An In Vitro Study on the Role of Cellulases and Xylanases of Bacillus subtilis in Dairy Cattle Nutrition
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacillus subtilis Strains
2.2. Bacterial Medium Preparation and Its Characterization
2.3. Bacillus subtilis Cultures
2.4. Cellulase and Xylanase Activity Assays
2.5. In Vitro Analyses
2.5.1. In Vitro NDF Degradability
2.5.2. In Vitro Gas Production
2.6. Calculations and Statistical Analyses
3. Results
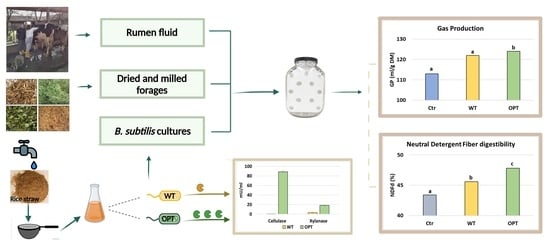
3.1. B. subtilis Feed Additive Preparation
3.2. Feed Treatment and Fiber Degradability
3.3. Gas Production and Ruminal Fermentative Profile
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Capper, J.L.; Bauman, D.E. The Role of Productivity in Improving the Environmental Sustainability of Ruminant Production Systems. Annu. Rev. Anim. Biosci. 2013, 1, 469–489. [Google Scholar] [CrossRef]
- Adesogan, A.T.; Arriola, K.G.; Jiang, Y.; Oyebade, A.; Paula, E.M.; Pech-Cervantes, A.A.; Romero, J.J.; Ferraretto, L.F.; Vyas, D. Symposium review: Technologies for improving fiber utilization. J. Dairy Sci. 2019, 102, 5726–5755. [Google Scholar] [CrossRef]
- Oba, M.; Kammes-Main, K. Symposium review: Effects of carbohydrate digestion on feed intake and fuel supply. J. Dairy Sci. 2023, 106, 2153–2160. [Google Scholar] [CrossRef]
- Donnelly, D.M.; de Resende, L.C.; Cook, D.E.; Atalla, R.H.; Combs, D.K. Technical note: A comparison of alkali treatment methods to improve neutral detergent fiber digestibility of corn stover. J. Dairy Sci. 2018, 101, 9058–9064. [Google Scholar] [CrossRef] [PubMed]
- Mor, P.; Bals, B.; Tyagi, A.K.; Teymouri, F.; Tyagi, N.; Kumar, S.; Bringi, V.; VandeHaar, M. Effect of ammonia fiber expansion on the available energy content of wheat straw fed to lactating cattle and buffalo in India. J. Dairy Sci. 2018, 101, 7990–8003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; McAllister, T.A. Rumen Microbes, Enzymes and Feed Digestion-A Review. Asian-Australas. J. Anim. Sci. 2002, 15, 1659–1676. [Google Scholar] [CrossRef]
- Tirado-González, D.N.; Miranda-Romero, L.A.; Ruíz-Flores, A.; Medina-Cuéllar, S.E.; Ramírez-Valverde, R.; Tirado-Estrada, G. Meta-analysis: Effects of exogenous fibrolytic enzymes in ruminant diets. J. Appl. Anim. Res. 2018, 46, 771–783. [Google Scholar] [CrossRef]
- Ferreira, R.G.; Azzoni, A.R.; Freitas, S. On the production cost of lignocellulose-degrading enzymes. Biofuels Bioprod. Biorefining 2021, 15, 85–99. [Google Scholar] [CrossRef]
- Ban, Y.; Guan, L.L. Implication and challenges of direct-fed microbial supplementation to improve ruminant production and health. J. Anim. Sci. Biotechnol. 2021, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Xu, Q.; Luo, Y.; Ding, S.-Y.; Lamed, R.; Bayer, E.A. Microbial enzyme systems for biomass conversion: Emerging paradigms. Biofuels 2010, 1, 323–341. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Factories 2020, 19, 173. [Google Scholar] [CrossRef]
- van Dijl, J.M.; Hecker, M. Bacillus subtilis: From soil bacterium to super-secreting cell factory. Microb. Cell Factories 2013, 12, 3. [Google Scholar] [CrossRef]
- Earl, A.M.; Losick, R.; Kolter, R. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 2008, 16, 269–275. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; de Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Update of the list of qualified presumption of safety (QPS) recommended microorganisms intentionally added to food or feed as notified to EFSA. EFSA J. 2023, 21, e07747. [Google Scholar] [CrossRef]
