The LiaSR Two-Component System Regulates Resistance to Chlorhexidine in Streptococcus mutans
Abstract
:1. Introduction
2. Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Minimum Inhibitory Concentration (MIC) Assay
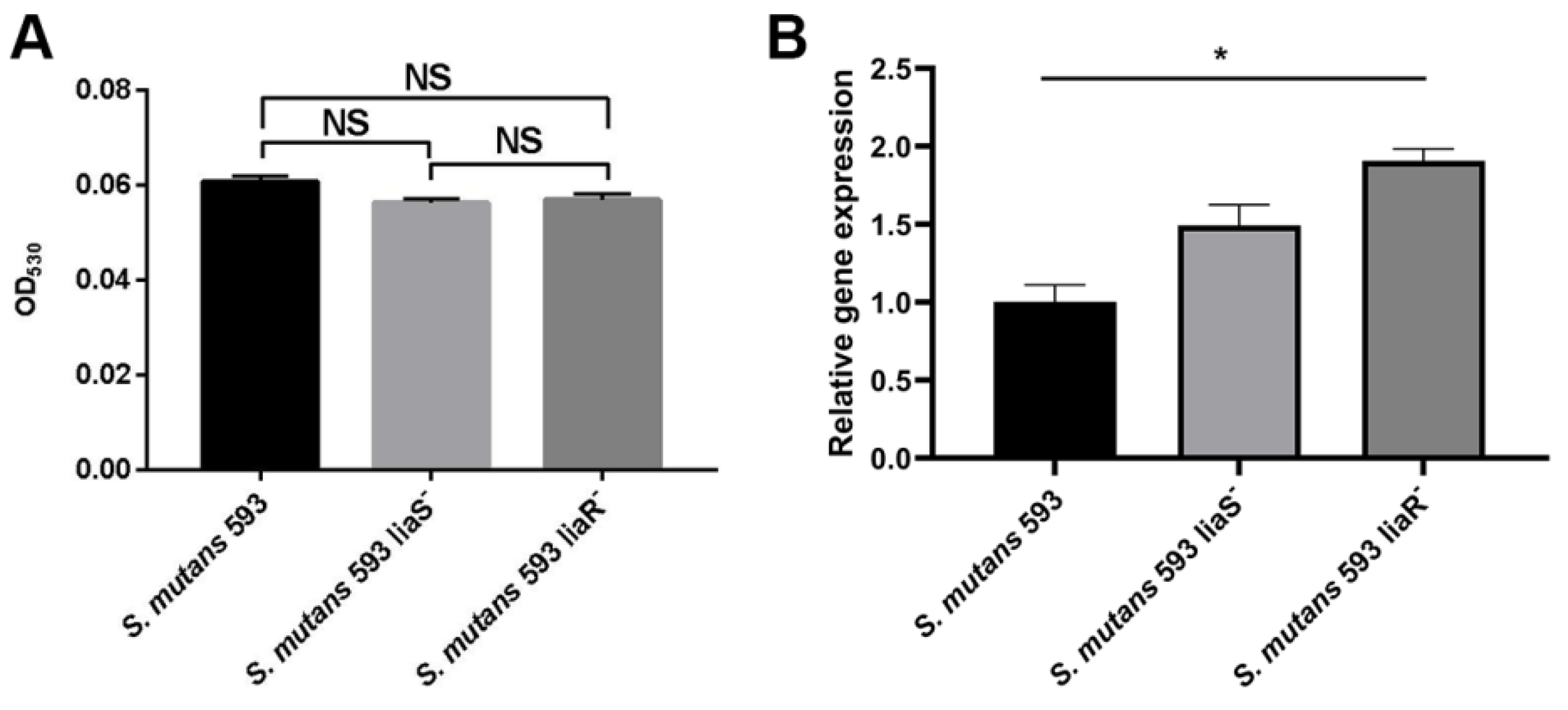
2.3. Biofilm Formation and Viability Assessment
2.4. Nile Red Efflux Assay
2.5. Transmission Electron Microscopy (TEM) Observation
2.6. Membrane Potential Assay
2.7. Assay for Hydrophobicity
2.8. Cytochrome C-Based Charge Determination of Biofilm-Grown Cells
2.9. RNA Isolation and Reverse-Transcription Quantitative PCR (RT-qPCR)
2.10. Statistical Analysis
3. Results
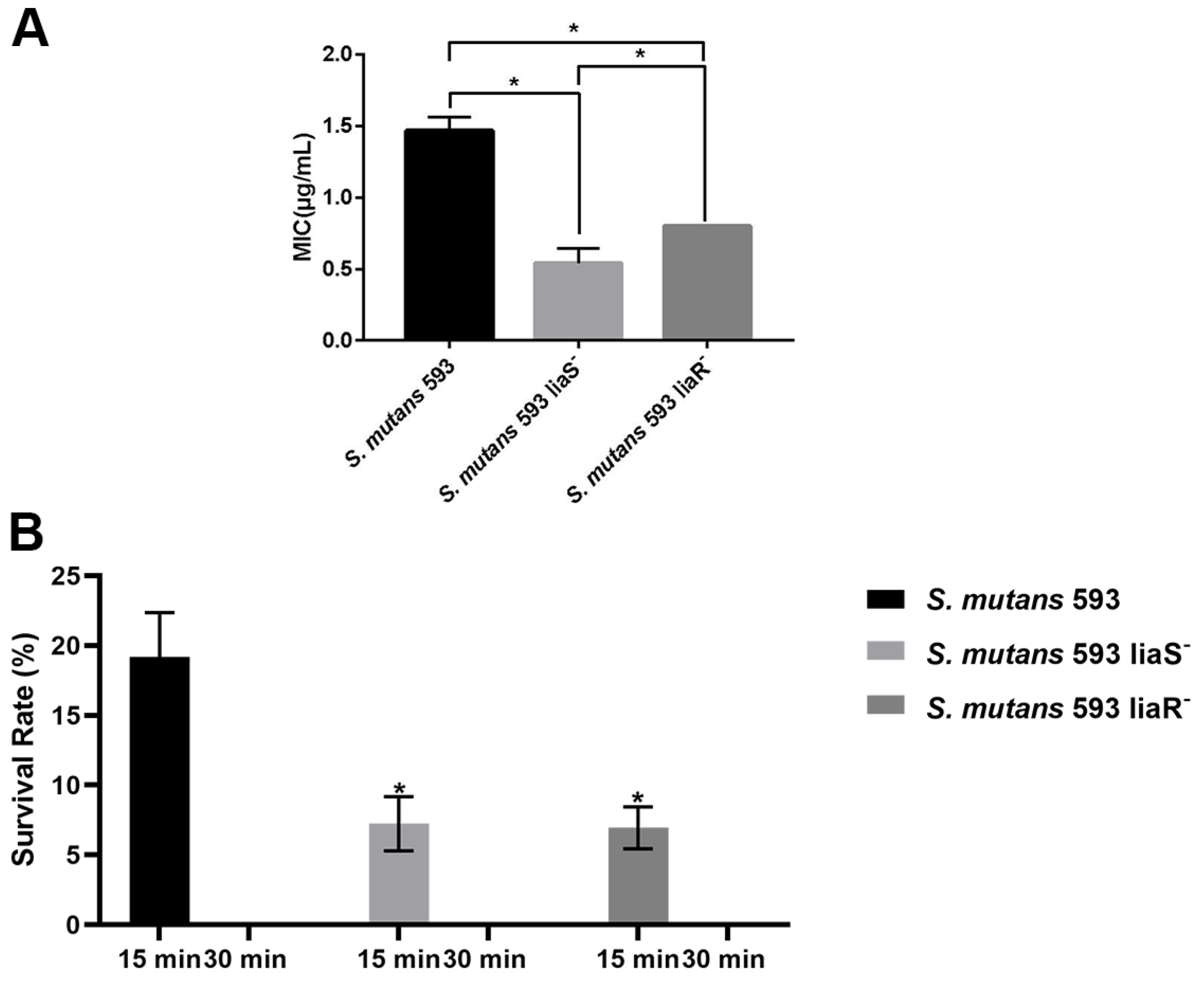
3.1. Sensitivity of S. mutans to CHX
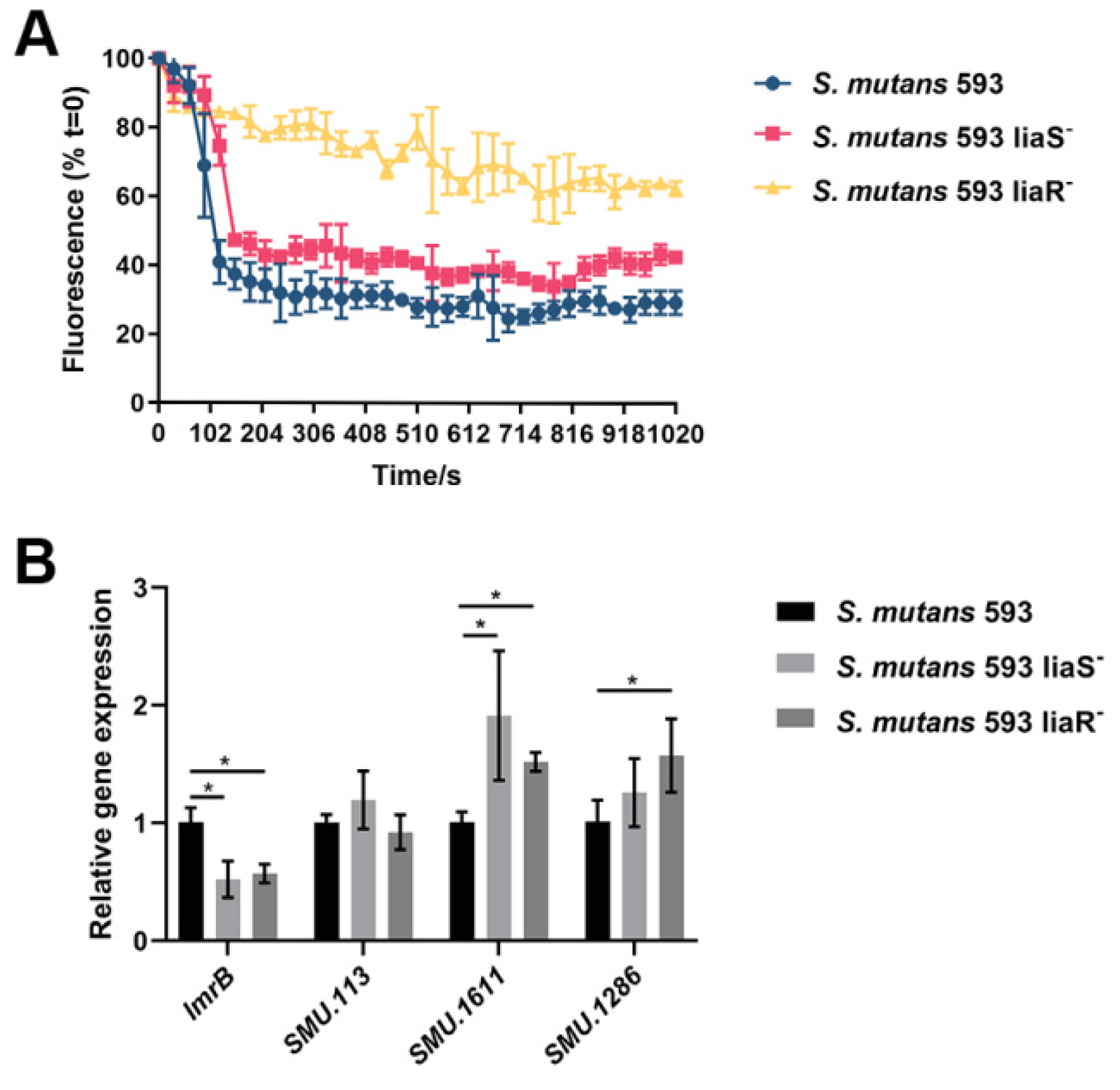
3.2. Efflux Activity of S. mutans
3.3. The Surface Charge of S. mutans
3.4. The Ability of CESRs in S. mutans
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.E.; Banerjee, R.; Doernberg, S.B.; Evans, S.R.; Komarow, L.; Satlin, M.J.; Schwager, N.; Simner, P.J.; Tillekeratne, L.G.; Patel, R.; et al. Priorities and Progress in Diagnostic Research by the Antibacterial Resistance Leadership Group. Clin. Infect. Dis. 2023, 77 (Suppl. 4), S314–S320. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Jing, M.; Gong, T.; Yan, J.; Wang, X.; Xu, M.; Zhou, X.; Zeng, J.; Li, Y. Regulatory mechanisms of exopolysaccharide synthesis and biofilm formation in Streptococcus mutans. J. Oral Microbiol. 2023, 15, 2225257. [Google Scholar] [CrossRef] [PubMed]
- Cugini, C.; Shanmugam, M.; Landge, N.; Ramasubbu, N. The Role of Exopolysaccharides in Oral Biofilms. J. Dent. Res. 2019, 98, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Huang, S.; Wu, M.; Chen, S.; Zhou, W.; Zhan, L.; Huang, X. Dlt operon regulates physiological function and cariogenic virulence in Streptococcus mutans. Future Microbiol. 2023, 18, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Brookes, Z.; McGrath, C.; McCullough, M. Antimicrobial Mouthwashes: An Overview of Mechanisms—What Do We Still Need to Know? Int. Dent. J. 2023, 73 (Suppl. 2), S64–S68. [Google Scholar] [CrossRef]
