Genome-Based Taxonomy of Species in the Pseudomonas syringae and Pseudomonas lutea Phylogenetic Groups and Proposal of Pseudomonas maioricensis sp. nov., Isolated from Agricultural Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pseudomonas Strains and Growth Conditions
2.2. Genome Sequencing
2.3. Phylogenomic Analysis
2.4. Genomic Insights
2.5. Characterisation of Strain S25T
3. Results
3.1. Phylogenomic Analysis
3.2. P. viridiflava Case Study
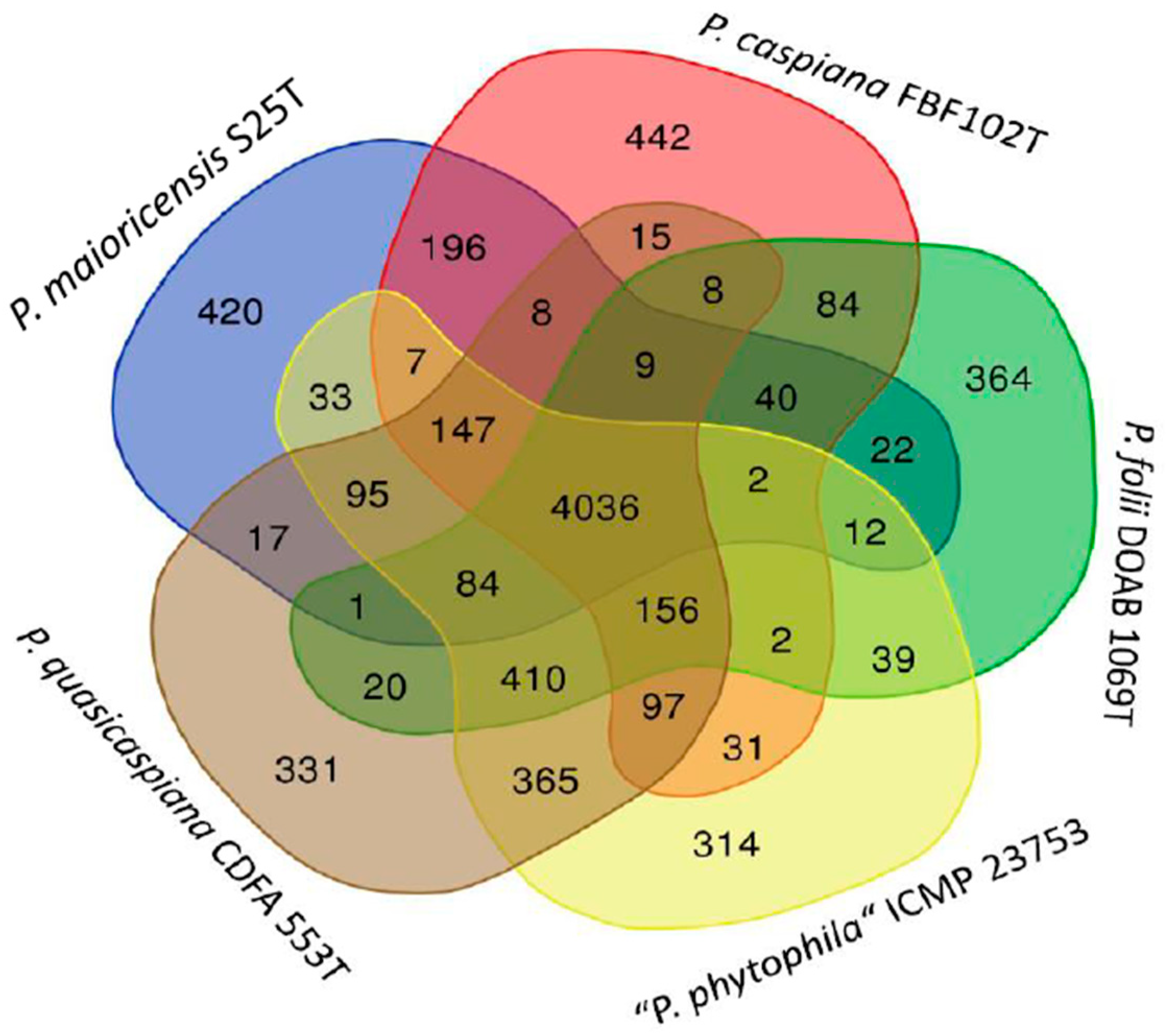
3.3. Gene Content Comparisons
3.4. Characterisation of P. maioricensis S25T sp. nov.
3.4.1. Genome Insights
3.4.2. Cell Morphology, Physiology and Biochemical Characterisation
3.4.3. Chemotaxonomic Analysis
4. Discussion
5. Description of Pseudomonas maioricensis sp. nov.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int. J. System. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Lalucat, J.; Gomila, M.; Mulet, M.; Zaruma, A.; García-Valdés, E. Past, present and future of the boundaries of the Pseudomonas genus: Proposal of Stutzerimonas gen. nov. Syst. Appl. Microbiol. 2022, 45, 126289. [Google Scholar] [CrossRef]
- Bashan, Y.; Okon, Y.; Henis, Y. Infection studies of Pseudomonas tomato, causal agent of bacterial speck of tomato. Phytoparasitica 1978, 6, 135–143. [Google Scholar] [CrossRef]
- Stefani, E.; Caffier, D.; Fiore, N. The economic impact of the bacterial blight of soybean under European agroclimatic conditions. J. Plant Pathol. 1998, 80, 211–221. [Google Scholar]
- Fatmi, M.B.; Collmer, A.; Sante Iacobellis, N.; Mansfield, J.W.; Murillo, J.; Schaad, N.W.; Ullrich, M. Pseudomonas syringae Pathovars and Related Pathogens—Identification, Epidemiology and Genomics; Springer: Dordrecht, The Netherlands, 2008; pp. 1–433. [Google Scholar]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Messéan, A.; Morris, C.E. Insights into epidemiology and control of diseases of annual plants caused by the Pseudomonas syringae species complex. J. Gen. Plant Pathol. 2015, 81, 331–350. [Google Scholar] [CrossRef]
- Berge, O.; Monteil, C.L.; Bartoli, C.; Chandeysson, C.; Guilbaud, C.; Sands, D.C.; Morris, C.E. A user’s guide to a data base of the diversity of Pseudomonas syringae and its application to classifying strains in this phylogenetic complex. PLoS ONE 2014, 9, e105547. [Google Scholar] [CrossRef] [PubMed]
- Gomila, M.; Busquets, A.; Mulet, M.; García-Valdés, E.; Lalucat, J. Clarification of Taxonomic Status within the Pseudomonas syringae Species Group Based on a Phylogenomic Analysis. J. Front. Microbiol. 2017, 8, 2422. [Google Scholar] [CrossRef]
- Lalucat, J.; Mulet, M.; Gomila, M.; García-Valdés, E. Genomics in Bacterial Taxonomy: Impact on the Genus Pseudomonas. Genes 2020, 11, 139. [Google Scholar] [CrossRef]
- Mulet, M.; Montaner, M.; Román, D.; Gomila, M.; Kittinger, C.; Zarfel, G.; Lalucat, J.; García-Valdés, E. Pseudomonas Species Diversity Along the Danube River Assessed by rpoD Gene Sequence and MALDI-TOF MS Analyses of Cultivated Strains. Front. Microbiol. 2020, 11, 2114. [Google Scholar] [CrossRef]
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory: New York, NY, USA, 1972. [Google Scholar]
- Richter, M.; Rosselló-Móra, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Avram, O.; Rapoport, D.; Portugez, S.; Pupko, T. M1CR0B1AL1Z3R-a user-friendly web server for the analysis of large-scale microbial genomics data. Nucleic Acids Res. 2019, 47, W88–W92. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 20 September 2023).
