Changes in Diversity and Abundance of Ammonia-Oxidizing Archaea and Bacteria along a Glacier Retreating Chronosequence in the Tianshan Mountains, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Sampling
2.2. Biogeochemical Properties of the Soil
2.3. DNA Extraction and PCR Amplification of the amoA Gene Fragment
2.4. Cloning and Sequence Analysis of amoA Gene
2.5. Quantitative PCR Anaylsis of amoA Gene
2.6. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Abundance of AOA and AOB
3.3. Diversity of AOA and AOB
3.4. Phylogenetic Analysis of AOA and AOB
3.5. Relationships between Soil Physicochemical Properties and AOA and AOB
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2023. [CrossRef]
- Bradley, J.A.; Singarayer, J.S.; Anesio, A.M. Microbial community dynamics in the forefield of glaciers. Proc. R. Soc. B. 2014, 281, 20140882. [Google Scholar] [CrossRef]
- Górniak, D.; Marszałek, H.; Kwaśniak-Kominek, M.; Rzepa, G.; Manecki, M. Soil formation and initial microbiological activity on a foreland of an Arctic glacier (SW Svalbard). Appl. Soil Ecol. 2017, 114, 34–44. [Google Scholar] [CrossRef]
- Kim, M.; Jung, J.Y.; Laffly, D.; Kwon, H.Y.; Lee, Y.K. Shifts in bacterial community structure during succession in a glacier foreland of the High Arctic. FEMS Microbiol. Ecol. 2016, 93, fiw213. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.Y.; Jung, J.Y.; Kim, O.-S.; Laffly, D.; Lim, H.S.; Lee, Y.K. Soil development and bacterial community shifts along the chronosequence of the Midtre Lovénbreen glacier foreland in Svalbard. J. Ecol. Environ. 2015, 38, 461–476. [Google Scholar] [CrossRef]
- Yan, W.; Ma, H.; Shi, G.; Li, Y.; Sun, B.; Xiao, X.; Zhang, Y. Independent Shifts of Abundant and Rare Bacterial Populations across East Antarctica Glacial Foreland. Front. Microbiol. 2017, 8, 1534. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.C.Q.; Godinho, V.M.; Silva, D.A.S.; de Paula, M.T.R.; Vitoreli, G.A.; Zani, C.L.; Alves, T.M.A.; Junior, P.A.S.; Murta, S.M.F.; Barbosa, E.C.; et al. Cultivable fungi present in Antarctic soils: Taxonomy, phylogeny, diversity, and bioprospecting of antiparasitic and herbicidal metabolites. Extremophiles 2018, 22, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Pessi, I.S.; Osorio-Forero, C.; Gálvez, E.J.C.; Simões, F.L.; Simões, J.C.; Junca, H.; Macedo, A.J. Distinct composition signatures of archaeal and bacterial phylotypes in the Wanda Glacier forefield, Antarctic Peninsula. FEMS Microbiol. Ecol. 2014, 91, 1–10. [Google Scholar] [CrossRef]
