Inhibiting F-Actin Polymerization Impairs the Internalization of Moraxella catarrhalis
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolates
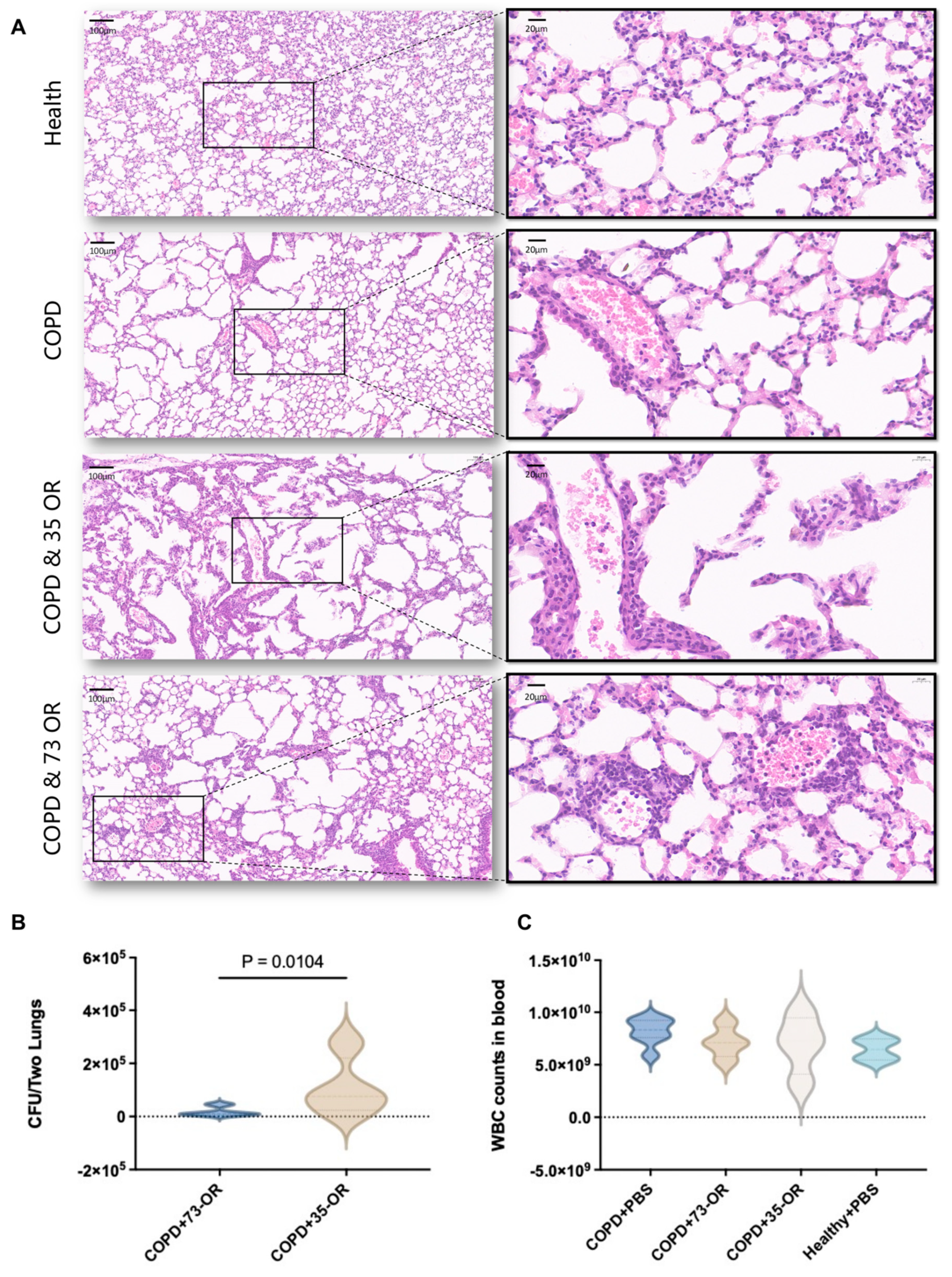
2.2. Mouse Model for COPD and Pulmonary Clearance Model
2.3. RNA Sequencing and Data Analysis
2.4. G-Actin/F-Actin In Vivo Analysis
2.5. Immunofluorescence and Imaging
2.6. Actin Polymerization Inhibition Assay
2.7. Statistical Analysis
3. Results
3.1. Murine COPD and Pulmonary Clearance Models
3.2. Genome-Wide Gene Expression Analysis
3.3. Functional Enrichment Analysis of DEGs
3.4. Actin Cytoskeleton Affects Lung Epithelial Cell Infection with M. catarrhalis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, Y.-C.; Singh, B.; Riesbeck, K. Moraxella catarrhalis: From interactions with the host immune system to vaccine development. Future Microbiol. 2012, 7, 1073–1100. [Google Scholar] [CrossRef]
- Murphy, T.F.; Brauer, A.L.; Grant, B.J.; Sethi, S. Moraxella catarrhalis in chronic obstructive pulmonary disease: Burden of disease and immune response. Am. J. Respir. Crit. Care Med. 2005, 172, 195–199. [Google Scholar] [CrossRef]
- Murphy, T.F.; Parameswaran, G.I. Moraxella catarrhalis, a human respiratory tract pathogen. Clin. Infect. Dis. 2009, 49, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.F.; Oliver, A.J.; Ioannou, C.; Billiard, J.; Dennison, J.; Van Den Berg, F.; Yang, S.; Chandrasekaran, V.; Young, G.C.; Lahiry, A.; et al. A Novel Inhaled Dry-Powder Formulation of Ribavirin Allows for Efficient Lung Delivery in Healthy Participants and Those with Chronic Obstructive Pulmonary Disease in a Phase 1 Study. Antimicrob. Agents Chemother. 2020, 64, e02267-19. [Google Scholar] [CrossRef] [PubMed]
- Rothnie, K.J.; Müllerová, H.; Smeeth, L.; Quint, J.K. Natural History of Chronic Obstructive Pulmonary Disease Exacerbations in a General Practice-based Population with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018, 198, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Murphy, T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- George, L.M.; Haigh, R.D.; Mistry, V.; Haldar, K.; Barer, M.R.; Oggioni, M.R.; Brightling, C.E. Sputum Moraxella catarrhalis strains exhibit diversity within and between COPD subjects. Int. J. Chron. Obstruct Pulmon Dis. 2018, 13, 3663–3667. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Shimada, Y.J.; Faridi, M.K.; Camargo, C.A.; Hasegawa, K. Incidence of Acute Cardiovascular Event After Acute Exacerbation of COPD. J. Gen. Intern. Med. 2018, 33, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Grossman, T.H.; Fyfe, C.; O’brien, W.; Hackel, M.; Minyard, M.B.; Waites, K.B.; Dubois, J.; Murphy, T.M.; Slee, A.M.; Weiss, W.J.; et al. Fluorocycline TP-271 Is Potent against Complicated Community-Acquired Bacterial Pneumonia Pathogens. mSphere 2017, 2, e00004-17. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhou, H.; Wang, F.; Liang, S.; Cheng, L.; Du, X.; Pang, F.; Tian, J.; Zhao, J.; Kan, B.; et al. Multilocus sequence typing-based analysis of Moraxella catarrhalis population structure reveals clonal spreading of drug-resistant strains isolated from childhood pneumonia. Infect. Genet. Evol. 2017, 56, 117–124. [Google Scholar] [CrossRef]