- Huson, D.H.; Bryant, D. Application of Phylogenetic Networks in Evolutionary Studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, J.C. Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams 2007–2015. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 20 September 2023).
- Winsor, G.L.; Griffiths, E.J.; Lo, R.; Dhillon, B.K.; Shay, J.A.; Brinkman, F.S. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 2016, 44, D646–D653. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Alanjary, M.; Steinke, K.; Ziemert, N. AutoMLST: An automated web server for generating multi-locus species trees highlighting natural product potential. Nucleic Acids Res. 2019, 47, W276–W282. [Google Scholar] [CrossRef]
- Parks, D.H.; Chuvochina, M.; Rinke, C.; Mussig, A.J.; Chaumeil, P.A.; Hugenholtz, P. GTDB: An ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 2021, 50, D785–D794. [Google Scholar] [CrossRef]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007, 35 (Suppl. S2), W182–W185. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.D.; Zhou, S.Y.; Chen, L.H.; Yang, J. VFDB 2022: A general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the comprehensive antibiotic resistance database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef]
- Lalucat, J. Analysis of Refractile (R) Bodies. In Methods in Microbiology—Electron Microscopy in Microbiology; Mayer, F., Ed.; Academic Press: London, UK, 1988; Volume 20, pp. 79–90. [Google Scholar]
- Beiki, F.; Busquets, A.; Gomila, M.; Rahimian, H.; Lalucat, J.; García-Valdés, E. New Pseudomonas spp. Are Pathogenic to Citrus. PLoS ONE 2016, 11, e0148796. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, D.; Mulet, M.; Rodríguez, A.C.; David, Z.; Lalucat, J.; García-Valdés, E. Pseudomonas aestusnigri sp. nov., isolated from crude oil-contaminated intertidal sand samples after the Prestige oil spill. Syst. Appl. Microbiol. 2014, 37, 89–94. [Google Scholar] [CrossRef]
- Sasser, M. Identification of Bacteria by Gas Chromatography of Cellular Fatty Acids; MIDI Technical Note 101; MIDI Inc.: Newark, DE, USA, 1990. [Google Scholar]
- MIDI Sherlock Microbial Identification System Operating Manual, Version 6. 1; MIDI Inc.: Newark, DE, USA, 2008. [Google Scholar]
- Karasov, T.L.; Almario, J.; Friedemann, C.; Ding, W.; Giolai, M.; Heavens, D.; Kersten, S.; Lundberg, D.S.; Neumann, M.; Regalado, J.; et al. Arabidopsis thaliana and Pseudomonas Pathogens exhibit stable associations over evolutionary timescales. Cell Host Microbe 2018, 24, 168–179.e4. [Google Scholar] [CrossRef] [PubMed]
- Busquets, A.; Gomila, M.; Beiki, F.; Mulet, M.; Rahimian, H.; García-Valdés, E.; Lalucat, J. Pseudomonas caspiana sp. nov., a citrus pathogen in the Pseudomonas syringae phylogenetic group. Syst. Appl. Microbiol. 2017, 40, 266–273. [Google Scholar] [CrossRef]
- Gonzalez, A.J.; Cleenwerck, I.; De Vos, P.; Fernandez-Sanz, A.M. Pseudomonas asturiensis sp. nov., isolated from soybean and weeds. Syst. Appl. Microbiol. 2013, 36, 320–324. [Google Scholar] [CrossRef]
- Gardan, L.; Shafik, H.; Belouin, S.; Broch, R.; Grimont, F.; Grimont, P.A. DNA relatedness among the pathovars of Pseudomonas syringae and description of Pseudomonas tremae sp. nov. and Pseudomonas cannabina sp. nov. (ex Sutic and Dowson 1959). Int. J. Syst. Bacteriol. 1999, 49 Pt 2, 469–478. [Google Scholar] [CrossRef]