- Huang, S.; Wu, M.; Li, Y.; Du, J.; Chen, S.; Jiang, S.; Huang, X.; Zhan, L. The dlt operon contributes to the resistance to chlorhexidine in Streptococcus mutans. Int. J. Antimicrob. Agents 2022, 59, 106540. [Google Scholar] [CrossRef]
- Kaspar, J.R.; Godwin, M.J.; Velsko, I.M.; Richards, V.P.; Burne, R.A. Spontaneously Arising Streptococcus mutans Variants with Reduced Susceptibility to Chlorhexidine Display Genetic Defects and Diminished Fitness. Antimicrob. Agents Chemother. 2019, 63, 7. [Google Scholar] [CrossRef]
- Wang, S.; Fang, L.; Zhou, H.; Wang, M.; Zheng, H.; Wang, Y.; Weir, M.D.; Masri, R.; Oates, T.W.; Cheng, L.; et al. Silica nanoparticles containing nano-silver and chlorhexidine respond to pH to suppress biofilm acids and modulate biofilms toward a non-cariogenic composition. Dent. Mater. 2024, 40, 179–189. [Google Scholar] [CrossRef]
- Boaro, L.C.C.; Campos, L.M.; Varca, G.H.C.; dos Santos, T.M.R.; Marques, P.A.; Sugii, M.M.; Saldanha, N.R.; Cogo-Müller, K.; Brandt, W.C.; Braga, R.R.; et al. Antibacterial resin-based composite containing chlorhexidine for dental applications. Dent. Mater. 2019, 35, 909–918. [Google Scholar] [CrossRef]
- Li, Y.-H.; Lau, P.C.Y.; Tang, N.; Svensäter, G.; Ellen, R.P.; Cvitkovitch, D.G. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J. Bacteriol. 2002, 184, 6333–6342. [Google Scholar] [CrossRef]
- Chong, P.; Drake, L.; Biswas, I. LiaS regulates virulence factor expression in Streptococcus mutans. Infect. Immun. 2008, 76, 3093–3099. [Google Scholar] [CrossRef]
