Mating Pheromone (Gamone 1) in Blepharisma: A Glycoprotein Responsible for Species Diversity in Unicellular Organisms (Alveolata, Ciliophora)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture
2.2. Preparation of Cell-Free Fluid (CFF)
2.3. Investigation on the Presence of Oligosaccharides in Gamone 1 of Two Species
2.3.1. Treatment of Samples Using Con-A Beads, and Preparation of Flow-Through Fraction and Elution Fraction
2.3.2. SDS-PAGE and Western Blotting
2.4. Preparation and Bioassay of Gamone 1 with and without N-Linked Oligosaccharides
2.4.1. Cleavage of N-Linked Oligosaccharides by Glycopeptidase F
2.4.2. Preparation of Gamone 1 with and without N-Linked Oligosaccharides
2.4.3. Bioassay of Conjugation-Inducing Activity
2.5. Extraction of Genomic DNA
2.6. Isolation of Gamone 1 Homologs and Sequencing
2.7. Hydrophobic Profile Search and Molecular Phylogenetic Analyses
3. Results
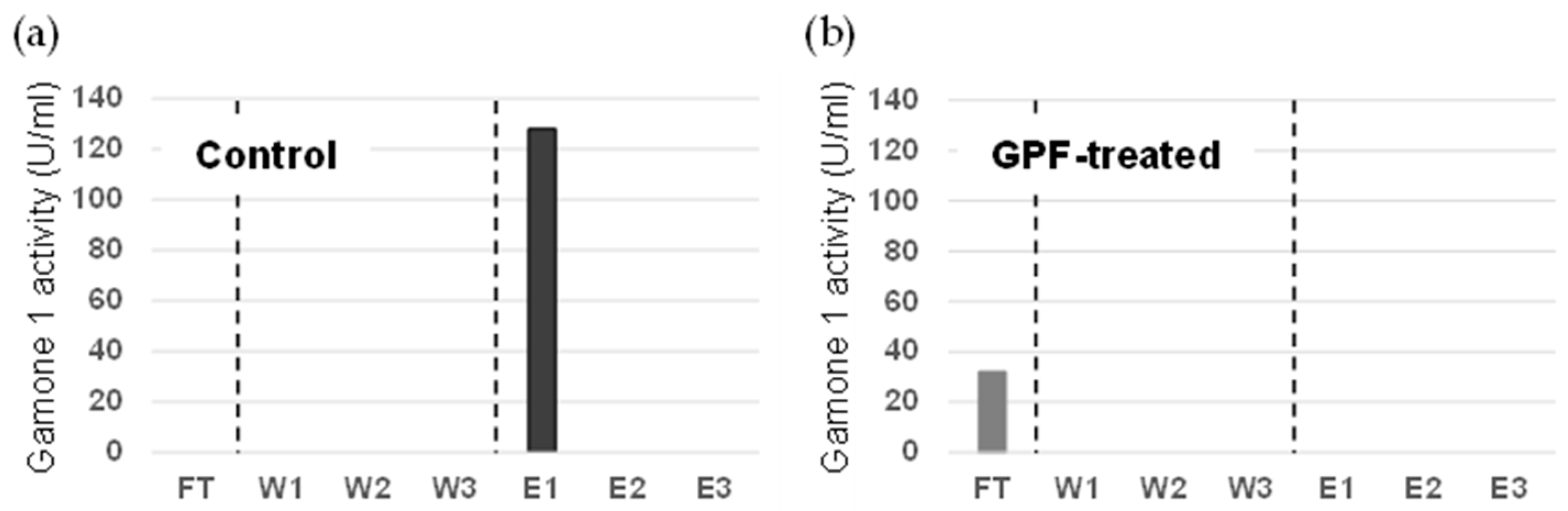
3.1. Investigation into the Involvement of Oligosaccharides in the Conjugation-Inducing Activity
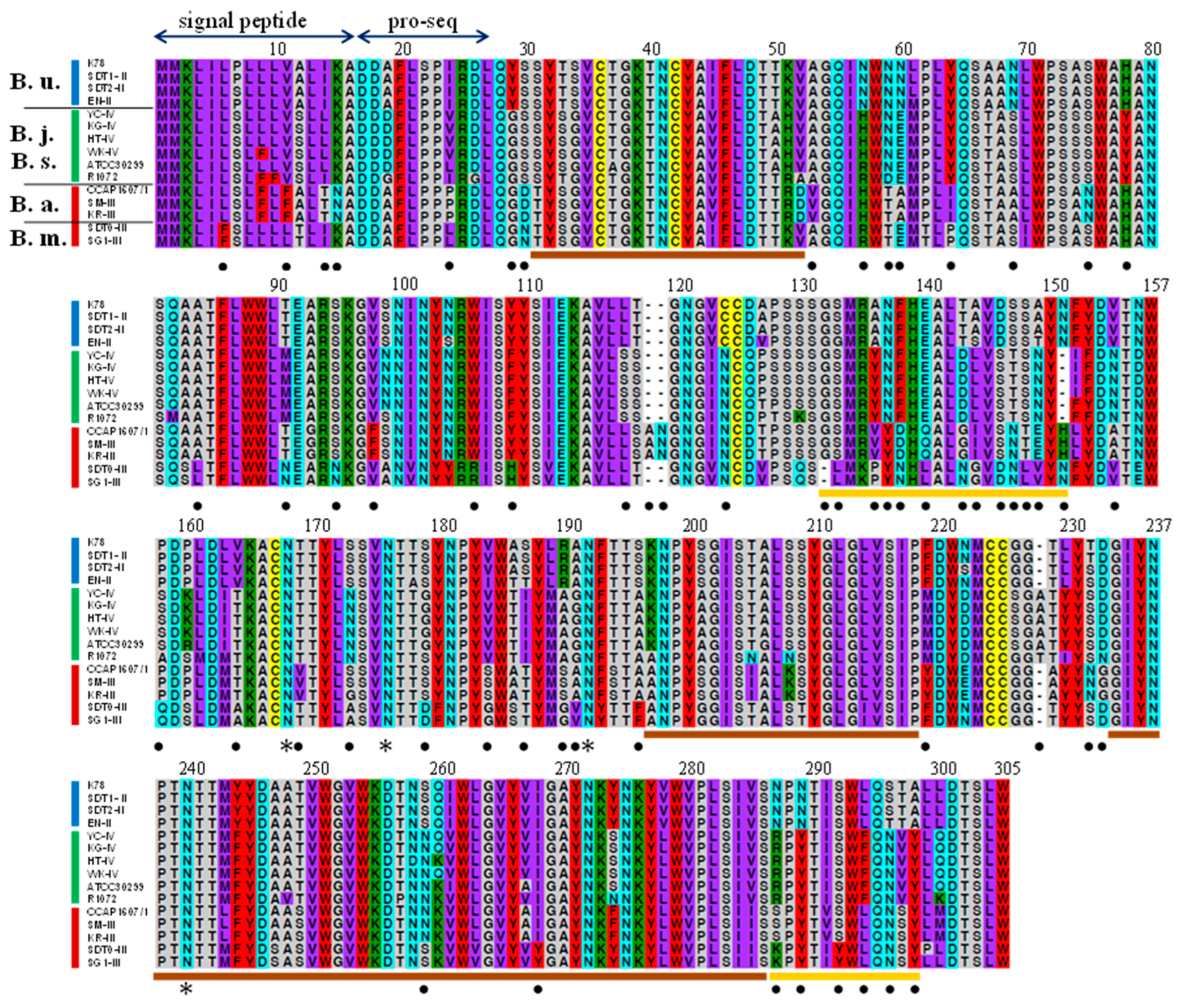
3.2. Comparison of Amino Acids Sequences of Gamone 1 among Species Belonging to Mk2, Mk3, and Mk4
3.2.1. Gamone 1 Genes in Mating Type I and II, and Newly Isolated Gamone 1 Homologs
3.2.2. ORF Length, Number of Estimated Oligosaccharide Attachment Sites, and Stop Codon in Gamone 1 Homologs of 15 Strains
3.2.3. Comparative Analysis of Gamone 1 Homologs and Amino Acid Variability among Species
3.2.4. Hydrophobicity Profiling and Estimated Sites for N-Linked Oligosaccharides
3.2.5. Molecular Phylogenetic Tree of Gamone 1
4. Discussion
4.1. Amino Acid Alterations of Gamone 1 and Possible Sites Responsible for Species Diversification
4.2. Three Steps for the Completion of Conjugation and Establishment of the Steps during Species Diversification
4.3. Species Problem in Paramecium and Blepharisma
4.4. Morphospecies and Biological Species, and Speciation in Blepharisma
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giese, A.C. Blepharisma. The Biology of a Light-Sensitive Protozoa; Stanford University Press: Redwood City, CA, USA, 1973. [Google Scholar]
- Small, E.; Lynn, D.H. Phylum Ciliophora Doflein. In An Illustrated Guide to the Protozoa; Lee, J.J., Hutner, S.H., Bovee, E.C., Eds.; Society of Protozoologists: Lawrence, KS, USA, 1985; pp. 393–575. [Google Scholar]