- Scortichini, M.; Marcelletti, S.; Ferrante, P.; Firrao, G. A genomic redefinition of Pseudomonas avellanae species. PLoS ONE 2013, 8, e75794. [Google Scholar] [CrossRef]
- Schaad, N.W.; Cunfer, B.M. Synonymy of Pseudomonas coronafaciens, Pseudomonas coronafaciens pathovars zeae, Pseudomonas coronafaciens subsp. atropurpurea, and Pseudomonas striafaciens. Int. J. Syst. Bacteriol. 1979, 29, 213–221. [Google Scholar] [CrossRef]
- Dutta, B.; Gitaitis, R.; Agarwal, G.; Coutinho, T.; Langston, D. Pseudomonas coronafaciens sp. nov., a new phytobacterial species diverse from Pseudomonas syringae. PLoS ONE 2018, 13, e0208271. [Google Scholar] [CrossRef] [PubMed]
- Gardan, L.; Cottin, S.; Bollet, C.; Hunault, G. Phenotypic heterogeneity of Pseudomonas syringae van Hall. Res. Microbiol. 1991, 142, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Sarris, P.F.; Trantas, E.A.; Skandalis, N.; Tampakaki, A.P.; Kapanidou, M.; Kokkinidis, M.; Panopoulos, N.J. Phytobacterial Type VI Secretion System—Gene Distribution, Phylogeny, Structure and Biological Functions. In Plant Pathology; Cumagun, C.J.R., Ed.; InTech: London, UK, 2012; pp. 53–84. [Google Scholar] [CrossRef]
- Boak, E.N.; Kirolos, S.; Pan, H.; Pierson, L.S.; Pierson, E.A. The Type VI Secretion Systems in Plant-Beneficial Bacteria Modulate Prokaryotic and Eukaryotic Interactions in the Rhizosphere. Front. Microbiol. 2022, 13, 843092. [Google Scholar] [CrossRef]
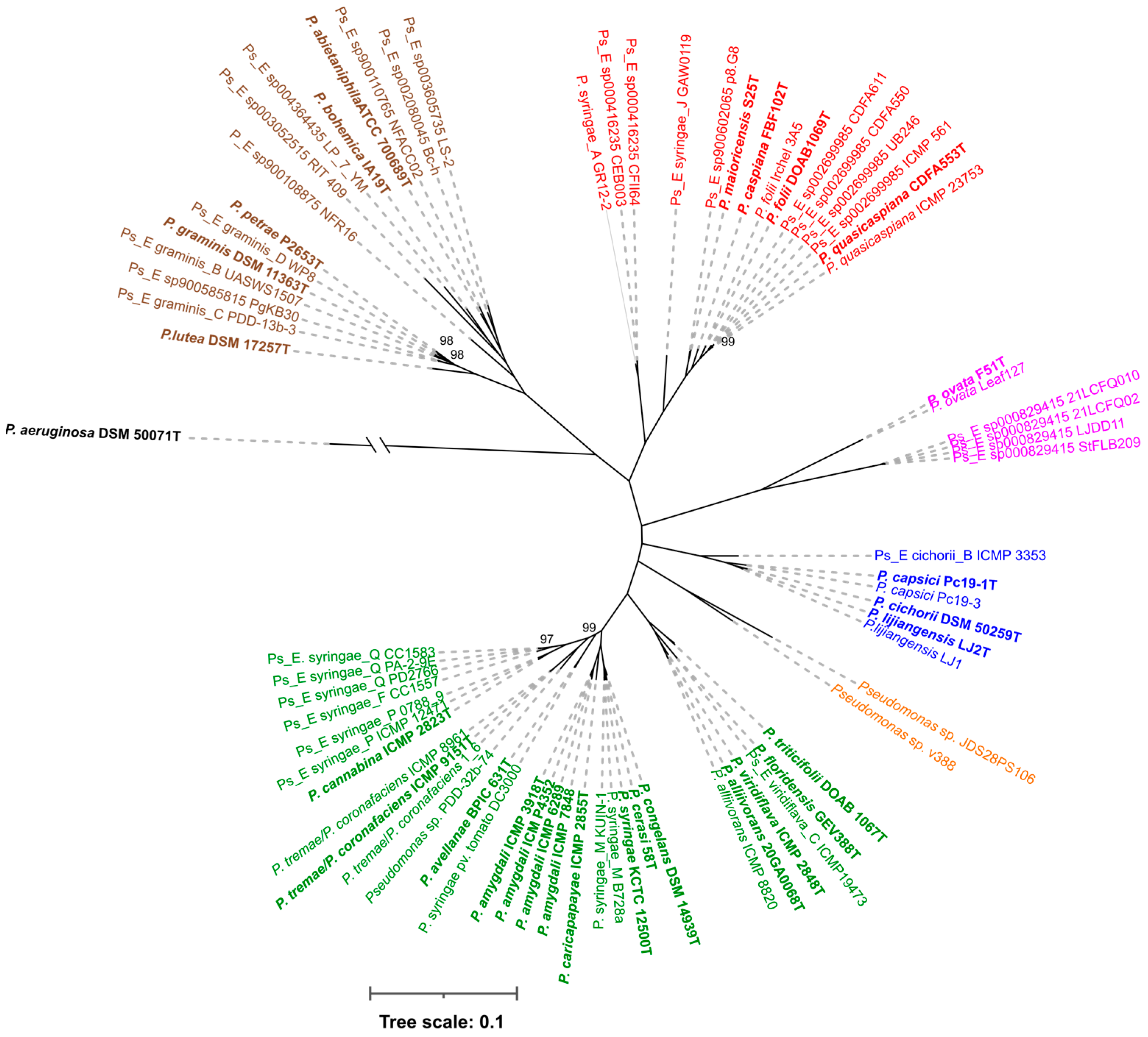
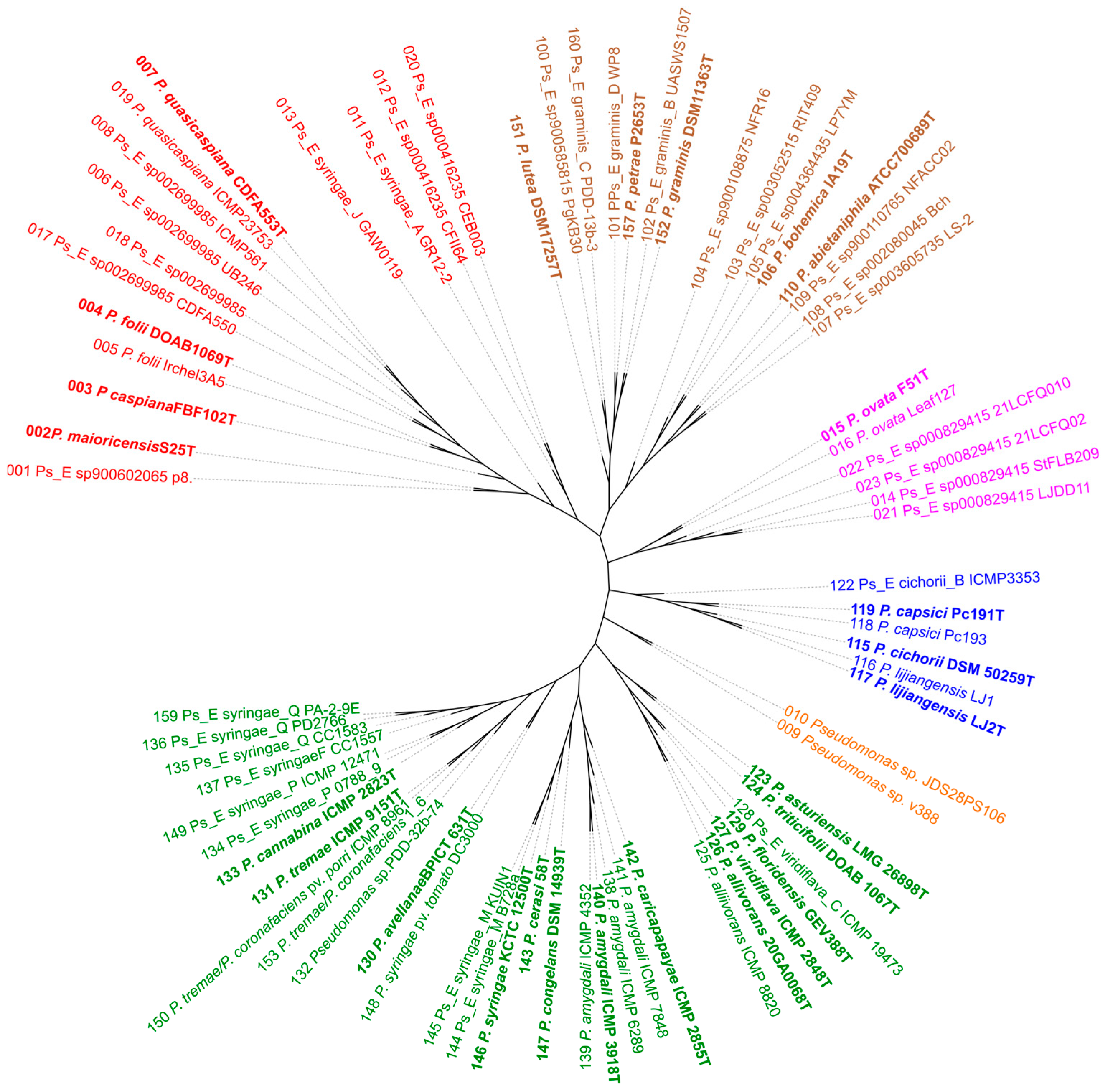
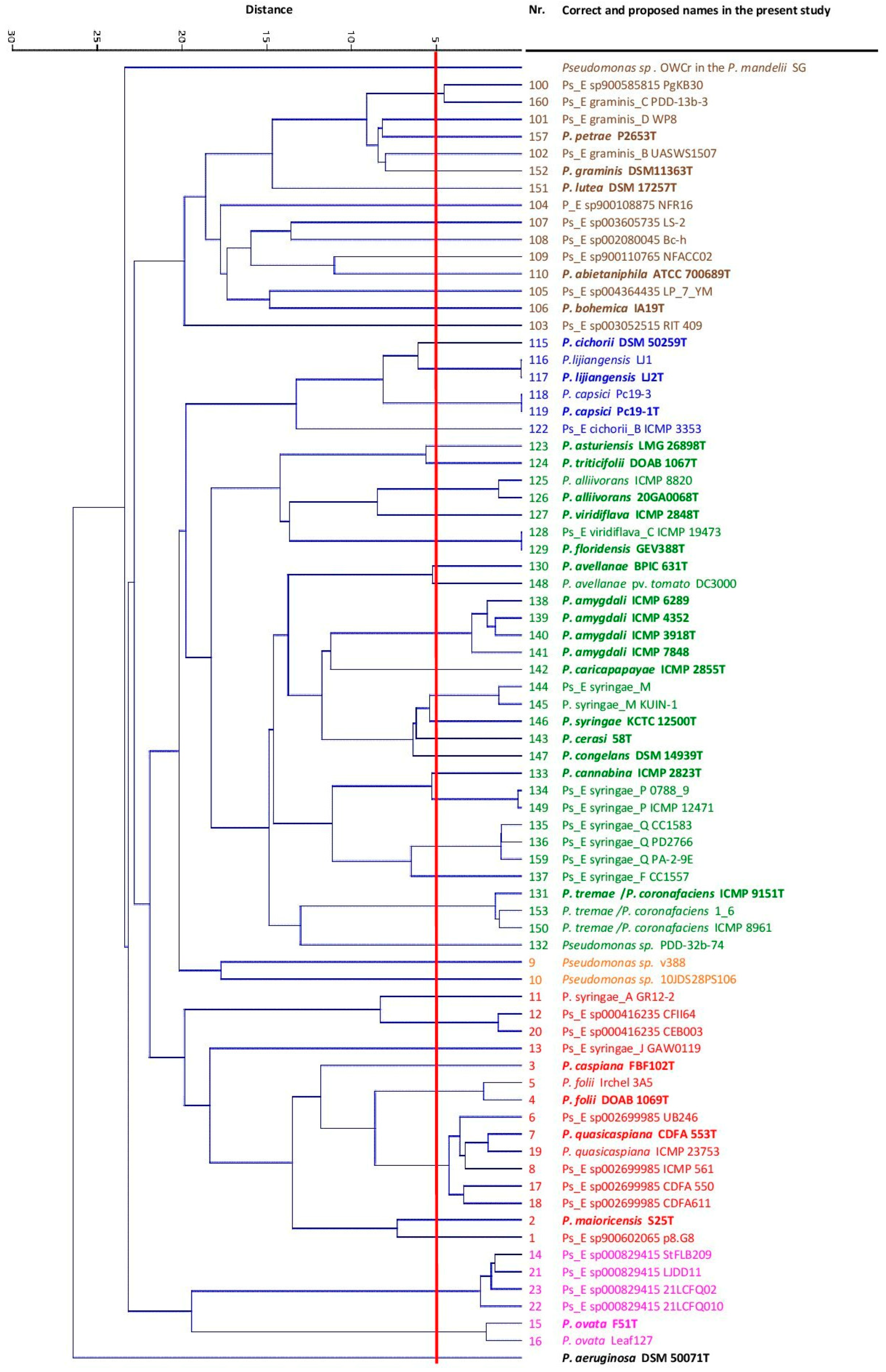


| Nr | Strain | NCBI Name | Assembly | GTDB Taxonomy | This Study | ||
|---|---|---|---|---|---|---|---|
| Proposed species name | Representative genome * | Strains in species cluster | Correct and proposed names in the present study | ||||
| -- | DSM 50071T | P. aeruginosa | GCF_001045685.1 | P. aeruginosa | false | 7037 | P. aeruginosa |
| -- | OWCr | P. caspiana | GCF_006439035.1 | Ps_E mandelii_C | false | 4 | Pseudomonas sp. in the P. mandelii SG |
| P. lutea SG | |||||||
| 100 | PgKB30 | P. graminis | GCF_013201545.1 | Ps_E sp900585815 | true | 5 | Ps_E sp900585815 |
| 160 | PDD-13b-3 | P. graminis | GCF_002093745.2 | Ps_E graminis_C | true | 3 | Ps_E graminis_C |
| 101 | WP8 | P. graminis | GCF_004364335.1 | Ps_E graminis_D | true | 1 | Ps_E graminis_D |
| 157 | P2653T | P. petrae | GCF_021728295.1 | Ps_E sp021728295 | true | 4 | P. petrae |
| 102 | UASWS1507 | P. graminis | GCF_001705435.1 | Ps_E graminis_B | true | 2 | Ps_E graminis_B |
| 152 | DSM11363T | P. graminis | GCF_900111735.1 | Ps_E graminis | true | 1 | P. graminis |
| 151 | DSM 17257T | P. lutea | GCF_000759445.1 | Ps_E lutea | true | 2 | P. lutea |
| 104 | NFR16 | Pseudomonas sp. | GCF_900108875.1 | Ps_E sp900108875 | true | 1 | P_E sp900108875 |
| 107 | LS-2 | Pseudomonas sp. | GCF_003605735.1 | Ps_E sp003605735 | true | 1 | Ps_E sp003605735 |
| 108 | Bc-h | Pseudomonas sp. | GCF_002080045.1 | Ps_E sp002080045 | true | 1 | Ps_E sp002080045 |
| 109 | NFACC02 | Pseudomonas sp. | GCF_900110765.1 | Ps_E sp900110765 | true | 1 | Ps_E sp900110765 |
