Blastocystis: A Mysterious Member of the Gut Microbiome
Abstract
:1. Introduction
2. Blastocystis and the Gut Microbiome
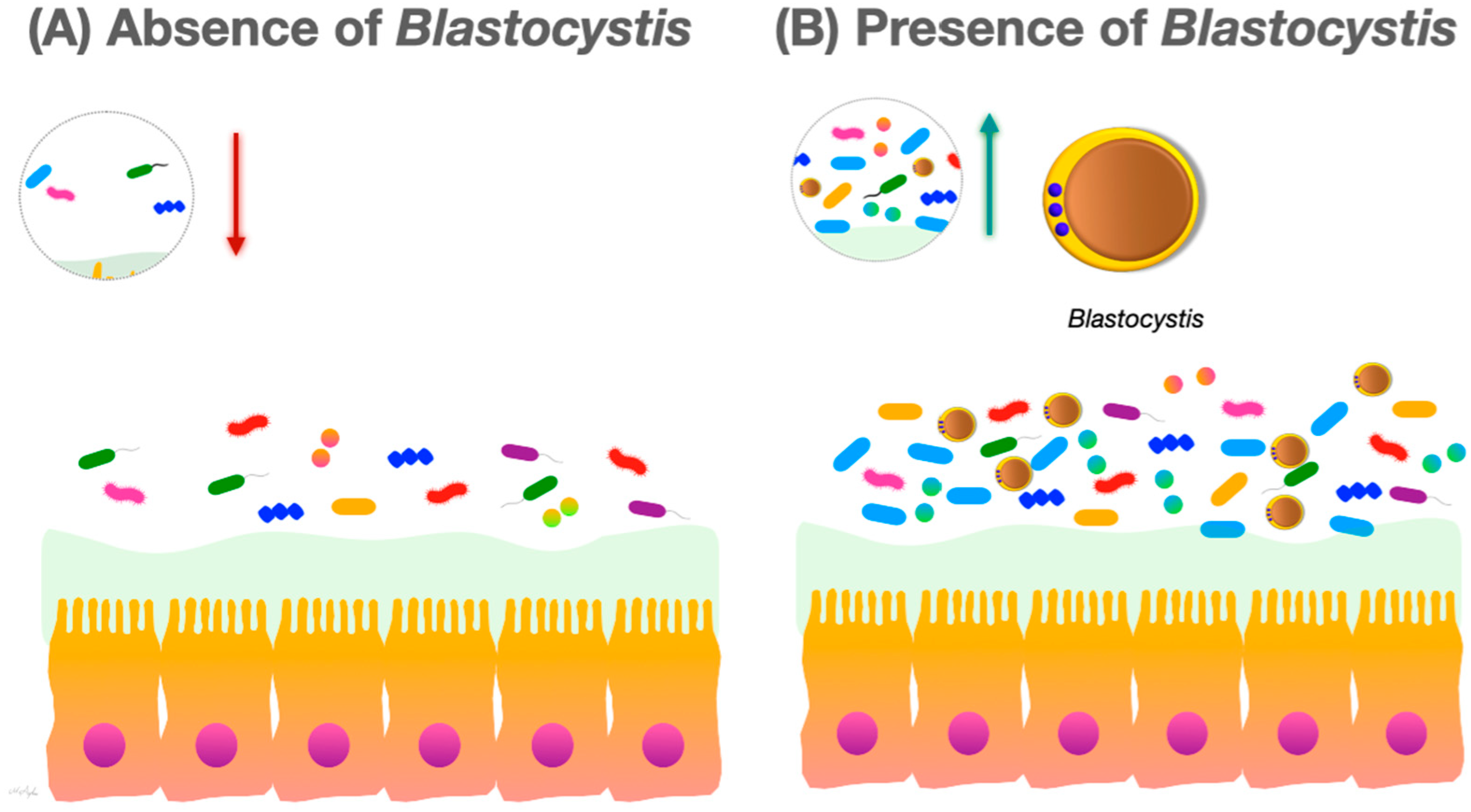
2.1. The Effect of Blastocystis on Gut Microbiome Modulation
2.2. The Effect of Blastocystis on Immune Modulation
2.3. The Interaction of Blastocystis and the Gut Microbiome in Autoimmune Diseases
3. Blastocystis and the Gut–Brain Axis
4. Blastocystis and Probiotics
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stensvold, C.R.; Tan, K.S.W.; Clark, C.G. Blastocystis. Trends Parasitol. 2020, 36, 315–316. [Google Scholar] [CrossRef]
- Tan, K.S. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 2008, 21, 639–665. [Google Scholar] [CrossRef]
- Clark, C.G.; Stensvold, C.R. Blastocystis: Isolation, Xenic Cultivation, and Cryopreservation. Curr. Protoc. Microbiol. 2016, 43, 20A.1.1–20A.1.8. [Google Scholar] [CrossRef]
- Tsaousis, A.D.; Hamblin, K.A.; Elliott, C.R.; Young, L.; Rosell-Hidalgo, A.; Gourlay, C.W.; Moore, A.L.; Van der Giezen, M. The human gut colonizer Blastocystis respires using complex II and alternative oxidase to buffer transient oxygen fluctuations in the gut. Front. Cell. Infect. Microbiol. 2018, 8, 371. [Google Scholar] [CrossRef]
- Tsaousis, A.D.; Ollagnier de Choudens, S.; Gentekaki, E.; Long, S.; Gaston, D.; Stechmann, A.; Vinella, D.; Py, B.; Fontecave, M.; Barras, F. Evolution of Fe/S cluster biogenesis in the anaerobic parasite Blastocystis. Proc. Natl. Acad. Sci. USA 2012, 109, 10426–10431. [Google Scholar] [CrossRef] [PubMed]
- Pyrihová, E.; King, M.S.; King, A.C.; Toleco, M.R.; van der Giezen, M.; Kunji, E.R. A mitochondrial carrier transports glycolytic intermediates to link cytosolic and mitochondrial glycolysis in the human gut parasite Blastocystis. bioRxiv 2023. [Google Scholar] [CrossRef]
- Popruk, S.; Pintong, A.-R.; Radomyos, P. Diversity of Blastocystis subtypes in humans. J. Trop. Med. Parasitol. 2013, 36, 88–97. [Google Scholar]
- El Safadi, D.; Cian, A.; Nourrisson, C.; Pereira, B.; Morelle, C.; Bastien, P.; Bellanger, A.-P.; Botterel, F.; Candolfi, E.; Desoubeaux, G. Prevalence, risk factors for infection and subtype distribution of the intestinal parasite Blastocystis sp. from a large-scale multi-center study in France. BMC Infect. Dis. 2016, 16, 451. [Google Scholar] [CrossRef] [PubMed]
- Alfellani, M.A.; Jacob, A.S.; Perea, N.O.; Krecek, R.C.; Taner-Mulla, D.; Verweij, J.J.; Levecke, B.; Tannich, E.; Clark, C.G.; Stensvold, C.R. Diversity and distribution of Blastocystis sp. subtypes in non-human primates. Parasitology 2013, 140, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Figueiredo, A.; Molokin, A.; George, N.S.; Köster, P.C.; Dashti, A.; González-Barrio, D.; Carmena, D.; Maloney, J.G. Division of Blastocystis ST10 into three new subtypes: ST42–ST44. J. Eukaryot. Microbiol. 2023, 71, e12998. [Google Scholar]
- Koehler, A.V.; Herath, H.D.; Hall, R.S.; Wilcox, S.; Gasser, R.B. Marked genetic diversity within Blastocystis in Australian wildlife revealed using a next generation sequencing–phylogenetic approach. Int. J. Parasitol. Parasites Wildl. 2024, 23, 100902. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Clark, C.G. Pre-empting Pandora’s Box: Blastocystis Subtypes Revisited. Trends Parasitol. 2020, 36, 229–232. [Google Scholar] [CrossRef]
- Higuera, A.; Salas-Leiva, D.E.; Curtis, B.; Patiño, L.H.; Zhao, D.; Jerlström-Hultqvist, J.; Dlutek, M.; Muñoz, M.; Roger, A.J.; Ramírez, J.D. Draft genomes of Blastocystis subtypes from human samples of Colombia. Parasites Vectors 2023, 16, 52. [Google Scholar] [CrossRef]
- Denoeud, F.; Roussel, M.; Noel, B.; Wawrzyniak, I.; Da Silva, C.; Diogon, M.; Viscogliosi, E.; Brochier-Armanet, C.; Couloux, A.; Poulain, J. Genome sequence of the stramenopile Blastocystis, a human anaerobic parasite. Genome Biol. 2011, 12, R29. [Google Scholar] [CrossRef] [PubMed]
- Gentekaki, E.; Curtis, B.A.; Stairs, C.W.; Klimes, V.; Elias, M.; Salas-Leiva, D.E.; Herman, E.K.; Eme, L.; Arias, M.C.; Henrissat, B.; et al. Extreme genome diversity in the hyper-prevalent parasitic eukaryote Blastocystis. PLoS Biol. 2017, 15, e2003769. [Google Scholar] [CrossRef] [PubMed]
- Robles-Cabrera, M.X.; Maguina, J.L.; Gonzales-Huerta, L.; Panduro-Correa, V.; Damaso-Mata, B.; Pecho-Silva, S.; Navarro-Solsol, A.C.; Rabaan, A.A.; Rodriguez-Morales, A.J.; Arteaga-Livias, K. Blastocystis species and Gastrointestinal Symptoms in Peruvian Adults Attended in a Public Hospital. Infect. Chemother. 2021, 53, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Cekin, A.H.; Cekin, Y.; Adakan, Y.; Tasdemir, E.; Koclar, F.G.; Yolcular, B.O. Blastocystosis in patients with gastrointestinal symptoms: A case–control study. BMC Gastroenterol. 2012, 12, 122. [Google Scholar] [CrossRef]
