Harnessing the Biocontrol Potential of Bradyrhizobium japonicum FCBP-SB-406 to Manage Charcoal Rot of Soybean with Increased Yield Response for the Development of Sustainable Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Procurement of Cultures of Macrophomina phaseolina and PGPRs
2.2. Metabolites Quantification of Selected PGPRs
2.2.1. Protease Activity
2.2.2. Catalase Activity
2.2.3. Hydrogen Cyanide (HCN) Production
2.3. In Vitro Screening of PGPRs for Antifungal Activity
2.4. Field Trial to Evaluate Disease Suppression Potential of Selected PGPRs
2.4.1. Preparation of PGPR Inoculum
2.4.2. Disease Induction
2.4.3. Field Experiment
2.4.4. Measurement of Photosynthetic Pigments
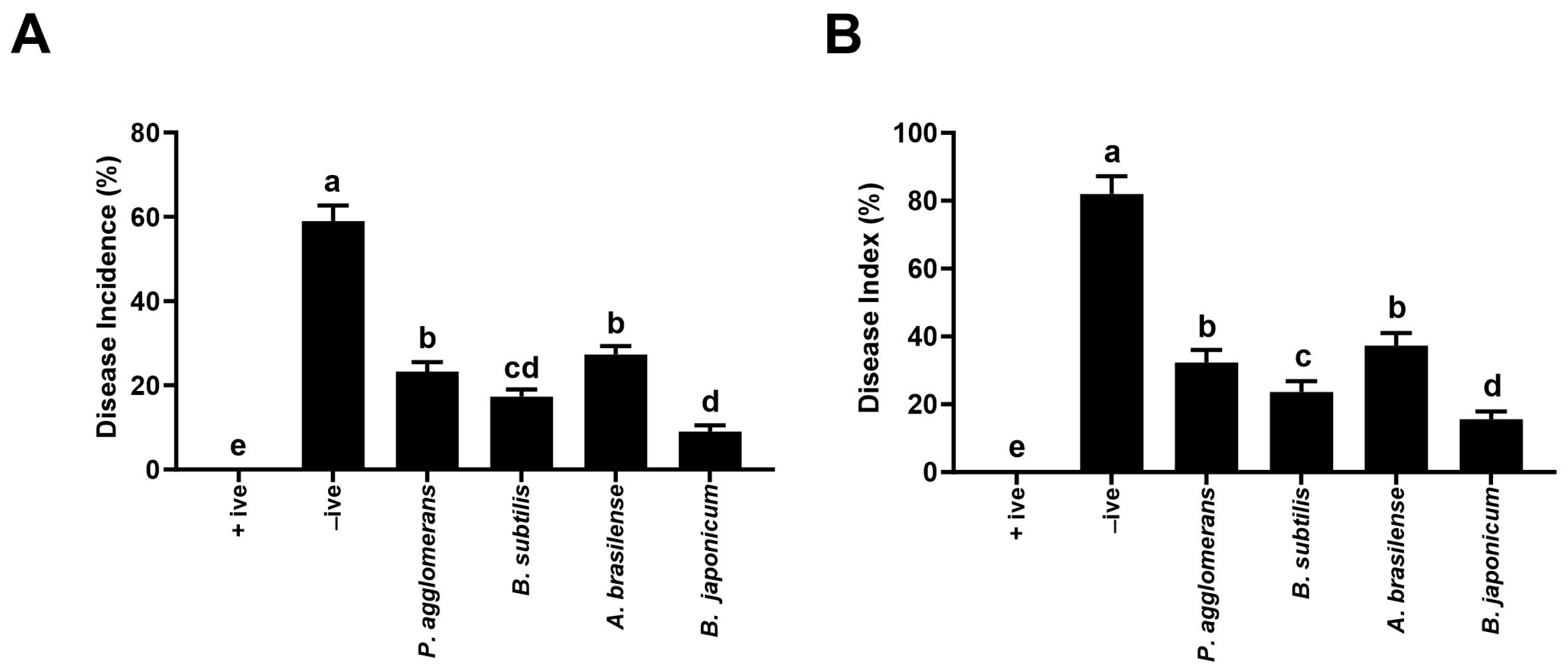
2.4.5. Calculation of Disease Incidence and Disease Severity
2.5. Evaluation of Defense-Related Enzymes
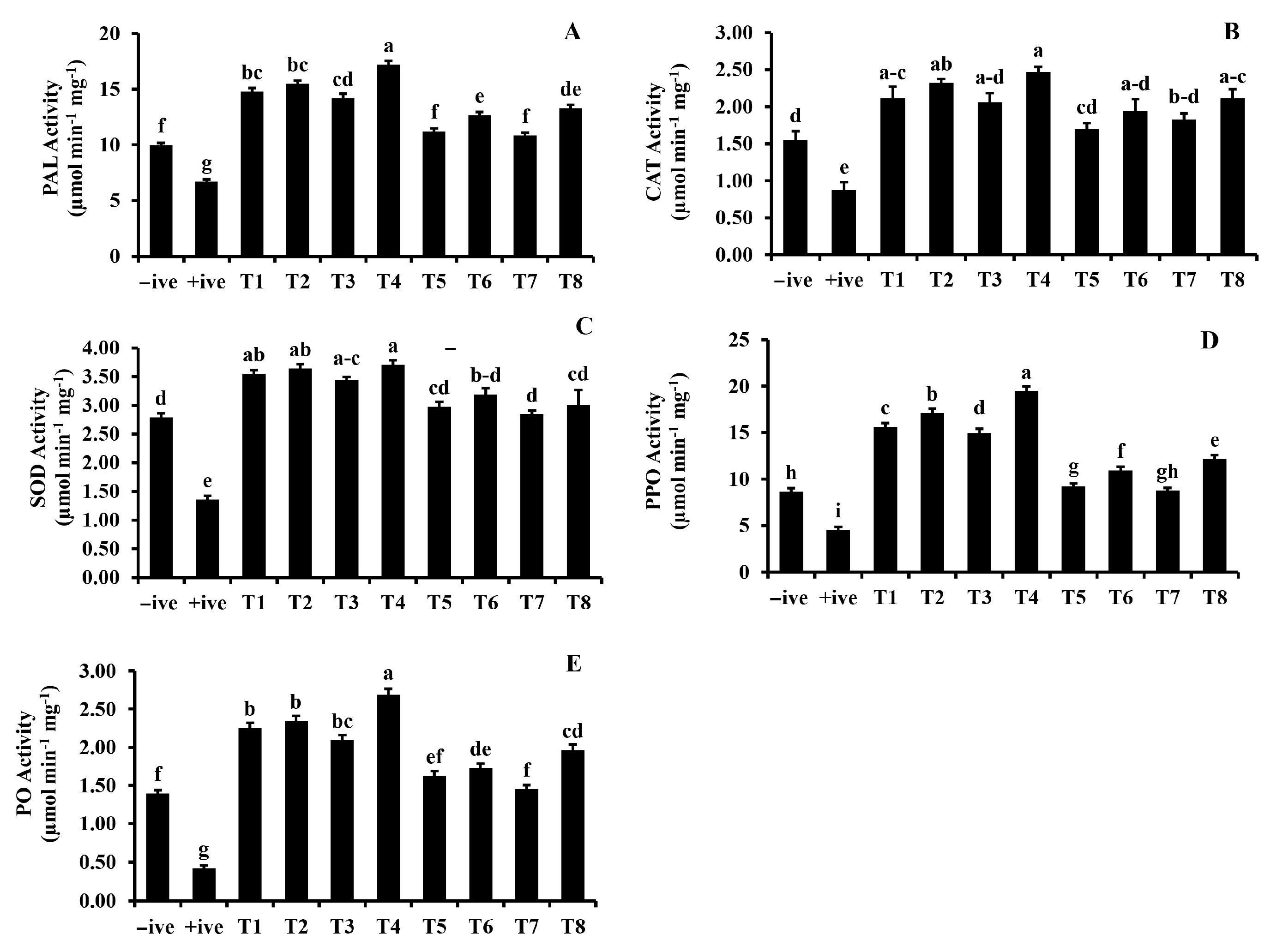
2.5.1. Peroxidase Activity
2.5.2. Polyphenol Oxidase Activity
2.5.3. Phenylalanine Ammonia Lyase Activity
2.5.4. Superoxide Dismutase Activity
2.5.5. Catalase Activity
2.6. Physico-Chemical Characterization of Field Soil
2.7. LC-Ms Profiling of Total Metabolites of Treated and Un-Treated Plants
2.8. Statistical Analysis
3. Results
3.1. Biochemical and Antifungal Activity of Selected PGPRs
3.2. Field Experiment
3.2.1. Plant Disease Incidence
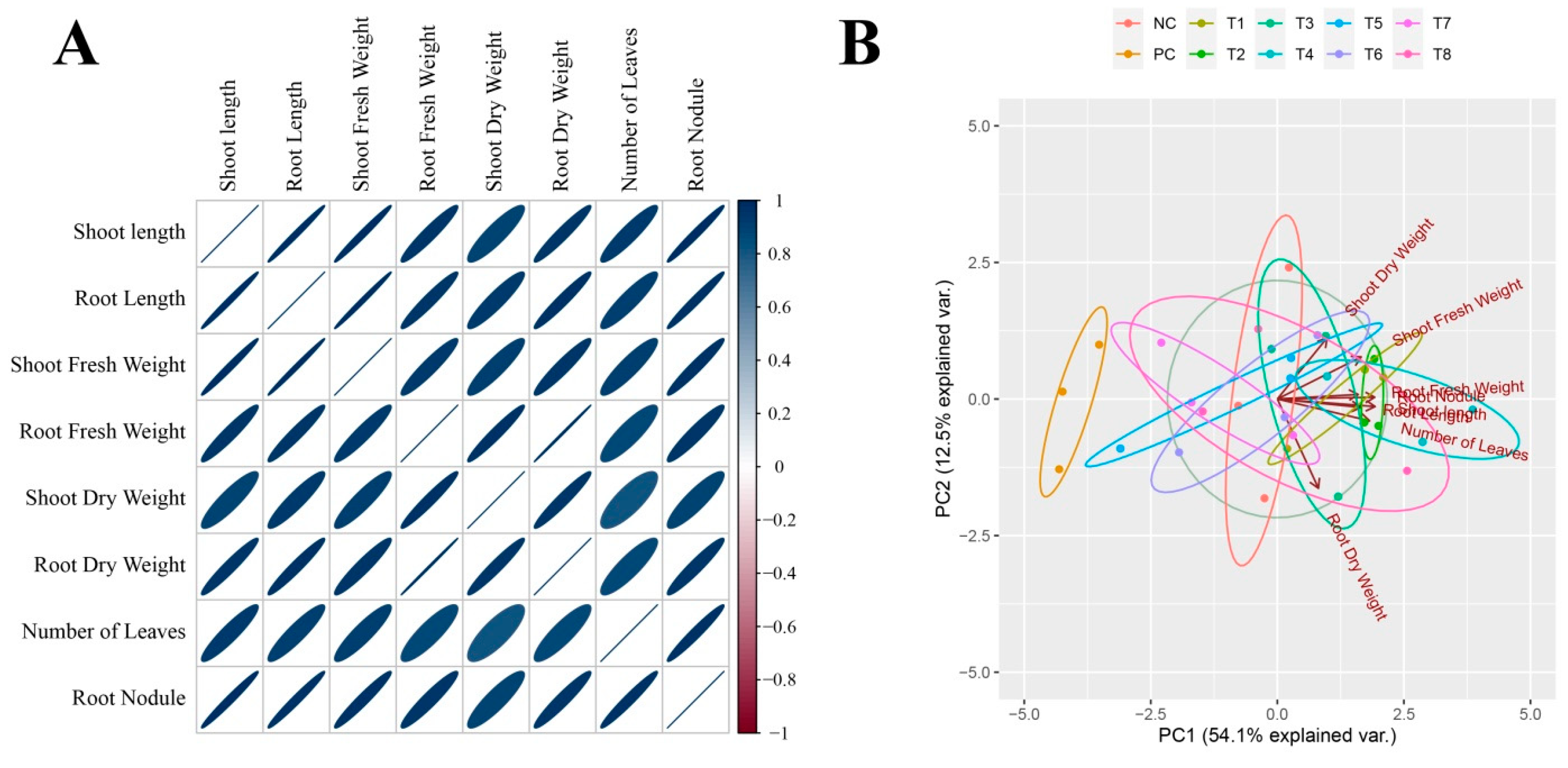
3.2.2. Agronomic Parameters and Photosynthetic Pigments
3.2.3. Soil Analysis
3.2.4. Analysis of Plant Defense Enzymes
3.3. Major Compounds Detected in Treated and Control Plants of Soybean through LC/MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Guan, R.; Liu, Z.; Ma, Y.; Wang, L.; Li, L.; Qiu, L. Genetic structure and diversity of cultivated soybean (Glycine max (L.) Merr.) landraces in China. Theor. Appl. Genet. 2008, 117, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Naamala, J.; Jaiswal, S.K.; Dakora, F.D. Microsymbiont diversity and phylogeny of native bradyrhizobia associated with soybean (Glycine max L. Merr.) nodulation in South African soils. Syst. Appl. Microbiol. 