Construction of Shale Gas Oil-Based Drilling Cuttings Degrading Bacterial Consortium and Their Degradation Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Diesel for Test
2.2. Physical and Chemical Characterization of Collected Samples
2.3. Culture Media
2.4. Isolation and Screening of Strains
2.5. Identification of a Strain
2.5.1. Morphological and Biochemical Characteristics
2.5.2. PCR Amplification of 16S rDNA
2.6. Construction of a Bacterial Consortium
2.7. Optimization of Degradation Conditions for the Strains
2.8. Validation of the Degradation Performance of the Bacterial Consortium
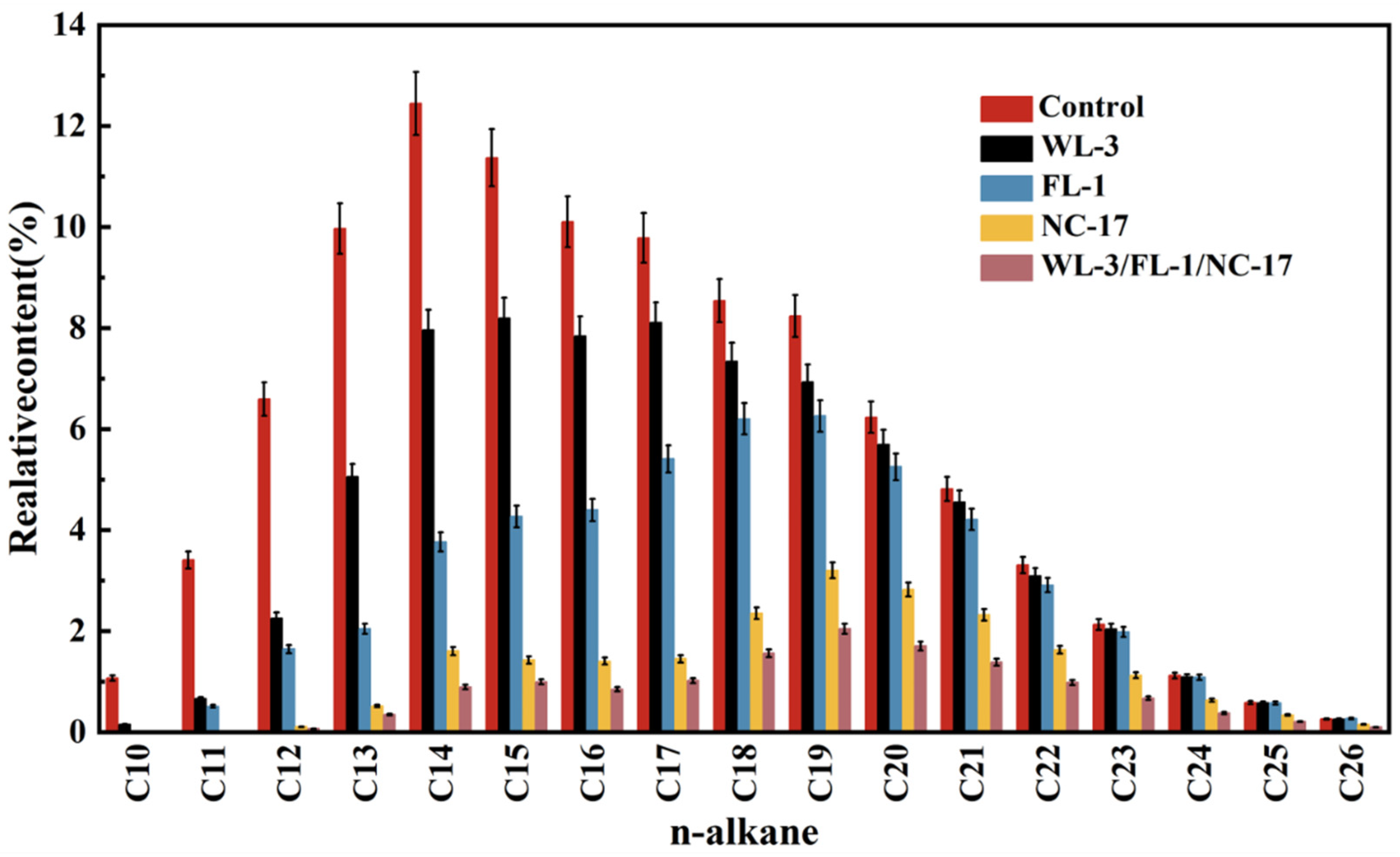
2.9. GC Evaluation of Oil Biodegradation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physical and Chemical Properties of Collected Samples
3.2. Isolation, Screening, and Identification of Strains
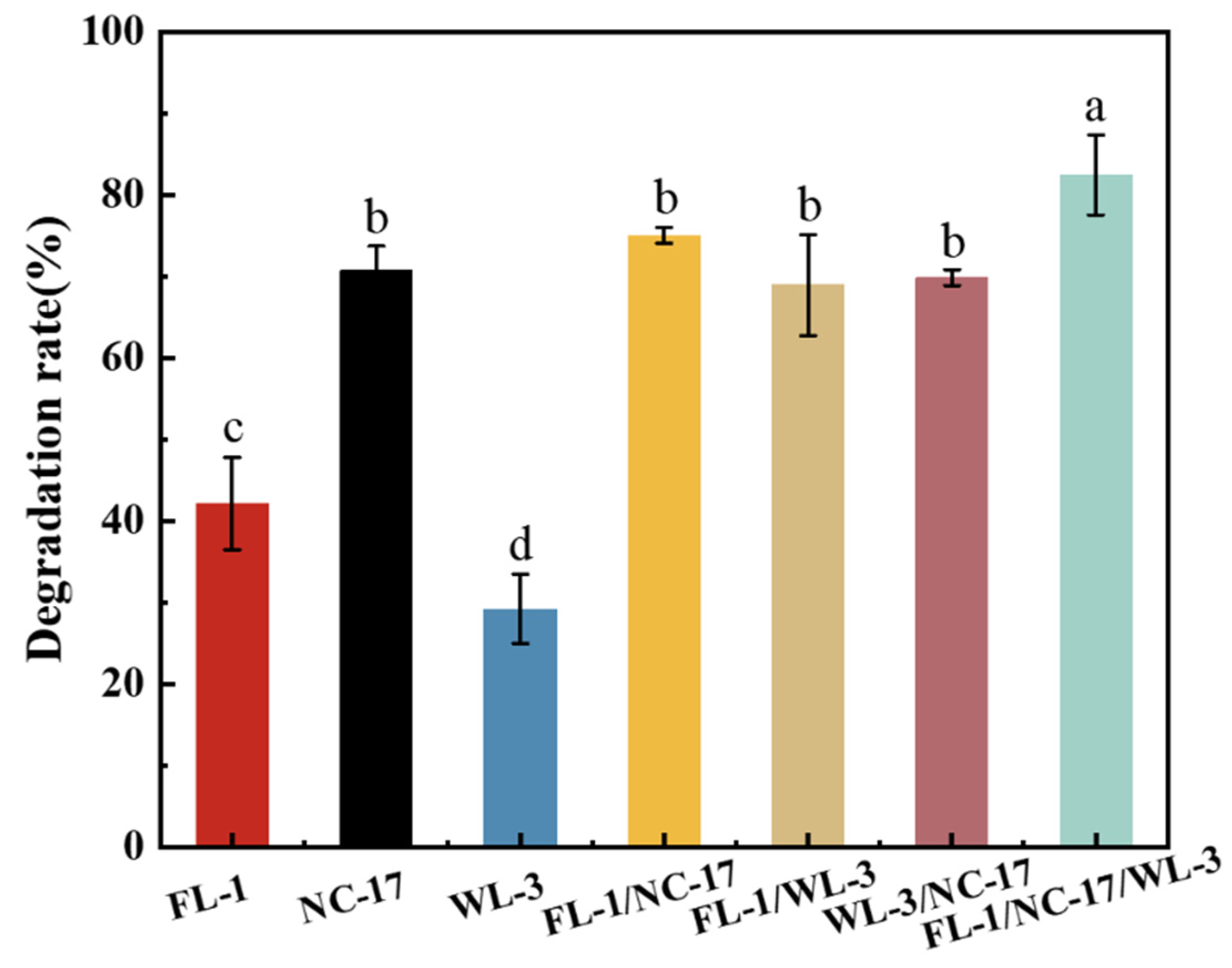
3.3. Construction of a Bacterial Consortium
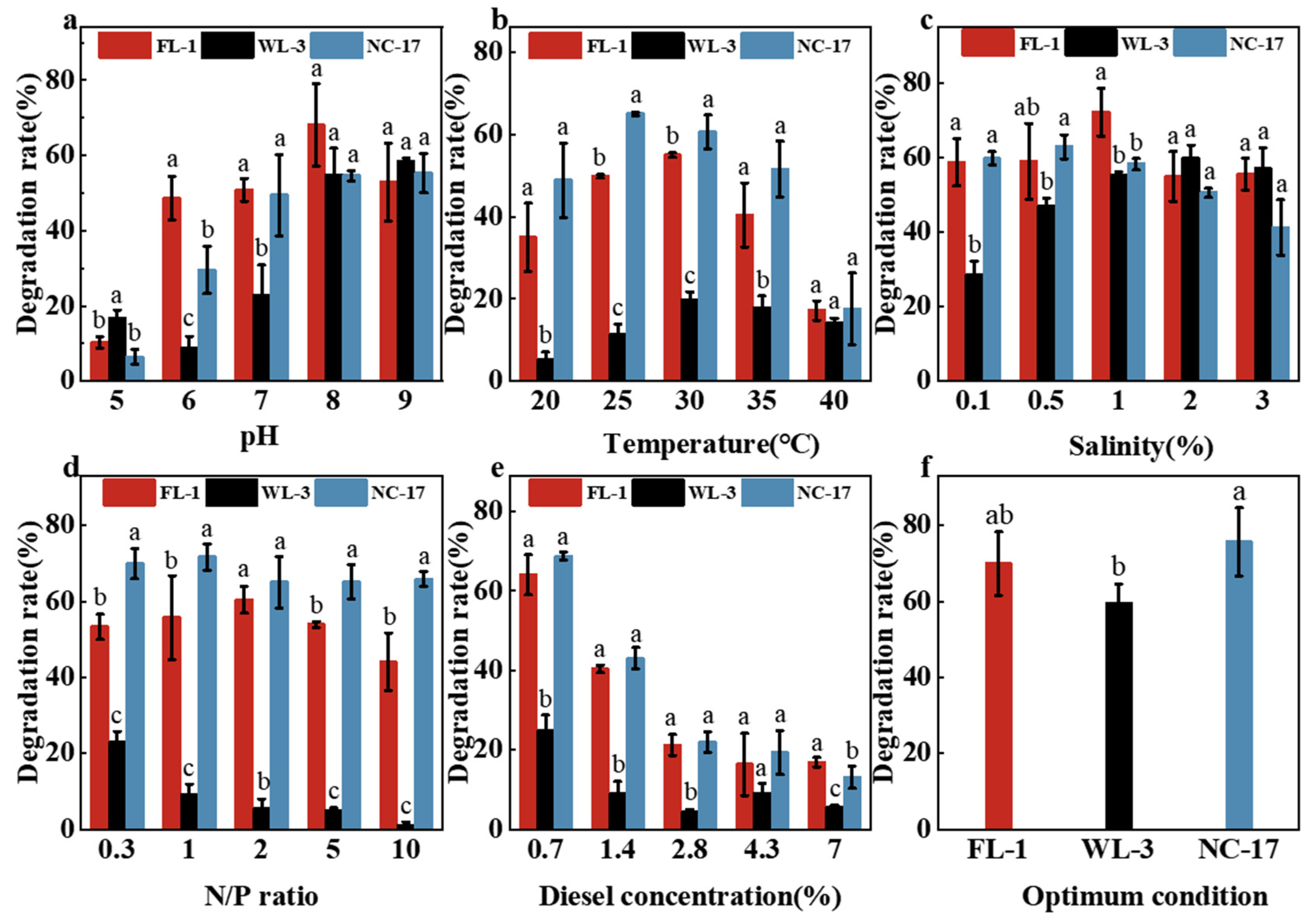
3.4. Optimization of Degradation Conditions
3.4.1. Effect of Initial pH
3.4.2. Effect of Temperature
3.4.3. Effect of Salinity
3.4.4. Effect of N/P Ratio
3.4.5. Effect of Diesel Concentration
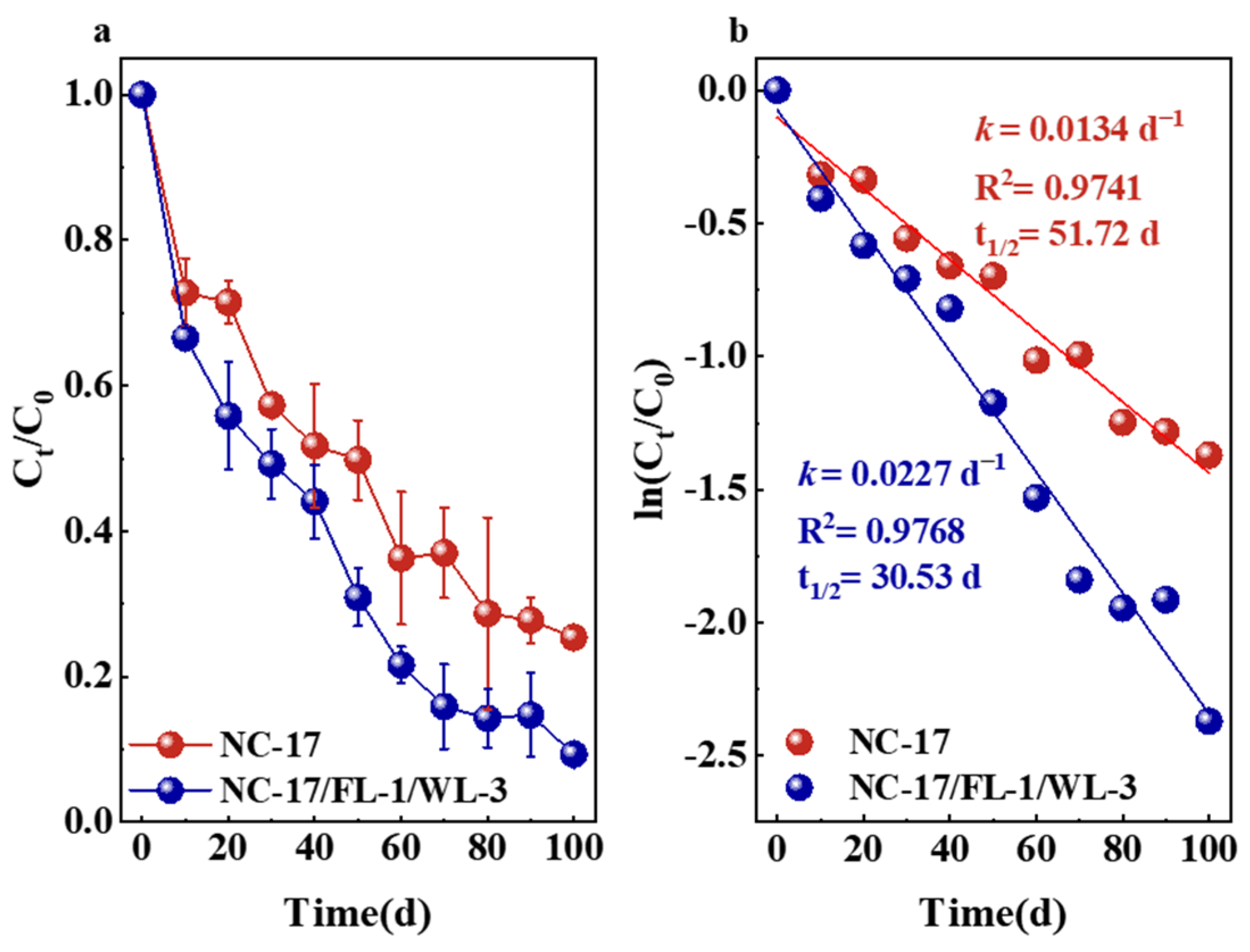
3.5. Validation of the Degradation Performance of the Bacterial Consortium
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- EPA. Shale Gas Production Drives World Natural Gas Production Growth. Available online: https://www.eia.gov/todayinenergy/detail.php?id=275122016-815 (accessed on 7 September 2023).
- Xie, B.; Qin, J.; Sun, H.; Wang, S.; Li, X. Release characteristics of polycyclic aromatic hydrocarbons (PAHs) leaching from oil-based drilling cuttings. Chemosphere 2022, 291, 132711. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Diao, H.; Zhang, Y.; Xia, S. Treatment and novel resource-utilization methods for shale gas oil based drill cuttings—A review. J. Environ. Manag. 2022, 317, 115462. [Google Scholar] [CrossRef] [PubMed]
- Ayati, B.; Molineux, C.; Newport, D.; Cheeseman, C. Manufacture and performance of lightweight aggregate from waste drill cuttings. J. Clean. Prod. 2019, 208, 252–260. [Google Scholar] [CrossRef]