- Perry, J.A.; Levesque, C.M.; Suntharaligam, P.; Mair, R.W.; Bu, M.; Cline, R.T.; Peterson, S.N.; Cvitkovitch, D.G. Involvement of Streptococcus mutans regulator RR11 in oxidative stress response during biofilm growth and in the development of genetic competence. Lett. Appl. Microbiol. 2008, 47, 439–444. [Google Scholar] [CrossRef]
- Suntharalingam, P.; Senadheera, M.D.; Mair, R.W.; Lévesque, C.M.; Cvitkovitch, D.G. The LiaFSR system regulates the cell envelope stress response in Streptococcus mutans. J. Bacteriol. 2009, 191, 2973–2984. [Google Scholar] [CrossRef]
- Kesel, S.; Mader, A.; Höfler, C.; Mascher, T.; Leisner, M. Immediate and heterogeneous response of the LiaFSR two-component system of Bacillus subtilis to the peptide antibiotic bacitracin. PLoS ONE 2013, 8, e53457. [Google Scholar] [CrossRef]
- Fernandes, P.B.; Reed, P.; Monteiro, J.M.; Pinho, M.G. Revisiting the Role of VraTSR in Staphylococcus aureus Response to Cell Wall-Targeting Antibiotics. J. Bacteriol. 2022, 204, e0016222. [Google Scholar] [CrossRef]
- Baseri, N.; Najar-Peerayeh, S.; Bakhshi, B. The effect of subinhibitory concentration of chlorhexidine on the evolution of vancomycin-intermediate Staphylococcus aureus and the induction of mutations in walKR and vraTSR systems. Infect. Genet. Evol. 2021, 87, 104628. [Google Scholar] [CrossRef]
- Zhang, J.; Biswas, I. A phenotypic microarray analysis of a Streptococcus mutans liaS mutant. Microbiology 2009, 155 Pt 1, 61–68. [Google Scholar] [CrossRef]
- Huang, X.; Liu, T.; Chen, G. Typing of Streptococcus mutans (serotype C) by arbitrarily primed polymerase chain reaction. Zhonghua Kou Qiang Yi Xue Za Zhi 2001, 36, 281–284. [Google Scholar]
- Huang, S.; Du, J.; Li, Y.; Wu, M.; Chen, S.; Jiang, S.; Zhan, L.; Huang, X. LiaSR two-component system modulates the oxidative stress response in Streptococcus mutans. Microb. Pathog. 2023, 185, 106404. [Google Scholar] [CrossRef]