2016, 39, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Asad, S.A.; Wahid, M.A.; Farina, S.; Ali, R.; Muhammad, F. Soybean production in Pakistan: Experiences, challenges and prospects. Int. J. Agric. Biol. 2020, 24, 995–1005. [Google Scholar]
- Mufti, R.; Bano, A. PGPR-induced defense responses in the soybean plant against charcoal rot disease. Eur. J. Plant Pathol. 2019, 155, 983–1000. [Google Scholar] [CrossRef]
- Abdel-Sattar, M.A.; Aly, A.A.; Omar, M.R. Some factors affecting the susceptiblity of cottonseed to Macrophomina phaseolina. J. Plant Prod. 2007, 32, 2455–2467. [Google Scholar] [CrossRef]
- Romero Luna, M.P.; Mueller, D.; Mengistu, A.; Singh, A.K.; Hartman, G.L.; Wise, K.A. Advancing our understanding of charcoal rot in soybeans. J. Integr. Pest Manag. 2017, 8, 8. [Google Scholar] [CrossRef]
- Rajput, L.S.; Kumar, S.; Nataraj, V.; Shivakumar, M.; Pathak, K.; Jaiswal, S.; Pandey, V. Recent advancement in management of soybean charcoal rot caused by Macrophomina phaseolina. In Macrophomina Phaseolina; Academic Press: Cambridge, MA, USA, 2023; pp. 55–74. [Google Scholar]
- Marquez, N.; Giachero, M.L.; Declerck, S.; Ducasse, D.A. Macrophomina phaseolina: General characteristics of pathogenicity and methods of control. Front. Plant Sci. 2021, 12, 634397. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Yi, H.S.; Yang, J.W.; Ryu, C.M. ISR meets SAR outside: Additive action of the endophyte Bacillus pumilus INR7 and the chemical inducer, benzothiadiazole, on induced resistance against bacterial spot in field-grown pepper. Front. Plant Sci. 2013, 4, 122. [Google Scholar] [CrossRef]