- Liu, P.; Xiao, Q.; Dai, N.; Liu, Z.; Wang, C. Study on Pyrolysis of Shale Gas Oil-Based Drilling Cuttings: Kinetics, Process Parameters, and Product Yield. ACS Omega 2023, 8, 13593–13604. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Mu, S.; Zhang, J.; Li, Q. Regional distribution, properties, treatment technologies, and resource utilization of oil-based drilling cuttings: A review. Chemosphere 2022, 308, 136145. [Google Scholar] [CrossRef] [PubMed]
- Kazamias, G.; Zorpas, A.A. Drill cuttings waste management from oil & gas exploitation industries through end-of-waste criteria in the framework of circular economy strategy. J. Clean. Prod. 2021, 322, 129098. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, L.; Zhang, C.; Shao, T.; Chen, W. Experimental study on the treatment of oil-based drill cutting by pulsed dielectric barrier discharge plasma at atmospheric pressure. J. Clean. Prod. 2022, 339, 130757. [Google Scholar] [CrossRef]
- Khanpour, R.; Sheikhi-Kouhsar, M.R.; Esmaeilzadeh, F.; Mowla, D. Removal of contaminants from polluted drilling mud using supercritical carbon dioxide extraction. J. Supercrit. Fluids 2014, 88, 1–7. [Google Scholar] [CrossRef]
- Yan, P.; Lu, M.; Guan, Y.; Zhang, W.; Zhang, Z. Remediation of oil-based drill cuttings through a biosurfactant-based washing followed by a biodegradation treatment. Bioresour. Technol. 2011, 102, 10252–10259. [Google Scholar] [CrossRef]
- Ball, A.; Stewart, R.; Schliephake, K. A review of the current options for the treatment and safe disposal of drill cuttings. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2011, 30, 457–473. [Google Scholar] [CrossRef]
- Hamidi, Y.; Ataei, S.A.; Sarrafi, A. A highly efficient method with low energy and water consumption in biodegradation of total petroleum hydrocarbons of oily sludge. J. Environ. Manag. 2021, 293, 112911. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, X.; Peng, S.; Wang, Z.; Zhou, R.; Tian, H. Isolation, screening, and crude oil degradation characteristics of hydrocarbons-degrading bacteria for treatment of oily wastewater. Water Sci. Technol. 2019, 78, 2626–2638. [Google Scholar] [CrossRef] [PubMed]