- Coyle, C.M.; Varughese, J.; Weiss, L.M.; Tanowitz, H.B. Blastocystis: To treat or not to treat. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 54, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Kurt, O.; Dogruman Al, F.; Tanyuksel, M. Eradication of Blastocystis in humans: Really necessary for all? Parasitol. Int. 2016, 65, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, F.; Babaei, E.; Badirzadeh, A.; Riabi, T.R.; Abdoli, A. Blastocystis, urticaria, and skin disorders: Review of the current evidences. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 39, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Aykur, M.; Camyar, A.; Turk, B.G.; Sin, A.Z.; Dagci, H. Evaluation of association with subtypes and alleles of Blastocystis with chronic spontaneous urticaria. Acta Trop. 2022, 231, 106455. [Google Scholar] [CrossRef]
- Nourrisson, C.; Wawrzyniak, I.; Cian, A.; Livrelli, V.; Viscogliosi, E.; Delbac, F.; Poirier, P. On Blastocystis secreted cysteine proteases: A legumain-activated cathepsin B increases paracellular permeability of intestinal Caco-2 cell monolayers. Parasitology 2016, 143, 1713–1722. [Google Scholar] [CrossRef]
- Krogsgaard, L.R.; Engsbro, A.L.; Stensvold, C.R.; Nielsen, H.V.; Bytzer, P. The prevalence of intestinal parasites is not greater among individuals with irritable bowel syndrome: A population-based case-control study. Clin. Gastroenterol. Hepatol. 2015, 13, 507–513.e2. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.; Stensvold, C.R.; Mirsepasi, H.; Engberg, J.; Friis-Moller, A.; Porsbo, L.J.; Hammerum, A.M.; Nordgaard-Lassen, I.; Nielsen, H.V.; Krogfelt, K.A. Active ulcerative colitis associated with low prevalence of Blastocystis and Dientamoeba fragilis infection. Scand. J. Gastroenterol. 2013, 48, 638–639. [Google Scholar] [CrossRef]
- Scanlan, P.D.; Stensvold, C.R.; Rajilic-Stojanovic, M.; Heilig, H.G.; De Vos, W.M.; O’Toole, P.W.; Cotter, P.D. The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol. Ecol. 2014, 90, 326–330. [Google Scholar] [CrossRef]
- Parfrey, L.W.; Walters, W.A.; Lauber, C.L.; Clemente, J.C.; Berg-Lyons, D.; Teiling, C.; Kodira, C.; Mohiuddin, M.; Brunelle, J.; Driscoll, M.; et al. Communities of microbial eukaryotes in the mammalian gut within the context of environmental eukaryotic diversity. Front. Microbiol. 2014, 5, 298. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; Ellis, J.; Harkness, J.; Marriott, D.; Stark, D. Treatment failure in patients with chronic Blastocystis infection. J. Med. Microbiol. 2014, 63, 252–257. [Google Scholar] [CrossRef]
- Poirier, P.; Wawrzyniak, I.; Albert, A.; El Alaoui, H.; Delbac, F.; Livrelli, V. Development and Evaluation of a Real-Time PCR Assay for Detection and Quantification of Blastocystis Parasites in Human Stool Samples: Prospective Study of Patients with Hematological Malignancies. J. Clin. Microbiol. 2011, 49, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Cahana, I.; Iraqi, F.A. Impact of host genetics on gut microbiome: Take-home lessons from human and mouse studies. Anim. Model. Exp. Med. 2020, 3, 229–236. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.B.; Tolonen, A.C.; Xavier, R.J. Human genetic variation and the gut microbiome in disease. Nat. Rev. Genet. 2017, 18, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Deidda, G.; Biazzo, M. Gut and Brain: Investigating Physiological and Pathological Interactions between Microbiota and Brain to Gain New Therapeutic Avenues for Brain Diseases. Front. Neurosci. 2021, 15, 753915. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, R.; Gundamaraju, R.; Shastri, M.D.; Shukla, S.D.; Kalpurath, K.; Ball, M.; Tristram, S.; Shankar, E.M.; Ahuja, K.; Eri, R. Gut Microbial Changes, Interactions, and Their Implications on Human Lifecycle: An Ageing Perspective. BioMed Res. Int. 2018, 2018, 4178607. [Google Scholar] [CrossRef]
- Gevers, D.; Knight, R.; Petrosino, J.F.; Huang, K.; McGuire, A.L.; Birren, B.W.; Nelson, K.E.; White, O.; Methe, B.A.; Huttenhower, C. The Human Microbiome Project: A community resource for the healthy human microbiome. PLoS Biol. 2012, 10, e1001377. [Google Scholar] [CrossRef]
- Ehrlich, S.D.; Consortium, M. MetaHIT: The European Union Project on Metagenomics of the Human Intestinal Tract. In Metagenomics of the Human Body; Springer: New York, NY, USA, 2011; pp. 307–316. [Google Scholar]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Zubeldia-Varela, E.; Barker-Tejeda, T.C.; Obeso, D.; Villasenor, A.; Barber, D.; Perez-Gordo, M. Microbiome and Allergy: New Insights and Perspectives. J. Investig. Allergol. Clin. Immunol. 2022, 32, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Gülden, E.; Wong, F.S.; Wen, L. The gut microbiota and type 1 diabetes. Clin. Immunol. 