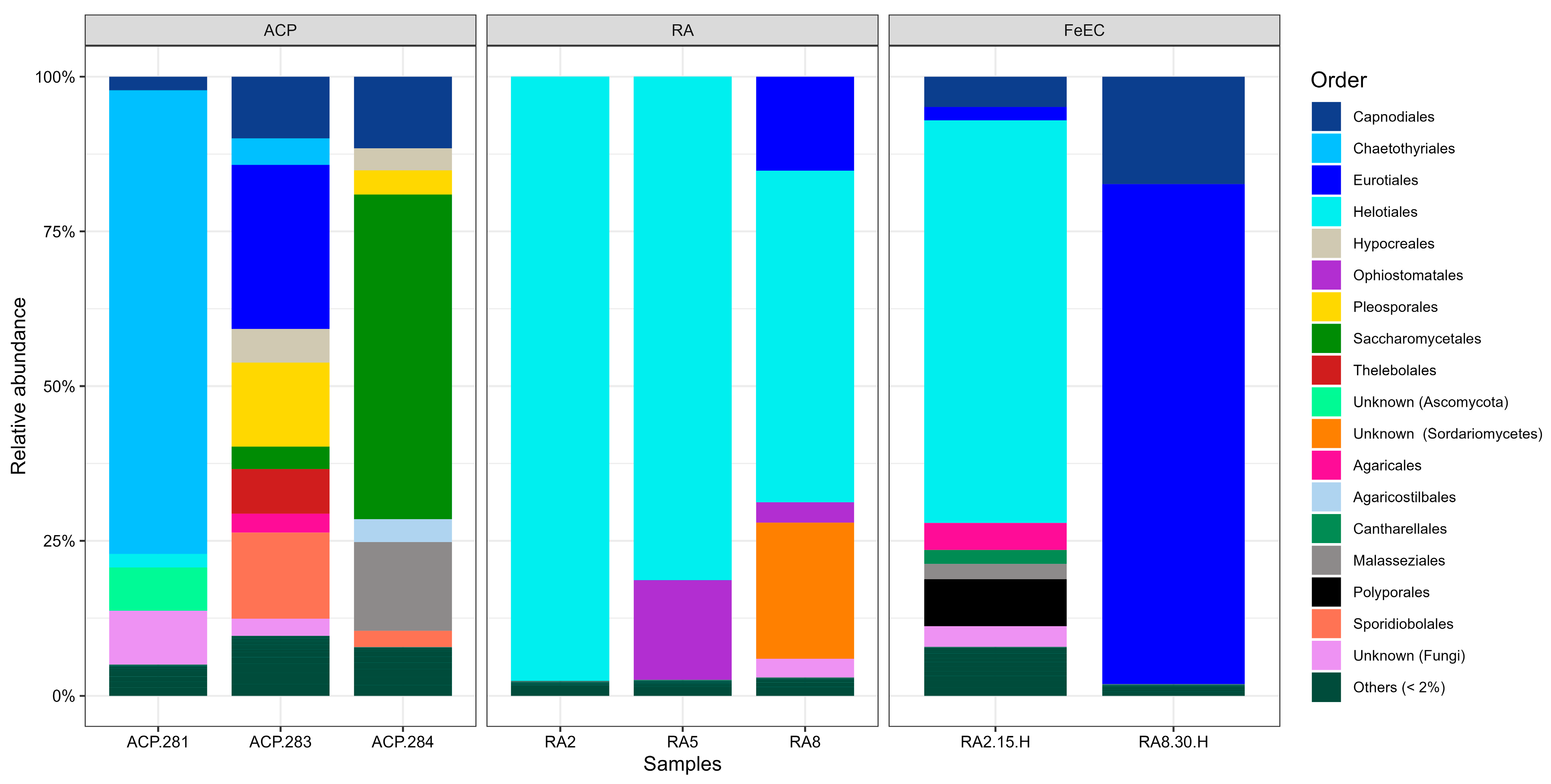
3.1.2. Fungal Diversity in the Amarillo River System and the Fe(II) Enrichment Cultures
4. Discussion
4.1. Species Involved in Iron Metabolism
4.2. Fungi in the Amarillo River
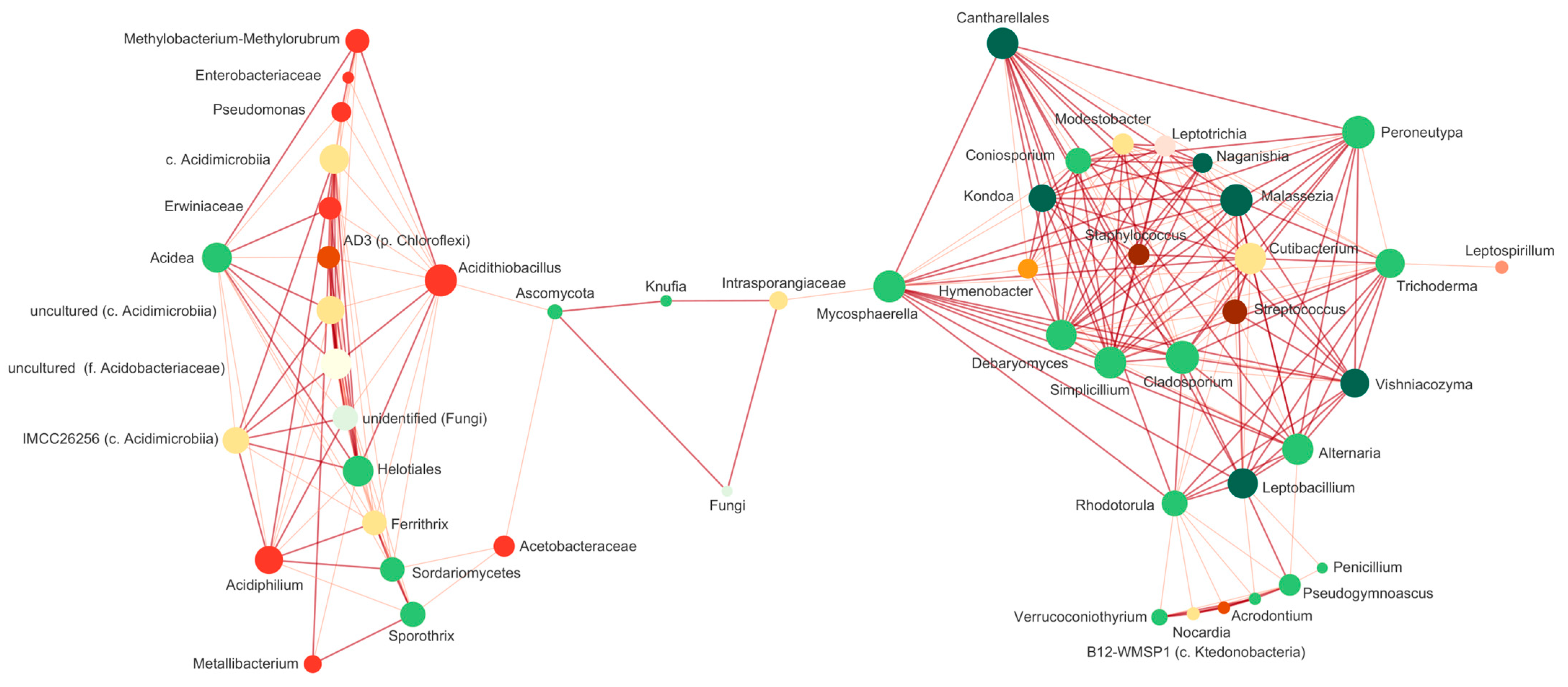
4.3. Common Species and Interactions
5. Final Comments and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hedrich, S.; Schipper, A. Distribution of acidophilic microorganisms in natural and man-made acidic environments. Curr. Issues Mol. Biol. 2021, 40, 25–48. [Google Scholar] [CrossRef] [PubMed]
- Amils, R. Lessons learned from thirty years of geomicrobiological studies of Río Tinto. Res. Microbiol. 2016, 167, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bedogni, G.; Massello, F.; Giaveno, A.; Donati, E.; Urbieta, M. A deeper look into the biodiversity of the extremely acidic Copahue volcano- Río Agrio system in Neuquén, Argentina. Microorganisms 2019, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.; Rivett, D.; Pakostova, E.; Creer, S.; Cotterell, T.; Johnson, D. Mineralogy affects prokaryotic community composition in an acidic metal mine. Microbiol. Res. 2023, 266, 127257. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; Huang, L.; Chen, L.; Hua, Z.; Li, S.; Hu, M.; Li, J.; Shu, W. Contemporary environmental variation determines microbial diversity patterns in acid mine drainage. ISME J. 2013, 7, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Brodtkorb, M.; Schalamuk, B. Yacimientos de cobre y oro de la Sierra de Famatina, La Rioja. In Recursos Minerales de la República Argentina; Zappettini, E.O., Ed.; Instituto de Geología y Recursos Minerales SEGEMAR: Buenos Aires, Argentina, 1999; pp. 1659–1663. [Google Scholar]
- Lecomte, K.; Maza, S.; Collo, G.; Sarmiento, A.; Depetris, P. Geochemical behavior of an acid drainage system: The case of the Amarillo River, Famatina (La Rioja, Argentina). Environ. Sci. Pollut. Res. 2017, 24, 1630–1647. [Google Scholar] [CrossRef] [PubMed]
- Maza, S.; Collo, G.; Astina, R.; Nieto, F.; Nieto, J.M. Holocene ochreous lacustrine sediments within the Famatina Belt, NW Argentina: A natural case for fossil damming of an acid drainage system. J. S. Am. Earth Sci. 2014, 52, 149–165. [Google Scholar] [CrossRef]
- Bernardelli, C.; Maza, S.; Lecomte, K.; Collo, G.; Astini, R.; Donati, E. Acidophilic microorganisms enhancing geochemical dynamics in an acidic drainage system, Amarillo River in La Rioja, Argentina. Chemosphere 2021, 263, 128098. [Google Scholar] [CrossRef]