- Ravi, A.; Ravuri, M.; Krishnan, R.; Narenkumar, J.; Anu, K.; Alsalhi, M.S.; Devanesan, S.; Kamala-Kannan, S.; Rajasekar, A. Characterization of petroleum degrading bacteria and its optimization conditions on effective utilization of petroleum hydrocarbons. Microbiol. Res. 2022, 265, 127184. [Google Scholar] [CrossRef] [PubMed]
- Viesser, J.A.; Sugai-Guerios, M.H.; Malucelli, L.C.; Pincerati, M.R.; Karp, S.G.; Maranho, L.T. Petroleum-Tolerant Rhizospheric Bacteria: Isolation, Characterization and Bioremediation Potential. Sci. Rep. 2020, 10, 2060. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, E.; Kuhn, L.; Marchal, R.; Jouanneau, Y. Proteomic investigation of enzymes involved in 2-ethylhexyl nitrate biodegradation in Mycobacterium austroafricanum IFP 2173. Res. Microbiol. 2009, 160, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ju, M.; Liu, J.; Wu, W.; Li, X. Isolation, identification, and crude oil degradation characteristics of a high-temperature, hydrocarbon-degrading strain. Mar. Pollut. Bull. 2016, 106, 301–307. [Google Scholar] [CrossRef]
- Xia, W.; Li, J.; Xia, Y.; Song, Z.; Zhou, J. Optimization of diesel oil biodegradation in seawater using statistical experimental methodology. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2012, 66, 1301–1309. [Google Scholar] [CrossRef]
- Nayak, A.S.; Vijaykumar, M.H.; Karegoudar, T.B. Characterization of biosurfactant produced by Pseudoxanthomonas sp. PNK-04 and its application in bioremediation. Int. Biodeterior. Biodegrad. 2009, 63, 73–79. [Google Scholar] [CrossRef]
- Zhang, K.; Hua, X.-F.; Han, H.-L.; Wang, J.; Miao, C.-C.; Xu, Y.-Y.; Huang, Z.-D.; Zhang, H.; Yang, J.-M.; Jin, W.-B.; et al. Enhanced bioaugmentation of petroleum- and salt-contaminated soil using wheat straw. Chemosphere 2008, 73, 1387–1392. [Google Scholar] [CrossRef]
- Khan, M.A.I.; Biswas, B.; Smith, E.; Naidu, R.; Megharaj, M. Toxicity assessment of fresh and weathered petroleum hydrocarbons in contaminated soil—A review. Chemosphere 2018, 212, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Ghorbannezhad, H.; Moghimi, H.; Dastgheib, S.M.M. Biodegradation of high molecular weight hydrocarbons under saline condition by halotolerant Bacillus subtilis and its mixed cultures with Pseudomonas species. Sci. Rep. 2022, 12, 13227. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Huang, L.; He, Y.; Zhao, T.; Han, B.; Jia, X. Combination of a crude oil-degrading bacterial consortium under the guidance of strain tolerance and a pilot-scale degradation test. Chin. J. Chem. Eng. 2017, 25, 1838–1846. [Google Scholar] [CrossRef]