- Myresiotis, C.K.; Karaoglanidis, G.S.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Evaluation of plant-growth-promoting rhizobacteria, acibenzolar-S-methyl and hymexazol for integrated control of Fusarium crown and root rot on tomato. Pest Manag. Sci. 2012, 68, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, H.; Naz, R.; Nosheen, A.; Hassan, M.N.; Ilyas, N.; Sajjad, M.; Geng, Z. Identification of new biocontrol agent against charcoal rot disease caused by Macrophomina phaseolina in soybean (Glycine max L.). Sustainability 2020, 12, 6856. [Google Scholar] [CrossRef]
- Borah, P.; Gogoi, N.; Asad, S.A.; Rabha, A.J.; Farooq, M. An insight into plant growth-promoting rhizobacteria-mediated mitigation of stresses in plant. J. Plant Growth Regul. 2023, 42, 3229–3256. [Google Scholar] [CrossRef]
- Boominadhan, U.; Rajakumar, R.; Sivakumaar PK, V.; Joe, M.M. Optimization of protease enzyme production using Bacillus sp. isolated from different wastes. Bot. Res. Int 2009, 2, 83–87. [Google Scholar]
- Korsten, L.; De Jager, E.S. Mode of action of Bacillus subtilis for control of avocado postharvest pathogens. S. Afr. Avocado Grow. Assoc. Yearb. 1995, 18, 124–130. [Google Scholar]
- Mihail, J.D.; Taylor, S.J. Interpreting variability among isolates of Macrophomina phaseolina in pathogenicity, pycnidium production, and chlorate utilization. Can. J. Bot. 1995, 73, 1596–1603. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Mengistu, A.; Ray, J.D.; Smith, J.R.; Paris, R.L. Charcoal rot disease assessment of soybean genotypes using a colony-forming unit index. Crop Sci. 2007, 47, 2453–2461. [Google Scholar] [CrossRef]
- Storer, D.A. A simple high sample volume ashing procedure for determination of soil organic matter. Commun. Soil Sci. Plant Anal. 1984, 15, 759–772. [Google Scholar] [CrossRef]
- Huynh, T.; Navi, S.S.; Yang, X.B. Efficacy of seed treatments with Bradyrhizobium japonicum to reduce occurrence of soybean sudden death syndrome in early-planted soybeans. In Fungal Diversity, Ecology and Control Management; Springer Nature: Singapore, 2022; pp. 415–437. [Google Scholar]