- Johnson, B.; Kanao, T.; Hedrich, S. Redox transformations of iron at extremely low pH: Fundamental and applied aspects. Front. Microbiol. 2012, 3, 96. [Google Scholar] [CrossRef]
- Tiquia-Arashiro, S.; Grube, M. (Eds.) Fungi in Extreme Environments: Ecological Role and Biotechnological Significance; Springer International Publishing: Cham, Switzerland; Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Coleine, C.; Stajich, J.; Selbmann, L. Fungi are key players in extreme ecosystems. Trends Ecol. Evol. 2022, 37, 517–528. [Google Scholar] [CrossRef]
- Aguilera, A.; Olsson, S.; Puerte-Sánchez, F. Physiological and Phylogenetic Diversity of Acidophilic Eukaryotes. In Acidophiles: Life in Extremely Acidic Environment; Quatrini, R., Johnson, D.B., Eds.; Caister Academic Press: Norfolk, VA, USA, 2016; pp. 107–118. [Google Scholar]
- Das, B.; Roy, A.; Koschorreck, M.; Mandal, S.; Wendt-Potthoff, K.; Bhattacharya, J. Occurrence and role of algae and fungi in acid mine drainage environment with special reference to metals and sulfate immobilization. Water Res. 2009, 43, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Hujslová, M.; Kubátová, A.; Kostovčík, M.; Blanchette, R.; Wilhelm de Beer, Z.; Chudíčková, M.; Kolařík, M. Three new genera of fungi from extremely acidic soils. Mycol. Progress 2014, 13, 819–831. [Google Scholar] [CrossRef]
- Hujslová, M.; Bystrianský, L.; Benada, O.; Gryndler, M. Fungi, a neglected component of acidophilic biofilms: Do they have a potential for biotechnology? Extrem. Life Under Extrem. Cond. 2019, 23, 267–275. [Google Scholar] [CrossRef]
- Oggerin, M.; Rodríguez, M.; del Moral, C.; Amils, R. Fungal jarosite biomineralization in Río Tinto. Res. Microbiol. 2014, 165, 719–725. [Google Scholar] [CrossRef]
- Marcos, O.; Zanettini, J. Geología y Exploración del Proyecto Nevados del Famatina; Servicio Geológico, Secretaría de Minería de la Nación, Informe Inédito: Buenos Aires, Argentina, 1982; 325p. [Google Scholar]
- Juarez, O.; Corbat, M.; Maza, S.; Collo, G.; Fucks, E.; Pannunzio, E. Transformación Mineral de los Cementos de Ferricretes de la Formación Cueva de Pérez, Asociados a un Drenaje ácido Natural (Famatina, La Rioja). In V Reunión Argentina de Geoquímica de la Superficie (RAGSU); Asociación Argentina de Sedimentología: Buenos Aires, Argentina, 2019; pp. 186–189. [Google Scholar]