- Jenic, D.; Waller, H.; Collins, H.; Erridge, C. Reversal of Tetracycline Resistance by Cepharanthine, Cinchonidine, Ellagic Acid and Propyl Gallate in a Multi-drug Resistant Escherichia coli. Nat. Prod. Bioprospect. 2021, 11, 345–355. [Google Scholar] [CrossRef]
- Schuster, S.; Vavra, M.; Greim, L.; Kern, W.V. Exploring the Contribution of the AcrB Homolog MdtF to Drug Resistance and Dye Efflux in a Multidrug Resistant E. coli Isolate. Antibiotics 2021, 10, 503. [Google Scholar] [CrossRef]
- Bohnert, J.A.; Karamian, B.; Nikaido, H. Optimized Nile Red efflux assay of AcrAB-TolC multidrug efflux system shows competition between substrates. Antimicrob. Agents Chemother. 2010, 54, 3770–3775. [Google Scholar] [CrossRef]
- Nilsson, M.; Rybtke, M.; Givskov, M.; Høiby, N.; Twetman, S.; Tolker-Nielsen, T. The dlt genes play a role in antimicrobial tolerance of Streptococcus mutans biofilms. Int. J. Antimicrob. Agents 2016, 48, 298–304. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Guo, L.; Zhao, W.; Hu, X.; Wei, X. Inactivation of a putative efflux pump (LmrB) in Streptococcus mutans results in altered biofilm structure and increased exopolysaccharide synthesis: Implications for biofilm resistance. Biofouling 2017, 33, 481–493. [Google Scholar] [CrossRef]
- Aleksandrowicz, A.; Kolenda, R.; Baraniewicz, K.; Thurston, T.L.M.; Suchański, J.; Grzymajlo, K. Membrane properties modulation by SanA: Implications for xenobiotic resistance in Salmonella Typhimurium. Front. Microbiol. 2023, 14, 1340143. [Google Scholar] [CrossRef]
- Feng, Z.; Luo, J.; Lyu, X.; Chen, Y.; Zhang, L. Selective antibacterial activity of a novel lactotransferrin-derived antimicrobial peptide LF-1 against Streptococcus mutans. Arch. Oral Biol. 2022, 139, 105446. [Google Scholar] [CrossRef]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral Bacteria—Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef]