- Essalmani, H.; Lahlou, H. In vitro antagonistic activity of some microorganisms towards Fusarium oxysporum f. sp. lentis (french). Cryptogam.-Mycol. 2002, 23, 221–234. [Google Scholar]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- El-Rahman, A.; Shaheen, H.A.; El-Aziz, A.; Rabab, M.; Ibrahim, D.S. Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne Incognita. Egypt. J. Biol. Pest Control 2019, 29, 41. [Google Scholar] [CrossRef]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda MD, C.; Glick, B.R. Rhizosphere colonization determinants by plant growth-promoting rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, P.; Banerjee, G.; Handique, P.J. Use of an abscisic acid-producing Bradyrhizobium japonicum isolate as biocontrol agent against bacterial wilt disease caused by Ralstonia solanacearum. J. Plant Dis. Prot. 2022, 129, 869–879. [Google Scholar] [CrossRef]
- Arfaoui, A.; El Hadrami, A.; Mabrouk, Y.; Sifi, B.; Boudabous, A.; El Hadrami, I.; Chérif, M. Treatment of chickpea with Rhizobium isolates enhances the expression of phenylpropanoid defense-related genes in response to infection by Fusarium oxysporum f. sp. ciceris. Plant Physiol. Biochem. 2007, 45, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Figueredo, M.S.; Tonelli, M.L.; Ibáñez, F.; Morla, F.; Cerioni, G.; del Carmen Tordable, M.; Fabra, A. Induced systemic resistance and symbiotic performance of peanut plants challenged with fungal pathogens and co-inoculated with the biocontrol agent Bacillus sp. CHEP5 and Bradyrhizobium sp. SEMIA6144. Microbiol. Res. 2017, 197, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Valle, A.; López-Calleja, A.C.; Alvarez-Venegas, R. Enhancement of pathogen resistance in common bean plants by inoculation with Rhizobium etli. Front. Plant Sci. 2019, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.L.; Figueredo, M.S.; Rodríguez, J.; Fabra, A.; Ibañez, F. Induced systemic resistance-like responses elicited by rhizobia. Plant Soil 2020, 448, 1–14. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X. Recent advances in polyphenol oxidase-mediated plant stress responses. Phytochemistry 2021, 181, 112588. [Google Scholar] [CrossRef]