- Rime, T.; Hartmann, M.; Brunner, I.; Widmer, F.; Zeyer, J.; Frey, B. Vertical distribution of the soil microbiota along a successional gradient in a glacier forefield. Mol. Ecol. 2015, 24, 1091–1108. [Google Scholar] [CrossRef]
- Hofmann, K.; Illmer, P. Temporal patterns of prokaryotic abundance, community structure and microbial activity in glacier foreland soils. Antonie Van Leeuwenhoek 2015, 108, 793–799. [Google Scholar] [CrossRef]
- Göransson, H.; Welc, M.; Bünemann, E.K.; Christl, I.; Venterink, H.O. Nitrogen and phosphorus availability at early stages of soil development in the Damma glacier forefield, Switzerland; implications for establishment of N2-fixing plants. Plant Soil 2016, 404, 251–261. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.A.; Pointing, S.B.; Pérez-Ortega, S.; Arróniz-Crespo, M.; Green, T.A.; Rozzi, R.; de Los Rios, A. Functional ecology of soil microbial communities along a glacier forefield in Tierra del Fuego (Chile). Int. Microbiol. 2016, 19, 161–173. [Google Scholar]
- Liu, J.; Kong, W.; Zhang, G.; Khan, A.; Guo, G.; Zhu, C.; Wei, X.; Kang, S.; Morgan-Kiss, R.M. Diversity and succession of autotrophic microbial community in high-elevation soils along deglaciation chronosequence. FEMS Microbiol. Ecol. 2016, 92, fiw160. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Wu, Y.-H.; Zhou, J.; Bing, H.-J. Variations of bacterial and fungal communities along a primary successional chronosequence in the Hailuogou glacier retreat area (Gongga Mountain, SW China). J. Mt. Sci. 2016, 13, 1621–1631. [Google Scholar] [CrossRef]
- Tian, J.; Qiao, Y.; Wu, B.; Chen, H.; Li, W.; Jiang, N.; Zhang, X.; Liu, X. Ecological Succession Pattern of Fungal Community in Soil along a Retreating Glacier. Front. Microbiol. 2017, 8, 1028. [Google Scholar] [CrossRef]
- Jiang, Y.; Lei, Y.; Yang, Y.; Korpelainen, H.; Niinemets; Li, C. Divergent assemblage patterns and driving forces for bacterial and fungal communities along a glacier forefield chronosequence. Soil Biol. Biochem. 2018, 118, 207–216. [Google Scholar] [CrossRef]