| 110 | ATCC 700689T | P. abietaniphila | GCF_900100795.1 | Ps_E abietaniphila | true | 1 | P. abietaniphila |
| 105 | LP_7_YM | Pseudomonas sp. | GCF_004364435.1 | Ps_E sp004364435 | true | 1 | Ps_E sp004364435 |
| 106 | IA19T | P. bohemica | GCF_002934685.1 | Ps_E bohemica | true | 1 | P. bohemica |
| 103 | RIT 409 | Pseudomonas sp. | GCF_003052515.2 | Ps_E sp003052515 | true | 2 | Ps_E sp003052515 |
| P. cichorii branch | |||||||
| 115 | DSM 50259T | P. cichorii | GCF_018343775.1 | Ps_E cichorii | true | 13 | P. cichorii |
| 116 | LJ1 | P. lijiangensis | GCF_019718915.1 | Ps_E cichorii_C | false | 23 | P.lijiangensis |
| 117 | LJ2T | P. lijiangensis | GCF_018968705.1 | Ps_E cichorii_C | true | 23 | P. lijiangensis |
| 118 | Pc19-3 | P. capsici | GCF_017165745.1 | Ps_E capsici | false | 16 | P. capsici |
| 119 | Pc19-1T | P. capsici | GCF_017165765.1 | Ps_E capsici | true | 16 | P. capsici |
| 122 | ICMP 3353 | P. cichorii | GCF_003700275.1 | Ps_E cichorii_B | true | 1 | Ps_E cichorii_B |
| P. syringae branch | |||||||
| 123 | LMG 26898T | P. asturiensis | GCF_900143095.1 | Ps_E asturiensis | true | 2 | P. asturiensis |
| 124 | DOAB 1067T | P. triticumensis | GCF_014358015.1 | Ps_E triticumensis | true | 3 | P. triticifolii |
| 125 | ICMP 8820 | P. viridiflava | GCF_002723575.1 | Ps_E alliivorans | false | 7 | P. alliivorans |
| 126 | 20GA0068T | P. alliivorans | GCF_017826695.1 | Ps_E alliivorans | true | 7 | P. alliivorans |
| 127 | ICMP 2848T | P. viridiflava | GCF_001642795.1 | Ps_E viridiflava | true | 1390 | P. viridiflava |
| 128 | ICMP 19473 | P. viridiflava | GCF_003702045.1 | Ps_E viridiflava_C | true | 3 | Ps_E viridiflava_C |
| 129 | GEV388T | P. viridiflava | GCF_002087235.1 | Ps_E floridensis | true | 1 | P. floridensis |
| 130 | BPIC 631T | P. avellanae | GCF_000444135.1 | Ps_E avellanae | true | 353 | P. avellanae |
| 148 | DC3000 | P. syringae pv. tomato | GCF_000007805.1 | Ps_E avellanae | false | 353 | P. syringae pv. tomato |
| 133 | ICMP 2823T | P. cannabina | GCF_900100365.1 | Ps_E cannabina | true | 17 | P. cannabina |
| 134 | 0788_9 | P. syringae pv. cilantro | GCF_001293775.1 | Ps_E syringae_P | true | 3 | Ps_E syringae_P |
| 149 | ICMP 12471 | P. syringae pv. coriandricola | GCF_001400185.1 | Ps_E syringae_P | false | 3 | Ps_E syringae_P |
| 135 | CC1583 | P. syringae | GCF_000452665.1 | Ps_E syringae_Q | false | 41 | Ps_E. syringae_Q |
| 136 | PD2766 | P. syringae pv. syringae | GCF_001466965.1 | Ps_E syringae_Q | false | 41 | Ps_E syringae_Q |
| 159 | PA-2-9E | P. syringae | GCF_023278085.1 | Ps_E syringae_Q | true | 41 | Ps_E syringae_Q |
| 137 | CC1557 | P. syringae | GCF_000452705.1 | Ps_E syringae_F | true | 2 | Ps_E syringae_F |
| 131 | ICMP 9151T | P. tremae | GCF_001401155.1 | Ps_E tremae | true | 85 | P. tremae |
| 153 | 1_6 | P. coronafaciens pv. oryzae | GCF_000156995.2 | Ps_E tremae | false | 85 | P. tremae/P. coronafaciens |
| 150 | ICMP 8961 | P. coronafaciens pv. porri | GCF_001400915.1 | Ps_E tremae | false | 85 | P. tremae/P. coronafaciens |
| 132 | PDD-32b-74 | P. syringae | GCF_002157375.1 | Ps_E graminis_C | false | 3 | Pseudomonas sp. |
| 138 | ICMP6289T | P. meliae | GCF_001400515.1 | Ps_E amygdali | false | 262 | P. amygdali |