- Wojtaszek, P. Oxidative burst: An early plant response to pathogen infection. Biochem. J. 1997, 322, 681–692. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Galeotti, F.; Barile, E.; Curir, P.; Dolci, M.; Lanzotti, V. Flavonoids from carnation (Dianthus caryophyllus) and their antifungal activity. Phytochem. Lett. 2008, 1, 44–48. [Google Scholar] [CrossRef]
- Chen, Z.; Tan, J.; Yang, G.; Miao, M.; Chen, Y.; Li, T. Isoflavones from the roots and stems of Nicotiana Tabacum and their anti-tobacco mosaic virus activities. Phytochem. Lett. 2012, 5, 233–235. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial activity of quercetin: An approach to its mechanistic principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Patel, D.K. The Potential Therapeutic Properties of Prunetin against Human Health Complications: A Review of Medicinal Importance and Pharmacological Activities. Drug Metab. Bioanal. Lett. Former. Drug Metab. Lett. 2022, 15, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Chen, W.H.; Guo, J.J.; Fu, Z.H.; Yi, C.; Zhang, M.; Na, X.L. Soy isoflavone supplementation could reduce body weight and improve glucose metabolism in non-Asian postmenopausal women—A meta-analysis. Nutrition 2013, 29, 8–14. [Google Scholar] [CrossRef]
- Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Labuschagne, N.; Dubery, I.A. Metabolic profiling of PGPR-treated tomato plants reveal priming-related adaptations of secondary metabolites and aromatic amino acids. Metabolites 2020, 10, 210. [Google Scholar] [CrossRef]