- Segawa, T.; Ishii, S.; Ohte, N.; Akiyoshi, A.; Yamada, A.; Maruyama, F.; Li, Z.; Hongoh, Y.; Takeuchi, N. The nitrogen cycle in cryoconites: Naturally occurring nitrification-denitrification granules on a glacier. Environ. Microbiol. 2014, 16, 3250–3262. [Google Scholar] [CrossRef]
- Ansari, A.H.; Hodson, A.J.; Heaton, T.H.E.; Kaiser, J.; Marca-Bell, A. Stable isotopic evidence for nitrification and denitrification in a High Arctic glacial ecosystem. Biogeochemistry 2012, 113, 341–357. [Google Scholar] [CrossRef]
- Telling, J.; Stibal, M.; Anesio, A.M.; Tranter, M.; Nias, I.; Cook, J.; Bellas, C.; Lis, G.; Wadham, J.L.; Sole, A.; et al. Microbial nitrogen cycling on the Greenland Ice Sheet. Biogeosciences 2012, 9, 2431–2442. [Google Scholar] [CrossRef]
- Cameron, K.A.; Hodson, A.J.; Osborn, A.M. Carbon and nitrogen biogeochemical cycling potentials of supraglacial cryoconite communities. Polar Biol. 2012, 35, 1375–1393. [Google Scholar] [CrossRef]
- Boyd, E.S.; Lange, R.K.; Mitchell, A.C.; Havig, J.R.; Hamilton, T.L.; Lafrenière, M.J.; Shock, E.L.; Peters, J.W.; Skidmore, M. Diversity, Abundance, and Potential Activity of Nitrifying and Nitrate-Reducing Microbial Assemblages in a Subglacial Eco-system. Appl. Environ. Microbiol. 2011, 77, 4778–4787. [Google Scholar] [CrossRef]
- Strauss, S.L.; Garcia-Pichel, F.; Day, T.A. Soil microbial carbon and nitrogen transformations at a glacial foreland on Anvers Island, Antarctic Peninsula. Polar Biol. 2012, 35, 1459–1471. [Google Scholar] [CrossRef]
- Pérez, C.A.; Silva, W.A.; Aravena, J.C.; Armesto, J.J. Limitations and Relevance of Biological Nitrogen Fixation during Postglacial Succession in Cordillera Darwin, Tierra del Fuego, Chile. Arct. Antarct. Alp. Res. 2017, 49, 29–42. [Google Scholar] [CrossRef]
- Brankatschk, R.; Töwe, S.; Kleineidam, K.; Schloter, M.; Zeyer, J. Abundances and potential activities of nitrogen cycling microbial communities along a chronosequence of a glacier forefield. ISME J. 2010, 5, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Duc, L.; Noll, M.; Meier, B.E.; Bürgmann, H.; Zeyer, J. High Diversity of Diazotrophs in the Forefield of a Receding Alpine Glacier. Microb. Ecol. 2008, 57, 179–190. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Anderson, S.P.; Cleveland, C.C.; Martin, A.P.; Miller, A.E.; Seimon, A.; Schmidt, S.K. Microbial Community Succession in an Unvegetated, Recently Deglaciated Soil. Microb. Ecol. 2006, 53, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.; Qin, D.; Jiang, G.; Duan, K.; Zhou, H. Atmospheric pollution of a remote area of Tianshan Mountain: Ice core record. J. Geophys. Res. Atmos. 2003, 108, 4406. [Google Scholar] [CrossRef]