| 139 | ICMP4352T | P. savastanoipv. savastanoi | GCF_001401285.1 | Ps_E amygdali | false | 262 | P. amygdali |
| 140 | ICMP3918T | P. amygdali | GCF_002699855.1 | Ps_E amygdali | true | 262 | P. amygdali |
| 141 | ICMP7848T | P. ficuserectae | GCF_001400815.1 | Ps_E amygdali | false | 262 | P. amygdali |
| 142 | ICMP2855T | P. caricapapayae | GCF_001400735.1 | Ps_E caricapapayae | true | 13 | P. caricapapayae |
| 144 | B728a | P. syringae pv. syringae | GCF_000012245.1 | Ps_E syringae_M | false | 128 | P. syringae_M |
| 145 | KUIN-1 | Pseudomonas sp. | GCF_009176725.1 | Ps_E syringae_M | true | 128 | P. syringae_M |
| 146 | KCTC 12500T | P. syringae | GCF_000507185.2 | Ps_E syringae | true | 173 | P. syringae |
| 143 | 58T | P. cerasi | GCF_900074915.1 | Ps_E cerasi | true | 73 | P. cerasi |
| 147 | DSM 14939T | P. congelans | GCF_900103225.1 | Ps_E congelans | true | 21 | P. congelans |
| No assigned branch | |||||||
| 9 | v388 | Pseudomonas sp. | GCF_003935425.1 | Ps_E sp003935425 | true | 1 | |
| 10 | JDS28PS106 | Pseudomonas sp. | not available | -- | -- | Pseudomonas sp. | |
| P. caspiana branch | |||||||
| 11 | GR12-2 | P. syringae | GCF_001698815.1 | Ps_E syringae_A | true | 1 | P. syringae_A |
| 12 | CFII64 | Pseudomonas sp. | GCF_000416235.1 | Ps_E sp000416235 | true | 2 | Ps_E sp000416235 |
| 20 | CEB003 | P. syringae | GCF_000737235.1 | Ps_E sp000416235 | false | 2 | Ps_E sp000416235 |
| 13 | GAW0119 | P. syringae | GCA_000737245.1 | Ps_E syringae_J | true | 1 | Ps_E syringae_J |
| 3 | FBF102T | P. caspiana | GCF_002158995.1 | Ps_E caspiana | true | 1 | P. caspiana |
| 5 | Irchel 3A5 | P. syringae | GCF_900187575.1 | Ps_E foliumensis | false | 2 | P. folii |
| 4 | DOAB 1069T | P. foliumensis | GCF_014357575.1 | Ps_E foliumensis | true | 2 | P. folii |
| 6 | UB246 | P. syringae | GCF_000452865.1 | Ps_E sp002699985 | false | 11 | Ps_E sp002699985 |
| 7 | CDFA 553T | P. quasicaspiana | GCF_021147825.1 | Ps_E sp002699985 | false | 11 | P. quasicaspiana |
| 19 | ICMP 23753 | ‘P. phytophila’ | GCF_025643095.1 | not available | -- | -- | P. quasicaspiana |
| 8 | ICMP 561 | Pseudomonas sp. | GCF_002699985.1 | Ps_E sp002699985 | true | 11 | Ps_E sp002699985 |
| 17 | CDFA 550 | Pseudomonas sp. | GCF_021147785.1 | Ps_E sp002699985 | false | 11 | Ps_E sp002699985 |
| 18 | CDFA611 | Pseudomonas sp. | GCF_021147805.1 | Ps_E sp002699985 | false | 11 | Ps_E sp002699985 |
| 2 | S25T | Pseudomonassp. | GCF_022790535.1 | Ps_E sp022790535 | true | 1 | P. maioricensis |
| 1 | p8.G8 | P. viridiflava | GCF_900602065.1 | Ps_E sp900602065 | true | 1 | Ps_E sp900602065 |
| P. ovata branch | |||||||
| 14 | StFLB209 | Pseudomonas sp. | GCF_000829415.1 | Ps_E sp000829415 | true | 4 | Ps_E sp000829415 |
| 21 | LJDD11 | Pseudomonas sp. | GCF_024584215.1 | Ps_E sp000829415 | false | 4 | Ps_E sp000829415 |
| 23 | 21LCFQ02 | Pseudomonas sp. | GCF_024129895.1 | Ps_E sp000829415 | false | 4 | Ps_E sp000829415 |
| 22 | 21LCFQ010 | Pseudomonas sp. | GCF_024129905.1 | Ps_E sp000829415 | false | 4 | Ps_E sp000829415 |
| 15 | F51T | P. ovata | GCF_003131185.1 | Ps_E ovata | true | 3 | P. ovata |
| 16 | Leaf127 | Pseudomonas sp. | GCF_001423155.1 | Ps_E ovata | false | 3 | P. ovata |
