Candida albicans Infection Disrupts the Metabolism of Vaginal Epithelial Cells and Inhibits Cellular Glycolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Groups and Treatment
2.3. Metabolomics Analysis
2.4. Scanning Electron Microscopy
2.5. Cellular Supernatant Index Assay
2.6. Immunofluorescence Staining
2.7. Statistical Analysis
3. Results
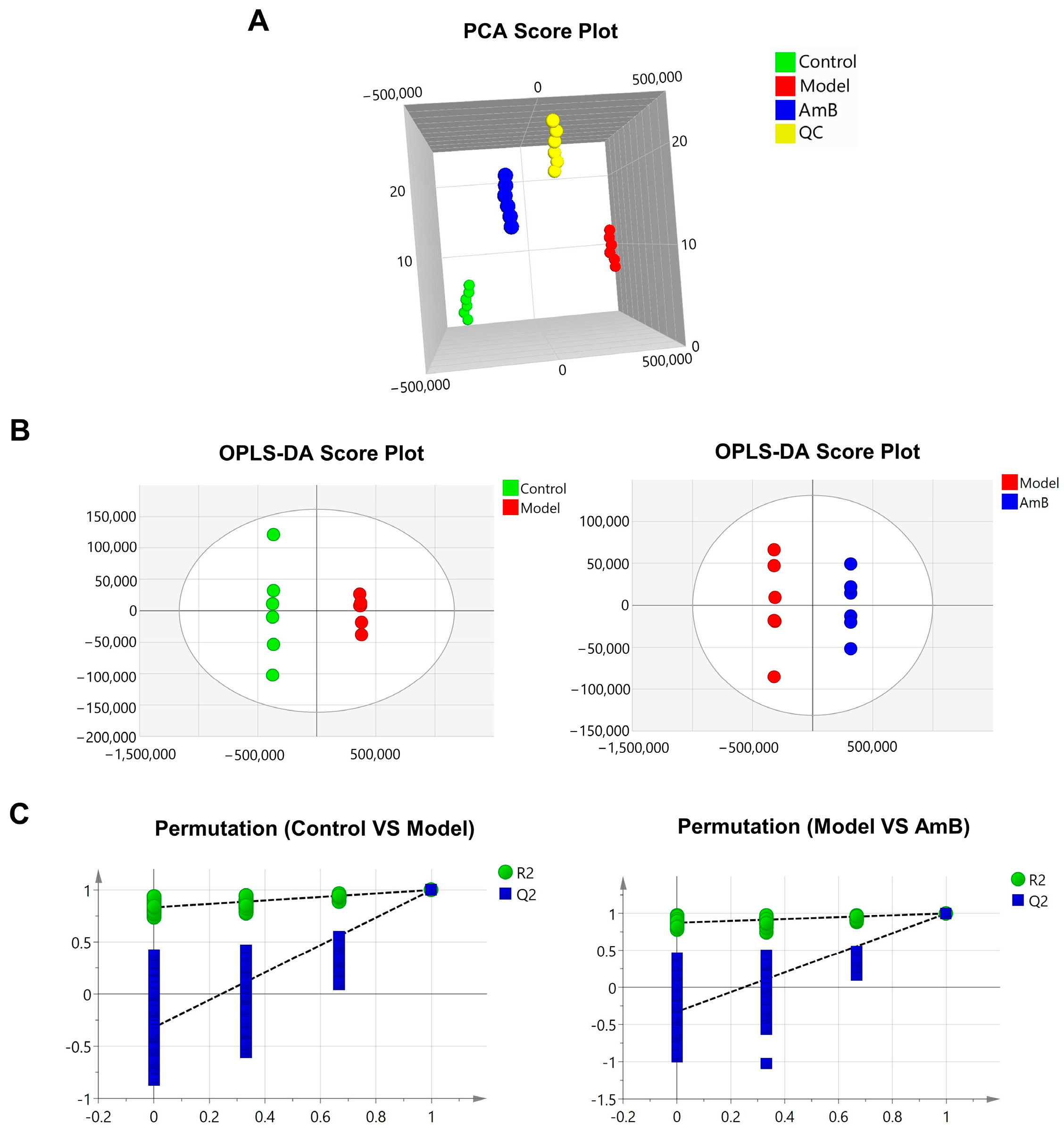
3.1. C. albicans Infection Disrupts the Metabolism of Vaginal Epithelial Cells
3.2. Changes in Metabolites after Administration of Antifungal Therapy
3.3. C. albicans Infection Disrupted Glycolysis-Related Metabolites and Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dovnik, A.; Golle, A.; Novak, D.; Arko, D.; Takač, I. Treatment of vulvovaginal candidiasis: A review of the literature. Acta Dermatovenerol. Alp. Pannonica Adriat. 2015, 24, 5–7. [Google Scholar] [CrossRef]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef]
- Rosati, D.; Bruno, M.; Jaeger, M.; Ten Oever, J.; Netea, M.G. Recurrent Vulvovaginal Candidiasis: An Immunological Perspective. Microorganisms 2020, 8, 144. [Google Scholar] [CrossRef]
- Baron, E.J.; Miller, J.M.; Weinstein, M.P.; Richter, S.S.; Gilligan, P.H.; Thomson, R.B., Jr.; Bourbeau, P.; Carroll, K.C.; Kehl, S.C.; Dunne, W.M.; et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 57, e22–e121. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Shroff, A.; Sequeira, R.; Reddy, K.V.R. Human vaginal epithelial cells augment autophagy marker genes in response to Candida albicans infection. Am. J. Reprod. Immunol. 2017, 77, e12639. [Google Scholar] [CrossRef]
- Roselletti, E.; Perito, S.; Sabbatini, S.; Monari, C.; Vecchiarelli, A. Vaginal Epithelial Cells Discriminate Between Yeast and Hyphae of Candida albicans in Women Who Are Colonized or Have Vaginal Candidiasis. J. Infect. Dis. 2019, 220, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef] [PubMed]
- Modrzewska, B.; Kurnatowski, P. Adherence of Candida sp. to host tissues and cells as one of its pathogenicity features. Ann. Parasitol. 2015, 61, 3–9. [Google Scholar]
- Dabiri, S.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Comparative analysis of proteinase, phospholipase, hydrophobicity and biofilm forming ability in Candida species isolated from clinical specimens. J. Mycol. Med. 2018, 28, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kadry, A.A.; El-Ganiny, A.M.; El-Baz, A.M. Relationship between Sap prevalence and biofilm formation among resistant clinical isolates of Candida albicans. Afr. Health Sci. 2018, 18, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.X.; Li, T.; Zhang, X.; Wang, S.X.; Liu, Z.H. Lactobacillus crispatus Modulates Vaginal Epithelial Cell Innate Response to Candida albicans. Chin. Med. J. 2017, 130, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Osis, G.; Traylor, A.M.; Black, L.M.; Spangler, D.; George, J.F.; Zarjou, A.; Verlander, J.W.; Agarwal, A. Expression of lactate dehydrogenase A and B isoforms in the mouse kidney. Am. J. Physiol. Renal Physiol. 