Abstract
Research on renewable biotechnology for renewable biofuel applications has reached new heights. This is highlighted by extensive biomining for novel enzymes to reduce the production costs from animal and insect gut microbiomes. This study explored the diversity and composition of hemicellulolytic fungi in the gut microbiota from dung beetles of the family Scarabaeidae (Pachylomerus femoralis, Anachalcos convexus and Euoniticellus intermedius). Two hundred and twenty-two filamentous fungi were isolated, purified and identified using rDNA sequencing of the ITS and D1/D2 regions. The fungal isolates were assigned to 12 genera and 25 species. Fungi associated with the genus Aspergillus was in abundance, with Hypocrea lixii predominantly isolated. Isolates that produced more than 3 U/mL of xylanase activity were evaluated further. The highest xylanase activity was of 23.6 and 23.5 U/mL for L1XYL9 (E. intermedius larvae) and Hypocrea lixii AB2A3 (A. convexus), respectively. Phylogeny of the fungal strains with xylanolytic activity was analysed using ITS rDNA sequences and revealed close genetic relatedness between isolates from the different dung beetle species. Fungal genera commonly found in the gut of both adult beetles and larvae included Aspergillus, Hypocrea, Talaromyces and Penicillium. The results obtained in this study suggest that the gut of Scarabaeidae dung beetles in South Africa is a rich source of xylanolytic fungi.
1. Introduction
Enzymes are preferred as the catalysts for many industrial applications, as chemical catalysts typically require extreme temperatures and pressure and these often have a negative impact on the environment [1]. Enzymes have recently gained attention for the breakdown of plant biomass into fermentable sugars for the production of products such as biofuels, animal feeds and chemicals [2]. Enzymes such as xylanases have received attention for their application in various industries such as animal feed, biofuel, baking, pulp and paper, liquefaction of fruits and vegetables, clarification of beer and juices, as well as bioremediation [3,4]. In the biofuel industry, xylanases are used for the efficient hydrolysis of hemicelluloses. The addition of xylanase during enzymatic hydrolysis of lignocellulose polymers increased the rate and the efficiency of the process [3,5], since xylanases help expose the cellulose to cellulase attack [6]. Enzymatic hydrolysis is a sustainable, effective and environmentally friendly method. This process entails breaking down glycosidic bonds within the polymers present in lignocellulosic materials to produce fermentable sugars. To achieve successful and efficient enzymatic hydrolysis of lignocellulosic plant biomass, xylanase enzymes play a pivotal role. Xylanases have been previously isolated from a diverse array of naturally occurring life forms including plants, animals, insects and microorganisms [7].
The symbiotic relationship between microorganisms and insects has been a subject of extensive study, aiming to gain insights into their evolutionary diversification. Fungal mutualism with insects holds significant importance in the developmental stages of insects and their overall fitness. This is achieved through the provision of nitrogen compounds, the degradation of high molecular weight molecules, and the production of pheromones for mating and communication [8]. Recently, these microorganisms have received attention for their lignocellulolytic activity, presenting promising applications in biofuel production [4,8]. Despite the widespread study of microorganisms associated with insects, their isolation from the gut of dung beetles remains limited. The gut microbiota of adult dung beetles and their larvae represent a unique source of xylanases. Dung beetles are a major group of insects (Order Coleoptera; Family Scarabaeidae) with approximately 370,000 species identified worldwide, of which 8000 of those species have been reported to be found in the Kruger National Park in South Africa [8,9]. Dung beetles are known to feed on wet and dry dung of herbivores which consists of 80% indigestible material such as cellulose, hemicellulose, lignin, tannin, chitin and other waste materials. This material is degraded by lignocellulolytic enzymes (xylanase and cellulase) produced by microorganisms in the digestive tract of the dung beetles [4]. These microorganisms further assist the digestion system of dung beetles by performing essential functions such as nutrient production and compound detoxification [10].
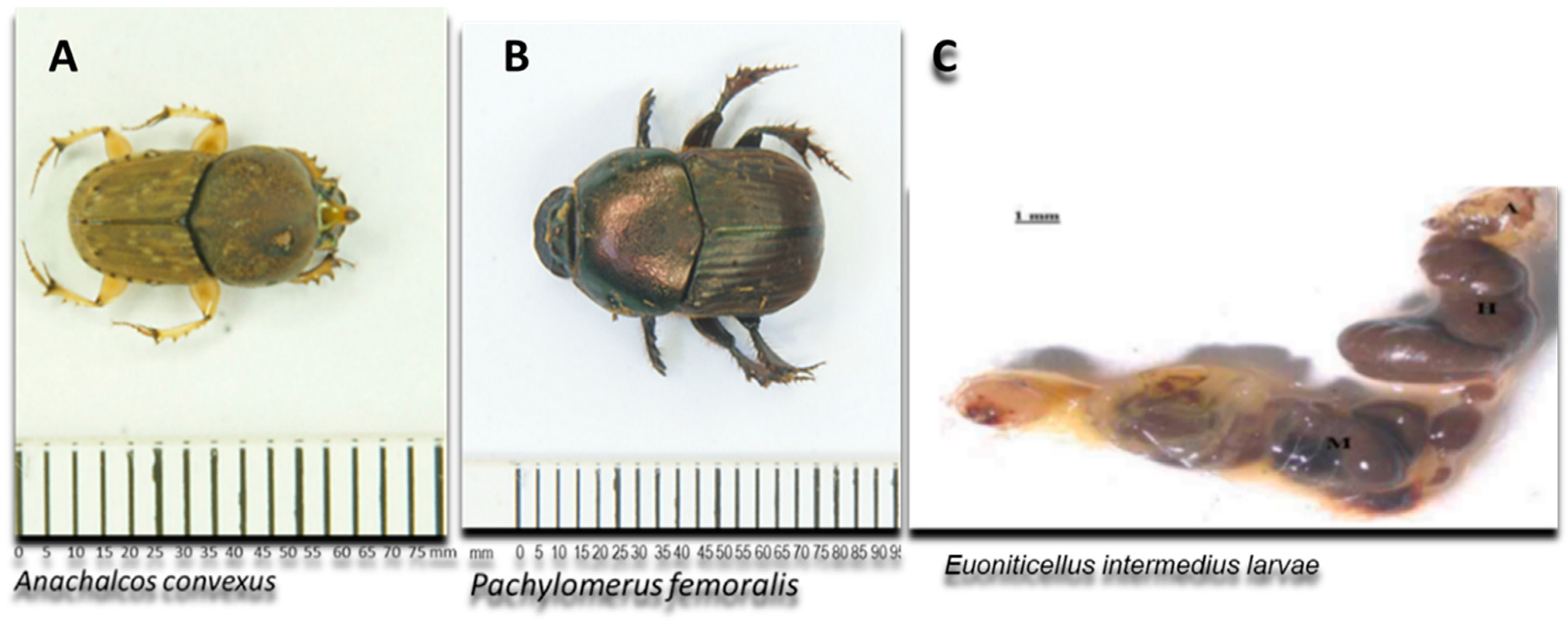
Currently, most research on gut microbiomes is mainly focused on termites, honeybees and mosquitoes [4,11,12]. The available information on the diversity and composition of the gut microbiota in dung beetles, particularly focusing on hemicellulolytic microbes, is currently limited. This knowledge gap extends to the fungal microbiota associated with these beetles [4,11]. Fungi are well known for their remarkable ability to produce extracellular enzymes important in biotechnological applications. Fungal species such as Aspergillus and Trichoderma are well-known for their ability to produce xylanase enzymes that are used commercially. Other fungal species include Talaromyces [5] and Rasamsonia emersonii [13]. In this study, the guts of two adult dung beetles of the Family Scarabaeidae (Pachylomerus femoralis and Anachalcos convexus) and gut of one dung beetle larva (Euoniticellus intermedius) (Figure 1) from the same family were screened for fungi with xylanolytic activity.
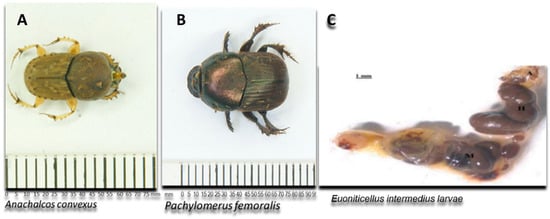
Figure 1.
Schematic representation of the adult dung beetles Pachylomerus femoralis and Anachalcos convexus and the gut of dung beetle larva Euoniticellus intermedius [14] used in this study.
2. Materials and Methods
2.1. Collection and Dissection of Dung Beetles
Dung beetle larva (Euoniticellus intermedius) and dung beetles (Pachylomerus femoralis and Anachalcos convexus) were donated by Prof Byrne, School of Animal, Plant and Environmental Sciences at the University of the Witwatersrand, South Africa. The dung beetles were maintained and anaesthetised following the method described in [15]. The beetles were surface sterilised by washing them in 70% ethanol for 10 min and subsequently washed with sterile distilled water before dissection. The dung beetles or dung beetle larvae were individually placed on a flame-sterilised glass slide and sterile forceps were used to remove the elytra, which allowed easy access to the gut content [12]. The entire gut of the dung beetles or dung beetle larva was homogenised by suspending the content in 10 mL of a 0.7% saline solution.
2.2. Isolation of Filamentous Fungi from the Gut of Dung Beetles/Larvae
One millilitre of the homogenised dung beetle or larva gut was plated on 0.67% YNB (Yeast Nitrogen Base, Difco) with amino acids containing 2% xylan as a carbon source and 0.2% chloramphenicol to inhibit bacterial growth [16]. Plates were incubated at 30 °C for up to 5 d. Different morphotypes were purified several times by placing 10 × 5 mm agar block repeatedly on YM plates (10 g/L glucose, 0.2 g/L chloramphenicol, 3 g/L malt extract, 3 g/L yeasts extract, 5 g/L peptone and 15 g/L bacteriological agar) [17]. The process was repeated until pure colonies were obtained. The purified fungal isolates were inoculated on YM plates and incubated at 30 °C for 5 d. Following incubation, 1 mL of sterile distilled water was added and spores were dislodged with a glass rod. The spore suspension was added to 1 mL 30% glycerol solution and stored at −80 °C until use.
2.3. ITS and D1/D2 Sequencing
All fungal isolates were sent to Inqaba Biotechnical Industries (Pty) Ltd., South Africa for sequencing of the ITS and D1/D2 rDNA regions. DNA was extracted using the ZR Fungal DNA MiniPrepTM Kit (Zymo Research) according to the manufacturer’s instructions. The ITS1-5.8S-ITS2 region was amplified using PCR primers ITS-1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS-4 (5′-TCC TCC GCT TAT TGA TAT GC-3′). Amplification was carried out in 25 µL reactions using the EconoTaq Plus Green Master Mix (Lucingen). The following PCR conditions were used: 35 cycles including an initial denaturation at 95 °C for 2 min. Subsequent denaturation at 95 °C for 30 s, annealing at 50 °C for 30 s and extension at 72 °C for 1 min. A final extension at 72 °C for 10 min was followed by holding at 4 °C. Additionally, the D1/D2 domain of the 26S rDNA region was amplified using primers NL1 (5‘-GCA TAT CAA TAA GCG GAG GAA AAG-3′) and NL4 (5′-GGT CCG TGT TTC AAG ACG G-3′) as described above. DNA sequencing was performed using ABI V3.1 BigDye according to the manufacturer’s instructions on an ABI 3500 XL Instrument [17].