- Williams, M.W.; Tonnessen, K.A.; Melack, J.M.; Daqing, Y. Sources and spatial variation of the chemical composition of snow in the Tien Shan, China. Ann. Glaciol. 1992, 16, 25–32. [Google Scholar] [CrossRef]
- Li, B.; Zhu, A.-X.; Zhang, Y.; Pei, T.; Qin, C.; Zhou, C. Glacier change over the past four decades in the middle Chinese Tien Shan. J. Glaciol. 2006, 52, 425–432. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.; Liu, G.; Yang, X.; Hu, P.; Chen, T.; Zhang, G.; Li, Z. Bacterial diversity in the foreland of the Tianshan No. 1 glacier, China. Environ. Res. Lett. 2012, 7, 014038. [Google Scholar] [CrossRef]
- Jiao, K.; Jing, Z.; Han, T.; Ye, B.; Yang, H.; Li, Z. Variation of the glacier No. 1 at the headwaters of the Urumqi river in the Tianshan mountains during the past 42 years and its trend prediction. J. Glaciol. Geocryol. 2004, 26, 253–260. [Google Scholar]
- Feng, F.; Li, Z.; Jin, S.; Dong, Z.; Wang, F. Hydrochemical characteristics and solute dynamics of meltwater runoff of Urumqi Glacier No. 1, eastern Tianshan, northwest China. J. Mt. Sci. 2012, 9, 472–482. [Google Scholar] [CrossRef]
- Chen, J. Preliminary researches on lichenometric chronology of Holocene glacial fluctuations and on other topics in the headwater of Urumqi River, Tian-Shan Mountains. Sci. China Chem. Engl. Ed. 1989, 12, 1487–1500. [Google Scholar]
- Liu, G.; Li, S.; Wu, X.; Zhang, B.; Zhang, B.; Long, H.; Tai, X.; Li, Z. Studies on the rule and mechanism of the succession of plant community in the retreat forefield of the Tianshan mountain glacier no. 1 at the headwaters of Urumqi river. J. Glaciol. Geocryol. 2012, 34, 1134–1141. [Google Scholar]
- Liu, G.-X.; Hu, P.; Zhang, W.; Wu, X.; Yang, X.; Chen, T.; Zhang, M.; Li, S.-W. Variations in soil culturable bacteria communities and biochemical characteristics in the Dongkemadi glacier forefield along a chronosequence. Folia Microbiol. 2012, 57, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.A.; Roberts, K.J.; Beman, J.M.; Santoro, A.E.; Oakley, B.B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef] [PubMed]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing evolutionary trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- GOOD, I.J. The Population Frequencies of Species and The Estimation of Population Parameters. Biometrika 1953, 40, 237–264. [Google Scholar] [CrossRef]