2015, 159, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Jalanka-Tuovinen, J.; Salojärvi, J.; Salonen, A.; Immonen, O.; Garsed, K.; Kelly, F.M.; Zaitoun, A.; Palva, A.; Spiller, R.C.; De Vos, W.M. Faecal microbiota composition and host–microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2014, 63, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Valdes, A.M. Role of the gut microbiome in chronic diseases: A narrative review. Eur. J. Clin. Nutr. 2022, 76, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.; Ash, A. Molecular epidemiology of Giardia and Cryptosporidium infections. Infect. Genet. Evol. 2016, 40, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Dubik, M.; Pilecki, B.; Moeller, J.B. Commensal Intestinal Protozoa—Underestimated Members of the Gut Microbial Community. Biology 2022, 11, 1742. [Google Scholar] [CrossRef] [PubMed]
- Hooshyar, H.; Rostamkhani, P.; Rezaeian, M. An Annotated checklist of the human and animal entamoeba (Amoebida: Endamoebidae) species—A review article. Iran. J. Parasitol. 2015, 10, 146. [Google Scholar]
- Lokmer, A.; Cian, A.; Froment, A.; Gantois, N.; Viscogliosi, E.; Chabé, M.; Ségurel, L. Use of shotgun metagenomics for the identification of protozoa in the gut microbiota of healthy individuals from worldwide populations with various industrialization levels. PLoS ONE 2019, 14, e0211139. [Google Scholar] [CrossRef]
- Garcia, L.S. Dientamoeba fragilis, One of the Neglected Intestinal Protozoa. J. Clin. Microbiol. 2016, 54, 2243–2250. [Google Scholar] [CrossRef]
- Shasha, D.; Grupel, D.; Treigerman, O.; Prajgrod, G.; Paran, Y.; Hacham, D.; Ben-Ami, R.; Albukrek, D.; Zacay, G. The clinical significance of Dientamoeba fragilis and Blastocystis in human stool—Retrospective cohort study. Clin. Microbiol. Infect. 2024, 30, 130–136. [Google Scholar] [CrossRef]
- Olyaiee, A.; Sadeghi, A.; Yadegar, A.; Mirsamadi, E.S.; Mirjalali, H. Gut Microbiota Shifting in Irritable Bowel Syndrome: The Mysterious Role of Blastocystis sp. Front. Med. 2022, 9, 890127. [Google Scholar] [CrossRef] [PubMed]
- Guangorena-Gomez, J.O.; Lozano, O., II; Rivera-Medina, I.L.; Mendez-Hernandez, A.; Espinosa-Fematt, J.A.; Munoz-Yanez, C. Relationship among Blastocystis, the Firmicutes/Bacteroidetes Ratio and Chronic Stress in Mexican University Students. Curr. Microbiol. 2022, 79, 72. [Google Scholar] [CrossRef]
- Even, G.; Lokmer, A.; Rodrigues, J.; Audebert, C.; Viscogliosi, E.; Segurel, L.; Chabe, M. Changes in the Human Gut Microbiota Associated with Colonization by Blastocystis sp. and Entamoeba spp. in Non-Industrialized Populations. Front. Cell. Infect. Microbiol. 2021, 11, 533528. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wojciech, L.; Gascoigne, N.R.J.; Peng, G.; Tan, K.S.W. New insights into the interactions between Blastocystis, the gut microbiota, and host immunity. PLoS Pathog. 2021, 17, e1009253. [Google Scholar] [CrossRef]
- Beghini, F.; Pasolli, E.; Truong, T.D.; Putignani, L.; Caccio, S.M.; Segata, N. Large-scale comparative metagenomics of Blastocystis, a common member of the human gut microbiome. ISME J. 2017, 11, 2848–2863. [Google Scholar] [CrossRef] [PubMed]
- Yanez, C.M.; Hernandez, A.M.; Sandoval, A.M.; Dominguez, M.A.M.; Muniz, S.A.Z.; Gomez, J.O.G. Prevalence of Blastocystis and its association with Firmicutes/Bacteroidetes ratio in clinically healthy and metabolically ill subjects. BMC Microbiol. 2021, 21, 339. [Google Scholar] [CrossRef]
- Nourrisson, C.; Scanzi, J.; Pereira, B.; NkoudMongo, C.; Wawrzyniak, I.; Cian, A.; Viscogliosi, E.; Livrelli, V.; Delbac, F.; Dapoigny, M.; et al. Blastocystis is associated with decrease of fecal microbiota protective bacteria: Comparative analysis between patients with irritable bowel syndrome and control subjects. PLoS ONE 2014, 9, e111868. [Google Scholar] [CrossRef]
- Shirvani, G.; Fasihi-Harandi, M.; Raiesi, O.; Bazargan, N.; Zahedi, M.J.; Sharifi, I.; Kalantari-Khandani, B.; Nooshadokht, M.; Shabandoust, H.; Mohammadi, M.A.; et al. Prevalence and Molecular Subtyping of Blastocystis from Patients with Irritable Bowel Syndrome, Inflammatory Bowel Disease and Chronic Urticaria in Iran. Acta Parasitol. 2020, 65, 90–96. [Google Scholar] [CrossRef]
- Yason, J.A.; Liang, Y.R.; Png, C.W.; Zhang, Y.; Tan, K.S.W. Interactions between a pathogenic Blastocystis subtype and gut microbiota: In vitro and in vivo studies. Microbiome 2019, 7, 30. [Google Scholar] [CrossRef]
- Billy, V.; Lhotska, Z.; Jirku, M.; Kadlecova, O.; Frgelecova, L.; Parfrey, L.W.; Pomajbikova, K.J. Blastocystis Colonization Alters the Gut Microbiome and, in Some Cases, Promotes Faster Recovery from Induced Colitis. Front. Microbiol. 2021, 12, 641483. [Google Scholar] [CrossRef]
- Behboud, S.; Solhjoo, K.; Erfanian, S.; Pirestani, M.; Abdoli, A. Alteration of gut bacteria composition among individuals with asymptomatic Blastocystis infection: A case-control study. Microb. Pathog. 2022, 169, 105639. [Google Scholar] [CrossRef]