2.4. Identification of Fungal Isolates and Phylogenetic Analysis
The ITS and D1/D2 sequencing data obtained were cleaned, trimmed and aligned using Bio-edit software version 7.0, http://www.mbio.ncsu.edu/bioedit/page2.html, accessed on 10 January 2020). Sequence alignment was performed using the Muscle software package as implemented in the MEGA 7 program [18]. Furthermore, the ITS and D1/D2 sequences were compared to sequences in the Genbank database using the basic local alignment search tool (BLAST) of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/, accessed on 10 March 2020). A phylogenetic tree was constructed based on the ITS domains using MEGA version 7.0 [18]. The evolutionary history was inferred using the neighbor-joining method [19]. Bootstrap analysis [20] was performed from 1000 replications to determine the confidence levels of clades, and only values >50% were recorded on the phylogenetic tree [19].
2.5. Screening Filamentous Fungal Isolates for Xylanase Activity
A 15 mm × 2 mm block of agar was cut from a 5-d-old fungal culture plate and inoculated into test tubes containing 5 mL 0.67% YNB with amino acids and 1% beechwood xylan as the carbon source. The test tubes were incubated on a rotary shaker at 30 °C for 72 h. After incubation, quantitative analysis for xylanase activity was performed using the DNS method [20]. The xylanase enzyme activity was expressed in katals per millilitre (U/mL), where one unit of xylanase is defined as the amount of enzyme that liberates 1 µmol of xylose equivalents per minute.
3. Results
In this study, the guts of three Scarabaeidae dung beetles (Pachylomerus femoralis and Anachalcos convexus) and one dung beetle larva (Euoniticellus intermedius) were isolated, identified and screened for xylanolytic fungi. A total of 222 filamentous fungi were isolated from P. femoralis, A. convexus and E. intermedius. A total of two hundred and seventeen xylanase-producing filamentous fungi were isolated from the guts of P. femoralis (35 fungal isolates), A. convexus (118 fungal isolates) and the larvaof the dung beetle E. intermedius (69 fungal isolates). The phylogenetic tree for all fungal isolates with xylanolytic activity was deduced to evaluate their relationship.
3.1. Identification and Characterisation of Fungal Isolates
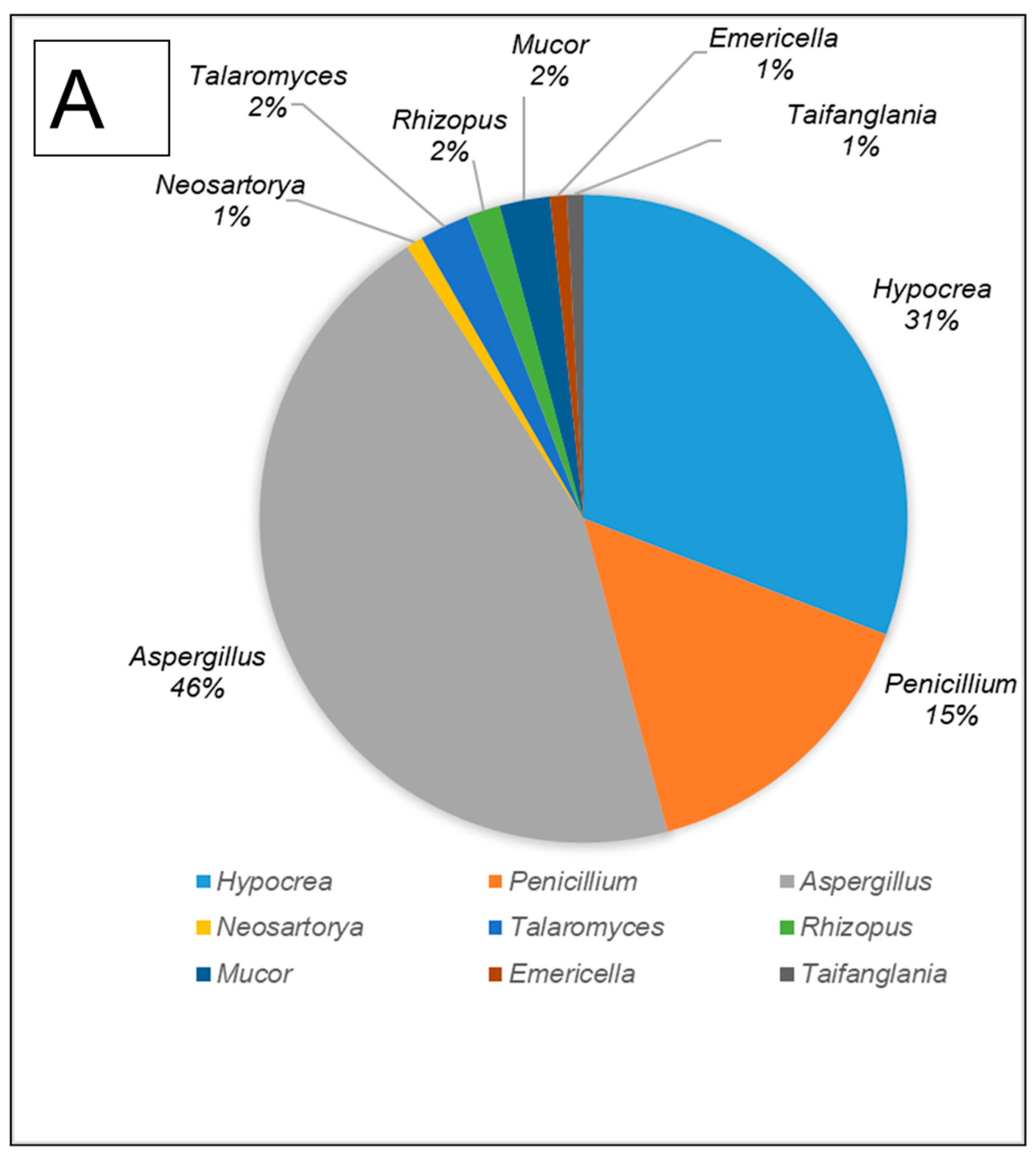
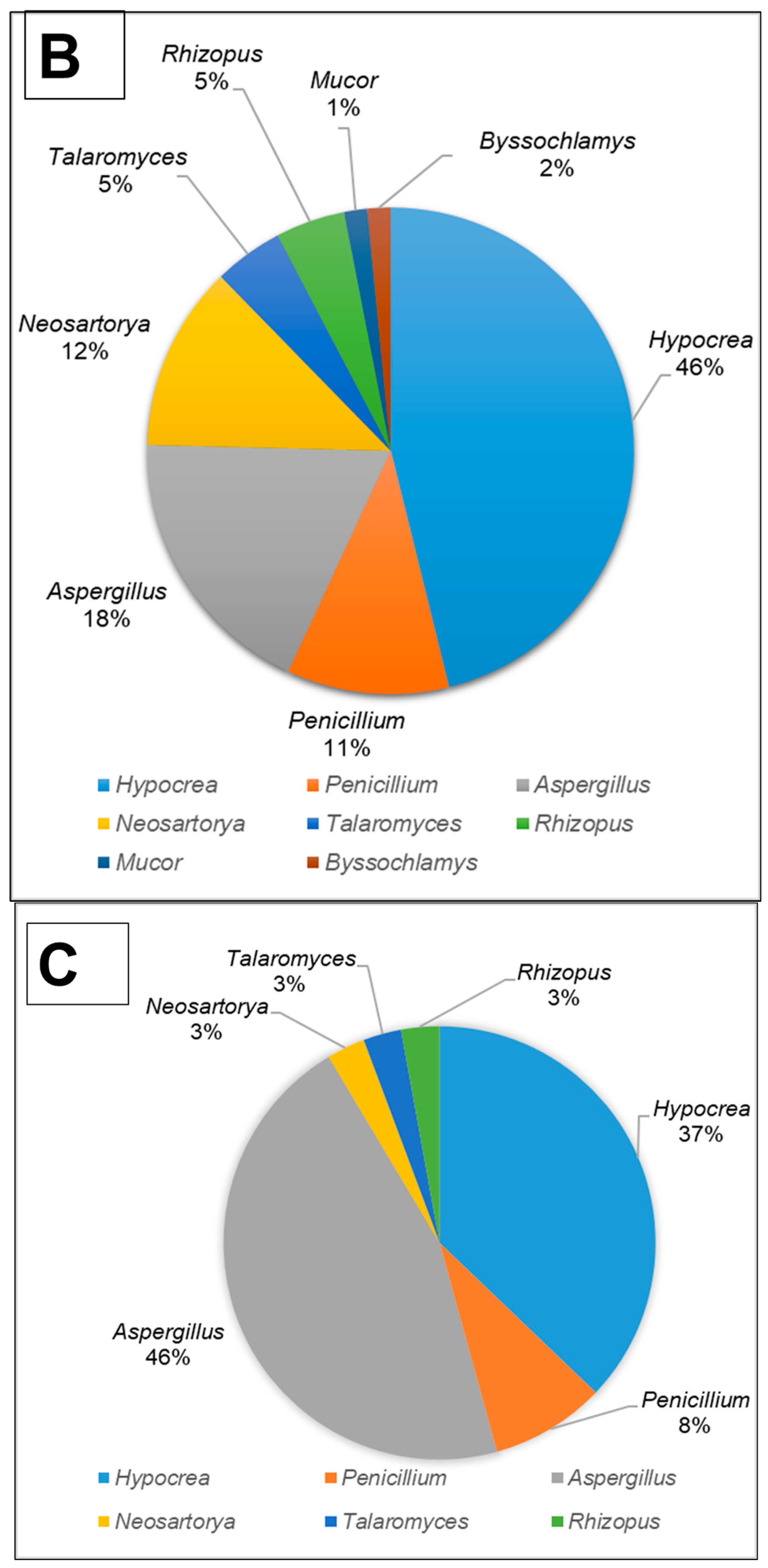
All fungal isolates were identified and characterised by sequencing the ITS and D1/D2 regions. The results revealed that the isolates belonged to ten different genera, namely Aspergillus, Hypocrea (previously Trichoderma), Penicillium, Mucor, Neosartorya, Rhizopus, Talaromyces, Taifanglania and Byssochlamys. The most prevalent genus was Aspergillus (89 strains) followed by Hypocrea (81 strains), Penicillium (28 strains) and Neosartorya (11 strains), while the less dominant isolates belonged to Talaromyces (7 strains) and Rhizopus (3 strains) with Byssochlamys, Taifanglania and Emericella represented by 1 strain each (Figure 2A–C).