- Lee, N.-K.; Kim, W.-S.; Paik, H.-D. Bacillus strains as human probiotics: Characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol. 2019, 28, 1297–1305. [Google Scholar] [CrossRef]
- Bernardeau, M.; Lehtinen, M.J.; Forssten, S.D.; Nurminen, P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 2017, 54, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Das, R.; Oak, S.; Mishra, P. Probiotics (Direct-Fed Microbials) in Poultry Nutrition and Their Effects on Nutrient Utilization, Growth and Laying Performance, and Gut Health: A Systematic Review. Animals 2020, 10, 1863. [Google Scholar] [CrossRef] [PubMed]
- Mun, D.; Kyoung, H.; Kong, M.; Ryu, S.; Jang, K.B.; Baek, J.; Park, K., II; Song, M.; Kim, Y. Effects of Bacillus-based probiotics on growth performance, nutrient digestibility, and intestinal health of weaned pigs. J. Anim. Sci. Technol. 2021, 63, 1314–1327. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, J.-Q.; Zhang, H.-T. Effects of supplementation of Bacillus subtilis natto Na and N1 strains on rumen development in dairy calves. Anim. Feed. Sci. Technol. 2011, 164, 154–160. [Google Scholar] [CrossRef]
- Sun, P.; Wang, J.Q.; Deng, L.F. Effects of Bacillus subtilis natto on milk production, rumen fermentation and ruminal microbiome of dairy cows. Animal 2013, 7, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.L.; Lopes, N.M.; Zacaroni, O.F.; Silveira, V.A.; Pereira, R.A.N.; Freitas, J.A.; Almeida, R.; Salvati, G.G.S.; Pereira, M.N. Lactation performance and diet digestibility of dairy cows in response to the supplementation of Bacillus subtilis spores. Livest. Sci. 2017, 200, 35–39. [Google Scholar] [CrossRef]
- Choonkham, W.; Schonewille, J.T.; Bernard, J.K.; Suriyasathaporn, W. Effects of on-farm supplemental feeding of probiotic Bacillus subtilis on milk production in lactating dairy cows under tropical conditions. J. Anim. Feed. Sci. 2020, 29, 199–205. [Google Scholar] [CrossRef]
- Jia, P.; Tu, Y.; Liu, Z.; Li, F.; Yan, T.; Ma, S.; Dong, L.; Diao, Q. Diets supplementation with Bacillus subtilis and Macleaya cordata extract improve production performance and the metabolism of energy and nitrogen, while reduce enteric methane emissions in dairy cows. Anim. Feed. Sci. Technol. 2022, 294, 115481. [Google Scholar] [CrossRef]
- Cappellozza, B.I.; Joergensen, J.N.; Copani, G.; Bryan, K.A.; Fantinati, P.; Bodin, J.-C.; Khahi, M.M.; DeGuzman, C.N.; Arriola, K.G.; Lima, L.O.; et al. Evaluation of a Bacillus-based direct-fed microbial probiotic on in vitro rumen gas production and nutrient digestibility of different feedstuffs and total mixed rations. Transl. Anim. Sci. 2023, 7, txad044. [Google Scholar] [CrossRef]
- Sun, P.; Li, J.; Bu, D.; Nan, X.; Du, H. Effects of Bacillus subtilis natto and Different Components in Culture on Rumen Fermentation and Rumen Functional Bacteria In Vitro. Curr. Microbiol. 2016, 72, 589–595. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Beauchemin, K.A.; Tang, S.; Zhou, C.; Han, X.; Wang, M.; Kang, J.; Odongo, N.E.; Tan, Z. Comparison of two live Bacillus species as feed additives for improving in vitro fermentation of cereal straws. Anim. Sci. J. 2016, 87, 27–36. [Google Scholar] [CrossRef]