- Schloss, P.D.; Handelsman, J. Introducing DOTUR, a Computer Program for Defining Operational Taxonomic Units and Estimating Species Richness. Appl. Environ. Microbiol. 2005, 71, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Reed, S.C.; Nemergut, D.R.; Grandy, A.S.; Cleveland, C.C.; Weintraub, M.N.; Hill, A.W.; Costello, E.K.; Meyer, A.; Neff, J.; et al. The earliest stages of ecosystem succession in high-elevation (5000 metres above sea level), recently deglaciated soils. Proc. R. Soc. B Biol. Sci. 2008, 275, 2793–2802. [Google Scholar] [CrossRef] [PubMed]
- Deiglmayr, K.; Philippot, L.; Tscherko, D.; Kandeler, E. Microbial succession of nitrate-reducing bacteria in the rhizosphere of Poa alpina across a glacier foreland in the Central Alps. Environ. Microbiol. 2006, 8, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Hämmerli, A.; Waldhuber, S.; Miniaci, C.; Zeyer, J.; Bunge, M. Local expansion and selection of soil bacteria in a glacier forefield. Eur. J. Soil Sci. 2007, 58, 1437–1445. [Google Scholar] [CrossRef]
- Hamilton, T.L.; Peters, J.W.; Skidmore, M.L.; Boyd, E.S. Molecular evidence for an active endogenous microbiome beneath glacial ice. ISME J. 2013, 7, 1402–1412. [Google Scholar] [CrossRef]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, L.; Zhu, Y.; Zhang, J.; He, J. Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ. Microbiol. 2008, 10, 1601–1611. [Google Scholar] [CrossRef]
- Hynes, H.M.; Germida, J.J. Relationship between ammonia oxidizing bacteria and bioavailable nitrogen in harvested forest soils of central Alberta. Soil Biol. Biochem. 2012, 46, 18–25. [Google Scholar] [CrossRef]
- Jia, Z.; Conrad, R. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 2009, 11, 1658–1671. [Google Scholar] [CrossRef]
- Martens-Habbena, W.; Berube, P.M.; Urakawa, H.; de la Torre, J.R.; Stahl, D.A. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 2009, 461, 976–979. [Google Scholar] [CrossRef]
- Offre, P.; Prosser, J.I.; Nicol, G.W. Growth of ammonia-oxidizing archaea in soil microcosms is inhibited by acetylene. FEMS Microbiol. Ecol. 2009, 70, 99–108. [Google Scholar] [CrossRef]