- Nieves-Ramirez, M.E.; Partida-Rodriguez, O.; Laforest-Lapointe, I.; Reynolds, L.A.; Brown, E.M.; Valdez-Salazar, A.; Moran-Silva, P.; Rojas-Velazquez, L.; Morien, E.; Parfrey, L.W.; et al. Asymptomatic Intestinal Colonization with Protist Blastocystis Is Strongly Associated with Distinct Microbiome Ecological Patterns. mSystems 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Di Cristanziano, V.; Farowski, F.; Berrilli, F.; Santoro, M.; Di Cave, D.; Gle, C.; Daeumer, M.; Thielen, A.; Wirtz, M.; Kaiser, R.; et al. Analysis of Human Gut Microbiota Composition Associated to the Presence of Commensal and Pathogen Microorganisms in Cote d’Ivoire. Microorganisms 2021, 9, 1763. [Google Scholar] [CrossRef]
- Audebert, C.; Even, G.; Cian, A.; The Blastocystis Investigation Group; Loywick, A.; Merlin, S.; Viscogliosi, E.; Chabe, M. Colonization with the enteric protozoa Blastocystis is associated with increased diversity of human gut bacterial microbiota. Sci. Rep. 2016, 6, 25255. [Google Scholar] [CrossRef] [PubMed]
- Kodio, A.; Coulibaly, D.; Kone, A.K.; Konate, S.; Doumbo, S.; Guindo, A.; Bittar, F.; Gouriet, F.; Raoult, D.; Thera, M.A.; et al. Blastocystis Colonization Is Associated with Increased Diversity and Altered Gut Bacterial Communities in Healthy Malian Children. Microorganisms 2019, 7, 649. [Google Scholar] [CrossRef] [PubMed]
- Alzate, J.F.; Toro-Londono, M.; Cabarcas, F.; Garcia-Montoya, G.; Galvan-Diaz, A. Contrasting microbiota profiles observed in children carrying either Blastocystis spp. or the commensal amoebas Entamoeba coli or Endolimax nana. Sci. Rep. 2020, 10, 15354. [Google Scholar] [CrossRef] [PubMed]
- Asghari, A.; Hassanipour, S.; Hatam, G. Comparative molecular prevalence and subtypes distribution of Blastocystis sp. a potentially zoonotic infection isolated from symptomatic and asymptomatic patients in Iran: A systematic review and meta-analysis. Acta Parasitol. 2021, 66, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.D.; Sanchez, A.; Hernandez, C.; Florez, C.; Bernal, M.C.; Giraldo, J.C.; Reyes, P.; Lopez, M.C.; Garcia, L.; Cooper, P.J.; et al. Geographic distribution of human Blastocystis subtypes in South America. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2016, 41, 32–35. [Google Scholar] [CrossRef]
- Yowang, A.; Tsaousis, A.D.; Chumphonsuk, T.; Thongsin, N.; Kullawong, N.; Popluechai, S.; Gentekaki, E. High diversity of Blastocystis subtypes isolated from asymptomatic adults living in Chiang Rai, Thailand. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2018, 65, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wojciech, L.; Png, C.W.; Kioh, Y.Q.D.; Ng, G.C.; Chan, E.C.Y.; Zhang, Y.; Gascoigne, N.R.J.; Tan, K.S.W. Colonization with ubiquitous protist Blastocystis ST1 ameliorates DSS-induced colitis and promotes beneficial microbiota and immune outcomes. NPJ Biofilms Microbiomes 2023, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.O.; Bonde, I.; Nielsen, H.B.; Stensvold, C.R. A retrospective metagenomics approach to studying Blastocystis. FEMS Microbiol. Ecol. 2015, 91, fiv072. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wojciech, L.; Png, C.W.; Koh, E.Y.; Aung, T.T.; Kioh, D.Y.Q.; Chan, E.C.Y.; Malleret, B.; Zhang, Y.; Peng, G.; et al. Experimental colonization with Blastocystis ST4 is associated with protective immune responses and modulation of gut microbiome in a DSS-induced colitis mouse model. Cell. Mol. Life Sci. 2022, 79, 245. [Google Scholar] [CrossRef] [PubMed]
- Feranmi, F. Blastocystis subtype 4 linked to gut microbiota stability. Lancet Microbe 2022, 3, e564. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Tan, K.S.W. Interactions between Blastocystis subtype ST4 and gut microbiota in vitro. Parasites Vectors 2022, 15, 80. [Google Scholar] [CrossRef]
- Forsell, J.; Bengtsson-Palme, J.; Angelin, M.; Johansson, A.; Evengard, B.; Granlund, M. The relation between Blastocystis and the intestinal microbiota in Swedish travellers. BMC Microbiol. 2017, 17, 231. [Google Scholar] [CrossRef]
- Tito, R.Y.; Chaffron, S.; Caenepeel, C.; Lima-Mendez, G.; Wang, J.; Vieira-Silva, S.; Falony, G.; Hildebrand, F.; Darzi, Y.; Rymenans, L.; et al. Population-level analysis of Blastocystis subtype prevalence and variation in the human gut microbiota. Gut 2019, 68, 1180–1189. [Google Scholar] [CrossRef]
- Hillestad, E.M.R.; van der Meeren, A.; Nagaraja, B.H.; Bjorsvik, B.R.; Haleem, N.; Benitez-Paez, A.; Sanz, Y.; Hausken, T.; Lied, G.A.; Lundervold, A.; et al. Gut bless you: The microbiota-gut-brain axis in irritable bowel syndrome. World J. Gastroenterol. 2022, 28, 412–431. [Google Scholar] [CrossRef]
- Sugahara, H.; Odamaki, T.; Fukuda, S.; Kato, T.; Xiao, J.Z.; Abe, F.; Kikuchi, J.; Ohno, H. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci. Rep. 2015, 5, 13548. [Google Scholar] [CrossRef]