- Rasti, A.; Ameri, A.; Riahi, M.A. Aerobic degradation of oil-based mud drilling fluid by in situ bacteria in the Hawizeh Marshes. J. Pet. Explor. Prod. Technol. 2021, 11, 3775–3783. [Google Scholar] [CrossRef]
- Zhang, B.; Shan, B.; Zhao, Y.; Zhang, L. Review of Formation and Gas Characteristics in Shale Gas Reservoirs. Energies 2020, 13, 5427. [Google Scholar] [CrossRef]
- Kogbara, R.B.; Ayotamuno, J.M.; Onuomah, I.; Ehio, V.; Damka, T.D. Stabilisation/solidification and bioaugmentation treatment of petroleum drill cuttings. Appl. Geochem. 2016, 71, 1–8. [Google Scholar] [CrossRef]
- Holt, S.G.; Kriey, N.R.; Sneath, P.H.A. Bergy’s Manual of Determinative for Bacteriology; Williams and Wilkins: New York, NY, USA, 1998. [Google Scholar]
- Ding, Z.; Wu, J.; You, A.; Huang, B.; Cao, C. Effects of heavy metals on soil microbial community structure and diversity in the rice (Oryza sativa L. subsp. Japonica, Food Crops Institute of Jiangsu Academy of Agricultural Sciences) rhizosphere. Soil Sci. Plant Nutr. 2017, 63, 75–83. [Google Scholar] [CrossRef]
- Li, C.; Quan, Q.; Gan, Y.; Dong, J.; Fang, J.; Wang, L.; Liu, J. Effects of heavy metals on microbial communities in sediments and establishment of bioindicators based on microbial taxa and function for environmental monitoring and management. Sci. Total Environ. 2020, 749, 141555. [Google Scholar] [CrossRef]
- Al-Marri, S.; Eldos, H.I.; Ashfaq, M.Y.; Saeed, S.; Skariah, S.; Varghese, L.; Mohamoud, Y.A.; Sultan, A.A.; Raja, M.M. Isolation, identification, and screening of biosurfactant-producing and hydrocarbon-degrading bacteria from oil and gas industrial waste. Biotechnol. Rep. 2023, 39, e00804. [Google Scholar] [CrossRef]
- Kavyanifard, A.; Ebrahimipour, G.; Ghasempour, A. Optimization of crude oil degradation by Dietzia cinnamea KA1, capable of biosurfactant production. J. Basic Microbiol. 2015, 56, 566–575. [Google Scholar] [CrossRef]