- Shi, W.; Wen, D.; Chen, C.; Yuan, L.; Gao, W.; Tang, P.; Cheng, X.; Yao, K. β-Lactamase production and antibiotic susceptibility pattern of Moraxella catarrhalis isolates collected from two county hospitals in China. BMC Microbiol. 2018, 18, 77. [Google Scholar] [CrossRef]
- Yamada, K.; Arai, K.; Saito, R. Antimicrobial susceptibility to β-lactam antibiotics and production of BRO β-lactamase in clinical isolates of Moraxella catarrhalis from a Japanese hospital. J. Microbiol. Immunol. Infect. 2017, 50, 386–389. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Xiao, M.; Cheng, J.-W.; Xu, H.-P.; Xu, Z.-P.; Ye, S.; Zhang, W.-J.; Kudinha, T.; Kong, F.; Xu, Y.-C. Macrolide-Resistant Isolates Are Highly Concentrated in Two MLST Clonal Complexes -CCN10 and CC363. Front. Microbiol. 2017, 8, 201. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Ding, R.; Jia, X.-M.; Huang, J.-J.; Yu, S.; Chan, H.T.; Li, W.; Mao, L.-L.; Zhang, L.; Zhang, X.-Y.; et al. Correlation of Moraxella catarrhalis macrolide susceptibility with the ability to adhere and invade human respiratory epithelial cells. Emerg. Microbes Infect. 2022, 11, 2055–2068. [Google Scholar] [CrossRef]
- Slevogt, H.; Seybold, J.; Tiwari, K.N.; Hocke, A.C.; Jonatat, C.; Dietel, S.; Hippenstiel, S.; Singer, B.B.; Bachmann, S.; Suttorp, N.; et al. Moraxella catarrhalis is internalized in respiratory epithelial cells by a trigger-like mechanism and initiates a TLR2- and partly NOD1-dependent inflammatory immune response. Cell Microbiol. 2007, 9, 694–707. [Google Scholar] [CrossRef]
- Yi, F.; Guo, J.; Dabbagh, D.; Spear, M.; He, S.; Kehn-Hall, K.; Fontenot, J.; Yin, Y.; Bibian, M.; Park, C.M.; et al. Discovery of Novel Small-Molecule Inhibitors of LIM Domain Kinase for Inhibiting HIV-1. J. Virol. 2017, 91, e02418-16. [Google Scholar] [CrossRef] [PubMed]
- Berenson, C.S.; Kruzel, R.L.; Eberhardt, E.; Dolnick, R.; Minderman, H.; Wallace, P.K.; Sethi, S. Impaired innate immune alveolar macrophage response and the predilection for COPD exacerbations. Thorax 2014, 69, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.S.; Yadav, R.; Arya, R. Altered Actin Dynamics in Cell Migration of GNE Mutant Cells. Front. Cell Dev. Biol. 2021, 9, 603742. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Kim, S.H.; Joetham, A.; Petrache, I.; Gelfand, E.W. Therapeutic benefits of recombinant alpha1-antitrypsin IgG1 Fc-fusion protein in experimental emphysema. Respir. Res. 2021, 22, 207. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Liu, Y.Q.; Chang, L.C.; Wang, C.Y.; Zhao, Y.L.; Zhao, X.B.; Qian, K.; Nan, X.; Yang, L.; Yang, X.M.; et al. Design, synthesis, mechanisms of action, and toxicity of novel 20(s)-sulfonylamidine derivatives of camptothecin as potent antitumor agents. J. Med. Chem. 2014, 57, 6008–6018. [Google Scholar] [CrossRef]