| Strain | Genome Accession | GTDB Nomenclature | Genomes in GTDB Species Cluster | Genomes in Bioproject PRJEB24450 | Assignation to Phylogenetic Group | Identification |
|---|---|---|---|---|---|---|
| p2.B6 | GCA_900589175.1 | Ps_E asturiensis | 2 | 1 | syringae G | P. asturiensis |
| p11.A4 | GCA_900576645.1 | Ps_e atacamensis | 60 | 21 | fluorescens G | P. atacamensis |
| p11.G1 | GCA_900580895.1 | Ps_E avellanae | 353 | 20 | syringae G | P. avellanae/’P. tomato’ |
| p11.H3 | GCA_900581025.1 | Ps_E baltica | 7 | 1 | P. rhizospherae/P. coleopterorum | P. baltica |
| p11.B7 | GCA_900580485.1 | Ps_E canadensis | 13 | 9 | fluorescens G | P. canadensis |
| p11.C6 | GCA_900580595.1 | Ps_E coleopterorum | 9 | 2 | P. rhizospherae/P. coleopterorum | P. coleopterorum |
| p13.B5 | GCA_900581935.1 | Ps_E congelans | 21 | 7 | syringae G | P. congelans |
| p24.A10 | GCA_900576715.1 | Ps_E gregormendelii | 10 | 6 | fluorescens G | P. gregormendelii |
| p4.F8 | GCA_900591125.1 | Ps_E lurida | 28 | 5 | fluorescens G | P. lurida |
| p2.E10 | GCA_900589445.1 | Ps_E marginalis | 19 | 1 | fluorescens G | P. marginalis |
| p2.D4 | GCA_900589345.1 | Ps_E orientalis_A | 22 | 12 | fluorescens G | Ps_E orientalis_A |
| p9.C4 | GCA_900602385.1 | Ps_E ovata | 3 | 1 | syringae G | P. ovata |
| p11.F1 | GCA_900580855.1 | Ps_E poae | 12 | 2 | fluorescens G | P. poae |
| p8.D4 | GCA_900601745.1 | Ps_E salomonii | 16 | 5 | fluorescens G | P. salomonii |
| p2.D10 | GCA_900589385.1 | Ps_E sivasensis | 14 | 6 | fluorescens G | P. sivasensis |
| p23.G3 | GCA_900586135.1 | Ps_E sp001297015 | 11 | 5 | fluorescens G | Ps_E sp001297015 |
| p7.A9 | GCA_900600635.1 | Ps_E sp002699985 | 11 | 2 | caspiana SG | P. quasicaspiana |
| p2.G9 | GCA_900589715.1 | Ps_E sp002843605 | 7 | 6 | fluorescens G | Ps_E sp002843605 |
| p11.A6 | GCA_900576665.1 | Ps_E sp002979555 | 11 | 11 | fluorescens G | Ps_E sp002979555 |
| p9.H9 | GCA_900573885.1 | Ps_E sp900573885 | 2 | 2 | putida-oleovorans G | Ps_E sp900573885 |
| p11.D4 | GCA_900580675.1 | Ps_E sp900580675 | 1 | 1 | fluorescens G | Ps_E sp900580675 |
| p11.F9 | GCA_900580865.1 | Ps_E sp900580865 | 1 | 1 | fluorescens G | Ps_E sp900580865 |
| p11.H11 | GCA_900581005.1 | Ps_E sp900581005 | 1 | 1 | fluorescens G | Ps_E sp900581005 |
| p13.D5 | GCA_900582195.1 | Ps_E sp900582195 | 2 | 2 | fluorescens G | Ps_E sp900582195 |
| p13.G10 | GCA_900582425.1 | Ps_E sp900582625 | 4 | 4 | putida- oleovorans G | Ps_E sp900582625 |
| p3.E1 | GCA_900590325.1 | Ps_E sp900583165 | 8 | 7 | fluorescens G | Ps_E sp900583165 |
| p26.D9 | GCA_900588365.1 | Ps_E sp900585815 | 5 | 4 | lutea SG | Ps_E sp900585815 |
| p23.C6 | GCA_900585905.1 | Ps_E sp900585905 | 1 | 1 | caspiana SG | Ps_E sp900585905 |
| p1.E6 | GCA_900583105.1 | Ps_E sp900589395 | 8 | 3 | syringae G | Ps_E sp900589395 |
| p4.B4 | GCA_900590755.1 | Ps_E sp900590755 | 1 | 1 | caspiana SG | Ps_E sp900590755 |
| p4.G3 | GCA_900591205.1 | Ps_E sp900591205 | 1 | 1 | fluorescens G | Ps_E sp900591205 |
| p2.G1 | GCA_900589655.1 | Ps_E sp900596015 | 8 | 5 | P. ryzospherae-P. coleopterorum | Ps_E sp900596015 |
| p8.G2 | GCA_900601905.1 | Ps_E sp900601905 | 1 | 1 | caspiana branch | Ps_E sp900601905 |
| p8.G8 | GCA_900602065.1 | Ps_E sp900602065 | 1 | 1 | caspiana branch | Ps_E sp900602065 |