- He, J.; Shen, J.; Zhang, L.; Zhu, Y.; Zheng, Y.; Xu, M.; Di, H. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol. 2007, 9, 2364–2374. [Google Scholar] [CrossRef]
- Nicol, G.W.; Leininger, S.; Schleper, C.; Prosser, J.I. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2008, 10, 2966–2978. [Google Scholar] [CrossRef] [PubMed]
- Di, H.J.; Cameron, K.C.; Shen, J.-P.; Winefield, C.S.; O’Callaghan, M.; Bowatte, S.; He, J.-Z. Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol. Ecol. 2010, 72, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Sahan, E.; Muyzer, G. Diversity and spatio-temporal distribution of ammonia-oxidizing Archaea and Bacteria in sediments of the Westerschelde estuary. FEMS Microbiol. Ecol. 2008, 64, 175–186. [Google Scholar] [CrossRef]
- Purkhold, U.; Pommerening-Röser, A.; Juretschko, S.; Schmid, M.C.; Koops, H.P.; Wagner, M. Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: Implications for molecular diversity surveys. Appl. Environ. Microbiol. 2000, 66, 5368–5382. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, A.E.; Colbert, D.; McManus, J.; Field, K.G. Microbial community dynamics based on 16S rRNA gene profiles in a Pacific Northwest estuary and its tributaries. FEMS Microbiol. Ecol. 2005, 52, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Head, I.M.; Hiorns, W.D.; Embley, T.M.; McCarthy, A.J.; Saunders, J.R. The phylogeny of autotrophic ammonia-oxidizing bacteria as determined by analysis of 16S ribosomal RNA gene sequences. J. Gen. Microbiol. 1993, 139, 1147–1153. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Gu, J.-D. Higher diversity of ammonia/ammonium-oxidizing prokaryotes in constructed freshwater wetland than natural coastal marine wetland. Appl. Microbiol. Biotechnol. 2012, 97, 7015–7033. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Wang, M.; Prosser, J.I.; Zheng, Y.-M.; He, J.-Z. Altitude ammonia-oxidizing bacteria and archaea in soils of Mount Everest. FEMS Microbiol. Ecol. 2009, 70, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Hastings, R.C.; Ceccherini, M.T.; Miclaus, N.; Saunders, J.R.; Bazzicalupo, M.; McCarthy, A.J. Direct molecular biological analysis of ammonia oxidising bacteria populations in cultivated soil plots treated with swine manure. FEMS Microbiol. Ecol. 1997, 23, 45–54. [Google Scholar] [CrossRef]