- Zeiss, D.R.; Piater, L.A.; Dubery, I.A. Hydroxycinnamate amides: Intriguing conjugates of plant protective metabolites. Trends Plant Sci. 2021, 26, 184–195. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Blechert, S.; Brodschelm, W.; Hölder, S.; Kammerer, L.; Kutchan, T.M.; Mueller, M.J.; Zenk, M.H. The octadecanoic pathway: Signal molecules for the regulation of secondary pathways. Proc. Natl. Acad. Sci. USA 1995, 92, 4099–4105. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. Phenylpropanoid Derivatives and Their Role in Plants’ Health and as antimicrobials. Curr. Microbiol. 2023, 80, 380. [Google Scholar] [CrossRef]

| Sr. No. | Bacterial Strains | Catalase | HCN | Protease | Antifungal Activity (%) |
|---|---|---|---|---|---|
| 1. | Bacillus subtilis | ++ | ++ | ++ | 55 |
| 2. | Bradyrhizobium japonicum | +++ | ++ | ++ | 62 |
| 3. | Azospirillum brasilense | + | + | + | 34 |
| 4. | Pantoea agglomerans | ++ | ++ | ++ | 49 |
| Treatments | Shoot Length (cm) | Root Length (cm) | Number of Leaves | Root Nodules | Shoot Fresh Weight (g) | Shoot Dry Weight (g) | Root Fresh Weight (g) | Shoot Fresh Weight (g) |
|---|---|---|---|---|---|---|---|---|
| −ive | 27.54 ± 0.99 d | 13.94 ± 0.19 cd | 27.67 ± 1.37 d | 44.33 ± 1.03 d | 3.35 ± 0.02 c | 0.67 ± 0.005 bc | 2.42 ± 0.06 c–e | 0.48 ± 0.01 c–e |
| +ive | 18.24 ± 0.42 f | 8.69 ± 0.44 f | 21.33 ± 1.37 e | 32.67 ± 1.37 e | 2.20 ± 0.16 e | 0.44 ± 0.03 e | 1.48 ± 0.07 f | 0.30 ± 0.01 f |
| T1 | 24.95 ± 0.56 e | 13.13 ± 0.29 de | 27.33 ± 0.52 d | 42.33 ± 0.52 d | 3.17 ± 0.02 cd | 0.63 ± 0.005 cd | 2.35 ± 0.09 de | 0.47 ± 0.02 de |
| T2 | 27.06 ± 0.44 d | 13.49 ± 0.37 de | 27.67 ± 1.37 d | 44.00 ± 0.89 d | 3.21 ± 0.02 cd | 0.64 ± 0.005 cd | 2.57 ± 0.09 b–d | 0.51 ± 0.02 b–d |
| T3 | 24.31 ± 0.55 e | 12.79 ± 0.29 e | 26.33 ± 1.03 d | 41.67 ± 1.03 d | 3.03 ± 0.07 d | 0.61 ± 0.01 d | 2.22 ± 0.05 e | 0.44 ± 0.01 e |
| T4 | 28.37 ± 0.49 d | 14.55 ± 0.31 c | 28.67 ± 0.52 cd | 47.33 ± 0.52 c | 3.33 ± 0.04 c | 0.67 ± 0.01 bc | 2.68 ± 0.16 a–c | 0.54 ± 0.03 a–c |
| T5 | 32.34 ± 0.3 bc | 16.49 ± 0.33 a | 31.33 ± 1.37 bc | 50.33 ± 1.03 b | 3.85 ± 0.04 ab | 0.67 ± 0.01b bc | 2.68 ± 0.16 a–c | 0.54 ± 0.03 a–c |