2021, 320, F706–F718. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liao, S.; Liang, L.; Deng, J.; Zhou, Y. The relationship between CD4(+) T cell glycolysis and their functions. Trends Endocrinol. Metab. 2023, 34, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. Glycolysis. Cold Spring Harb. Perspect. Biol. 2021, 13, a040535. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Choi, S.C.; Xu, Z.; Perry, D.J.; Seay, H.; Croker, B.P.; Sobel, E.S.; Brusko, T.M.; Morel, L. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl. Med. 2015, 7, 274ra218. [Google Scholar] [CrossRef]
- Desai, J.V.; Urban, A.; Swaim, D.Z.; Colton, B.; Kibathi, L.W.; Ferrè, E.M.N.; Stratton, P.; Merideth, M.A.; Hunsberger, S.; Matkovits, T.; et al. Efficacy of Cochleated Amphotericin B in Mouse and Human Mucocutaneous Candidiasis. Antimicrob. Agents Chemother. 2022, 66, e0030822. [Google Scholar] [CrossRef]
- Bezerra, L.S.; Silva, J.A.D.; Santos-Veloso, M.A.O.; Lima, S.G.; Chaves-Markman, Â.V.; Jucá, M.B. Antifungal Efficacy of Amphotericin B in Candida Albicans Endocarditis Therapy: Systematic Review. Braz. J. Cardiovasc. Surg. 2020, 35, 789–796. [Google Scholar] [CrossRef]
- Ceccarani, C.; Foschi, C.; Parolin, C.; D’Antuono, A.; Gaspari, V.; Consolandi, C.; Laghi, L.; Camboni, T.; Vitali, B.; Severgnini, M.; et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019, 9, 14095. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Ilhan, Z.E.; Łaniewski, P.; Thomas, N.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. EBioMedicine 2019, 44, 675–690. [Google Scholar] [CrossRef]
- Sigmundsdóttir, G.; Christensson, B.; Björklund, L.J.; Håkansson, K.; Pehrson, C.; Larsson, L. Urine D-arabinitol/L-arabinitol ratio in diagnosis of invasive candidiasis in newborn infants. J. Clin. Microbiol. 2000, 38, 3039–3042. [Google Scholar] [CrossRef]
- Knuplez, E.; Marsche, G. An Updated Review of Pro- and Anti-Inflammatory Properties of Plasma Lysophosphatidylcholines in the Vascular System. Int. J. Mol. Sci. 2020, 21, 4501. [Google Scholar] [CrossRef]
- Yang, K.; Xia, B.; Wang, W.; Cheng, J.; Yin, M.; Xie, H.; Li, J.; Ma, L.; Yang, C.; Li, A.; et al. A Comprehensive Analysis of Metabolomics and Transcriptomics in Cervical Cancer. Sci. Rep. 2017, 7, 43353. [Google Scholar] [CrossRef]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef]