- Kowalchuk, G.A.; Stienstra, A.W.; Heilig, G.H.J.; Stephen, J.R.; Woldendorp, J.W. Molecular analysis of ammonia-oxidising bacteria in soil of successional grasslands of the Drentsche A (The Netherlands). FEMS Microbiol. Ecol. 2000, 3, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Di, H.J.; Cameron, K.C.; Shen, J.P.; Winefield, C.S.; O’callaghan, M.; Bowatte, S.; He, J.Z. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat. Geosci. 2009, 2, 621–624. [Google Scholar] [CrossRef]
- Allison, S.; Prosser, J. Ammonia oxidation at low pH by attached populations of nitrifying bacteria. Soil Biol. Biochem. 1993, 25, 935–941. [Google Scholar] [CrossRef]
- Hansel, C.M.; Fendorf, S.; Jardine, P.M.; Francis, C.A. Changes in Bacterial and Archaeal Community Structure and Functional Diversity along a Geochemically Variable Soil Profile. Appl. Environ. Microbiol. 2008, 74, 1620–1633. [Google Scholar] [CrossRef]
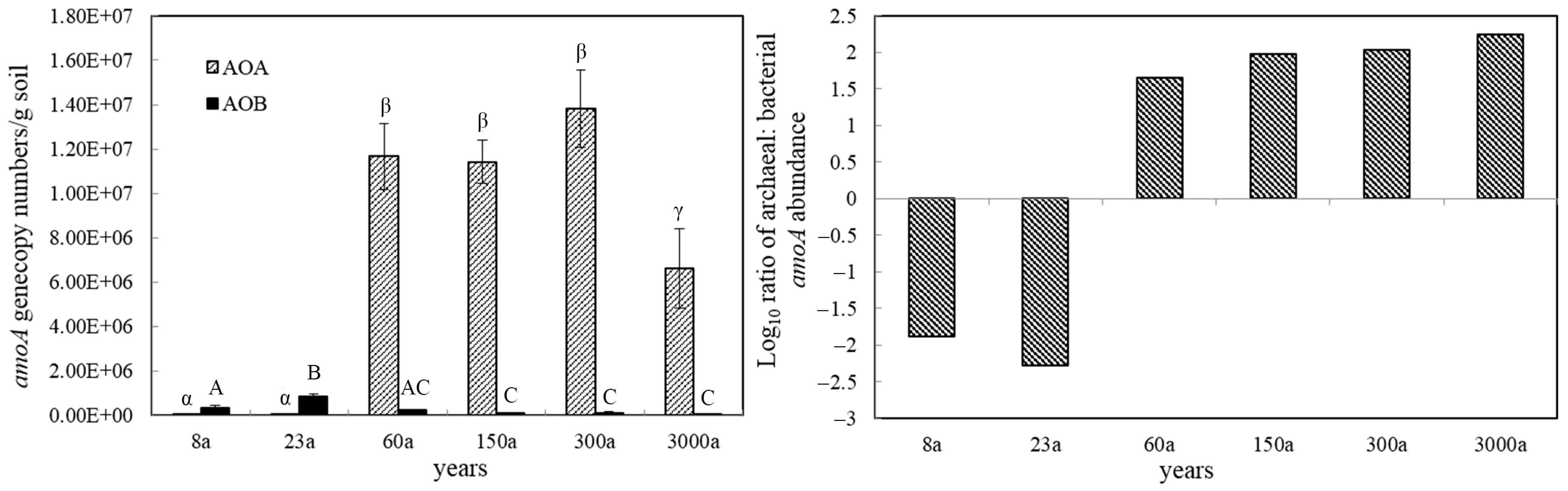
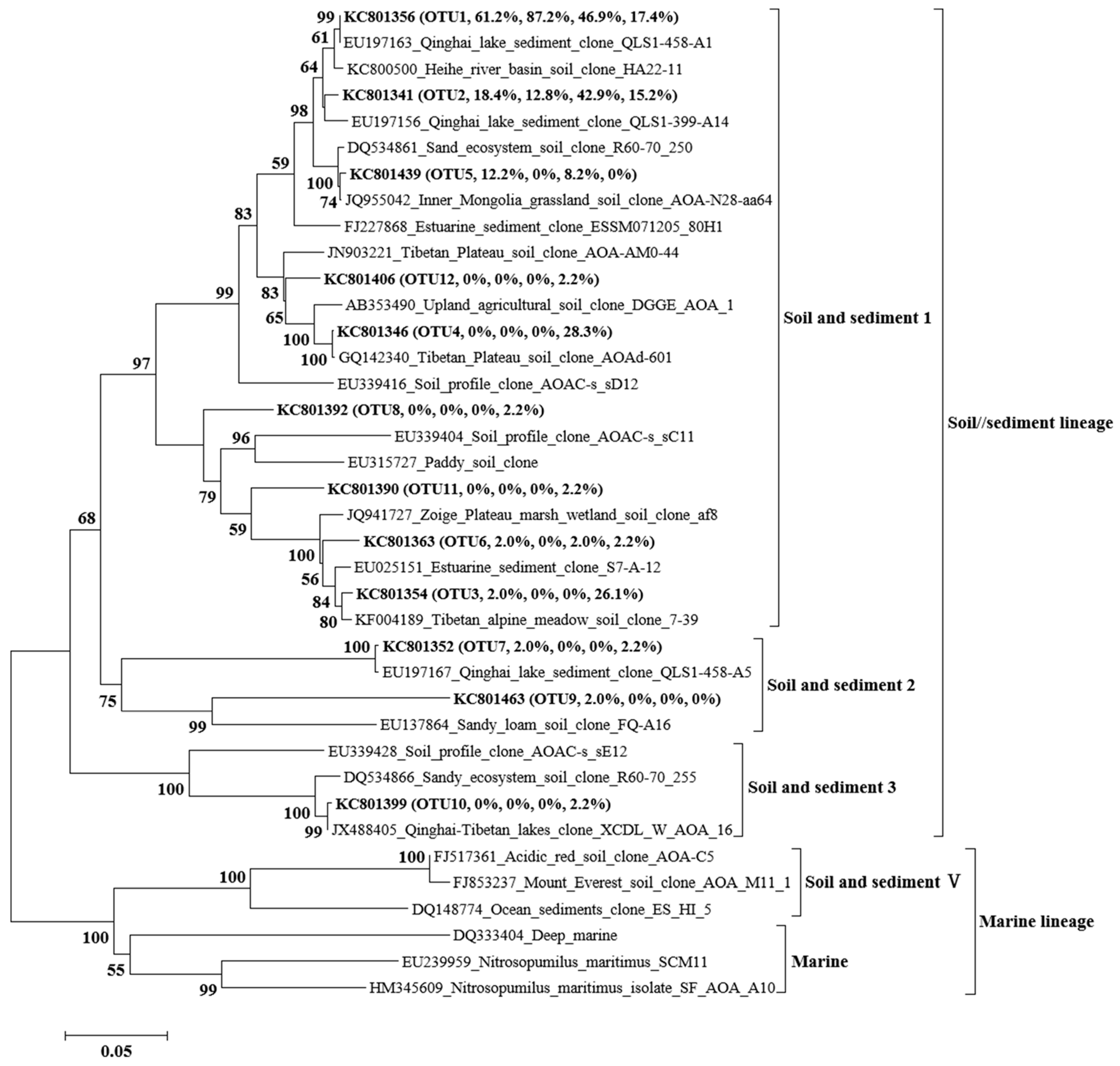
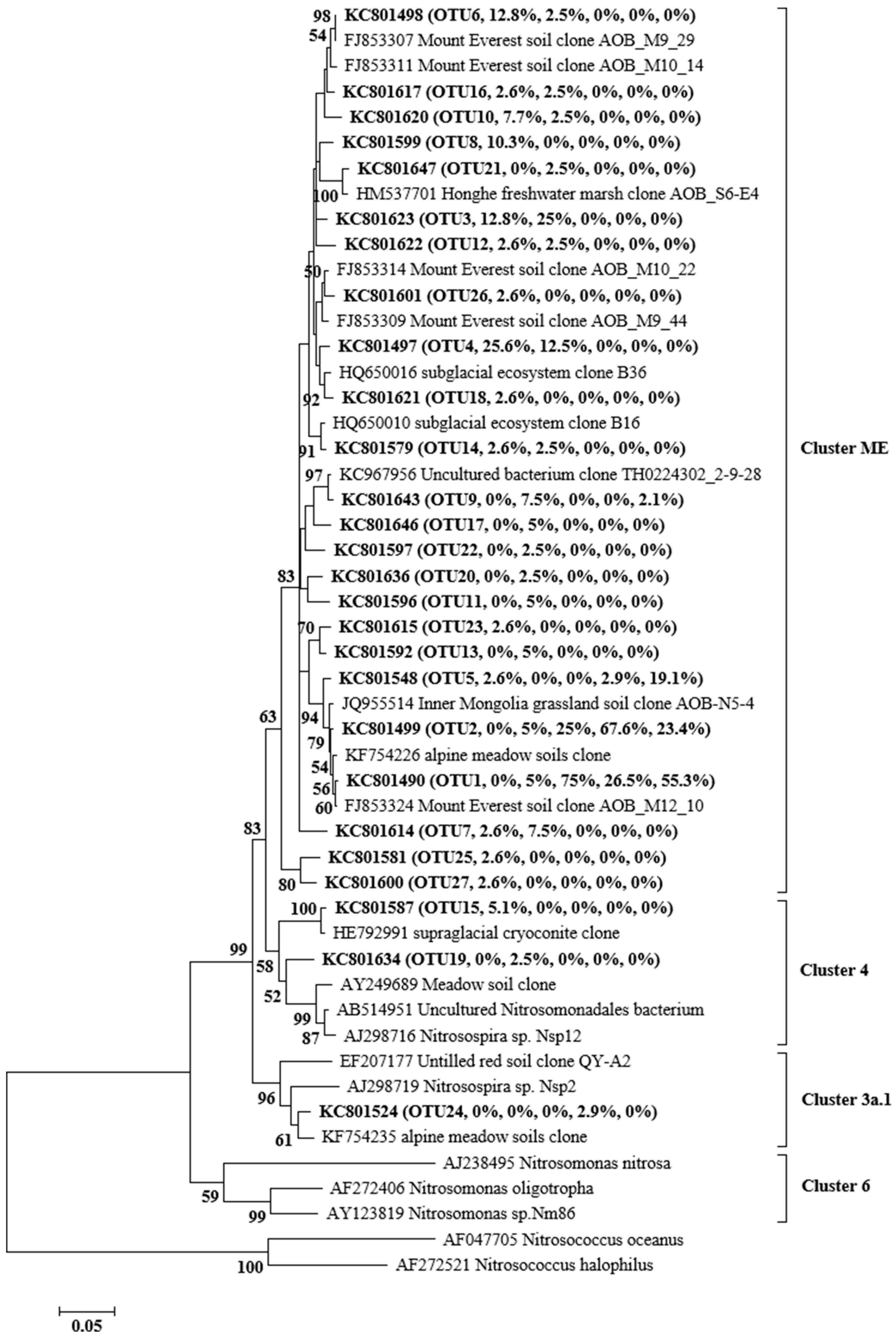

| Distance from the Glacier Front | 15 m | 34 m | 250 m | 450 m | 600 m | 750 m |
|---|---|---|---|---|---|---|
| Retreated time | 8a | 23a | 60a | 150a | 300a | 3000a |
| Distance from the Glacier Front (m) | Soil Age after Deglaciation (years) | pH | Total Organic C (% dw) | Total N (% dw) | Ammonium (mg/kg dw) | Nitrate (mg/kg dw) |
|---|---|---|---|---|---|---|
| 15 | 8a | 8.41 ± 0.25 a | 0.286 ± 0.009 a | 0.026 ± 0.006 a | 10.516 ± 0.036 a | 1.031 ± 0.017 a |
| 34 | 23a | 7.75 ± 0.17 b | 0.454 ± 0.067 a | 0.055 ± 0.008 a | 9.327 ± 0.143 a | 0.961 ± 0.095 a |
| 250 | 60a | 7.70 ± 0.03 b | 0.524 ± 0.065 a | 0.040 ± 0.007 a | 9.512 ± 0.038 a | 0.829 ± 0.149 a |
| 450 | 150a | 7.17 ± 0.21 b | 1.229 ± 0.019 b | 0.129 ± 0.003 b | 12.996 ± 0.056 b | 0.925 ± 0.067 a |
| 600 | 300a | 7.32 ± 0.26 b | 1.387 ± 0.038 b | 0.149 ± 0.007 b | 14.915 ± 0.106 b | 1.051 ± 0.094 a |
| 750 | 3000a | 7.37 ± 0.26 b | 2.577 ± 0.281 c | 0.361 ± 0.045 c | 14.427 ± 0.406 b | 4.271 ± 1.082 b |
| Succession Years | OTUs | Coverage | Shannon Index | Simpson Index | ||||
|---|---|---|---|---|---|---|---|---|
| AOA | AOB | AOA | AOB | AOA | AOB | AOA | AOB | |
| 8a | ND | 16 | ND | 74.4% | ND | 2.40 | ND | 0.88 |
| 23a | ND | 18 | ND | 77.5% | ND | 2.57 | ND | 0.89 |