- Walter, J. Ecological role of lactobacilli in the gastrointestinal tract: Implications for fundamental and biomedical research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wojciech, L.; Png, C.W.; Kioh, D.Y.Q.; Gu, Y.; Aung, T.T.; Malleret, B.; Chan, E.C.Y.; Peng, G.; Zhang, Y. Colonization with two different Blastocystis subtypes in DSS-induced colitis mice is associated with strikingly different microbiome and pathological features. Theranostics 2023, 13, 1165. [Google Scholar] [CrossRef] [PubMed]
- Nagel, R.; Traub, R.J.; Allcock, R.J.; Kwan, M.M.; Bielefeldt-Ohmann, H. Comparison of faecal microbiota in Blastocystis-positive and Blastocystis-negative irritable bowel syndrome patients. Microbiome 2016, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, S.; Dogan, I.; Dogruman-Al, F.; Nalbantoglu, U.; Ustek, D.; Sarzhanov, F.; Yildirim, S. Association of Enteric Protist Blastocystis spp. and Gut Microbiota with Hepatic Encephalopathy. J. Gastrointest. Liver Dis. 2016, 25, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Passaro, G.; Gasbarrini, A.; Landolfi, R.; Montalto, M. Modulation of microbiota as treatment for intestinal inflammatory disorders: An uptodate. World J. Gastroenterol. 2016, 22, 7186–7202. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Velázquez, L.; Morán, P.; Serrano-Vázquez, A.; Portillo-Bobadilla, T.; González, E.; Pérez-Juárez, H.; Hernández, E.; Partida-Rodríguez, O.; Nieves-Ramírez, M.; Padilla, A.; et al. The regulatory function of Blastocystis spp. on the immune inflammatory response in the gut microbiome. Front. Cell. Infect. Microbiol. 2022, 12, 967724. [Google Scholar] [CrossRef] [PubMed]
- Betts, E.L.; Newton, J.M.; Thompson, G.S.; Sarzhanov, F.; Jinatham, V.; Kim, M.-J.; Popluechai, S.; Dogruman-Al, F.; Won, E.-J.; Gentekaki, E. Metabolic fluctuations in the human stool obtained from Blastocystis carriers and non-carriers. Metabolites 2021, 11, 883. [Google Scholar] [CrossRef]
- Tan, K.S.W.; Mirza, H. Blastocystis–Host Interactions: Insights from In Vitro Model Systems. In Blastocystis: Pathogen or Passenger? An Evaluation of 101 Years of Research; Mehlhorn, H., Tan, K.S.W., Yoshikawa, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 51–63. [Google Scholar] [CrossRef]
- Partida-Rodriguez, O.; Serrano-Vazquez, A.; Nieves-Ramirez, M.E.; Moran, P.; Rojas, L.; Portillo, T.; Gonzalez, E.; Hernandez, E.; Finlay, B.B.; Ximenez, C. Human Intestinal Microbiota: Interaction between Parasites and the Host Immune Response. Arch. Med. Res. 2017, 48, 690–700. [Google Scholar] [CrossRef]
- Reynolds, L.A.; Finlay, B.B.; Maizels, R.M. Cohabitation in the Intestine: Interactions among Helminth Parasites, Bacterial Microbiota, and Host Immunity. J. Immunol. 2015, 195, 4059–4066. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Ahrodia, T.; Das, S.; Bakshi, S.; Das, B. Structure, functions, and diversity of the healthy human microbiome. Prog. Mol. Biol. Transl. 2022, 191, 53–82. [Google Scholar] [CrossRef]
- Belzer, C.; Chia, L.W.; Aalvink, S.; Chamlagain, B.; Piironen, V.; Knol, J.; de Vos, W.M. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B12 production by intestinal symbionts. Mbio 2017, 8, e00770-17. [Google Scholar] [CrossRef] [PubMed]
- Llinás-Caballero, K.; Caraballo, L. Helminths and bacterial microbiota: The interactions of two of humans’ “old friends”. Int. J. Mol. Sci. 2022, 23, 13358. [Google Scholar] [CrossRef] [PubMed]
- Schonfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Woof, J.M.; Kerr, M.A. The function of immunoglobulin A in immunity. J. Pathol. J. Pathol. Soc. Great Br. Irel. 2006, 208, 270–282. [Google Scholar] [CrossRef]
- Puthia, M.K.; Vaithilingam, A.; Lu, J.; Tan, K.S. Degradation of human secretory immunoglobulin A by Blastocystis. Parasitol. Res. 2005, 97, 386–389. [Google Scholar] [CrossRef]
- Puthia, M.K.; Lu, J.; Tan, K.S. Blastocystis ratti contains cysteine proteases that mediate interleukin-8 response from human intestinal epithelial cells in an NF-κB-dependent manner. Eukaryot. Cell 2008, 7, 435–443. [Google Scholar] [CrossRef]
- Long, H.; Handschack, A.; König, W.; Ambrosch, A. Blastocystis hominis modulates immune responses and cytokine release in colonic epithelial cells. Parasitol. Res. 2001, 87, 1029–1030. [Google Scholar] [CrossRef]
- Lim, M.X.; Png, C.W.; Tay, C.Y.; Teo, J.D.; Jiao, H.; Lehming, N.; Tan, K.S.; Zhang, Y. Differential regulation of proinflammatory cytokine expression by mitogen-activated protein kinases in macrophages in response to intestinal parasite infection. Infect. Immun. 2014, 82, 4789–4801. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Deng, Z. Imbalanced shift of cytokine expression between T helper 1 and T helper 2 (Th1/Th2) in intestinal mucosa of patients with post-infectious irritable bowel syndrome. BMC Gastroenterol. 2012, 12, 91. [Google Scholar] [CrossRef]