- Horner, C.; Mawer, D.; Wilcox, M. Reduced susceptibility to chlorhexidine in Staphylococci: Is it increasing and does it matter? J. Antimicrob. Chemother. 2012, 67, 2547–2559. [Google Scholar] [CrossRef]
- Morita, Y.; Tomida, J.; Kawamura, Y. Responses of Pseudomonas aeruginosa to antimicrobials. Front. Microbiol. 2014, 4, 422. [Google Scholar] [CrossRef]
- Wand, M.E.; Bock, L.J.; Bonney, L.C.; Sutton, J.M. Mechanisms of Increased Resistance to Chlorhexidine and Cross-Resistance to Colistin following Exposure of Klebsiella pneumoniae Clinical Isolates to Chlorhexidine. Antimicrob. Agents Chemother. 2017, 61, 1. [Google Scholar] [CrossRef]
- Du, D.; Wang-Kan, X.; Neuberger, A.; Van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef]
- Huang, S.; Du, J.Y.; Li, Y.J.; Wu, M.J.; Chen, S.; Jiang, S.; Huang, X.J. Role and related mechanisms of LiaSR two-component system in acid tolerance and biofilm formation of Streptococcus mutans. Zhonghua Kou Qiang Yi Xue Za Zhi 2024, 59, 54–63. [Google Scholar]
- Schrank, C.L.; Wilt, I.K.; Ortiz, C.M.; Haney, B.A.; Wuest, W.M. Using membrane perturbing small molecules to target chronic persistent infections. RSC Med. Chem. 2021, 12, 1312–1324. [Google Scholar] [CrossRef]
- Baker, J.L.; Saputo, S.; Faustoferri, R.C.; Quivey, R.G., Jr. Streptococcus mutans SpxA2 relays the signal of cell envelope stress from LiaR to effectors that maintain cell wall and membrane homeostasis. Mol. Oral Microbiol. 2020, 35, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.H.; Pick, K.; Raivio, T.L. Bacterial envelope stress responses: Essential adaptors and attractive targets. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119387. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Lach, S.R.; Konovalova, A. Homeostasis of the Gram-negative cell envelope. Curr. Opin. Microbiol. 2021, 61, 99–106. [Google Scholar] [CrossRef]