- Pan, L.; Harper, K.; Queiroz, O.; Copani, G.; Cappellozza, B.I. Effects of a Bacillus-based direct-fed microbial on in vitro nutrient digestibility of forage and high-starch concentrate substrates. Transl. Anim. Sci. 2022, 6, txac067. [Google Scholar] [CrossRef]
- Dhakal, R.; Copani, G.; Cappellozza, B.I.; Milora, N.; Hansen, H.H. The Effect of Direct-Fed Microbials on In-Vitro Rumen Fermentation of Grass or Maize Silage. Fermentation 2023, 9, 347. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2013, 42, D490–D495. [Google Scholar] [CrossRef]
- Krämer-Schmid, M.; Lund, P.; Weisbjerg, M.R. Importance of NDF digestibility of whole crop maize silage for dry matter intake and milk production in dairy cows. Anim. Feed. Sci. Technol. 2016, 219, 68–76. [Google Scholar] [CrossRef]
- Srivatsan, A.; Han, Y.; Peng, J.; Tehranchi, A.K.; Gibbs, R.; Wang, J.D.; Chen, R. High-Precision, Whole-Genome Sequencing of Laboratory Strains Facilitates Genetic Studies. PLoS Genet. 2008, 4, e1000139. [Google Scholar] [CrossRef] [PubMed]
- Doria, E.; Buonocore, D.; Marra, A.; Bontà, V.; Gazzola, A.; Dossena, M.; Verri, M.; Calvio, C. Bacterial-Assisted Extraction of Bioactive Compounds from Cauliflower. Plants 2022, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Das, P.; Ragauskas, A.J. Rice straw as a feedstock for biofuels: Availability, recalcitrance, and chemical properties. Biofuels Bioprod. Biorefining 2018, 12, 83–107. [Google Scholar] [CrossRef]
- Smith, J.L.; Goldberg, J.M.; Grossman, A.D. Complete Genome Sequences of Bacillus subtilis subsp. subtilis Laboratory Strains JH642 (AG174) and AG1839. Genome Announc. 2014, 2, e00663-14. [Google Scholar] [CrossRef] [PubMed]
- Belal, E.B. Bioethanol production from rice straw residue. Braz. J. Microbiol. 2013, 44, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Krishania, M.; Kumar, V.; Sangwan, R.S. Integrated approach for extraction of xylose, cellulose, lignin and silica from rice straw. Bioresour. Technol. Rep. 2018, 1, 89–93. [Google Scholar] [CrossRef]
- Bergstrand, M.; Karlsson, M.O. Handling Data Below the Limit of Quantification in Mixed Effect Models. AAPS J. 2009, 11, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Ermoli, F.; Bontà, V.; Vitali, G.; Calvio, C. SwrA as global modulator of the two-component system DegSU in Bacillus subtilis. Res. Microbiol. 2021, 172, 103877. [Google Scholar] [CrossRef]
- Spanghero, M.; Chiaravalli, M.; Colombini, S.; Fabro, C.; Froldi, F.; Mason, F.; Moschini, M.; Sarnataro, C.; Schiavon, S.; Tagliapietra, F. Rumen Inoculum Collected from Cows at Slaughter or from a Continuous Fermenter and Preserved in Warm, Refrigerated, Chilled or Freeze-Dried Environments for in Vitro Tests. Animals 2019, 9, 815. [Google Scholar] [CrossRef]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar] [CrossRef]
- Pirondini, M.; Colombini, S.; Malagutti, L.; Rapetti, L.; Galassi, G.; Zanchi, R.; Crovetto, G.M. Effects of a selection of additives on in vitro ruminal methanogenesis and in situ and in vivo NDF digestibility. Anim. Sci. J. 2015, 86, 59–68. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed. Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Colombini, S.; Rota Graziosi, A.; Parma, P.; Iriti, M.; Vitalini, S.; Sarnataro, C.; Spanghero, M. Evaluation of dietary addition of 2 essential oils from Achillea moschata, or their components (bornyl acetate, camphor, and eucalyptol) on in vitro ruminal fermentation and microbial community composition. Anim. Nutr. 2021, 7, 224–231. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, S.; Vancov, T. Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw. Biomass Bioenergy 2011, 35, 3094–3103. [Google Scholar] [CrossRef]