- Lukeš, J.; Stensvold, C.R.; Jirků-Pomajbíková, K.; Wegener Parfrey, L. Are human intestinal eukaryotes beneficial or commensals? PLoS Pathog. 2015, 11, e1005039. [Google Scholar] [CrossRef]
- Shaheen, W.A.; Quraishi, M.N.; Iqbal, T.H. Gut microbiome and autoimmune disorders. Clin. Exp. Immunol. 2022, 209, 161–174. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ismail, K.A.; Khalifa, O.M.; Abdel-wahab, M.M.; Hagag, H.M.; Mahmoud, M.K. Molecular Identification of Blastocystis hominis Isolates in Patients with Autoimmune Diseases. Appl. Microbiol. 2023, 3, 417–428. [Google Scholar] [CrossRef]
- Keshawy, M.M.; Alabbassy, M.M. Systemic Lupus Erythematosus and Irritable Bowel Syndrome: Is Blastocystis Hominis the Missing Piece of the Puzzle. Suez Canal Univ. Med. J. 2018, 21, 88–98. [Google Scholar] [CrossRef]
- Jimenez-Balderas, F.; Camargo-Coronel, A.; Gargia-Jaimes, J.; Zonana-Nacach, A.; Alcantara-Anguianoi, I.; Carrillo-Becerril, L.; Tapia-Romero, R.; Gonzalez, T.; Villalobos-Gomez, F.; Martinez-Hernandez, S. A study on parasites in Mexican rheumatic disease patients. J. Egypt. Soc. Parasitol. 2012, 42, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Alamlih, L.; Abufaied, M.; Al-Allaf, A.-W. Classical reactive arthritis (ReA) due to Blastocystis infection: A very unusual trigger. Rheumatology 2019, 58, kez108.019. [Google Scholar] [CrossRef]
- Nieto-Clavijo, C.; Morales, L.; Marquez-Ortíz, R.A.; Romero-Sánchez, C.; Ramos-Casallas, A.; Escobar-Perez, J.; Bautista-Molano, W.; Bello-Gualtero, J.M.; Chaparro-Olaya, J. Differential gut microbiome in spondyloarthritis patients associated to Blastocystis colonization. Sci. Rep. 2023, 13, 13480. [Google Scholar] [CrossRef] [PubMed]
- Bakland, G.; Nossent, H.C. Epidemiology of spondyloarthritis: A review. Curr. Rheumatol. Rep. 2013, 15, 351. [Google Scholar] [CrossRef] [PubMed]
- Stoll, M.L.; Weiss, P.F.; Weiss, J.E.; Nigrovic, P.A.; Edelheit, B.S.; Bridges, S.L., Jr.; Danila, M.I.; Spencer, C.H.; Punaro, M.G.; Schikler, K.; et al. Age and fecal microbial strain-specific differences in patients with spondyloarthritis. Arthritis Res. Ther. 2018, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Zhang, P.; Song, C.; Pan, F.; Li, G.; Peng, L.; Yang, Y.; Wei, Z.; Huang, F. Gut microbiota changes in patients with spondyloarthritis: A systematic review. Semin. Arthritis Rheum. 2022, 52, 151925. [Google Scholar] [CrossRef] [PubMed]
- Tito, R.Y.; Cypers, H.; Joossens, M.; Varkas, G.; Van Praet, L.; Glorieus, E.; Van den Bosch, F.; De Vos, M.; Raes, J.; Elewaut, D. Brief Report: Dialister as a Microbial Marker of Disease Activity in Spondyloarthritis. Arthritis Rheumatol. 2017, 69, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Breban, M.; Tap, J.; Leboime, A.; Said-Nahal, R.; Langella, P.; Chiocchia, G.; Furet, J.P.; Sokol, H. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann. Rheum. Dis. 2017, 76, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zheng, Z.; Shao, T.; Liu, L.; Xie, Z.; Le Chatelier, E.; He, Z.; Zhong, W.; Fan, Y.; Zhang, L.; et al. Quantitative metagenomics reveals unique gut microbiome biomarkers in ankylosing spondylitis. Genome Biol. 2017, 18, 142. [Google Scholar] [CrossRef]
- Shaikh, S.D.; Sun, N.; Canakis, A.; Park, W.Y.; Weber, H.C. Irritable Bowel Syndrome and the Gut Microbiome: A Comprehensive Review. J. Clin. Med. 2023, 12, 2558. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.S.; Chang, P.F.; Liao, C.H.; Lee, T.H.; Chen, Y.; Lee, Y.C.; Wu, M.S.; Wang, H.P.; Ni, Y.H. Differences of microbiota in small bowel and faeces between irritable bowel syndrome patients and healthy subjects. Scand. J. Gastroenterol. 2016, 51, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Carroll, I.M.; Ringel-Kulka, T.; Siddle, J.P.; Ringel, Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2012, 24, 521-e248. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut Microbiota in Patients with Irritable Bowel Syndrome—A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef]
- Liu, H.N.; Wu, H.; Chen, Y.Z.; Chen, Y.J.; Shen, X.Z.; Liu, T.T. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig. Liver Dis. 2017, 49, 331–337. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef]
- Costea, P.I.; Hildebrand, F.; Arumugam, M.; Backhed, F.; Blaser, M.J.; Bushman, F.D.; de Vos, W.M.; Ehrlich, S.D.; Fraser, C.M.; Hattori, M.; et al. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol. 2018, 3, 8–16. [Google Scholar] [CrossRef]
- Ou, J.; Carbonero, F.; Zoetendal, E.G.; DeLany, J.P.; Wang, M.; Newton, K.; Gaskins, H.R.; O’Keefe, S.J. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin. Nutr. 2013, 98, 111–120. [Google Scholar] [CrossRef]