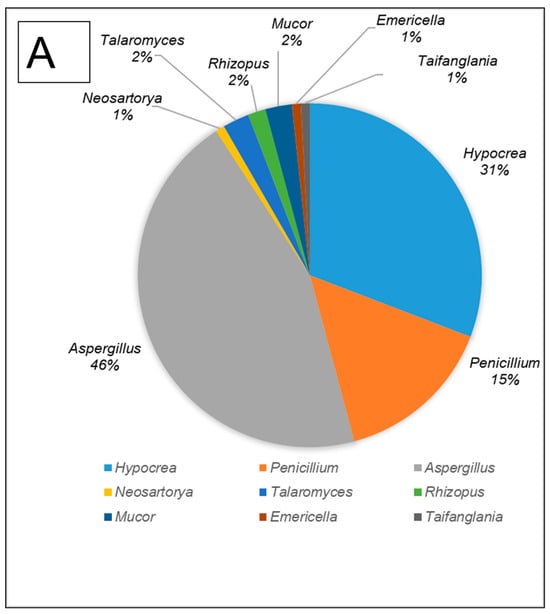
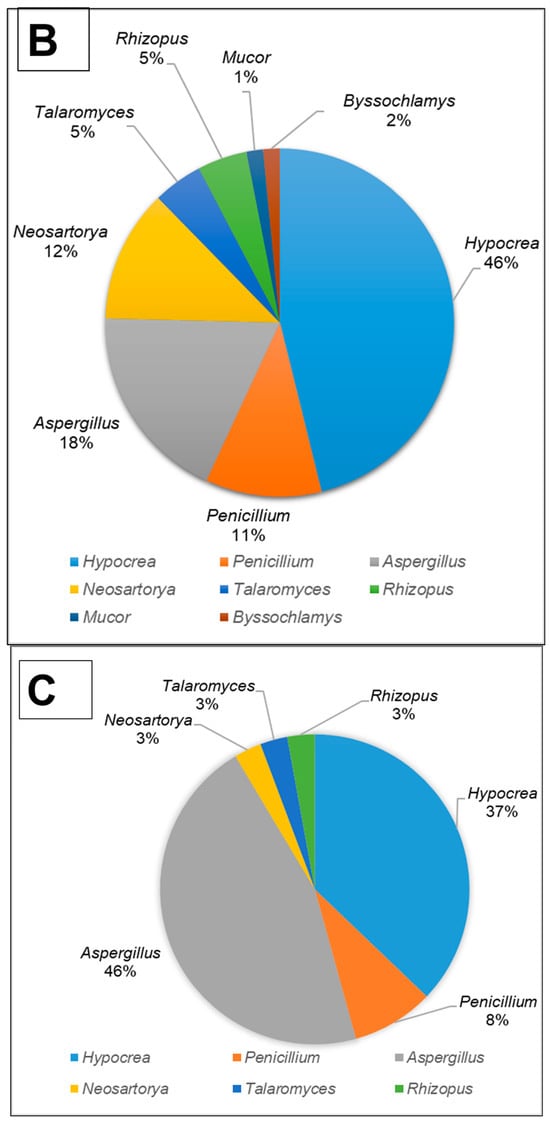
Figure 2.
The filamentous fungi isolated from the gut of dung beetles and dung beetle larva. (A) gut microbiome from A. convexus (B) gut microbiome from E. intermedius larva and (C) gut microbiome from P. femoralis.
The gut of A. convexus yielded 118 strains, of which Aspergillus was the most abundant followed by Hypocrea and Penicillium (Figure 2A). The gut of dung beetle larva yielded 64 strains represented by 10 different genera. The most dominant genus in the larva gut was Hypocrea, followed by Aspergillus and Neosartorya (Figure 2B). Thirty-five isolates were obtained from the gut of P. femoralis, of which Aspergillus was also the dominant genus, followed by Trichoderma and Penicillium (Figure 2C).
3.2. Xylanase Production by Fungal Isolates
All isolates obtained in this study were screened for xylanase activity using xylan as a substrate. Two hundred and seventeen were xylanolytic, with 203 isolates showing xylanase activity higher than 3 U/mL (Figure 3, Figure 4 and Figure 5). All isolates with more than 3 U/mL were -evaluated further. Xylanolytic isolates belonged to 10 genera, namely Aspergillus (78), Hypocrea (77), Penicillium (27), Talaromyces (4), Neosartorya (6), Mucor (4), Geotrichum (3), Rhizopus (2), Byssochlamys and Taifanglania (1).
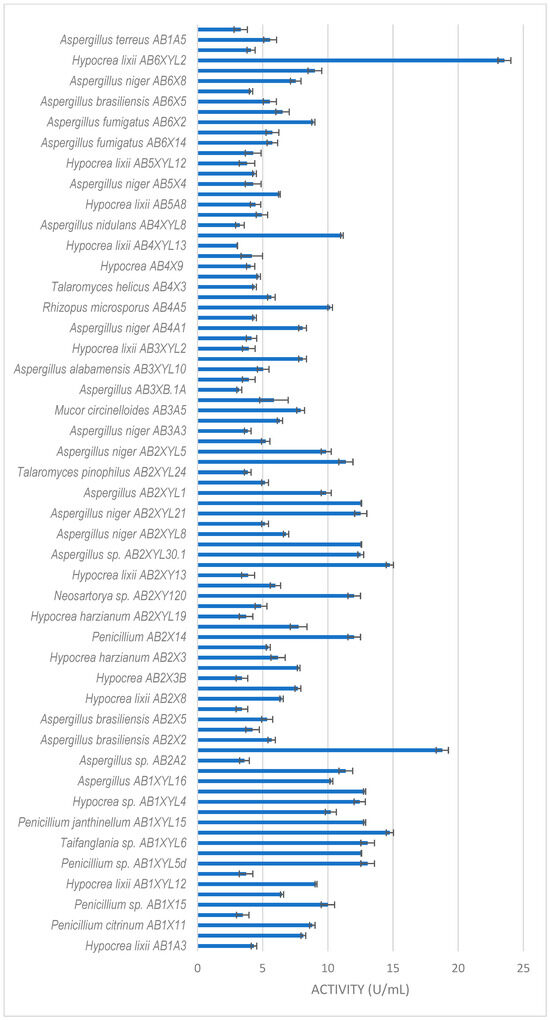
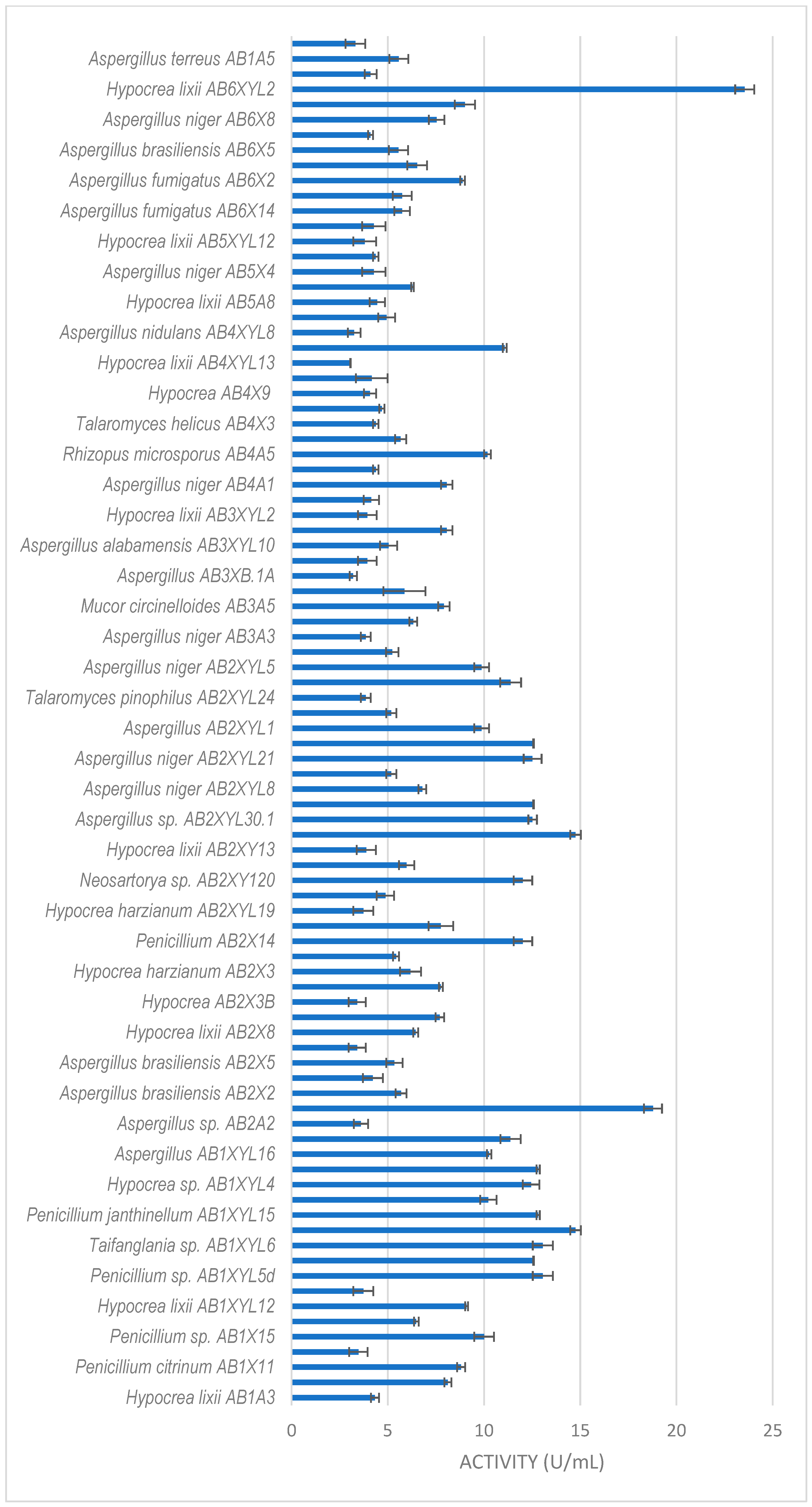
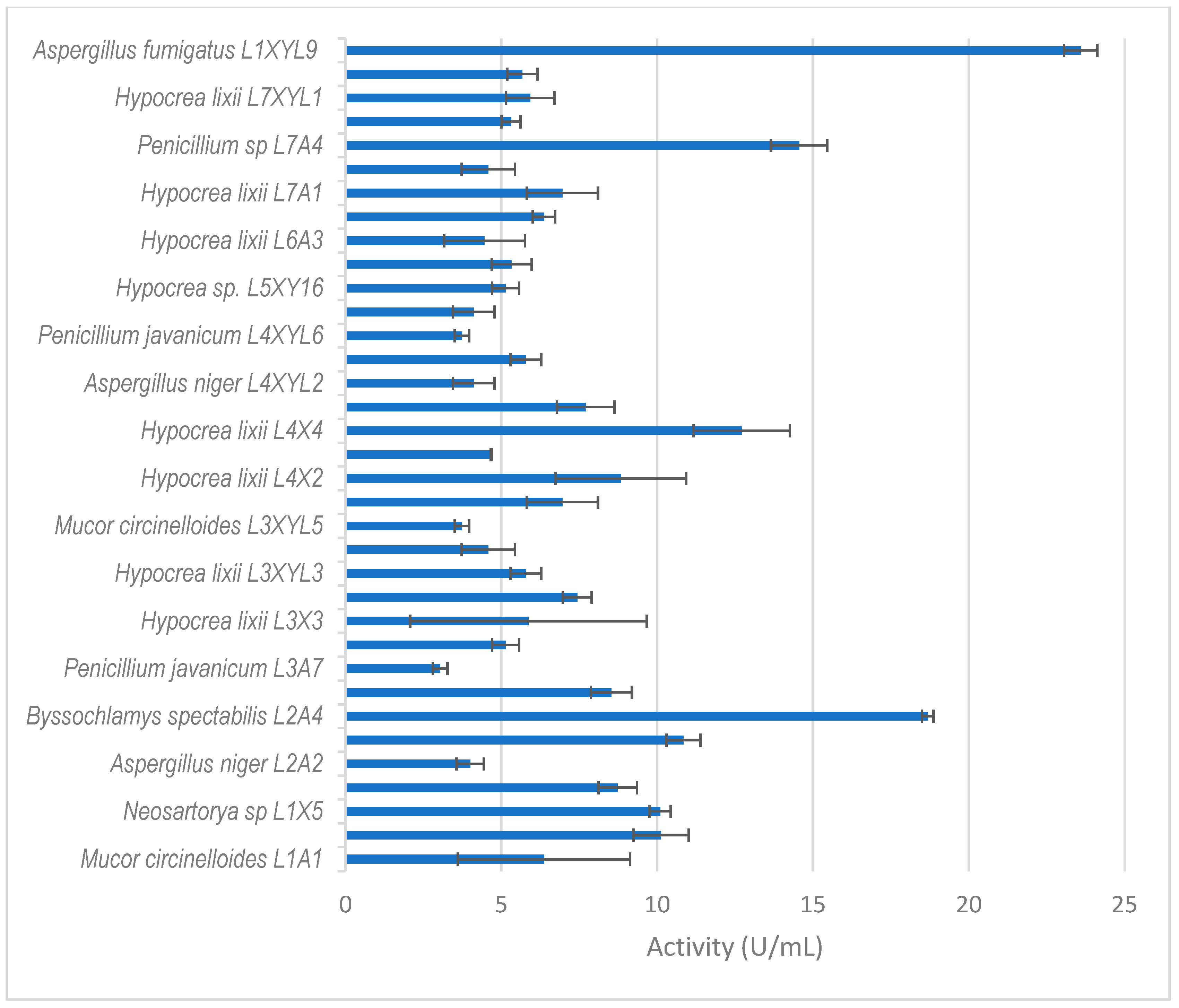
Figure 3.