- Makkar, R.S.; Cameotra, S.S. Effects of various nutritional supplements on biosurfactant production by a strain of Bacillus subtilis at 45 °C. J. Surfactants Deterg. 2002, 5, 11–17. [Google Scholar] [CrossRef]
- Kiran, G.S.; Nishanth, L.A.; Priyadharshini, S.; Anitha, K.; Selvin, J. Effect of Fe nanoparticle on growth and glycolipid biosurfactant production under solid state culture by marine Nocardiopsissp. MSA13A. BMC Biotechnol. 2014, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, Z.-X.; Jia, X.-Q.; Chen, Y.; Liu, J.; Wen, J.-P. Biodegradation of Crude Oil by a Newly Isolated Strain Rhodococcus sp. JZX-01. Appl. Biochem. Biotechnol. 2013, 171, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Nkem, B.M.; Halimoon, N.; Yusoff, F.M.; Johari, W.L.W.; Zakaria, M.P.; Medipally, S.R.; Kannan, N. Isolation, identification and diesel-oil biodegradation capacities of indigenous hydrocarbon-degrading strains of Cellulosimicrobium cellulans and Acinetobacter baumannii from tarball at Terengganu beach, Malaysia. Mar. Pollut. Bull. 2016, 107, 261–268. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef] [PubMed]
- Roslee, A.F.A.; Zakaria, N.N.; Convey, P.; Zulkharnain, A.; Lee, G.L.Y.; Gomez-Fuentes, C.; Ahmad, S.A. Statistical optimisation of growth conditions and diesel degradation by the Antarctic bacterium, Rhodococcus sp. strain AQ5–07. Extremophiles 2020, 24, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-L.; JiangYang, J.-H.; Nie, Y.; Wu, X.-L. Regulation of the Alkane Hydroxylase CYP153 Gene in a Gram-Positive Alkane-Degrading Bacterium, Dietzia sp. Strain DQ12-45-1b. Appl. Environ. Microbiol. 2016, 82, 608–619. [Google Scholar] [CrossRef]
- Nie, Y.; Liang, J.-L.; Fang, H.; Tang, Y.-Q.; Wu, X.-L. Characterization of a CYP153 alkane hydroxylase gene in a Gram-positive Dietzia sp. DQ12-45-1b and its “team role” with alkW1 in alkane degradation. Appl. Microbiol. Biotechnol. 2014, 98, 163–173. [Google Scholar] [CrossRef]
- Binazadeh, M.; Karimi, I.A.; Li, Z. Fast biodegradation of long chain n-alkanes and crude oil at high concentrations with Rhodococcus sp. Moj-3449. Enzym. Microb. Technol. 2009, 45, 195–202. [Google Scholar] [CrossRef]