- Mackintosh, M. Nitrogen Fixation by Thiobacillus ferrooxidans. Microbiology 1978, 105, 215–218. [Google Scholar] [CrossRef]
- Callahan, B.; McMurdie, P.; Rosen, M.; Han, A.; Johnson, A.; Holmes, S. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Martin-Pozas, T.; Fernandez-Cortes, A.; Cuezva, S.; Cañaveras, J.; Benavente, D.; Duarte, E.; Sanchez-Moral, S. New insights into the structure, microbial diversity and ecology of yellow biofilms in a Paleolithic rock art cave (Pindal Cave, Asturias, Spain). Sci. Total Environ. 2023, 897, 165218. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Jiang, Z.; Liang, Z.; Wang, P.; Liu, Z.; Jiang, C. Key factors governing microbial community in extremely acidic mine drainage (pH < 3). Front. Microbiol. 2021, 12, 761579. [Google Scholar]
- Demir, E.; Yaman, B.; Çelik, P.; Sahinkaya, E. Iron oxidation in a ceramic membrane bioreactor using acidophilic bacteria isolated from an acid mine drainage. J. Water Process Eng. 2020, 38, 101610. [Google Scholar] [CrossRef]
- Souza-Egipsy, V.; González-Toril, E.; Zettler, E.; Amaral-Zettler, L.; Aguilera, A.; Amils, R. Prokaryotic community structure in algal photosynthetic biofilms from extreme acidic streams in Río Tinto (Huelva, Spain). Int. Microbiol. 2008, 11, 251–260. [Google Scholar] [PubMed]
- Gavrilov, S.; Korzhenkov, A.; Kublanov, I.; Bargiela, R.; Zamana, L.; Popova, A.; Golyshina, O. Microbial communities of polymetallic deposits’ acidic ecosystems of continental climatic zone with high temperature contrasts. Front. Microbiol. 2019, 10, 1573. [Google Scholar] [CrossRef] [PubMed]
- Geremia, R.; Pușcaș, M.; Zinger, L.; Bonneville, J.; Choler, P. Contrasting microbial biogeographical patterns between anthropogenic subalpine grasslands and natural alpine grasslands. New Phytol. 2016, 209, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- de Mesquita, C.; Sartwell, S.; Ordemann, E.; Porazinska, D.; Farrer, E.; King, A.; Schmidt, S. Patterns of root colonization by arbuscular mycorrhizal fungi and dark septate endophytes across a mostly unvegetated, high-elevation landscape. Fungal Ecol. 2018, 36, 63–74. [Google Scholar] [CrossRef]