- Al-Rasheid, K.A.S.; Nilsson, J.R.; Larsen, H.F. Blepharisma intermedium Padmavathi, 1959 (Ciliophora: Heterotrichida) from Al-Hassa inland hypersaline oasis in Saudi Arabia. Acta Protozool. 2001, 40, 63–69. [Google Scholar]
- Post, F.J.; Borowitzka, L.J.; Borowitzka, M.A.; Mackay, B.; Moulton, T. The protozoa of a western Australian hypersaline lagoon. Hydrobiologia 1983, 105, 95–113. [Google Scholar] [CrossRef]
- Fernandes, N.M.; Dias, R.J.; Senra, M.V.; Soares, C.A.; da Silva Neto, I.D. Morphology and 18S rDNA gene sequence of Blepharisma sinuosum Sawaya, 1940 (Ciliophora: Heterotrichea) from Brazil. Eur. J. Protistol. 2013, 49, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Fan, Y.; Chen, X.; Li, L.; Warren, A.; Al-Farraj, S.A.; Song, W. Taxonomy and phylogeny of three heterotrich ciliates (Protozoa, Ciliophora), with description of a new Blepharisma species. Zool. J. Linn. Soc. 2016, 177, 320–334. [Google Scholar] [CrossRef]
- Chen, X.; Kim, J.H.; Shazib, S.U.A.; Kwon, C.B.; Shin, M.K. Morphology and molecular phylogeny of three hetero-trichid species (Ciliophora, Heterotrichea), including a new species of Anigsteinia. Eur. J. Protistol. 2017, 61, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Li, B.; Song, Y.; Warren, A.; Pan, X. Taxonomy and molecular phylogeny of two new Blepharisma species (Ciliophora, Heterotrichea) from northeastern China. Eur. J. Protistol. 2022, 85, 125908. [Google Scholar] [CrossRef]
- Pan, H.; Stoeck, T. Redescription of the halophile ciliate, Blepharisma halophilum Ruinen, 1938 (Ciliophora, Heterotrichea, Heterotrichida) shows that the genus Blepharisma is non-monophyletic. Eur. J. Protistol. 2017, 61, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Hirshfield, H.I.; Isquith, I.R.; DiLorenzo, A.M. Classification, distribution, and evolution. In Blepharisma; Giese, C., Ed.; Stanford University Press: Stanford, CA, USA, 1973; pp. 304–332. [Google Scholar]
- Leary, G.P.; Allen, J.E.; Bunger, P.L.; Luginbill, J.B.; Linn, C.E., Jr.; Macallister, I.E.; Kavanaugh, M.P.; Wanner, K.W. Single mutation to a sex pheromone receptor provides adaptive specificity between closely related moth species. Proc. Natl. Acad. Sci. USA 2012, 109, 14081–14086. [Google Scholar] [CrossRef] [PubMed]