- Jiang, Z.; He, H.; Liu, H.; Thayumanavan, S. Cellular Uptake Evaluation of Amphiphilic Polymer Assemblies: Importance of Interplay between Pharmacological and Genetic Approaches. Biomacromolecules 2019, 20, 4407–4418. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.; Sanders, K.L.; Edwards, J.L.; Ye, J.; Si, F.; Gao, A.; Huang, L.; Hsueh, E.C.; Ford, D.A.; et al. TLR8-Mediated Metabolic Control of Human Treg Function: A Mechanistic Target for Cancer Immunotherapy. Cell Metab. 2019, 29, 103–123.e5. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Raduka, A.; Rezaee, F. Respiratory syncytial virus disrupts the airway epithelial barrier by decreasing cortactin and destabilizing F-actin. J. Cell Sci. 2022, 135, jcs259871. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Su, Y.-C.; Riesbeck, K. Vitronectin in bacterial pathogenesis: A host protein used in complement escape and cellular invasion. Mol. Microbiol. 2010, 78, 545–560. [Google Scholar] [CrossRef]


| Category | GO ID | Description | Gene Ratio | BgRatio | p-Value | p adj | Gene Names |
|---|---|---|---|---|---|---|---|
| Molecular function | GO:0001228 | Transcriptional activator activity, RNA polymerase II transcription regulatory region sequence-specific DNA binding | 7/47 | 356/14430 | 0.0001 | 0.0186 | NFATC2/KLF7/MAFF/FOSB/IRF1/CSRNP1/EGR1 |
| Molecular function | GO:0032036 | Myosin heavy chain binding | 2/47 | 10/14430 | 0.0004 | 0.0303 | SPTBN5/CORO1A |
| Molecular function | GO:0017022 | Myosin binding | 3/47 | 59/14430 | 0.0009 | 0.0370 | SPTBN5/NPC1L1/CORO1A |
| Molecular function | GO:0001077 | Transcriptional activator activity, RNA polymerase II proximal promoter sequence-specific DNA binding | 5/47 | 243/14430 | 0.0011 | 0.0370 | NFATC2/KLF7/FOSB/IRF1/EGR1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Huang, J.; Ding, R.; Xu, Y.; Liu, Y. Inhibiting F-Actin Polymerization Impairs the Internalization of Moraxella catarrhalis. Microorganisms 2024, 12, 291. https://doi.org/10.3390/microorganisms12020291
Yu J, Huang J, Ding R, Xu Y, Liu Y. Inhibiting F-Actin Polymerization Impairs the Internalization of Moraxella catarrhalis. Microorganisms. 2024; 12(2):291. https://doi.org/10.3390/microorganisms12020291
Chicago/Turabian StyleYu, Jinhan, Jingjing Huang, Rui Ding, Yingchun Xu, and Yali Liu. 2024. "Inhibiting F-Actin Polymerization Impairs the Internalization of Moraxella catarrhalis" Microorganisms 12, no. 2: 291. https://doi.org/10.3390/microorganisms12020291
APA StyleYu, J., Huang, J., Ding, R., Xu, Y., & Liu, Y. (2024). Inhibiting F-Actin Polymerization Impairs the Internalization of Moraxella catarrhalis. Microorganisms, 12(2), 291. https://doi.org/10.3390/microorganisms12020291