- Breitenbach, R.; Silbernagl, D.; Toepel, J.; Sturm, H.; Broughton, W.J.; Sassaki, G.; Gorbushina, A. Corrosive extracellular polysaccharides of the rock-inhabiting model fungus Knufia petricola. Extremophiles 2018, 22, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, L.; Xia, Y.; Zhuang, X.; Chu, W. Isolation, identification of carotenoid-producing Rhodotorula sp. from marine environment and optimization for carotenoid production. Mar. Drugs 2019, 17, 161. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Z.; Chen, S.; Wang, T.; Jiang, L.; Wang, M.; Li, Z. Biochemical changes of polysaccharides and proteins within EPS under Pb (II) stress in Rhodotorula mucilaginosa. Ecotoxicol. Environ. Saf. 2019, 174, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, P.; Vásquez, G.; Gil-Durán, C.; Oliva, V.; Díaz, A.; Henríquez, M.; Álvarez, E.; Laich, F.; Chávez, R.; Vaca, I. Description of the First Four Species of the Genus Pseudogymnoascus from Antarctica. Front. Microbiol. 2021, 12, 713189. [Google Scholar] [CrossRef]
- Anastasi, A.; Spina, F.; Prigione, V.; Tigini, V.; Giansanti, P.; Varese, G. Scale-up of a bioprocess for textile wastewater treatment using Bjerkandera adusta. Bioresour. Technol. 2010, 101, 3067–3075. [Google Scholar] [CrossRef]
- Vázquez-Campos, X.; Kinsela, A.; Waite, T.; Collins, R.; Neilan, B. Fodinomyces uranophilus gen. nov. sp. nov. and Coniochaeta fodinicola sp. nov., two uranium mine-inhabiting Ascomycota fungi from northern Australia. Mycologia 2014, 106, 1073–1089. [Google Scholar] [CrossRef]
- Hallberg, K.; González-Toril, E.; Johnson, D. Acidithiobacillus ferrivorans, sp. nov.; facultatively anaerobic, psychrotolerant iron-, and sulfur-oxidizing acidophiles isolated from metal mine-impacted environments. Extremophiles 2010, 14, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Barahona, S.; Castro-Severyn, J.; Dorador, C.; Saavedra, C.; Remonsellez, F. Determinants of Copper Resistance in Acidithiobacillus ferrivorans ACH Isolated from the Chilean Altiplano. Genes 2020, 11, 844–863. [Google Scholar] [CrossRef] [PubMed]
- Liljeqvist, M.; Valdes, J.; Holmes, D.; Dopson, M. Draft genome of the psychrotolerant acidophile Acidithiobacillus ferrivorans SS3. J. Bacteriol. 2011, 193, 4304–4305. [Google Scholar] [CrossRef] [PubMed]