- Kikuyama, S.; Toyoda, F.; Ohmiya, Y.; Matsuda, K.; Tanaka, S.; Hayashi, H. Sodefrin: A female-attracting peptide pheromone in newt cloacal glands. Science 1995, 267, 1643–1645. [Google Scholar] [CrossRef]
- Kikuyama, S.; Toyoda, F. Sodefrin: A novel sex pheromone in a newt. Rev. Reprod. 1999, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Seike, T.; Nakamura, T.; Shimoda, C. Molecular coevolution of a sex pheromone and its receptor triggers reproductive isolation in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 2015, 112, 4405–4410. [Google Scholar] [CrossRef]
- Miyake, A.; Beyer, J. Cell interaction by means of soluble factors (gamones) in conjugation of Blepharisma intermedium. Exptl. Cell Res. 1973, 76, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A. Cell interaction by gamones in Blepharisma. In Sexual Interaction in Eukaryotic Microbes; O’Day, D.H., Horgan, P.A., Eds.; Academic Press: New York, NY, USA, 1981; pp. 95–129. [Google Scholar]
- Miyake, A.; Miyake, A. Fertilization and sexuality in ciliates. In Ciliates: Cells as Organisms; Hausmann, K., Bradbury, P.C., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1996; pp. 243–290. [Google Scholar]
- Miyake, A.; Beyer, J. Blepharmone: A conjugation-inducing glycoprotein in the ciliate Blepharisma. Science 1974, 185, 621–623. [Google Scholar] [CrossRef]
- Kubota, T.; Tokoroyama, T.; Tsukuda, Y.; Koyama, H.; Miyake, A. Isolation and structure determination of blepharismin, a conjugation initiating gamone in the ciliate Blepharisma. Science 1973, 179, 400–402. [Google Scholar] [CrossRef]
- Braun, V.; Miyake, A. Composition of blepharmone, a conjugation-inducing glycoprotein of the ciliate Blepharisma. FEBS Lett. 1975, 53, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Harumoto, T. Identification, characterization, and complete amino acid sequence of the conjugation-inducing glycoprotein (blepharmone) in the ciliate Blepharisma japonicum. Proc. Natl. Acad. Sci. USA 2001, 98, 14446–14451. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Bleyman, L.K. Gamones and mating types in the genus Blepharisma and their possible taxonomic application. Genet. Res. Camb. 1976, 27, 67–275. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Miura, M.; Takusagawa, M.; Sugiura, M.; Harumoto, T. Two possible barriers blocking conjugation between different megakaryotypes of Blepharisma. Zool. Sci. 2015, 32, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Sugino, A.; Kobayashi, M.; Sugiura, M.; Harumoto, T. Possible third step preventing conjugation between different species of Blepharisma. Microorganisms 2023, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Harumoto, T.; Suzaki, T. Analysis of gamone 1 receptor in the ciliate Blepharisma japonicum using a gamone 1 antibody. Jpn. J. Protozool. 2009, 42, 34–35. [Google Scholar]
- Epstein, L.M.; Forney, J.D. Mendelian and non-Mendelian mutations affecting surface antigen expression in Paramecium tetraurelia. Mol. Cell Biol. 1984, 4, 1583–1590. [Google Scholar] [CrossRef]
- Forney, P.J.; Epstein, L.M.; Preer, L.B.; Rudman, B.M.; Widmayer, D.J.; Klein, W.H.; Preer, J.R., Jr. Structure and expression of genes for surface protein in Paramecium. Mol. Cell Biol. 1983, 3, 466–474. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Whelan, S.; Goldman, N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 2001, 18, 691–699. [Google Scholar] [CrossRef]
- Yang, Z. Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: Approximate methods. J. Mol. Evol. 1994, 39, 306–314. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kishino, H.; Hasegawa, M. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in hominoidea. J. Mol. Evol. 1989, 29, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, H. An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 2002, 51, 492–508. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, H.; Hasegawa, M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol. Biol. Evol. 1999, 16, 1114–1116. [Google Scholar] [CrossRef]
- Lozupone, A.; Knight, R.D.; Landweber, L.F. The molecular basis of nuclear genetic code change in ciliates. Curr. Biol. 2001, 11, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Tanaka, Y.; Suzaki, T.; Harumoto, T. Alternative gene expression in type I and type II cells may enable further nuclear changes during conjugation of Blepharisma japonicum. Protist 2012, 163, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Miyazawa, S.; Yasunaga, T. Two types of amino acid substitutions in protein evolution. J. Mol. Evol. 1979, 12, 219–236. [Google Scholar] [CrossRef]
- Schloegel, J.J. From anomaly to unification: Tracy Sonneborn and the species problem in protozoa, 1954–1957. J. Hist. Biol. 1999, 32, 93–132. [Google Scholar] [CrossRef]