Xylanase activity by filamentous fungi isolated from the gut of the dung beetle A. convexus. The results are the mean of three independent experiments with standard deviation values.
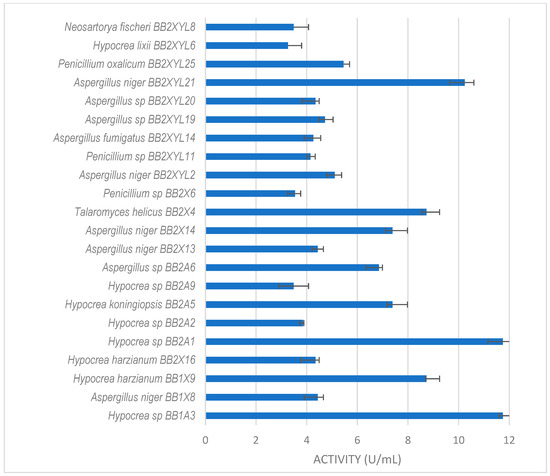
Figure 4.
Xylanase activity by filamentous fungi isolated from the gut of the dung beetle P. femoralis. The results are the mean of three independent experiments with standard deviation values.
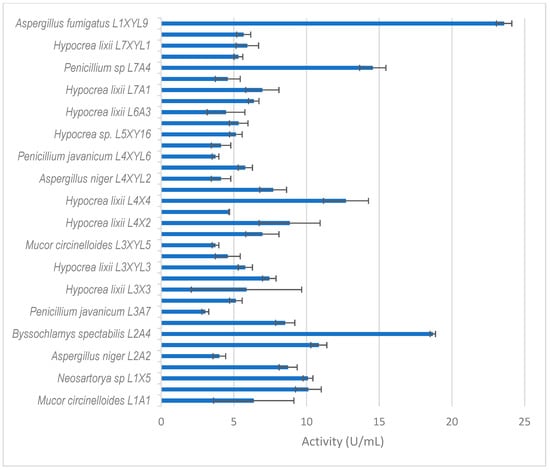
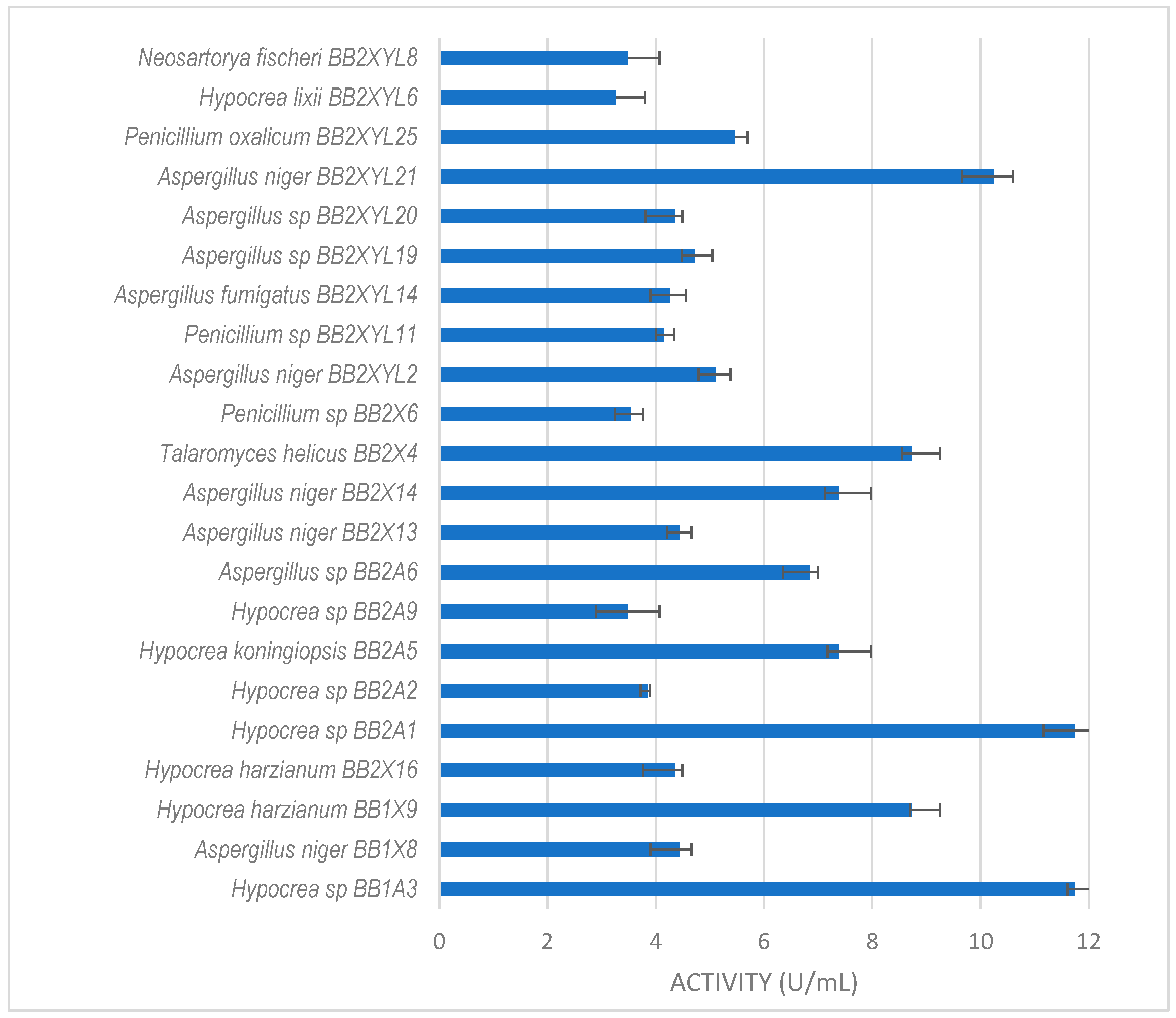
Figure 5.
Xylanase activity by filamentous fungi isolated from the dung beetle E. intermedius larva. The results are the mean of three independent experiments with standard deviation values.
The dung beetle A. convexus yielded the most fungi, with xylanase activity higher than 3 U/mL (Figure 3). The best producers were Hypocrea lixii AB6XYL2 and AB2A3 with 24 and 19 U/mL, respectively. This was followed by Aspergillus sp. AB2XYL30 (15 U/mL), Penicillium janthinellum AB1XYL14 (15 U/mL) and Hypocrea sp. AB1XYL4 (12 U/mL).
Hypocrea sp. (BB2A1 and BB1A3) from the gut of P. femoralis (Figure 4) both produced the highest xylanase activity of 12 U/mL. This was followed by A. niger BB2XYL21 (10 U/mL) and T. helicus BB2X4 (9 U/mL).
Thirty-five isolates from E. intermedius showed xylanase activity higher than 3 U/mL (Figure 5). H. lixii was the most dominant species, with its xylanase activity ranging from 4–13 U/mL. H. lixii is the teleomorph of Trichoderma harzianum. H. lixii was an abundant species in the gut of both adult and dung beetle larva. A. fumigatus L1XYL9 produced the highest xylanase activity of 24 U/mL followed by B. spectabilis L2A4 producing 19 U/mL.