- Talla, E.; Hedrich, S.; Mangenot, S.; Ji, B.; Johnson, D.; Barbe, V.; Bonnefoy, V. Insights into the pathways of iron-and sulfur-oxidation, and biofilm formation from the chemolithotrophic acidophile Acidithiobacillus ferrivorans CF27. Res. Microbiol. 2014, 165, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, A.; Imhoff, J. Acidiphilium. In Bergey’s Manual of Systematics of Archaea and Bacteria; American Cancer Society: New York, NY, USA, 2015; pp. 1–14. [Google Scholar]
- Ullrich, S.; Poehlein, A.; Voget, S.; Hoppert, M.; Daniel, R.; Leimbach, A.; Mühling, M. Permanent draft genome sequence of Acidiphilium sp. JA12-A1. Stand. Genom. Sci. 2015, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Cha, G.; Gao, B. A phylogenomic and molecular markers based analysis of the class Acidimicrobiia. Front. Microbiol. 2018, 9, 987. [Google Scholar] [CrossRef]
- Johnson, D.; Bacelar-Nicolau, P.; Okibe, N.; Thomas, A.; Hallberg, K. Ferrimicrobium acidiphilum gen. nov., sp. nov. and Ferrithrix thermotolerans gen. nov., sp. nov.: Heterotrophic, iron-oxidizing, extremely acidophilic actinobacteria. Int. J. Syst. Evol. Microbiol. 2009, 59, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, Z.; Zhou, Z.; Li, L.; Meng, D.; Li, X.; Yin, H. Mobile genetic elements mediate the mixotrophic evolution of novel Alicyclobacillus species for acid mine drainage adaptation. Environ. Microbiol. 2021, 23, 3896–3912. [Google Scholar] [CrossRef]
- Panyushkina, A.; Muravyov, M.; Fomchenko, N. A Case of Predominance of Alicyclobacillus tolerans in Microbial Community during Bioleaching of Pentlandite-Chalcopyrite Concentrate. Minerals 2022, 12, 396. [Google Scholar] [CrossRef]
- Rowe, O.; Johnson, D. Comparison of ferric iron generation by different species of acidophilic bacteria immobilized in packed-bed reactors. Syst. Appl. Microbiol. 2008, 31, 68–77. [Google Scholar] [CrossRef]
- Mehrotra, A.; Sreekrishnan, T. Heavy metal bioleaching and sludge stabilization in a single-stage reactor using indigenous acidophilic heterotrophs. Environ. Technol. 2017, 38, 2709–2724. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Johnson, D. Iron kinetics and evolution of microbial populations in low-pH, ferrous iron-oxidizing bioreactors. Environ. Sci. Technol. 2016, 50, 8239–8245. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Zhuang, X.; Wu, J.; Cui, M.; Lv, D.; Liu, C.; Zhuang, G. Ascomycota members dominate fungal communities during straw residue decomposition in arable soil. PLoS ONE 2013, 8, e66146. [Google Scholar] [CrossRef] [PubMed]