- Park, J.-y.; Seyama, T.; Shiroma, R.; Ike, M.; Srichuwong, S.; Nagata, K.; Arai-Sanoh, Y.; Kondo, M.; Tokuyasu, K. Efficient Recovery of Glucose and Fructose via Enzymatic Saccharification of Rice Straw with Soft Carbohydrates. Biosci. Biotechnol. Biochem. 2009, 73, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.T.; Duarte, M.E.; Holanda, D.M.; Kim, S.W. Friend or Foe? Impacts of Dietary Xylans, Xylooligosaccharides, and Xylanases on Intestinal Health and Growth Performance of Monogastric Animals. Animals 2021, 11, 609. [Google Scholar] [CrossRef]


| Before Hydrolysis | After Hydrolysis | |||
|---|---|---|---|---|
| Carbon Source | Glucose 1 | Xylose 1 | Glucose 1 | Xylose 1 |
| WL (mg/mL) | 0.542 ± 0.012 | 0.500 ± 0.000 | 2.183 ± 0.519 | 0.700 ± 0.047 |
| Treatment 1 | SE 2 | p-Value 3 | |||||
|---|---|---|---|---|---|---|---|
| Control | PB5700 | PB2OPT | T | Feed | T × Feed | ||
| NDFd 4 (%) | 43.4 a | 45.6 b | 47.8 c | 1.30 | <0.001 | <0.001 | 0.028 |
| GP 5 0–24 h (mL/g DM) | 83.3 a | 89.6 b | 91.1 b | 5.07 | 0.019 | <0.001 | 0.099 |
| GP 24–48 h (mL/g DM) | 29.3 a | 32.2 b | 32.8 b | 1.56 | 0.016 | <0.001 | 0.757 |
| GP 0–48 h (mL/g DM) | 113 a | 122 b | 124 b | 5.25 | 0.010 | <0.001 | 0.217 |
| pH | 6.26 | 6.29 | 6.28 | 0.161 | 0.239 | <0.001 | 0.392 |
| N-NH3 6 (mg/dL) | 49.1 | 47.9 | 47.6 | 7.59 | 0.556 | 0.005 | 0.938 |
| Total VFA 7 (mmol/L) | 92.4 | 97.4 | 94.4 | 13.1 | 0.455 | 0.255 | 0.342 |
| VFA (% of total VFA) | |||||||
| Acetic acid (%) | 56.7 | 57.3 | 57.9 | 0.839 | 0.151 | <0.001 | 0.922 |
| Propionic acid (%) | 21.4 | 20.2 | 20.1 | 2.74 | 0.063 | 0.051 | 0.528 |
| Butyric acid (%) | 17.0 | 17.5 | 16.7 | 1.48 | 0.490 | <0.001 | 0.459 |
| Valeric acid (%) | 4.86 | 5.05 | 5.20 | 0.570 | 0.551 | 0.301 | 0.545 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bontà, V.; Battelli, M.; Rama, E.; Casanova, M.; Pasotti, L.; Galassi, G.; Colombini, S.; Calvio, C. An In Vitro Study on the Role of Cellulases and Xylanases of Bacillus subtilis in Dairy Cattle Nutrition. Microorganisms 2024, 12, 300. https://doi.org/10.3390/microorganisms12020300
Bontà V, Battelli M, Rama E, Casanova M, Pasotti L, Galassi G, Colombini S, Calvio C. An In Vitro Study on the Role of Cellulases and Xylanases of Bacillus subtilis in Dairy Cattle Nutrition. Microorganisms. 2024; 12(2):300. https://doi.org/10.3390/microorganisms12020300
Chicago/Turabian StyleBontà, Valeria, Marco Battelli, Erlinda Rama, Michela Casanova, Lorenzo Pasotti, Gianluca Galassi, Stefania Colombini, and Cinzia Calvio. 2024. "An In Vitro Study on the Role of Cellulases and Xylanases of Bacillus subtilis in Dairy Cattle Nutrition" Microorganisms 12, no. 2: 300. https://doi.org/10.3390/microorganisms12020300
APA StyleBontà, V., Battelli, M., Rama, E., Casanova, M., Pasotti, L., Galassi, G., Colombini, S., & Calvio, C. (2024). An In Vitro Study on the Role of Cellulases and Xylanases of Bacillus subtilis in Dairy Cattle Nutrition. Microorganisms, 12(2), 300. https://doi.org/10.3390/microorganisms12020300