- Hews, C.L.; Cho, T.; Rowley, G.; Raivio, T.L. Maintaining Integrity Under Stress: Envelope Stress Response Regulation of Pathogenesis in Gram-Negative Bacteria. Front. Cell. Infect. Microbiol. 2019, 9, 313. [Google Scholar] [CrossRef]

| Strain or Plasmid | Relevant Characteristics | Source |
|---|---|---|
| S. mutans 593 | Wild-type, serotype c | [21] |
| S. mutans 593 liaS− | Defined liaS deletion mutant | [22] |
| S. mutans 593 liaR− | Defined liaR deletion mutant |
| Primers | Sequence (5′→3′) |
|---|---|
| dltC-F | ACCAAGGCTGTACTTAATCAGG |
| dltC-R | AGGATGCAGATAAAGGAAGCC |
| dagK-F | GGGATCCTTCTCATGTTCTCTC |
| dagK-R | CATCTGCATTGACTTCCATCATT |
| murB-F | GCAGTGCTAAGACTCCCGAATC |
| murB-R | TTGCGGAAGTGTGGAGATTGGC |
| rgpB-F | GGAATCGTTAGAAGCAAAAGGTG |
| rgpB-R | TGACCAAAGCAGTAACCCTC |
| lmrB-F | CGTCATCTCTTTATCGCAAGCATC |
| lmrB-R | CAGTATGTTCCAGCACTTCAGCC |
| SMU_113-F | AAATACGGTGACACTTGCTGGTAAG |
| SMU_113-R | TCAATAGATGGCACAACGGTAATC |
| SMU_1611-F | ACCGCTATGCTAAGGCTCTC |
| SMU_1611-R | AACCTATCTGAAGACGCTTGTC |
| SMU_1286-F | TGCCACAGGATGCAGAAGG |
| SMU_1286-R | TGTTGCACATCCTTACCCGT |
| 16S-F | AGCGTTGTCCGGATTTATTG |
| 16S-R | CTACGCATTTCACCGCTACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Huang, J.; Du, J.; Li, Y.; Wu, M.; Chen, S.; Zhan, L.; Huang, X. The LiaSR Two-Component System Regulates Resistance to Chlorhexidine in Streptococcus mutans. Microorganisms 2024, 12, 468. https://doi.org/10.3390/microorganisms12030468
Huang S, Huang J, Du J, Li Y, Wu M, Chen S, Zhan L, Huang X. The LiaSR Two-Component System Regulates Resistance to Chlorhexidine in Streptococcus mutans. Microorganisms. 2024; 12(3):468. https://doi.org/10.3390/microorganisms12030468
Chicago/Turabian StyleHuang, Shan, Jing Huang, Jingyun Du, Yijun Li, Minjing Wu, Shuai Chen, Ling Zhan, and Xiaojing Huang. 2024. "The LiaSR Two-Component System Regulates Resistance to Chlorhexidine in Streptococcus mutans" Microorganisms 12, no. 3: 468. https://doi.org/10.3390/microorganisms12030468