- Méndez-García, C.; Peláez, A.; Mesa, V.; Sánchez, J.; Golyshina, O.; Ferrer, M. Microbial diversity and metabolic networks in acid mine drainage habitats. Front. Microbiol. 2015, 6, 475. [Google Scholar]
- Aguilera, A.; González-Toril, E. Eukaryotic Life in Extreme Environments: Acidophilic Fungi. In Fungi in Extreme Environments: Ecological Role and Biotechnological Significance; Tiquia-Arashiro, S., Grube, M., Eds.; Springer: New York, NY, USA, 2019; pp. 21–38. [Google Scholar]
- Hujslová, M.; Gryndlerová, H.; Bystrianský, L.; Hršelová, H.; Gryndler, M. Biofilm and planktonic microbial communities in highly acidic soil (pH < 3) in the Soos National Nature Reserve, Czech Republic. Extremophiles 2020, 24, 577–591. [Google Scholar]
- Rousk, J.; Bååth, E.; Brookes, P.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Stępniewska, H.; Uzarowicz, Ł.; Błońska, E.; Kwasowski, W.; Słodczyk, Z.; Gałka, D.; Hebda, A. Fungal abundance and diversity as influenced by properties of Technosols developed from mine wastes containing iron sulphides: A case study from abandoned iron sulphide and uranium mine in Rudki, south-central Poland. Appl. Soil Ecol. 2020, 145, 103349. [Google Scholar] [CrossRef]
- Cai, H.; Shi, P.; Luo, H.; Bai, Y.; Huang, H.; Yang, P.; Yao, B. Acidic β-mannanase from Penicillium pinophilum C1: Cloning, characterization and assessment of its potential for animal feed application. J. Biosci. Bioeng. 2011, 112, 551–557. [Google Scholar] [CrossRef]
- López-Archilla, A.; González, A.; Terrón, M.; Amils, R. Ecological study of the fungal populations of the acidic Tinto River in southwestern Spain. Can. J. Microbiol. 2004, 50, 923–934. [Google Scholar] [CrossRef]
- Jin, Y.; Qin, S.; Gao, H.; Zhu, G.; Wang, W.; Zhu, W.; Wang, Y. An anti-HBV anthraquinone from aciduric fungus Penicillium sp. OUCMDZ-4736 under low pH stress. Extremophiles 2018, 22, 39–45. [Google Scholar] [CrossRef]
- Dalsgaard, P.; Larsen, T.; Christophersen, C. Bioactive cyclic peptides from the psychrotolerant fungus Penicillium algidum. J. Antibiot. 2005, 58, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, S.; Dianese, J.; Fell, J.; Gunde-Cimerman, N.; Zalar, P. Unusual fungal niches. Mycologia 2011, 103, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.; Godinho, V.; Silva, D.; de Paula, M.T.R.; Vitoreli, G.A.; Zani, C.L.; Alves, T.M.A.; Junior, P.A.S.; Murta, S.M.F.; Barbosa, E.C.; et al. Cultivable fungi present in Antarctic