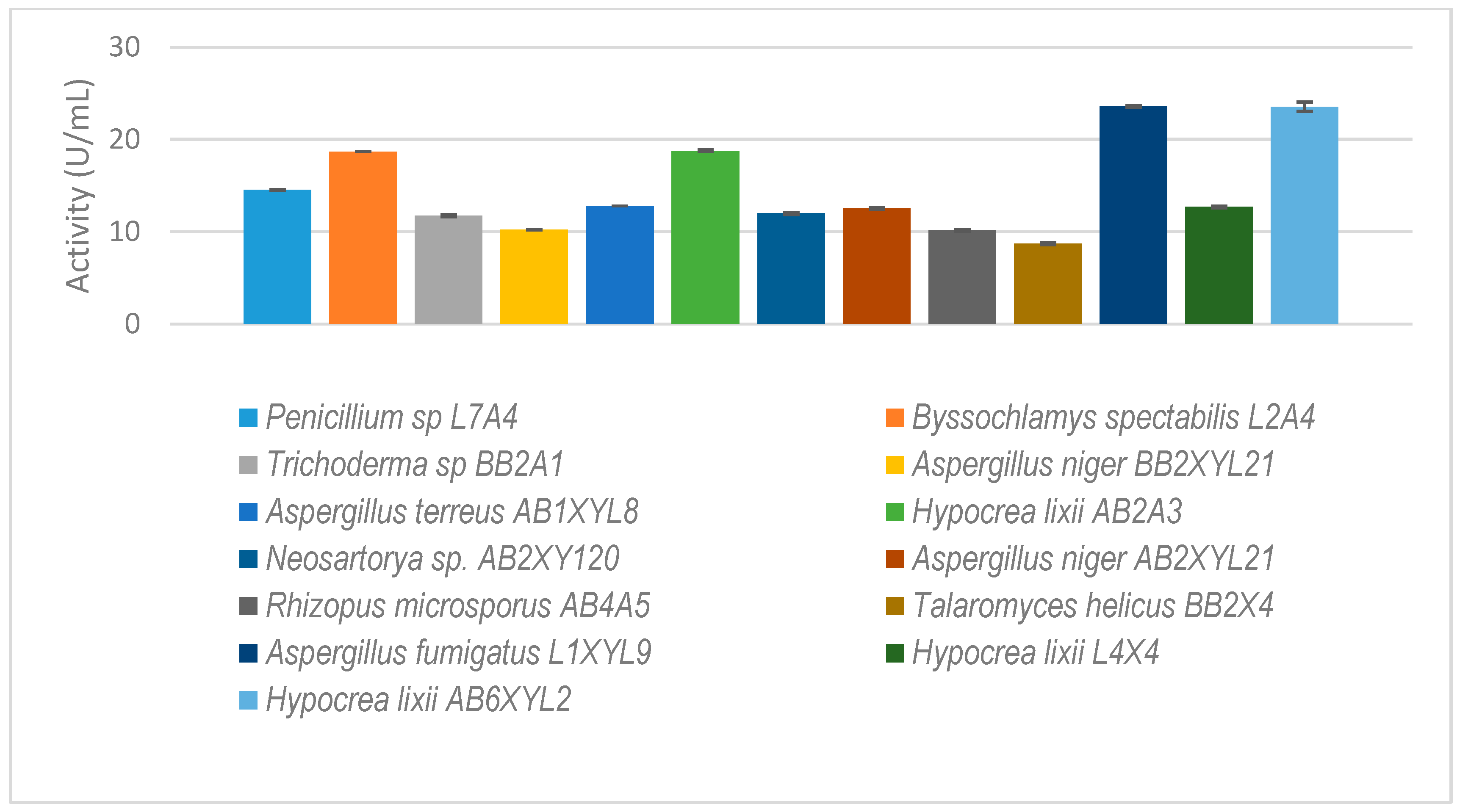
Thirteen fungal isolates that showed higher xylanolytic activity as compared to other species were selected for further study (Figure 6). Some species, such as Rhizopus microsporus (AB4A5), were not selected because of their higher xylanolytic activity but because they were unique gut microbiome species.
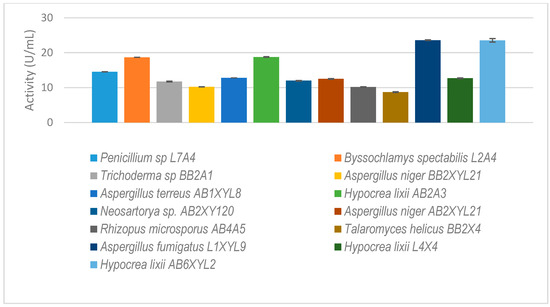
Figure 6.
The isolated gut-inhabiting fungi from P. femoralis and A. convexus and dung beetle larva E. intermedius selected for further study. The results are the mean of three independent experiments with standard deviation values.
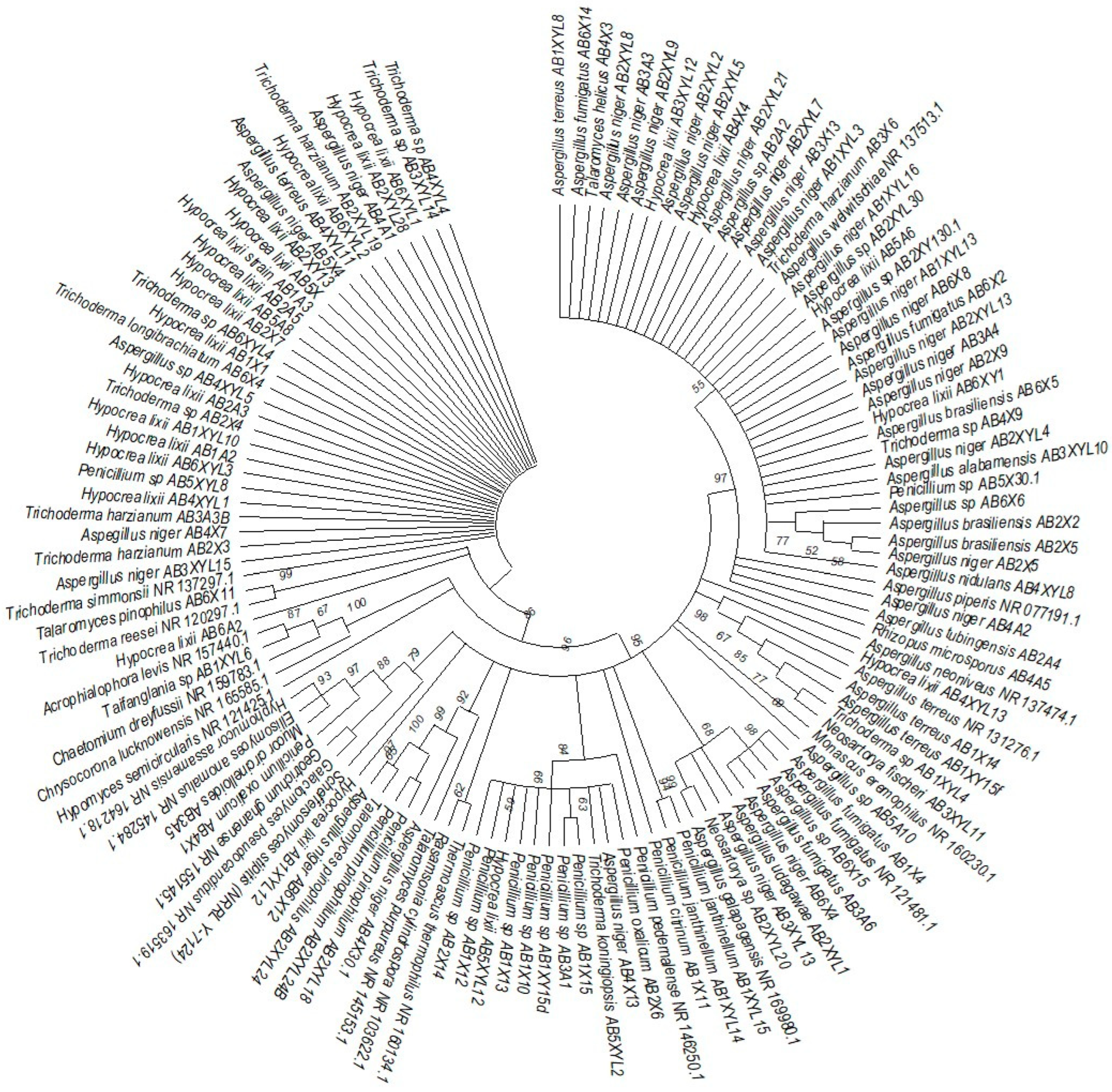
3.3. Phylogenetic Analysis
The phylogenetic trees were prepared using ITS sequencing data for the individual isolates (Figure 7, Figure 8, Figure 9 and Figure 10). Three phylogenetic trees were constructed, representing the species with xylanolytic activity from the gut of A. convexus, P. femoralis and E. intermedius.
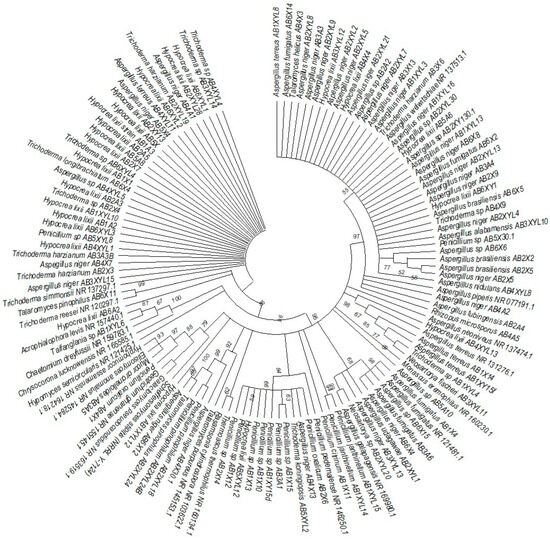
Figure 7.
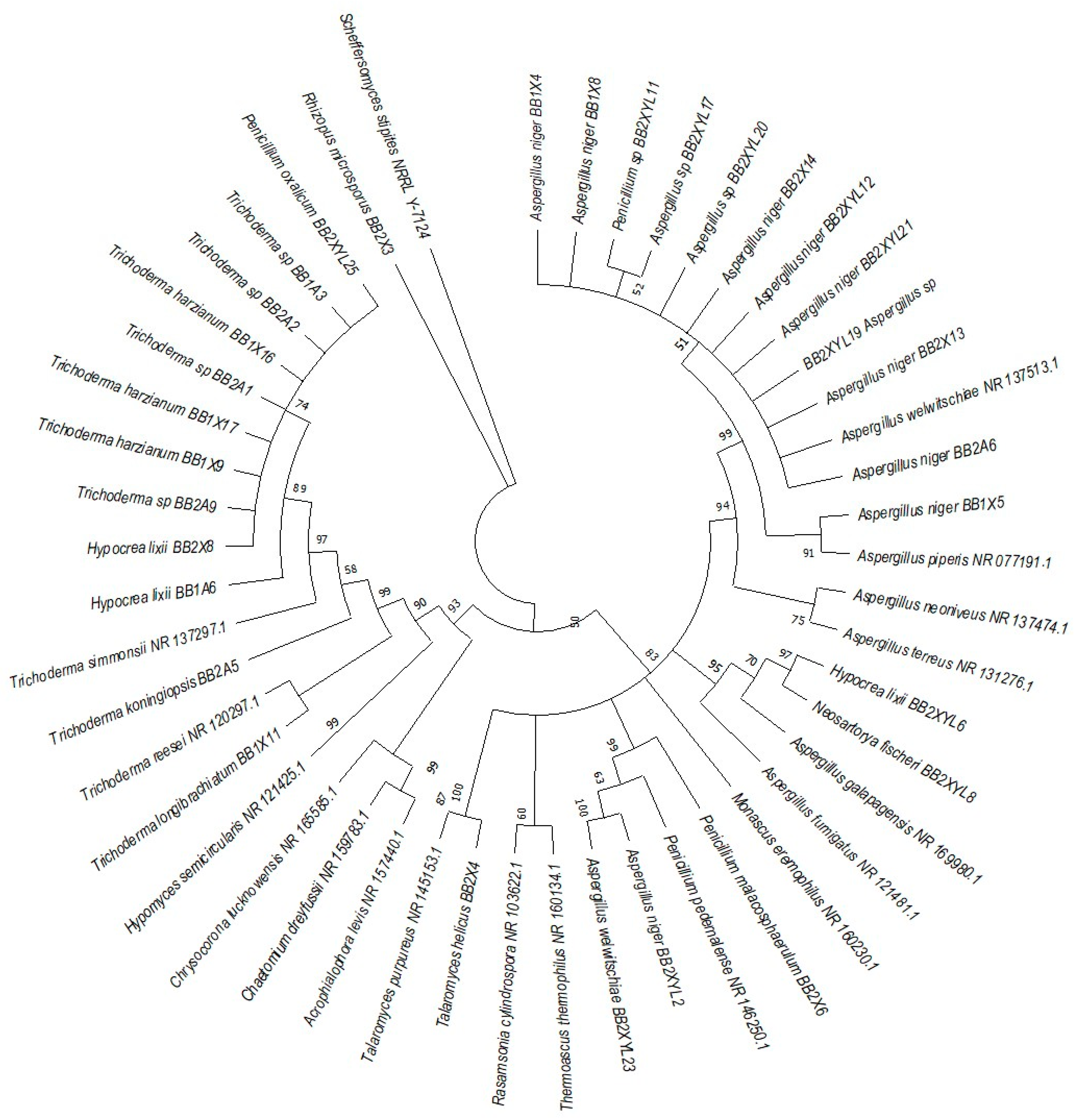
Neighbor-joining tree deduced using an ITS sequence of different strains isolated from the gut of the dung beetle A. convexus. Only branches with more than 50% bootstrap support are shown. S. stipitis NRRL-Y-7124 was used as an outgroup.