| T6 | 32.92 ± 0.76 ab | 16.71 ± 0.41 a | 34.33 ± 1.37 b | 52.67 ± 1.03 b | 3.95 ± 0.04 a | 0.69 ± 0.006 ab | 2.78 ± 0.07 ab | 0.56 ± 0.01 ab |
| T7 | 30.74 ± 0.34 c | 15.54 ± 0.24 b | 28.33 ± 1.37 cd | 47.67 ± 0.52 c | 3.74 ± 0.04 b | 0.66 ± 0.01 bc | 2.63 ± 0.07 a–d | 0.53 ± 0.01 a–d |
| T8 | 34.25 ± 1.05 a | 17.13 ± 0.53 a | 37.67 ± 1.370 a | 56.33 ± 1.37 a | 3.98 ± 0.04 a | 0.71 ± 0.02 a | 2.90 ± 0.13 a | 0.58 ± 0.03 a |
| Treatments | Chlorophyll a (mg/g FW) | Chlorophyll b (mg/g FW) | Total Chl (mg/g FW) | Carotenoids (mg/g FW) |
|---|---|---|---|---|
| −ive | 0.193 ± 0.015 c | 0.13 ± 0.012 c | 0.32 ± 0.026 d | 0.11 ± 0.012 cd |
| +ive | 0.093 ± 0.015 d | 0.057 ± 0.007 d | 0.15 ± 0.021 e | 0.05 ± 0.006 e |
| T1 | 0.233 ± 0.02 bc | 0.12 ± 0.012 c | 0.35 ± 0.032 cd | 0.11 ± 0.009 cd |
| T2 | 0.277 ± 0.02 abc | 0.133 ± 0.009 bc | 0.41 ± 0.012 bcd | 0.13 ± 0.012 bcd |
| T3 | 0.213 ± 0.02 c | 0.11 ± 0.012 cd | 0.32 ± 0.0032 d | 0.10 ± 0.012 d |
| T4 | 0.303 ± 0.012 ab | 0.153 ± 0.015 bc | 0.46 ± 0.026 bc | 0.15 ± 0.012 abc |
| T5 | 0.307 ± 0.02 ab | 0.143 ± 0.009 bc | 0.45 ± 0.012 bc | 0.13 ± 0.009 bcd |
| T6 | 0.316 ± 0.023 ab | 0.187 ± 0.012 ab | 0.50 ± 0.035 ab | 0.16 ± 0.009 ab |
| T7 | 0.247 ± 0.02 bc | 0.133 ± 0.009 bc | 0.38 ± 0.029 bcd | 0.12 ± 0.009 ab |
| T8 | 0.353 ± 0.02 a | 0.227 ± 0.018 a | 0.58 ± 0.038 a | 0.18 ± 0.012 a |
| Treatments | TDS (mgL−1) | EC (dSm−1) | Organic Matter (%) | Soil pH | Field Capacity % |
|---|---|---|---|---|---|
| −ive | 79.13 | 0.74 | 0.51 | 7.6 | 30.5 |
| +ive | 78.65 | 0.72 | 0.52 | 7.5 | 26.8 |
| T1 | 73.51 | 0.41 | 1.23 | 6.7 | 27.7 |
| T2 | 69.82 | 0.31 | 1.34 | 6.3 | 28.6 |
| T3 | 68.04 | 0.36 | 1.11 | 6.1 | 28.5 |
| T4 | 66.46 | 0.27 | 1.13 | 6.3 | 30.6 |
| T5 | 71.53 | 0.37 | 1.17 | 6.8 | 28.3 |
| T6 | 68.27 | 0.29 | 1.34 | 6.3 | 30.9 |
| T7 | 68.83 | 0.32 | 1.34 | 6.3 | 28.5 |
| T8 | 65.65 | 0.26 | 1.39 | 6.2 | 31.5 |
| Retention Time | Compound Name | Retention Time | Compound Name |
|---|---|---|---|
| 9.122 | Carbamic acid | 18.102 | Tetra methoxy isoflavonone |