- von der Weid, I.; Marques, J.M.; Cunha, C.D.; Lippi, R.K.; dos Santos, S.C.C.; Rosado, A.S.; Lins, U.; Seldin, L. Identification and biodegradation potential of a novel strain of Dietzia cinnamea isolated from a petroleum-contaminated tropical soil. Syst. Appl. Microbiol. 2007, 30, 331–339. [Google Scholar] [CrossRef]
- Chen, W.; Kong, Y.; Li, J.; Sun, Y.; Min, J.; Hu, X. Enhanced biodegradation of crude oil by constructed bacterial consortium comprising salt-tolerant petroleum degraders and biosurfactant producers. Int. Biodeterior. Biodegrad. 2020, 154, 105047. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Adam, M. Biodegradation of marine crude oil pollution using a salt-tolerant bacterial consortium isolated from Bohai Bay, China. Mar. Pollut. Bull. 2016, 105, 43–50. [Google Scholar] [CrossRef]
- Phulpoto, I.A.; Hu, B.; Wang, Y.; Ndayisenga, F.; Li, J.; Yu, Z. Effect of natural microbiome and culturable biosurfactants-producing bacterial consortia of freshwater lake on petroleum-hydrocarbon degradation. Sci. Total Environ. 2021, 751, 141720. [Google Scholar] [CrossRef]
- Singh, K.; Chandra, S. Treatment of Petroleum Hydrocarbon Polluted Environment Through Bioremediation: A Review. Pak. J. Biol. Sci. PJBS 2014, 17, 1–8. [Google Scholar] [CrossRef]
- Van Hong Thi, P.; Chaudhary, D.K.; Jeong, S.-W.; Kim, J. Oil-degrading properties of a psychrotolerant bacterial strain, Rhodococcus sp. Y2-2, in liquid and soil media. World J. Microbiol. Biotechnol. 2018, 34, 33. [Google Scholar] [CrossRef]
- Chen, X.; Shan, G.; Shen, J.; Zhang, F.; Liu, Y.; Cui, C. In situ bioremediation of petroleum hydrocarbon–contaminated soil: Isolation and application of a Rhodococcus strain. Int. Microbiol. 2023, 26, 411–421. [Google Scholar] [CrossRef]
- Koshlaf, E.; Ball, A. Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS Microbiol. 2017, 3, 25–49. [Google Scholar] [CrossRef]
- Adams, G.; Tawari-Fufeyin, P.; Okoro, S.; Ehinomen, I. Bioremediation, Biostimulation and Bioaugmention: A Review. Int. J. Environ. Bioremediat. Biodegrad. 2015, 3, 28–39. [Google Scholar] [CrossRef]
- Dindar, E.; Topaç, F.O.; BaŞKaya, H. Biodegradation of used engine oil in a wastewater sludge-amended agricultural soil. Turk. J. Agric. For. 2016, 40, 631–641. [Google Scholar] [CrossRef]