| p4.G2 | GCA_900591195.1 | Ps_E synxantha_A | 16 | 1 | fluorescens G | Ps_E synxantha_A |
| p26.C10 | GCA_900588235.1 | Ps_E syringae | 163 | 2 | syringae G | P. syringae |
| p4.D11 | GCA_900590885.1 | Ps_E viridiflava_D | 1 | 3 | fluorescens G | Ps_E viridiflava_D |
| Characteristic | P. maioricensis S25T | P. caspiana FBF102T | P. quasicaspiana LMG 32434T | P. folii LMG 32142T | P. syringae ATCC 19310T |
|---|---|---|---|---|---|
| API 20 NE: Hydrolysis of gelatine | − | − | − | − | + |
| BIOLOG GENIII tests: | |||||
| Carbon source utilisation assays | |||||
| D-Sorbitol | − | + | + | − | + |
| Pectin | + | − | − | − | − |
| D-Galacturonic Acid | + | + | + | + | − |
| Methyl Pyruvate | + | + | + | + | − |
| D-Galactonic Acid lactone | + | + | + | + | − |
| D-Trehalose | + | − | − | − | − |
| Beta-Methyl-D-Glucoside | + | − | − | − | w |
| Myo-Inositol | − | − | + | − | + |
| L-arginine | + | + | + | + | − |
| D-Cellobiose | + | − | − | − | + |
| D-Salicin | + | − | − | − | − |
| L-Aspartic Acid | + | + | + | + | − |
| D-Glucuronic Acid | + | + | + | + | w |
| Gentiobiose | + | − | − | − | − |
| Acetoacetic Acid | + | − | − | − | − |
| Sucrose | + | − | − | − | + |
| L-Histidine | + | + | + | + | − |
| D-Malic Acid | + | + | + | + | − |
| Propionic Acid | + | − | + | w | + |
| D-Aspartic Acid | + | − | − | − | − |
| Quinic acid | + | + | + | + | − |
| L-Malic Acid | + | + | + | + | w |
| D-Serine | w | + | − | w | + |
| Formic Acid | + | + | + | + | - |
| Chemical sensitivity assays (growth): | |||||
| Nalidixic Acid | − | + | + | + | w |
| Guanidine HCl | + | + | + | + | − |
| Lithium Chloride | + | − | + | − | + |
| Sodium Butyrate | + | w | w | w | − |
| 8% NaCl | + | + | w | w | − |
| Minocycline | + | w | − | − | − |
| Sodium Bromate | + | − | − | − | − |
| Species name | Pseudomonas maioricensis |
| Species etymology | P. maioricensis (mai.or.i.cen’sis. M.L. masc./fem. Adj. maioricensis, pertaining to the island of Mallorca, where the type strain of the species was isolated) sp. nov. |
| Species status | |
| Designation of the type strain | S25 |
| Strain collection numbers | CCUG 69272, CECT 30911 |
| 16S rRNA gene accession number | OR891488 |
| Alternative housekeeping genes | rpoD gene (OR900883) |
| Genome accession number | LOHG01 (GCF_022790535.1) |
| Genome status | Complete |
| Genome size (pb) | 5.911.519 bp |
| GC mol % | 57 |
| Country of origin | Spain |
| Region of origin | Balearic Islands |
| Date of isolation | 2010 |
| Source of isolation | Agricultural soil |
| Sampling date | 2010 |
| Geographic location | Mallorca |
| Latitude and Longitude | 39°38′11.8″ N, 2°38′50.1″ E |
| Growth medium, incubation conditions used for standard cultivation | Lysis broth (LB) at 30 °C |
| Gram stain | Negative |
| Cell shape Cell size (length or diameter) | Rod 1.9–2.5 µm long and 0.7–1.1 µm wide |
| Motility Colony morphology | Motile with one polar flagellum Round (1–1.5 mm of diameter) flat and beige coloured, regular margins and translucent |
| Temperature range for growth | 4–30 |
| Temperature optimum | 30 |
| pH range for growth NaCl range for growth | 5–9 0–6% |
| Metabolism | Aerobic, strictly respiratory |