soils: Taxonomy, phylogeny, diversity, and bioprospecting of antiparasitic and herbicidal metabolites. Extremophiles 2018, 22, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Cavello, I.; Garmendia, G.; Rufo, C.; Cavalitto, S.; Vero, S. Yeasts from sub-Antarctic region: Biodiversity, enzymatic activities and their potential as oleaginous microorganisms. Extremophiles 2016, 20, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Scardoni, G.; Laudanna, C. Centralities Based Analysis of Complex Networks. In New Frontiers in Graph Theory; IntechOpen: London, UK, 2012; pp. 323–348. [Google Scholar]
- Chung, A.; Lopes, A.; Nobre, M.; Morais, P. Hymenobacter perfusus sp. nov., Hymenobacter flocculans sp. nov. and Hymenobacter metalli sp. nov. three new species isolated from an uranium mine waste water treatment system. Syst. Appl. Microbiol. 2010, 33, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Busarakam, K.; Bull, A.; Trujillo, M.; Riesco, R.; Sangal, V.; van Wezel, G.; Goodfellow, M. Modestobacter caceresii sp. nov., novel actinobacteria with an insight into their adaptive mechanisms for survival in extreme hyper-arid Atacama Desert soils. Syst. Appl. Microbiol. 2016, 39, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.; King, A.; Farías, M.; Sowell, P.; Vitry, C.; Schmidt, S. The potential for microbial life in the highest-elevation (>6000 masl) mineral soils of the Atacama region. J. Geophys. Res. Biogeosci. 2012, 117, G02028. [Google Scholar] [CrossRef]
- Ziegler, S.; Waidner, B.; Itoh, T.; Schumann, P.; Spring, S.; Gescher, J. Metallibacterium scheffleri gen. nov., sp. nov., an alkalinizing gammaproteobacterium isolated from an acidic biofilm. Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 4, 1499–1504. [Google Scholar] [CrossRef]
- Zakharova, K.; Tesei, D.; Marzban, G.; Dijksterhuis, J.; Wyatt, T.; Sterflinger, K. Microcolonial fungi on rocks: A life in constant drought? Mycopathologia 2013, 175, 537–547. [Google Scholar] [CrossRef]
- Tesei, D.; Chiang, A.; Kalkum, M.; Stajich, J.; Mohan, G.; Sterflinger, K.; Venkateswaran, K. Effects of simulated microgravity on the proteome and secretome of the polyextremotolerant black fungus Knufia chersonesos. Front. Genet. 2021, 12, 638708. [Google Scholar] [CrossRef]
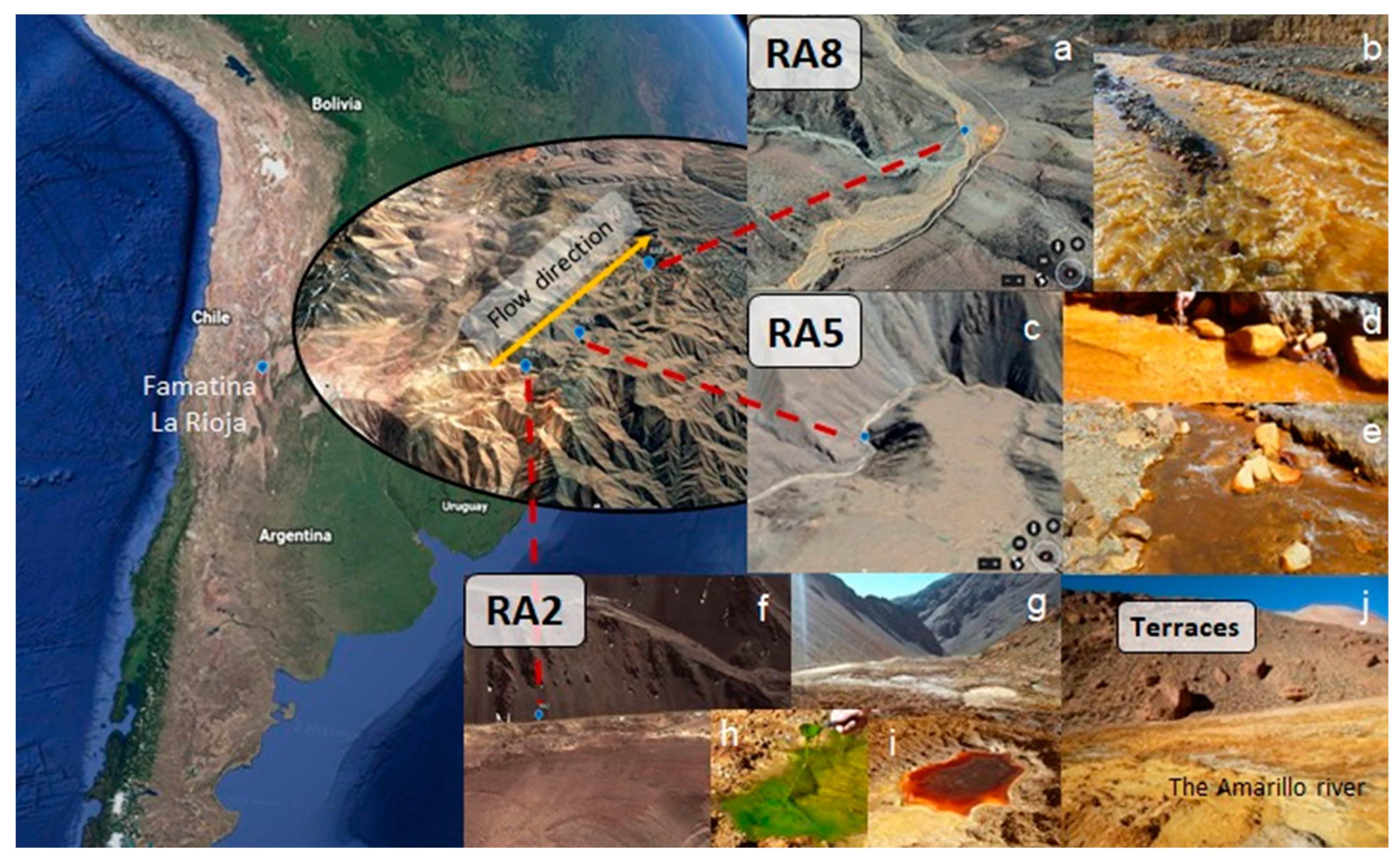



| Sampling Point Name | RA2 | RA5 | RA8 |
|---|---|---|---|