- Sobhani, I.; Tap, J.; Roudot-Thoraval, F.; Roperch, J.P.; Letulle, S.; Langella, P.; Corthier, G.; Tran Van Nhieu, J.; Furet, J.P. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS ONE 2011, 6, e16393. [Google Scholar] [CrossRef]
- De Palma, G.; Nadal, I.; Medina, M.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010, 10, 63. [Google Scholar] [CrossRef]
- Liu, L.; Huh, J.R.; Shah, K. Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. EBioMedicine 2022, 77, 103908. [Google Scholar] [CrossRef]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. [Google Scholar] [CrossRef]
- Fulling, C.; Dinan, T.G.; Cryan, J.F. Gut Microbe to Brain Signaling: What Happens in Vagus. Neuron 2019, 101, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Blecharz-Klin, K.; Swierczynska, M.; Piechal, A.; Wawer, A.; Joniec-Maciejak, I.; Pyrzanowska, J.; Wojnar, E.; Zawistowska-Deniziak, A.; Sulima-Celinska, A.; Mlocicki, D.; et al. Infection with intestinal helminth (Hymenolepis diminuta) impacts exploratory behavior and cognitive processes in rats by changing the central level of neurotransmitters. PLoS Pathog. 2022, 18, e1010330. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- McKenney, E.A.; Williamson, L.; Yoder, A.D.; Rawls, J.F.; Bilbo, S.D.; Parker, W. Alteration of the rat cecal microbiome during colonization with the helminth Hymenolepis diminuta. Gut Microbes 2015, 6, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhu, Y.; Zhou, L.; Lu, Y.; Feng, T.; Dai, M.; Liu, J.; Xu, W.; Cheng, W.; Sun, F.; et al. Parasite-Derived Excretory-Secretory Products Alleviate Gut Microbiota Dysbiosis and Improve Cognitive Impairment Induced by a High-Fat Diet. Front. Immunol. 2021, 12, 710513. [Google Scholar] [CrossRef] [PubMed]
- Defaye, M.; Nourrisson, C.; Baudu, E.; Lashermes, A.; Meynier, M.; Meleine, M.; Wawrzyniak, I.; Bonnin, V.; Barbier, J.; Chassaing, B.; et al. Fecal dysbiosis associated with colonic hypersensitivity and behavioral alterations in chronically Blastocystis-infected rats. Sci. Rep. 2020, 10, 9146. [Google Scholar] [CrossRef] [PubMed]
- Mayneris-Perxachs, J.; Arnoriaga-Rodriguez, M.; Garre-Olmo, J.; Puig, J.; Ramos, R.; Trelis, M.; Burokas, A.; Coll, C.; Zapata-Tona, C.; Pedraza, S.; et al. Presence of Blastocystis in gut microbiota is associated with cognitive traits and decreased executive function. ISME J. 2022, 16, 2181–2197. [Google Scholar] [CrossRef]
- Borre, Y.E.; Moloney, R.D.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The impact of microbiota on brain and behavior: Mechanisms & therapeutic potential. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Springer: New York, NY, USA, 2014; pp. 373–403. [Google Scholar]
- Leonardi, S.S.; Tan, K.S.-W. Blastocystis: View from atop the gut–brain iceberg. Trends Parasitol. 2023, 40, 1–4. [Google Scholar] [CrossRef]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef]
- Khine, W.W.T.; Voong, M.L.; Ng, T.K.S.; Feng, L.; Rane, G.A.; Kumar, A.P.; Kua, E.H.; Mahendran, R.; Mahendran, R.; Lee, Y.K. Mental awareness improved mild cognitive impairment and modulated gut microbiome. Aging 2020, 12, 24371–24393. [Google Scholar] [CrossRef]
- Stensvold, C.R.; van der Giezen, M. Associations between Gut Microbiota and Common Luminal Intestinal Parasites. Trends Parasitol. 2018, 34, 369–377. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Tryptophan metabolism in depression: A narrative review with a focus on serotonin and kynurenine pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef]
- Nozawa, K.; Kawabata-Shoda, E.; Doihara, H.; Kojima, R.; Okada, H.; Mochizuki, S.; Sano, Y.; Inamura, K.; Matsushime, H.; Koizumi, T. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3408–3413. [Google Scholar] [CrossRef]
- Travers, M.-A.; Florent, I.; Kohl, L.; Grellier, P. Probiotics for the control of parasites: An overview. J. Parasitol. Res. 2011, 2011, 610769. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Vitetta, L.; Saltzman, E.T.; Nikov, T.; Ibrahim, I.; Hall, S. Modulating the gut micro-environment in the treatment of intestinal parasites. J. Clin. Med. 2016, 5, 102. [Google Scholar] [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Tsigalou, C.; Dalamaga, M. Probiotics, Prebiotics, Synbiotics, Postbiotics, and Obesity: Current Evidence, Controversies, and Perspectives. Curr. Obes. Rep. 2020, 9, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Sarid, L.; Zanditenas, E.; Ye, J.; Trebicz-Geffen, M.; Ankri, S. Insights into the mechanisms of Lactobacillus acidophilus activity against Entamoeba histolytica by using thiol redox proteomics. Antioxidants 2022, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, prebiotics and immunomodulation of gut mucosal defences: Homeostasis and immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.R.; Sardi, J.d.C.O.; de Souza Pitangui, N.; Roque, S.M.; da Silva, A.C.B.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Berrilli, F.; Di Cave, D.; Cavallero, S.; D’Amelio, S. Interactions between parasites and microbial communities in the human gut. Front. Cell. Infect. Microbiol. 2012, 2, 141. [Google Scholar] [CrossRef]