| 60a | 7 | 2 | 91.8% | 100.0% | 1.19 | 0.56 | 0.57 | 0.38 |
| 150a | 2 | 4 | 100.0% | 94.1% | 0.19 | 0.82 | 0.22 | 0.47 |
| 300a | 4 | 4 | 98.0% | 97.9% | 1.00 | 1.07 | 0.59 | 0.60 |
| 3000a | 10 | ND | 87.0% | ND | 1.80 | ND | 0.80 | ND |
| OTU | Shannon Index | Simpson Index | Copy Numbers | Copy Numbers Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|
| AOA | AOB | AOA | AOB | AOA | AOB | AOA | AOB | AOA/AOB | |
| Year | 0.770 | −0.518 | 0.754 | −0.625 | 0.679 | −0.804 | 0.020 | −0.478 | 0.792 |
| TOC | 0.735 | −0.702 | 0.745 | −0.755 | 0.761 | −0.833 * | 0.364 | −0.672 | 0.968 ** |
| TN | 0.728 | −0.621 | 0.732 | −0.695 | 0.719 | −0.814 * | 0.229 | −0.591 | 0.923 ** |
| pH | −0.455 | 0.710 | −0.449 | 0.690 | −0.574 | 0.604 | −0.731 | 0.491 | −0.762 |
| Ammonium | 0.434 | −0.619 | 0.510 | −0.586 | 0.614 | −0.539 | 0.540 | −0.762 | 0.876 * |
| Nitrate | 0.727 | −0.446 | 0.710 | −0.562 | 0.621 | −0.758 | −0.067 | −0.426 | 0.739 |
| C/N ratio | −0.100 | −0.020 | −0.138 | 0.054 | −0.120 | 0.217 | 0.226 | −0.015 | −0.501 |
| A/N ratio | −0.534 | 0.078 | −0.493 | 0.227 | −0.326 | 0.493 | 0.462 | 0.018 | −0.332 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Zhang, W.; Liu, G.; Chen, T.; Li, Z. Changes in Diversity and Abundance of Ammonia-Oxidizing Archaea and Bacteria along a Glacier Retreating Chronosequence in the Tianshan Mountains, China. Microorganisms 2023, 11, 2871. https://doi.org/10.3390/microorganisms11122871
Wu X, Zhang W, Liu G, Chen T, Li Z. Changes in Diversity and Abundance of Ammonia-Oxidizing Archaea and Bacteria along a Glacier Retreating Chronosequence in the Tianshan Mountains, China. Microorganisms. 2023; 11(12):2871. https://doi.org/10.3390/microorganisms11122871
Chicago/Turabian StyleWu, Xiukun, Wei Zhang, Guangxiu Liu, Tuo Chen, and Zhongqin Li. 2023. "Changes in Diversity and Abundance of Ammonia-Oxidizing Archaea and Bacteria along a Glacier Retreating Chronosequence in the Tianshan Mountains, China" Microorganisms 11, no. 12: 2871. https://doi.org/10.3390/microorganisms11122871
APA StyleWu, X., Zhang, W., Liu, G., Chen, T., & Li, Z. (2023). Changes in Diversity and Abundance of Ammonia-Oxidizing Archaea and Bacteria along a Glacier Retreating Chronosequence in the Tianshan Mountains, China. Microorganisms, 11(12), 2871. https://doi.org/10.3390/microorganisms11122871