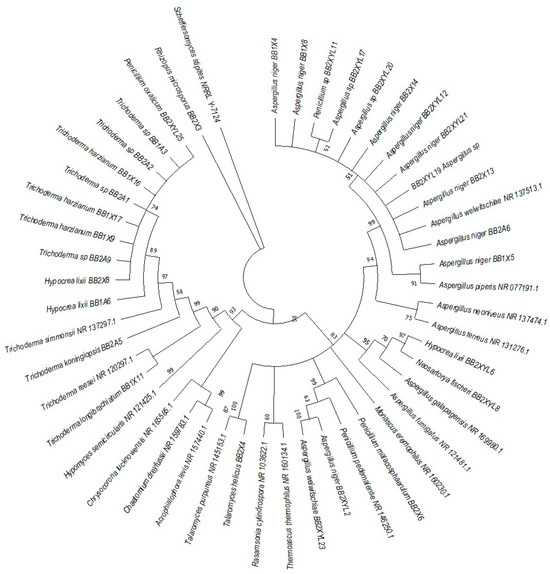
Figure 8.
Neighbor-joining tree was deduced using an ITS sequence of different strains isolated from the gut of the dung beetle P. femoralis. Only branches with more than 50% bootstrap support are shown. S. stipitis NRRL-Y-7124 was used as an outgroup.
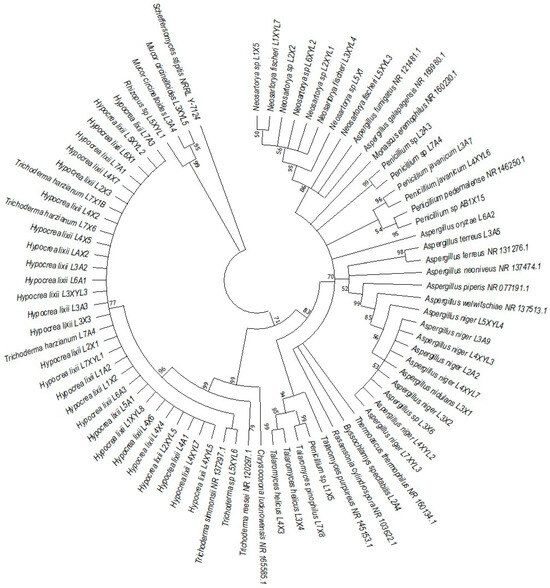
Figure 9.
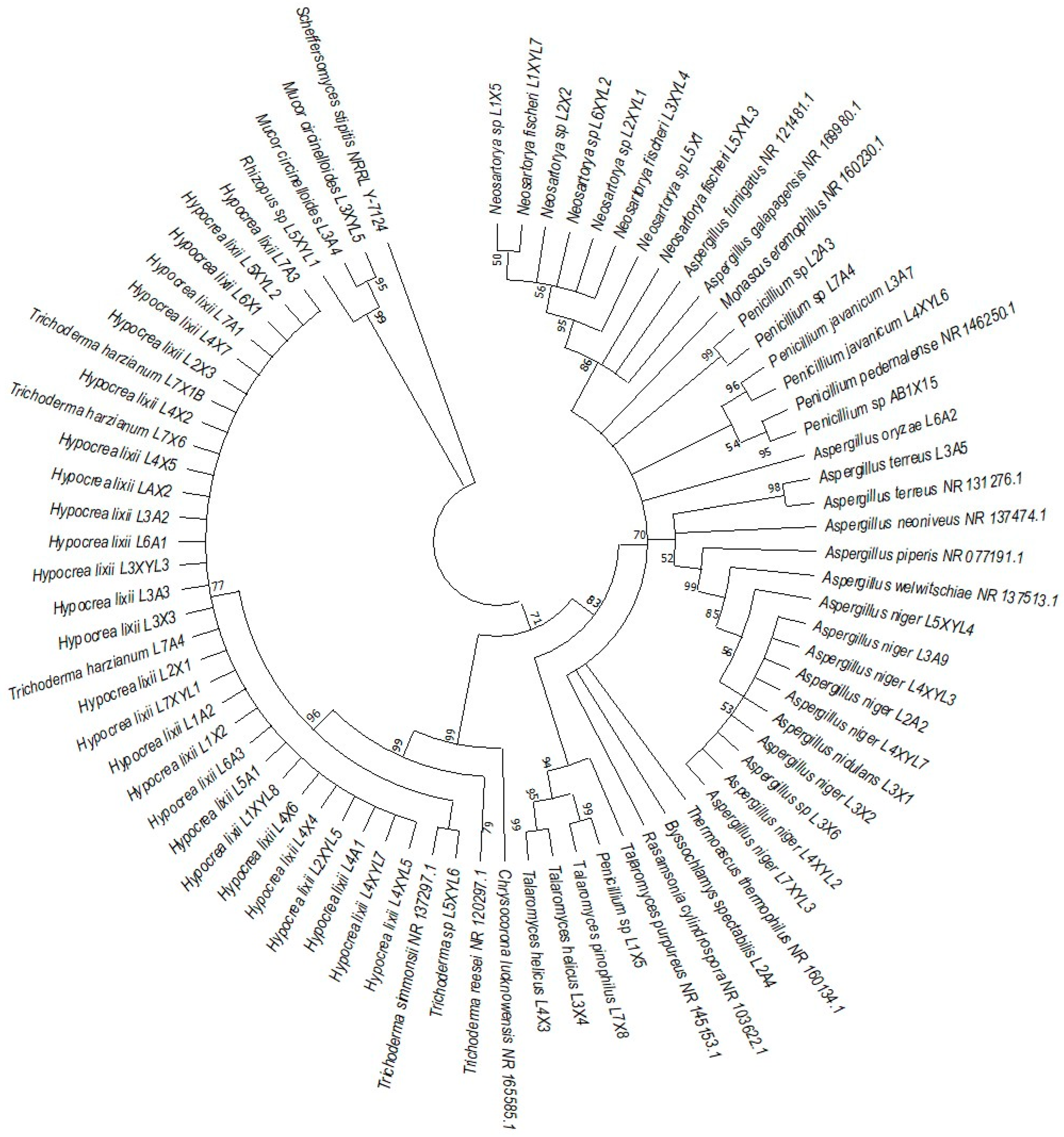
Neighbor-joining tree deduced using the ITS sequence of different strains isolated from the gut of the dung beetle larva E. intermedius. Only branches with more than 50% bootstrap support are shown. S. stipitis NRRL-Y-7124 was used as an outgroup.
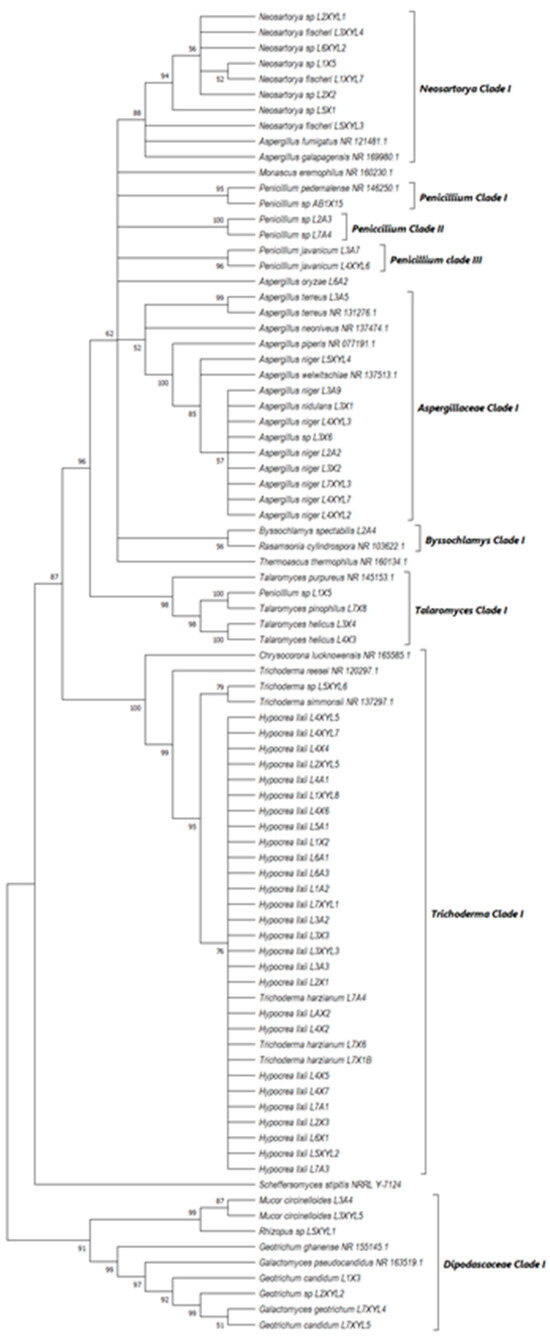
Figure 10.