| 10.013 | Fructose | 18.891 | Glycitin |
| 10.205 | Ascorbate | 19.492 | Quercetin |
| 10.417 | Glucose-6-phosphate | 20.363 | Malic acid |
| 11.701 | Glutamic acid | 20.131 | Octadecanoic acid |
| 12.003 | Malate | 21.411 | Phenyl alanine |
| 12.412 | Pyruvate | 21.912 | Tryptophan |
| 12.843 | Ascorbic acid | 24.624 | Arginine |
| 14.731 | β-Cryptoxanthin | 27.479 | Phenylpropanoic acid |
| 14.895 | Acetic acid | 30.413 | Genistein |
| 17.907 | Prunetin | 32.411 | 7-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, U.; Aftab, Z.-e.-H.; Anjum, T.; Bokhari, N.A.; Akram, W.; Anwar, W. Harnessing the Biocontrol Potential of Bradyrhizobium japonicum FCBP-SB-406 to Manage Charcoal Rot of Soybean with Increased Yield Response for the Development of Sustainable Agriculture. Microorganisms 2024, 12, 304. https://doi.org/10.3390/microorganisms12020304
Khalid U, Aftab Z-e-H, Anjum T, Bokhari NA, Akram W, Anwar W. Harnessing the Biocontrol Potential of Bradyrhizobium japonicum FCBP-SB-406 to Manage Charcoal Rot of Soybean with Increased Yield Response for the Development of Sustainable Agriculture. Microorganisms. 2024; 12(2):304. https://doi.org/10.3390/microorganisms12020304
Chicago/Turabian StyleKhalid, Umar, Zill-e-Huma Aftab, Tehmina Anjum, Najat A. Bokhari, Waheed Akram, and Waheed Anwar. 2024. "Harnessing the Biocontrol Potential of Bradyrhizobium japonicum FCBP-SB-406 to Manage Charcoal Rot of Soybean with Increased Yield Response for the Development of Sustainable Agriculture" Microorganisms 12, no. 2: 304. https://doi.org/10.3390/microorganisms12020304
APA StyleKhalid, U., Aftab, Z.-e.-H., Anjum, T., Bokhari, N. A., Akram, W., & Anwar, W. (2024). Harnessing the Biocontrol Potential of Bradyrhizobium japonicum FCBP-SB-406 to Manage Charcoal Rot of Soybean with Increased Yield Response for the Development of Sustainable Agriculture. Microorganisms, 12(2), 304. https://doi.org/10.3390/microorganisms12020304