- Schlegel, M.; Meisterfeld, R. The species problem in protozoa revisited. Europ. J. Protistol. 2003, 39, 349–355. [Google Scholar] [CrossRef]
- Sonneborn, T.M. The Paramecium aurelia complex of fourteen sibling species. Trans. Am. Micros. Soc. 1975, 94, 155–178. [Google Scholar] [CrossRef]
- Aufderheide, K.J.; Daggett, P.-M.; Nerad, T.A. Paramecium sonneborni n. sp., a new member of the Paramecium aurelia species-complex. J. Protozool. 1983, 30, 128–131. [Google Scholar] [CrossRef]
- Tsukii, Y.; Hiwatashi, K. Genes controlling mating-type specificity in Paramecium caudatum: Three loci revealed by intersyngenic crosses. Genetics 1983, 104, 41–62. [Google Scholar] [CrossRef]
- Tsukii, Y.; Hiwatashi, K. Meiotic nondisjunction and aneuploids in intersyngenic hybrids of Paramecium caudatum. Genetics 1985, 111, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Tsukii, Y. Genetic and chromosomal instability and induction of aneuploidy in Paramecium. In Aneuploidy, Part B: Induction and Test System; Alan R. Liss, Inc.: New York, NY, USA, 1988; pp. 209–225. [Google Scholar]
- Inaba, F. Conjugation between two strains of Blepharisma. J. Protozool. 1965, 12, 146–151. [Google Scholar] [CrossRef]



| Mk | Species | Mating Type | Strain * | Long Diameter | Short Diameter | Collection Site |
|---|---|---|---|---|---|---|
| 2 | B. undulans | I | EN-II | 137 | 60 | Ibaraki, Japan |
| SDT2-II | 130 | 48 | Minamidaito Island, Japan | |||
| K78 | 119 | 49 | Ishikawa, Japan | |||
| II | SDT1-II | 111 | 22 | Minamidaito Island, Japan | ||
| 3 | B. americanum | I | CCAP1607/1 * | 233 | 105 | USA |
| SM-III * | 148 | 61 | unidentified | |||
| II | KR-III * | 148 | 44 | Gyeongju, South Korea | ||
| B. musculus | I | SDT0-III * | 166 | 64 | Minamidaito Island, Japan | |
| SG1-III * | 145 | 58 | Ibaraki, Japan | |||
| 4 | B. japonicum | I | R1072 | 340 | 164 | Bangalore, India |
| II | YC-IV | 232 | 104 | Yamaguchi Japan | ||
| KG-IV * | 251 | 103 | Nara, Japan | |||
| B. stoltei | I | ATCC30299 | 211 | 86 | Lake Federsee, Germany | |
| II | HT-IV | 199 | 75 | Aichi, Japan | ||
| WK-IV * | 176 | 62 | Hokkaido, Japan |
| Megakaryotype 2 | Megakaryotype 3 | Megakaryotype 4 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. undulans | B. americanum | B. musculus | B. japonicum | B. stoltei | ||||||||||||
| K78 | SDT2-II | SDT1-II | CCAP 1607/1 | SM-III | KR-III | SG1-III | SDT0-III | R1072 | KG-IV | YC-IV | ATCC 30299 | HT-IV | WK-IV | |||