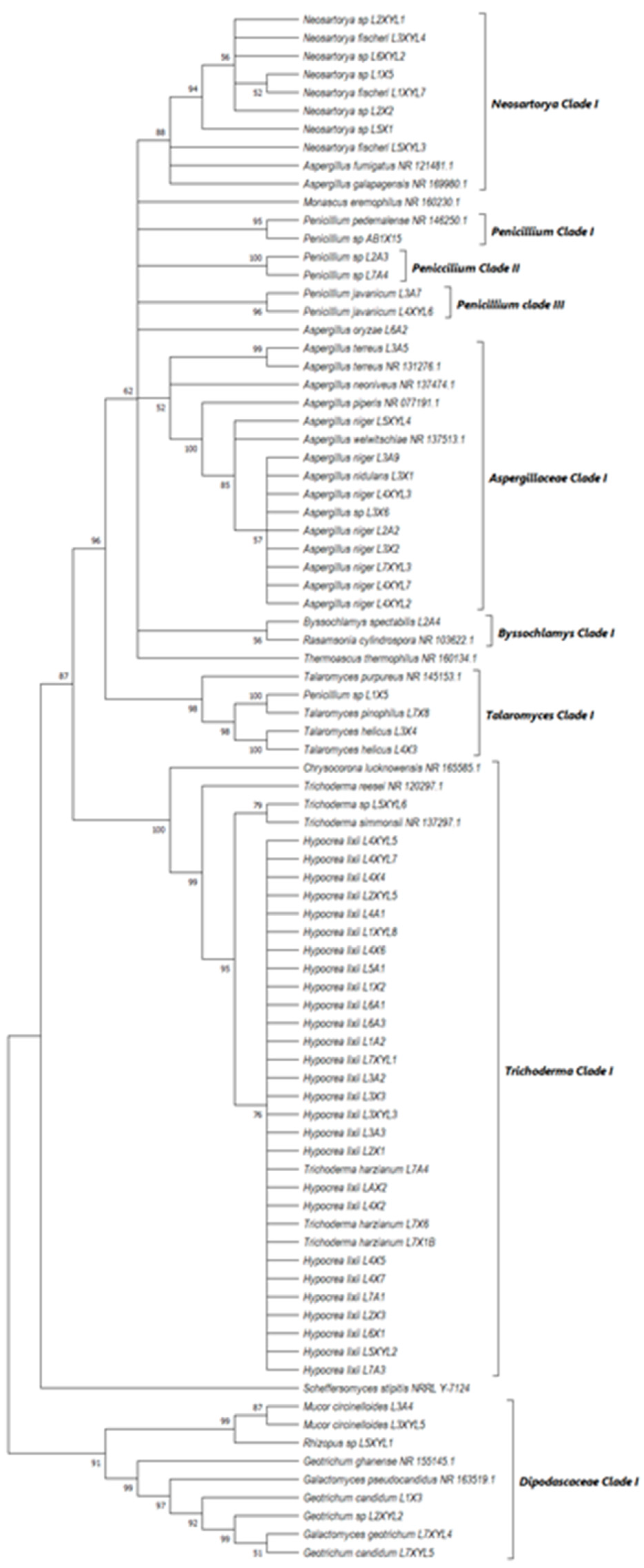
Neighbor-joining tree deduced using the ITS sequence of fungal strains isolated from the guts of the dung beetles P. femoralis and A. convexus and the dung beetle larva E. intermedius. Only branches with more than 50% bootstrap support are shown. S. stipitis NRRL-Y-7124 was used as an outgroup.
The species isolated from the gut of A. convexus represent several clades, namely Trichocomaceae, Penicillium, Aspergillus and Trichoderma (Figure 7). The family Trichocomaceae split into three separate families, which were Aspergillaceae, Trichocomaceae and Thermoascaceae. This is reflected in the phylogenetic data of the Trichocomaceae clade I, composed of Talaromyces, Aspergillus and Penicillium species. The Mucoraceae clade was represented by Hyphomucor, Mucor and Rhizopus genera. The relationship among the species was well supported by branches > 50%.
P. femoralis represented two clades, namely Trichocomaceae and Trichoderma (Figure 8). Phylogeny of E. intermedius isolates deduced three monophyly groups of Penicillium. Other clades were Neosartorya, Aspergillaceae, Talaromyces, Trichoderma and Byssochlamyces (Figure 9). These clades, besides Hypocrea, are part of the Trichocomaceace family. The other clade deduced was the Mucoraceae clade made up of Mucor circinelloides and Rhizopus sp.
All the fungal isolates with xylanolytic activity from the gut of A. convexus, P. femoralis and E. intermedius were used to construct a best-scoring neighbor-joining tree to evaluate the relatedness of their fungal species. Fungal species such as H. lixii, Hypocrea sp., A. niger, Neosartorya fischeri and Talaromyces helicus from the gut of E. intermedius showed relatedness as they formed clades with fungal species from the gut of A. convexus and P. femoralis. The deduced branches were well-supported with a bootstrap of > 50%. The deduced phylogenetic tree showed that the fungal species isolated from A. convexus, P. femoralis and E. intermedius are closely related since most species formed clades with bootstrap values > 50% (Figure 10).
4. Discussion
Lignocellulose is the predominant component of woody plant material, soil organic material and decaying wood, making it the most abundant form of biomass in terrestrial ecosystems. Lignocellulose is a cheap, abundant, renewable substrate that can be used for the production of biofuel. Lignocellulose is composed of cellulose and hemicellulose polymers that can be broken down into their monomers, and these can be fermented to ethanol. Currently, several constraints are affecting the use of biomass for biofuel. One of these constraints is finding cost-efficient enzymes that degrade lignocellulose polymers into monomers. The degradation efficiency could be improved with the inclusion of the endo-xylanase enzyme. The gut of the dung beetle is a rich source of hemicellulolytic microorganisms [4,12]. In this study, the guts of two Scarabaeidae dung beetles (A. convexus and P. femoralis) and one dung beetle larva (E. intermedius) were screened for xylanolytic fungi. A total of 222 filamentous fungi were isolated from A. convexus, P. femoralis and E. intermedius. Two-hundred and seventeen were found to have xylanase activity.
The abundance of genera such as Aspergillus, Hypocrea, Penicillium and Neosartorya in the guts of A. convexus and P. femoralis and one dung beetle larva (E. intermedius) indicates that the gut of dung beetles is a good source for the isolation of xylanolytic microorganisms. These genera are one of the well-known cellulase and xylanase producers, with Hypocrea jecorina (previously Trichoderma reesei) and Aspergillus niger being the most studied species and the species widely used for the production of a commercial enzyme [16,21,22]. The species isolated from the adult dung beetles (A. convexus and P. femoralis) were similar but differed from those isolated from the dung beetle larva (E. intermedius). The difference in species composition between the adult beetles and the larva could be caused by the moulting process that occurs several times during the development stage. During this process, the larva shed not only the hind- and foregut lining but also the inhabiting microorganisms [11]. In addition, species composition is expected to differ between different developmental stages [10]. According to [10], the difference in species composition could also be due to the difference in diet [11], the environment [23], and social interactions [24].
Most filamentous fungal isolates had xylanolytic activity and only a few did not exhibit any xylanolytic activity. Filamentous fungi are well-known for their ability to produce xylanase enzymes and are widely used in industry for enzyme production, especially species such as Hypocrea jecorina and Aspergillus niger [25,26]. The fungal isolates that showed the highest xylanase activity were A. fumigatus L1XYL9 isolated from dung beetle larva (E. intermedius) with xylanase activity of 24 U/mL. The xylanase activity produced by A. fumigatus L1XYL9 in this study was higher than that by A. fumigatus GGV – BT 03 (0,1 U/mL) reported by [27], A. fumigatus Z5 (15 U/mL) reported by [28] and A. Fumigatus MA28 (9 U/mL) reported by [29]. This was the same as xylanase activity exhibited by H. lixii AB6XYL2 isolated from the gut of the adult dung beetle A. convexus. The xylanase activity produced by H. lixii AB6XYL2 in this study was higher than that produced by H. lixii reported by [30], with 24 U/mL of xylanase activity. Hypocrea species are well-known hyper-producers of industrially important enzymes including lignocellulolytic enzymes. Hypocrea and Aspergillus species are well-known for their ability to degrade polymers (cellulose and hemicellulose) [25,26]. The xylanase activity of these gut microbiota is notably lower compared to the commercial xylanase [31]. This disparity arises from the absence of an optimization strategy, a crucial factor that could substantially elevate xylanase activity. Implementing a targeted optimization approach is essential to unlock the full potential of the gut microbiota’s xylanase capabilities, thereby bridging the performance gap with commercial counterparts. The dominance of these fungal species in the gut of these dung beetles revealed that the gut of dung beetles/larvae is a good source for the isolation of fungal species that will play an important role in the biofuel industry.
The dominant fungal species in the guts of the adult dung beetles was A. niger. Its presence in the gut of dung beetles was in agreement with the study by [32] which found A. niger to dominate the gut of the three insect species collected from Assiut Governorate. Most of the A niger isolates in this study were able to produce xylanases. The co-operative action between A. niger and H. lixii in degrading polymers such as cellulose and hemicellulose reflects a symbiotic association in the gut microbiomes of dung beetles. Given the variations in composition, determining whether it is more beneficial to isolate the microbiota from the larvae or the gut becomes challenging. Further species isolated in this study included species such as A. terreus [32], R. oryzae [33], P. echinulatum [34] and T. helices [35] that have been used in the biofuel industry. In this study, these isolates showed good xylanase activity. The deduced phylogenetic tree reflected a close relationship between the fungi with xylanolytic activity from the gut of the two adult dung beetle and the larvae species. This further shows that the species that showed lower xylanolytic activity could be enhanced through evolutionary adaptation. Evolutionary adaptation has been extensively employed for strain improvement in industrial applications. Fungal strains exhibiting lower xylanase activity in this study can be enhanced through evolutionary adaptation, following an initial optimization strategy aimed at increasing xylanase production [36]. The fungal species such as Taifanglania sp. AB1XYL6 isolated in this study did not show any xylanase activity, but did, however, form a paraphyletic group with the genus Acrophialophora, which is a thermotolerant soil fungus that is widely distributed in temperate and tropical regions. Acrophialophora nainiana has been reported for its ability to produce cellulase and xylanase enzymes [37]. Since it shares a common recent ancestor with Taifanglania sp., they may be paraphyletic because Taifanglania sp, lost some of its characteristics (lignocellulolytic activity) that it shared with Acrophialophora. The genus Taifanglania further forms a monophyletic group with the genus Chaetomium. The genus Chaetomium is known for its ability to colonise different substrates. Most species from this genus are capable of producing cellulase that degrades cellulose, resulting in the production of different bioactive metabolites [38].
It is very difficult to be certain if the isolated species are endosymbionts of the gut of the Scarabaeidae dung beetle or if this species will differ from one Scarabaeidae species to the other since the microbial species could be transitory inhabitants associated with host feeding habits or might even be using the insect as a dispersal mechanism [13].
In conclusion, the results of this study show that the filamentous fungi associated with the guts of dung beetle/larvae are highly diverse in terms of the number of species, while phylogenetic analysis showed their close relatedness. The findings are in agreement with the report that the gut of the dung beetle is a rich source of hemicellulolytic microorganisms. The gut habitats have consortia that are acting synergistically to provide many of the nutritional needs of the beetle host. In addition, the degradation of lignocellulosic materials is reflected by the high percentage of filamentous fungi with xylanolytic ability. This is the first study to report the distribution of xylanase-producing fungal species inhabiting the gut of the A. convexus, P. femoralis and E. intermedius larvae. This study recommends that the highest xylanase-producing fungi should be investigated further to improve the xylanase activity and be screened for other enzymes related to lignocellulose degradation. These fungal isolates could be ideal in the hydrolysis of cheap lignocellulose substrates, such as grasses.