- Li, X.; Berg, N.K.; Mills, T.; Zhang, K.; Eltzschig, H.K.; Yuan, X. Adenosine at the Interphase of Hypoxia and Inflammation in Lung Injury. Front. Immunol. 2020, 11, 604944. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Haskó, G. Immunity, inflammation and cancer: A leading role for adenosine. Nat. Rev. Cancer 2013, 13, 842–857. [Google Scholar] [CrossRef]
- Smith, T.J.; Johnson, C.R.; Koshy, R.; Hess, S.Y.; Qureshi, U.A.; Mynak, M.L.; Fischer, P.R. Thiamine deficiency disorders: A clinical perspective. Ann. N. Y. Acad. Sci. 2021, 1498, 9–28. [Google Scholar] [CrossRef]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Köhler, G.A.; Assefa, S.; Reid, G. Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect. Dis. Obstet. Gynecol. 2012, 2012, 636474. [Google Scholar] [CrossRef]
- Cassone, A. Vulvovaginal Candida albicans infections: Pathogenesis, immunity and vaccine prospects. BJOG 2015, 122, 785–794. [Google Scholar] [CrossRef]
- Baldewijns, S.; Sillen, M.; Palmans, I.; Vandecruys, P.; Van Dijck, P.; Demuyser, L. The Role of Fatty Acid Metabolites in Vaginal Health and Disease: Application to Candidiasis. Front. Microbiol. 2021, 12, 705779. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, D.; Jeanson, Y.; Portais, J.C.; Galinier, A.; Ader, I.; Casteilla, L.; Carrière, A. Lactate Fluxes and Plasticity of Adipose Tissues: A Redox Perspective. Front. Physiol. 2021, 12, 689747. [Google Scholar] [CrossRef]
- Chu, X.; Raju, R.P. Regulation of NAD(+) metabolism in aging and disease. Metabolism 2022, 126, 154923. [Google Scholar] [CrossRef]
- Fania, L.; Mazzanti, C.; Campione, E.; Candi, E.; Abeni, D.; Dellambra, E. Role of Nicotinamide in Genomic Stability and Skin Cancer Chemoprevention. Int. J. Mol. Sci. 2019, 20, 5946. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Bernier, M.; Aon, M.A.; Cortassa, S.; Kim, E.Y.; Fang, E.F.; Palacios, H.H.; Ali, A.; Navas-Enamorado, I.; Di Francesco, A.; et al. Nicotinamide Improves Aspects of Healthspan, but Not Lifespan, in Mice. Cell Metab. 2018, 27, 667–676.e664. [Google Scholar] [CrossRef]
- Xing, X.; Liao, Z.; Tan, F.; Zhu, Z.; Jiang, Y.; Cao, Y. Effect of Nicotinamide Against Candida albicans. Front. Microbiol. 2019, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liao, Z.; Shen, J.; Zhu, Z.; Cao, Y. Nicotinamide potentiates amphotericin B activity against Candida albicans. Virulence 2022, 13, 1533–1542. [Google Scholar] [CrossRef]







| No. | Name | HMDB | Formula | m/z | RT (Min) | Model vs. Control | AmB vs. Model |
|---|---|---|---|---|---|---|---|
| 1 | LysoPC (18:2(9Z,12Z)) | HMDB0010386 | C26H50NO7P | 519.33 | 10.71 | ↑ | ↓ |
| 2 | Adenylsuccinic acid | HMDB0000536 | C14H18N5O11P | 463.07 | 2.74 | ↑ | ↓ |