| Latitude (S) | 28°59′51.6″ | 28°58′34.5″ | 28°55′36.7″ |
| Longitude (W) | 67°44′21.4″ | 67°42′53.9″ | 67°40′34.0″ |
| Height (m.a.s.l.) | 3869 | 3419 | 2838 |
| Downstream distance (Km) | 4.89 | 8.83 | 17.09 |
| pH | 2.77 (* 3.00) | * 2.92 | 2.94 (* 3.33) |
| Eh (mV) | 575 (* 667) | * 664 | 716 (* 634) |
| Conductivity (mS·cm−1) | 6.7 (* 5.78) | * 4.15 | 1.63 (* 2.09) |
| Al (mg·L−1) | 690 (* 542) | * 419 | 102 (* 185) |
| As (mg·L−1) | 1.05 (* 0.51) | * 0.12 | b.d.l. |
| K (mg·L−1) | 58.8 (* 50) | * 13.6 | 7.4 (* 6.9) |
| Mg (mg·L−1) | 76.6 (* 94.2) | * 73.9 | 29 (* 44) |
| Mn (mg·L−1) | 47.7 (* 65.2) | * 42.3 | 11.4 (* 19.6) |
| Si (mg·L−1) | 36 (* 39.7) | * 36.7 | 14.6 (* 21.2) |
| Ca (mg·L−1) | 51.8 (* 41.6) | * 38.5 | 26.4 (* 33.2) |
| Cu (mg·L−1) | 63.3 (* 39.9) | * 32.5 | 7.89 (* 13.6) |
| FeTOT (mg·L−1) | 1430 (* 1220) | * 761 | 108 (* 196) |
| Zn (mg·L−1) | 40.3 (* 50.9) | * 31.8 | 7.46 (* 14.2) |
| SO4−2 (mg·L−1) | 2340 (* 5241) | * 4013 | 363 (* 1845) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardelli, C.E.; Colman, D.; Donati, E.R.; Urbieta, M.S. The First Description of the Microbial Diversity in the Amarillo River (La Rioja, Argentina), a Natural Extreme Environment Where the Whole Microbial Community Paints the Landscape Yellow. Microorganisms 2024, 12, 235. https://doi.org/10.3390/microorganisms12020235
Bernardelli CE, Colman D, Donati ER, Urbieta MS. The First Description of the Microbial Diversity in the Amarillo River (La Rioja, Argentina), a Natural Extreme Environment Where the Whole Microbial Community Paints the Landscape Yellow. Microorganisms. 2024; 12(2):235. https://doi.org/10.3390/microorganisms12020235
Chicago/Turabian StyleBernardelli, Cecilia Elena, Deborah Colman, Edgardo Ruben Donati, and María Sofía Urbieta. 2024. "The First Description of the Microbial Diversity in the Amarillo River (La Rioja, Argentina), a Natural Extreme Environment Where the Whole Microbial Community Paints the Landscape Yellow" Microorganisms 12, no. 2: 235. https://doi.org/10.3390/microorganisms12020235
APA StyleBernardelli, C. E., Colman, D., Donati, E. R., & Urbieta, M. S. (2024). The First Description of the Microbial Diversity in the Amarillo River (La Rioja, Argentina), a Natural Extreme Environment Where the Whole Microbial Community Paints the Landscape Yellow. Microorganisms, 12(2), 235. https://doi.org/10.3390/microorganisms12020235