Author Contributions
Conceptualization, L.M.; Funding acquisition, E.L.J.v.R. and D.C.L.G.; Investigation, L.M.; Methodology, D.C.L.G. and K.L.M.M.; Project administration, E.L.J.v.R.; Resources, E.L.J.v.R.; Supervision, E.L.J.v.R.; Validation, K.L.M.M. and E.L.J.v.R.; Writing—original draft, L.M.; Writing—review and editing, D.C.L.G., K.L.M.M., M.J.M. and N.N.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by South African National Research Funding and the APC was funded by the University of Limpopo.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
This study is dedicated to the late I. Ncube, may his soul rest in peace. Further, we extend appreciation to V.G Mbazima (mentor). The authors are grateful for the financial support offered by the National Research Foundation and the University of Limpopo.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- De Souza, J.B.; Francisco, M.M.; Amâncio, L.R.; Vital Brazile, O.A.; Maria de Lourdes, T.M.P.; Ruzenee, D.S.; Silvae, D.P.; Mendonça, M.d.C.; López, A.J. Sunflower stalk as a carbon source inductive for fungal xylanase production. Ind. Crops Prod. 2020, 153, 112268. [Google Scholar] [CrossRef]
- Binod, P.; Gnansounou, E.; Sindhu, R.; Pandey, A. Enzymes for second-generation biofuels: Recent developments and future perspectives. Bioresour. Technol. Rep. 2019, 5, 317–325. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef]
- Nwaefuna, A.E.; Rumbold, K.; Boekhout, T.; Zhou, N. Bioethanolic yeasts from dung beetles: Tapping the potential of extremophilic yeasts for improvement of lignocellulolytic feedstock fermentation. Biotechnol. Biofuels Bioprod. 2021, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Orencio-Trejo, M.; Torres-Granados, J.; Rangel-Lara, A.; Beltrán-Guerrero, E.; García-Aguilar, S.; Moss-Acosta, C.; Valenzuela-Soto, H.; De la Torre-Zavala, S.; Gastelum-Arellanez, A.; Martinez, A.; et al. Cellulase and Xylanase Production by the Mexican Strain Talaromyces stollii LV186 and Its Application in the Saccharification of Pretreated Corn and Sorghum Stover. Bioenergy Res. 2016, 9, 1034–1045. [Google Scholar] [CrossRef]
- Tian, S.; Zhao, R.; Chen, Z. Review of the pretreatment and bioconversion of lignocellulosic biomass from wheat straw materials. Renew. Sustain. Energy Rev. 2018, 91, 483–489. [Google Scholar] [CrossRef]
- de Souza, T.S.; Kawagut, H.Y. Cellulases, Hemicellulases, and Pectinases: Applications in the Food and Beverage Industry. Food Bioprocess Technol. 2021, 14, 1446–1477. [Google Scholar] [CrossRef]
- Thiyonila, B.; Naveen, P.R.; Mani, K.; Srinivasan, S.; Krishnan, M. Dung beetle gut microbes: Diversity, metabolic and immunity-related roles in host system. Int. J. Sci. Innov. 2018, 4, 77–83. [Google Scholar]
- Rushworth, D. Siyabona Africa: Dung Beetles Perspective. 2022. Available online: https://www.krugerpark.co.za/krugerpark-times-20-dung-beetle-18101.html (accessed on 2 September 2022).
- Suárez-Moo, P.; Cruz-Rosales, M.; Ibarra-Laclette, E.; Desgarennes, D.; Huerta, C.; Lamelas, A. Diversity and Composition of the Gut Microbiota in the Developmental Stages of the Dung Beetle Copris incertus Say (Coleoptera, Scarabaeidae). Front. Microbiol. 2020, 11, 1698. [Google Scholar] [CrossRef] [PubMed]
- Franzini, P.Z.N.; Ramond, J.B.; Scholtz, C.H.; Sole, C.L.; Ronca, S.; Cowan, D.A. The gut Microbiomes of two Pachysoma Macleay desert dung beetle species (Coleoptera: Scarabaeidae: Scarabaeinae) feeding on different diets. PLoS ONE 2016, 11, 3–19. [Google Scholar]
- Rojas-Jiménez, K.; Hernández, M. Isolation of fungi and bacteria associated with the guts of tropical wood-feeding coleoptera and determination of their lignocellulolytic activities. Int. J. Microbiol. 2015, 2015, 285018. [Google Scholar] [CrossRef]
- Martínez, P.M.; Appeldoorn, M.M.; Gruppen, H.; Kabel, M.A. The two Rasamsonia emersonii α-glucuronidases, ReGH67 and ReGH115, show a different mode-of-action towards glucuronoxylan and glucuronoxylo- oligosaccharides. Biotechnol. Biofuels 2016, 9, 105. [Google Scholar] [CrossRef]
- Mabhegedhe, M. Cellulolytic Activities of the Dung Beetle, Euoniticellus Intermedius, Larva Gut Micro-Flora. Open Biotechnol. J. 2017, 11, 105–113. [Google Scholar] [CrossRef]
- Lemke, T.; Stingl, U.; Egert, M.; Friedrich, M.W.; Brune, A. Physicochemical conditions and microbial activities in the highly alkaline gut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 2003, 69, 6650–6658. [Google Scholar] [CrossRef]
- Urbina, H.; Schuster, J.; Blackwell, M. The gut of Guatemalan passalid beetles: A habitat colonized by cellobiose- and xylose-fermenting yeasts. Fungal Ecol. 2013, 6, 339–355. [Google Scholar] [CrossRef]
- Makhuvele, R.; Ncube, I.; Jansen van Rensburg, E.L.; La Grange, D.C. Isolation of fungi from the dung of wild herbivores for application in bioethanol production. Braz. J. Microbiol. 2017, 48, 648–655. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbour-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Reczey, K.; Szengyel, Z.; Eklund, R.; Zacchi, G. Cellulase production by T. reesei. Bioresour. Technol. 1996, 57, 25–30. [Google Scholar] [CrossRef]
- Singhania, R.R.; Sukumaran, R.K.; Pillai, A.; Prema, P.; Szakacs, P. Solid-state fermentation of lignocellulosic substrates for cellulase production by Trichoderma reesei NRRL 11460. Indian J. Biotechnol. 2006, 5, 332–336. [Google Scholar]
- Ng, S.H.; Stat, M.; Bunce, M.; Simmons, L.W. The influence of diet and environment on the gut microbial community of field crickets. Ecol. Evol. 2018, 8, 4704–4720. [Google Scholar] [CrossRef]
- Martinson, V.G.; Moy, J.; Moran, N.A. Establishment of characteristic gut bacteria during the development of the honeybee worker. Appl. Environ. Microbiol. 2012, 78, 2830–2840. [Google Scholar] [CrossRef]
- Bischof, R.H.; Ramoni, J.; Seiboth, B. Cellulases and beyond: The first 70 years of the enzyme producer Trichoderma reesei. Microb. Cell Fact. 2016, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, J.; Du, G.; Chen, J.; Takahashi, S.; Liu, S. Developing Aspergillus niger as a cell factory for food enzyme production. Biotechnol. Adv. 2020, 44, 107630. [Google Scholar] [CrossRef] [PubMed]
- Ire, S.; Chima, I.J.; Ezebuiro, V. Biocatalysis and Agricultural Biotechnology Enhanced xylanase production from UV-mutated Aspergillus niger grown on corn cob and sawdust. Biocatal. Agric. Biotechnol. 2021, 31, 101869. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Zhao, S. Secretome diversity and quantitative analysis of cellulolytic Aspergillus fumigatus Z5 in the presence of different carbon sources. Biotechnol. Biofuels 2013, 6, 149. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, B.K.; Abbass, M. Studies on an alkali-thermostable xylanase from Aspergillus fumigatus MA28. 3 Biotech 2011, 1, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Gómez García, R.; Medina Morales, M.A.; Rodrìguez, R.; Farruggia, B.M.; Picó, G.A.; Aguilar, C.N. Production of a xylanase by Trichoderma harzianum (Hypocrea lixii) in solid-state fermentation and its recovery by an aqueous two-phase system. Can. J. Biotechnol. 2018, 2, 2560–8304. [Google Scholar] [CrossRef]
- Yan, S.; Xu, Y.; Yu, X.W. Rational engineering of xylanase hyper-producing system in Trichoderma reesei for efficient biomass degradation. Biotechnol. Biofuels 2021, 14, 90. [Google Scholar] [CrossRef]
- Moubasher, A.H.; Abdel-Sater, M.A.; Soliman, Z. Yeasts and filamentous fungi inhabiting guts of three insect species in Assiut, Egypt. Mycosphere 2017, 8, 1297–1316. [Google Scholar] [CrossRef]
- Pandey, A.K.; Edgard, G.; Negi, S. Optimization of concomitant production of cellulase and xylanase from Rhizopus oryzae SN5 through EVOP-factorial design technique and application in Sorghum Stover based bioethanol production. Renew. Energy 2016, 98, 51–56. [Google Scholar] [CrossRef]
- Scholl, A.L.; Menegol, D.; Pitarelo, A.P.; Fontana, R.C.; Filho, A.Z.; Ramos, L.P.; Camassola, M. Elephant grass pretreated by the steam explosion for inducing secretion of cellulases and xylanases by Penicillium echinulatum S1M29 solid-state cultivation. Ind. Crops Prod. 2015, 77, 97–107. [Google Scholar] [CrossRef]
- Varriale, S.; Houbraken, J.; Granchi, Z.; Pepe, O.; Grigoriev, I.V.; Henrissat, B.V.; Ronald, P.D. Talaromyces borbonicus sp. nov., a novel fungus from biodegraded Arundo donax with potential abilities in lignocellulose conversion. Mycologia 2018, 110, 316–324. [Google Scholar] [CrossRef]
- Lubbers, R.J.M.; Liwanag, A.J.; Peng, M.; Dilokpimol, A.; Benoit-Gelber, I.; de Vries, R.P. Evolutionary adaptation of Aspergillus niger for increased ferulic acid tolerance. J. Appl. Microbiol. 2020, 128, 735–746. [Google Scholar] [CrossRef]
- Barros, R.R.; Oliveira, R.A.; Gottschalk, L.M.; Bon, E.P. Production of cellulolytic enzymes by fungi Acrophialophora nainiana and Ceratocystis paradoxa using different carbon sources. Appl. Biochem. Biotechnol. 2010, 161, 448–454. [Google Scholar] [CrossRef]
- Wang, X.W.; Houbraken, J.; Groenewald, J.Z.; Meijer, M.; Andersen, B.; Nielsen, K.F.; Crous, P.W.; Samson, R.A. Diversity and taxonomy of Chaetomium and chaetomium-like fungi from indoor environments. Stud. Mycol. 2016, 84, 145–224. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).