| 3 | Indole | HMDB0000738 | C8H7N | 117.06 | 5.67 | ↑ | ↓ |
| 4 | Palmitic acid | HMDB0000220 | C16H32O2 | 273.27 | 8.91 | ↑ | ↓ |
| 5 | 2-Furoic acid | HMDB0000617 | C5H4O3 | 112.01 | 1.47 | ↑ | ↓ |
| 6 | Guanosine monophosphate | HMDB0001397 | C10H14N5O8P | 381.07 | 1.69 | ↑ | ↓ |
| 7 | Betaine | HMDB0000043 | C5H11NO2 | 117.08 | 1.06 | ↑ | ↓ |
| 8 | Cysteinylglycine | HMDB0000078 | C5H10N2O3S | 161.01 | 1.45 | ↑ | ↓ |
| 9 | Pipecolic acid | HMDB0000070 | C6H11NO2 | 147.09 | 1.45 | ↑ | ↓ |
| 10 | Guanine | HMDB0000132 | C5H5N5O | 134.02 | 1.69 | ↑ | ↓ |
| 11 | Pyruvic acid | HMDB0000243 | C3H4O3 | 106.03 | 1.53 | ↑ | ↓ |
| 12 | Ophthalmic acid | HMDB0005765 | C11H19N3O6 | 289.13 | 1.51 | ↑ | ↓ |
| 13 | D-Arabitol | HMDB0000568 | C5H12O5 | 152.07 | 1.02 | ↑ | ↓ |
| 14 | Diacetyl | HMDB0003407 | C4H6O2 | 104.05 | 1.49 | ↑ | ↓ |
| 15 | cis-Aconitic acid | HMDB0000072 | C6H6O6 | 174.02 | 1.08 | ↑ | ↓ |
| 16 | Thiamine | HMDB0000235 | C12H16N4OS | 264.10 | 1.22 | ↓ | ↑ |
| 17 | Nicotinamide | HMDB0001406 | C6H6N2O | 122.05 | 1.43 | ↓ | ↑ |
| 18 | Hypoxanthine | HMDB0000157 | C5H4N4O | 136.04 | 1.43 | ↓ | ↑ |
| 19 | N-Acetyl-L-aspartic acid | HMDB0000812 | C6H9NO5 | 175.05 | 1.12 | ↓ | ↑ |
| 20 | Inosine | HMDB0000195 | C10H12N4O5 | 268.08 | 1.70 | ↓ | ↑ |
| 21 | Uracil | HMDB0000300 | C4H4N2O2 | 95.00 | 1.50 | ↓ | ↑ |
| 22 | Nicotinic acid | HMDB0001488 | C6H5NO2 | 123.03 | 1.18 | ↓ | ↑ |
| 23 | Allantoic acid | HMDB0001209 | C4H8N4O4 | 176.05 | 0.92 | ↓ | ↑ |
| 24 | Beta-Citryl-L-glutamic acid | HMDB0013220 | C11H15NO10 | 321.07 | 1.60 | ↓ | ↑ |
| 25 | L-Lactic acid | HMDB0000190 | C3H6O3 | 90.03 | 1.43 | ↓ | ↑ |
| 26 | 2-Phosphoglyceric acid | HMDB0000362 | C3H7O7P | 185.99 | 1.26 | ↓ | ↑ |
| 27 | Phosphoenolpyruvic acid | HMDB0000263 | C3H5O6P | 167.98 | 1.39 | ↓ | ↑ |
| 28 | Octadecanamide | HMDB0034146 | C18H37NO | 283.29 | 14.69 | ↓ | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, P.; Sun, X.; Zhao, M.; Chen, Y.; Gao, X. Candida albicans Infection Disrupts the Metabolism of Vaginal Epithelial Cells and Inhibits Cellular Glycolysis. Microorganisms 2024, 12, 292. https://doi.org/10.3390/microorganisms12020292
Zhao Y, Wang P, Sun X, Zhao M, Chen Y, Gao X. Candida albicans Infection Disrupts the Metabolism of Vaginal Epithelial Cells and Inhibits Cellular Glycolysis. Microorganisms. 2024; 12(2):292. https://doi.org/10.3390/microorganisms12020292
Chicago/Turabian StyleZhao, Yanni, Pengjiao Wang, Xiaodong Sun, Mei Zhao, Yixuan Chen, and Xiuli Gao. 2024. "Candida albicans Infection Disrupts the Metabolism of Vaginal Epithelial Cells and Inhibits Cellular Glycolysis" Microorganisms 12, no. 2: 292. https://doi.org/10.3390/microorganisms12020292