- Ferreira, T.; Coelho, M.A.; Rocha-Leão, M.H. Factors influencing crude oil biodegradation by Yarrowia lipolytica. Braz. Arch. Biol. Technol. 2012, 55, 785–791. [Google Scholar] [CrossRef]
- Gatti, M.; García-Usach, F.; Seco, A.; Ferrer, J. Wastewater COD Characterization: Analysis of Respirometric and Physical-Chemical Methods for Determining Biodegradable Organic Matter Fractions. J. Chem. Technol. Biotechnol. 2010, 85, 536–544. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Y.; Zhang, Z.; Wang, H. Salinity effect on the metabolic pathway and microbial function in phenanthrene degradation by a halophilic consortium. AMB Express 2018, 8, 67. [Google Scholar] [CrossRef]
- Borzenkov, I.A.; Milekhina, E.; Gotoeva, M.; Rozanova, E.; Beliaev, S. The properties of hydrocarbon-oxidizing bacteria isolated from the oilfields of Tatarstan, Western Siberia, and Vietnam. Mikrobiologiia 2006, 75, 82–89. [Google Scholar] [CrossRef]
- Ouriache, H.; Moumed, I.; Arrar, J.; Abdelkader, N.; Lounici, H. Influence of C/N/P ratio evolution on biodegradation of petroleum hydrocarbons-contaminated soil. Alger. J. Environ. Sci. Technol. 2020, 6, 1604–1611. [Google Scholar]
- Al Disi, Z.; Jaoua, S.; Al-Thani, D.; Almeer, S.; Zouari, N. Isolation, Screening and Activity of Hydrocarbon-Degrading Bacteria from Harsh Soils. In Proceedings of the World Congress on Civil, Structural, and Environmental Engineering, Prague, Czech Republic, 30–31 March 2016. [Google Scholar]
- Rossetti, I.; Conte, F.; Ramis, G. Kinetic Modelling of Biodegradability Data of Commercial Polymers Obtained under Aerobic Composting Conditions. Eng 2021, 2, 54–68. [Google Scholar] [CrossRef]

| Sample | Oil Content | Water Content | Solid Content | pH |
|---|---|---|---|---|
| FL | 11.16 | 9.80 | 75.95 | 9.75 |
| WL | 6.60 | 5.03 | 85.57 | 9.23 |
| NC | 10.50 | 14.05 | 73.60 | 9.24 |
| DZ | 8.43 | 9.57 | 78.33 | 9.62 |
| Parameters | FL | WL | NC | DZ |
|---|---|---|---|---|
| Benzene | 0.07 | - | 0.002 | - |
| Toluene | 0.73 | - | 0.09 | 0.03 |
| Ethyl Benzene | 1.05 | - | 0.59 | 0.06 |
| Xylenes | 6.33 | - | 4.71 | 0.49 |
| PAHs | 1.25 | - | - | 0.24 |
| TPHs | 2.67 × 104 | 7.93 × 104 | 5.47 × 104 | 2.99 × 104 |
| Metals | FL | WL | NC | DZ |