| BIOLOG GENIII positive tests | Alfa-d-glucose, pectin, Tween 40, d-mannose, d-mannitol, glycyl-L-proline, d-galacturonic acid, methyl pyruvate, gamma-amino-butyric acid, d-fructose, d-arabitol, L-alanine, d-galactonic acid lactone, alfa-hydroxy butyric acid, d-trehalose, beta-methyl-d-glucoside, d-galactose, L-arginine, d-gluconic acid, L-lactic acid, beta-hydroxy-d,L butyric acid, d-cellobiose, d-salicin, glycogen, L-aspartic acid, d-glucuronic acid, citric acid, alfa-keto-butyric acid, gentiobiose, d-fucose, L-glutamic acid, glucuronamide, alfa-keto glutaric acid, acetoacetic acid, sucrose, L-histidine, mucic acid, d-malic acid, propionic acid, L-rhamnose, d-aspartic acid, L-pyroglutamic acid, quinic acid, L-malic Acid, acetic acid, inosine, L-serine, d-saccharic acid, formic acid |
| Negative tests with BIOLOG GENIII | d-raffinose, d-sorbitol, gelatin, p-hydroxy-phenylacetic acid, dextrin, alfa-d-lactose, d-maltose, d-lactic acid methyl ester, myo-inositol, 3-methyl glucose, N-acetyl-d-glucosamine, d-glucose-6-PO4, N-acetyl-beta-d-mannosamine, L-fucose, d-fructose-6-PO4, turanose, N-acetyl-d-galactosamine, stachyose, N-acetyl-neuraminic acid, bromo-succinic acid |
| Positive tests with API 20NE | Hydrolysis of aesculin, assimilation of glucose, arabinose, mannose, mannitol, gluconate, caprate, adipate, malate, citrate |
| Negative tests with API 20NE | Assimilation of N-acetyl-d-glucosamine, maltose, adipate, phenylacetate; reduction of nitrate to nitrite; reduction of nitrite to N2; indole production; glucose fermentation; presence of arginine dihydrolase and urease |
| Energy metabolism | Chemoorganotrophic, strictly respiratory. |
| Oxidase | Positive |
| Catalase | Positive |
| Pigment production on King A | Positive |
| Major fatty acids of the type strain | C10:0 3-OH (3.0%), C12:0 (6.3%), C12:0 2-OH (2.7%), C12:0 3-OH (5.3%), C16:0 (24.6%), C18:0 (1.5%), summed feature 3 C16:1 w7c/C16:1 w6c (342%) and summed feature 8 C18:1 w7c/C18:1 w6c (18.8%). C17:0 cyclo was present in low amounts (1.9%) |
| Biosafety level | 1 |
| Habitat | Soil |
| Biotic relationship | Free-living |
| Known pathogenicity | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulet, M.; Gomila, M.; Busquets, A.; Sánchez, D.; Lalucat, J.; García-Valdés, E. Genome-Based Taxonomy of Species in the Pseudomonas syringae and Pseudomonas lutea Phylogenetic Groups and Proposal of Pseudomonas maioricensis sp. nov., Isolated from Agricultural Soil. Microorganisms 2024, 12, 460. https://doi.org/10.3390/microorganisms12030460
Mulet M, Gomila M, Busquets A, Sánchez D, Lalucat J, García-Valdés E. Genome-Based Taxonomy of Species in the Pseudomonas syringae and Pseudomonas lutea Phylogenetic Groups and Proposal of Pseudomonas maioricensis sp. nov., Isolated from Agricultural Soil. Microorganisms. 2024; 12(3):460. https://doi.org/10.3390/microorganisms12030460
Chicago/Turabian StyleMulet, Magdalena, Margarita Gomila, Antonio Busquets, David Sánchez, Jorge Lalucat, and Elena García-Valdés. 2024. "Genome-Based Taxonomy of Species in the Pseudomonas syringae and Pseudomonas lutea Phylogenetic Groups and Proposal of Pseudomonas maioricensis sp. nov., Isolated from Agricultural Soil" Microorganisms 12, no. 3: 460. https://doi.org/10.3390/microorganisms12030460