- Pryshliak, O.Y.; Protsyk, A.L.; Semaniv, M.V.; Boichuk, O.P.; Gerych, P.R. Effect of probiotics on the intestinal microbiota of patients with giardiasis and ascariasis. J. Med. Life 2022, 15, 1278. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.; Mohamed, S.H.; Saleh, M.M.; Yehia, H.M. Preventive role of probiotic bacteria against gastrointestinal diseases in mice caused by Giardia lamblia. Biosci. Rep. 2021, 41, BSR20204114. [Google Scholar] [CrossRef]
- Del Coco, V.F.; Sparo, M.D.; Sidoti, A.; Santín, M.; Basualdo, J.A.; Córdoba, M.A. Effects of Enterococcus faecalis CECT 7121 on Cryptosporidium parvum infection in mice. Parasitol. Res. 2016, 115, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.; Sharma, A.; Bhatia, R.; Sharma, M. Prophylactic potential of synbiotic (Lactobacillus casei and Inulin) in malnourished murine giardiasis: An immunological and ultrastructural study. Probiotics Antimicrob. Proteins 2019, 11, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Saracino, M.P.; Vila, C.C.; Baldi, P.C.; Gonzalez Maglio, D.H. Searching for the one (s): Using probiotics as anthelmintic treatments. Front. Pharmacol. 2021, 12, 714198. [Google Scholar] [CrossRef]
- Rooney, J.; Cantacessi, C.; Sotillo, J.; Cortés, A. Gastrointestinal worms and bacteria: From association to intervention. Parasite Immunol. 2023, 45, e12955. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.O.; Stensvold, C.R. Blastocystis in Health and Disease: Are We Moving from a Clinical to a Public Health Perspective? J. Clin. Microbiol. 2016, 54, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Mirza, H.; Wu, Z.; Kidwai, F.; Tan, K.S.W. A Metronidazole-Resistant Isolate of Blastocystis spp. Is Susceptible to Nitric Oxide and Downregulates Intestinal Epithelial Inducible Nitric Oxide Synthase by a Novel Parasite Survival Mechanism. Infect. Immun. 2011, 79, 5019–5026. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.A.; Tan, K.S.; Vanelle, P.; Juspin, T.; Crozet, M.D.; Terme, T.; Upcroft, P.; Upcroft, J.A. Development of metronidazole-resistant lines of Blastocystis sp. Parasitol. Res. 2012, 111, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Sekar, U.; Shanthi, M. Blastocystis: Consensus of treatment and controversies. Trop. Parasitol. 2013, 3, 35–39. [Google Scholar] [CrossRef]
- Roberts, T.; Stark, D.; Harkness, J.; Ellis, J. Update on the pathogenic potential and treatment options for Blastocystis sp. Gut Pathog. 2014, 6, 17. [Google Scholar] [CrossRef]
- Batista, L.; Perez Jove, J.; Rosinach, M.; Gonzalo, V.; Sainz, E.; Loras, C.; Forne, M.; Esteve, M.; Fernandez-Banares, F. Low efficacy of metronidazole in the eradication of Blastocystis hominis in symptomatic patients: Case series and systematic literature review. Gastroenterol. Hepatol. 2017, 40, 381–387. [Google Scholar] [CrossRef]
- Dinleyici, E.C.; Eren, M.; Dogan, N.; Reyhanioglu, S.; Yargic, Z.A.; Vandenplas, Y. Clinical efficacy of Saccharomyces boulardii or metronidazole in symptomatic children with Blastocystis hominis infection. Parasitol. Res. 2011, 108, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Angelici, M.C.; Nardis, C.; Scarpelli, R.; Ade, P. Blastocystis hominis transmission by non-potable water: A case report in Italy. New Microbiol. 2018, 41, 173–177. [Google Scholar] [PubMed]
- Méabed, E.M.; Abdelhafez, D.N.; Abdelaliem, Y.F. Saccharomyces boulardii inhibits the expression of pro-inflammatory cytokines and inducible nitric oxide synthase genes in the colonic mucosa of rats experimentally-infected with Blastocystis subtype-3 cysts. Parasitology 2019, 146, 1532–1540. [Google Scholar] [CrossRef]
- Lepczynska, M.; Dzika, E. The influence of probiotic bacteria and human gut microorganisms causing opportunistic infections on Blastocystis ST3. Gut Pathog. 2019, 11, 6. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aykur, M.; Malatyalı, E.; Demirel, F.; Cömert-Koçak, B.; Gentekaki, E.; Tsaousis, A.D.; Dogruman-Al, F. Blastocystis: A Mysterious Member of the Gut Microbiome. Microorganisms 2024, 12, 461. https://doi.org/10.3390/microorganisms12030461
Aykur M, Malatyalı E, Demirel F, Cömert-Koçak B, Gentekaki E, Tsaousis AD, Dogruman-Al F. Blastocystis: A Mysterious Member of the Gut Microbiome. Microorganisms. 2024; 12(3):461. https://doi.org/10.3390/microorganisms12030461
Chicago/Turabian StyleAykur, Mehmet, Erdoğan Malatyalı, Filiz Demirel, Burçak Cömert-Koçak, Eleni Gentekaki, Anastasios D. Tsaousis, and Funda Dogruman-Al. 2024. "Blastocystis: A Mysterious Member of the Gut Microbiome" Microorganisms 12, no. 3: 461. https://doi.org/10.3390/microorganisms12030461