|---|---|---|---|---|
| Barium (Ba) | 2.21 × 105 | 1.91 × 105 | 1.91 × 105 | 1.73 × 105 |
| Strontium (Sr) | 2.29 × 103 | 1.86 × 103 | 1.97 × 103 | 2.10 × 103 |
| Cadmium (Cd) | 119.47 | 107.00 | 93.85 | 5.21 |
| Arsenic (As) | 12.30 | 16.8 | 7.43 | 11.95 |
| Plumbum (Pb) | 51.55 | 12.5 | 52.55 | 29.35 |
| Chromium (Cr) | 144.52 | 117.33 | 93.49 | 79.4 |
| Cuprum (Cu) | 71.62 | 67.16 | 76.55 | 83.8 |
| Zinc (Zn) | 267.75 | 140.30 | 232.00 | 400.50 |
| Nickel (Ni) | 45.90 | 37.13 | 49.00 | 49.95 |
| Vanadium (V) | 575.20 | 517.70 | 607.50 | 652.00 |
| Manganese (Mn) | 646.50 | 528.33 | 506.20 | 248.00 |
| Cobalt (Co) | 11.80 | 11.70 | 7.87 | 6.71 |
| Titanium (Ti) | 11.02 | 14.76 | 12.05 | 10.99 |
| Characteristics | FL-1 | WL-3 | NC-17 |
|---|---|---|---|
| Colony form | Inerratic | Inerratic | Irregular |
| Cell shape | Bacilliform | Rounded | Bacilliform |
| Colony color | White | Yellow | White |
| Smoothness | Smooth | Smooth | Rough |
| Gram stain | + | + | + |
| Gelatin liquefaction | - | - | - |
| Hydrolysis of starch | - | - | - |
| Nitrate to nitrite | - | + | + |
| Indol test | - | - | - |
| Citrate test | + | - | + |
| Glucose fermentation | + | + | + |
| Strain | pH | Temperature (°C) | Salinity (%) | N/P Ratio | Diesel Concentration (%) |
|---|---|---|---|---|---|
| FL-1 | 8 | 30 | 1 | 2 | 0.7 |
| WL-3 | 9 | 25 | 2 | 0.3 | 0.7 |
| NC-17 | 9 | 30 | 0.5 | 1 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Gong, X.; Lv, Q.; Bin, D.; Wang, L. Construction of Shale Gas Oil-Based Drilling Cuttings Degrading Bacterial Consortium and Their Degradation Characteristics. Microorganisms 2024, 12, 318. https://doi.org/10.3390/microorganisms12020318
Fan L, Gong X, Lv Q, Bin D, Wang L. Construction of Shale Gas Oil-Based Drilling Cuttings Degrading Bacterial Consortium and Their Degradation Characteristics. Microorganisms. 2024; 12(2):318. https://doi.org/10.3390/microorganisms12020318
Chicago/Turabian StyleFan, Li, Xianhe Gong, Quanwei Lv, Denghui Bin, and Li’Ao Wang. 2024. "Construction of Shale Gas Oil-Based Drilling Cuttings Degrading Bacterial Consortium and Their Degradation Characteristics" Microorganisms 12, no. 2: 318. https://doi.org/10.3390/microorganisms12020318