| Mk2 | B. undulans | EN-II | 95.7 | 95.7 | 96.1 | 73.1 | 73.1 | 72.8 | 73.7 | 73.4 | 73.1 | 75.1 | 75.1 | 75.1 | 74.4 | 74.8 |
| K78 | - | 98.7 | 99.7 | 73.4 | 73.4 | 73.1 | 72.4 | 72.0 | 73.1 | 75.1 | 75.1 | 75.1 | 74.4 | 74.8 | ||
| SDT2-II | - | - | 99.0 | 74.1 | 74.1 | 73.8 | 73.0 | 72.7 | 74.1 | 76.1 | 76.1 | 76.1 | 75.4 | 75.7 | ||
| SDT1-II | - | - | - | 73.8 | 73.8 | 73.4 | 72.7 | 72.4 | 73.4 | 75.4 | 75.4 | 75.4 | 74.8 | 75.1 | ||
| Mk3 | B. americanum | CCAP1607/1 | - | - | - | - | 100 | 99.7 | 70.4 | 70.1 | 72.1 | 72.1 | 72.1 | 72.8 | 72.1 | 72.5 |
| SM-III | - | - | - | - | - | 99.7 | 70.4 | 70.1 | 72.1 | 72.1 | 72.1 | 72.8 | 72.1 | 72.5 | ||
| KR-III | - | - | - | - | - | - | 70.4 | 70.1 | 72.1 | 72.1 | 72.1 | 72.8 | 72.1 | 72.5 | ||
| B. musculus | SG1-III | - | - | - | - | - | - | - | 99.7 | 68.1 | 69.1 | 69.1 | 69.7 | 69.1 | 68.8 | |
| SDT0-III | - | - | - | - | - | - | - | - | 67.8 | 68.8 | 68.8 | 69.4 | 68.8 | 68.4 | ||
| Mk4 | B. japonicum | R1072 | - | - | - | - | - | - | - | - | - | 88.2 | 88.2 | 87.5 | 88.2 | 88.5 |
| KG-IV | - | - | - | - | - | - | - | - | - | - | 100 | 95.7 | 99.3 | 99.7 | ||
| YC-IV | - | - | - | - | - | - | - | - | - | - | - | 95.7 | 99.3 | 99.7 | ||
| B. stoltei | ATCC30299 | - | - | - | - | - | - | - | - | - | - | - | - | 95.7 | 95.4 | |
| HT-IV | - | - | - | - | - | - | - | - | - | - | - | - | - | 99.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, M.; Sugiura, M.; Iwasaki, S.; Iwabe, N.; Harumoto, T. Mating Pheromone (Gamone 1) in Blepharisma: A Glycoprotein Responsible for Species Diversity in Unicellular Organisms (Alveolata, Ciliophora). Microorganisms 2024, 12, 299. https://doi.org/10.3390/microorganisms12020299
Kobayashi M, Sugiura M, Iwasaki S, Iwabe N, Harumoto T. Mating Pheromone (Gamone 1) in Blepharisma: A Glycoprotein Responsible for Species Diversity in Unicellular Organisms (Alveolata, Ciliophora). Microorganisms. 2024; 12(2):299. https://doi.org/10.3390/microorganisms12020299
Chicago/Turabian StyleKobayashi, Mayumi, Mayumi Sugiura, Shoko Iwasaki, Naoyuki Iwabe, and Terue Harumoto. 2024. "Mating Pheromone (Gamone 1) in Blepharisma: A Glycoprotein Responsible for Species Diversity in Unicellular Organisms (Alveolata, Ciliophora)" Microorganisms 12, no. 2: 299. https://doi.org/10.3390/microorganisms12020299




