Exploration of the Vermiculite-Induced Bacterial Community and Co-Network Successions during Sludge–Waste Mushroom Co-Composting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composting and Analysis of Physicochemical Property
2.2. DNA Extraction, High-Throughput Sequencing
2.3. Quality Control and Statistical Analysis
3. Results and Discussion
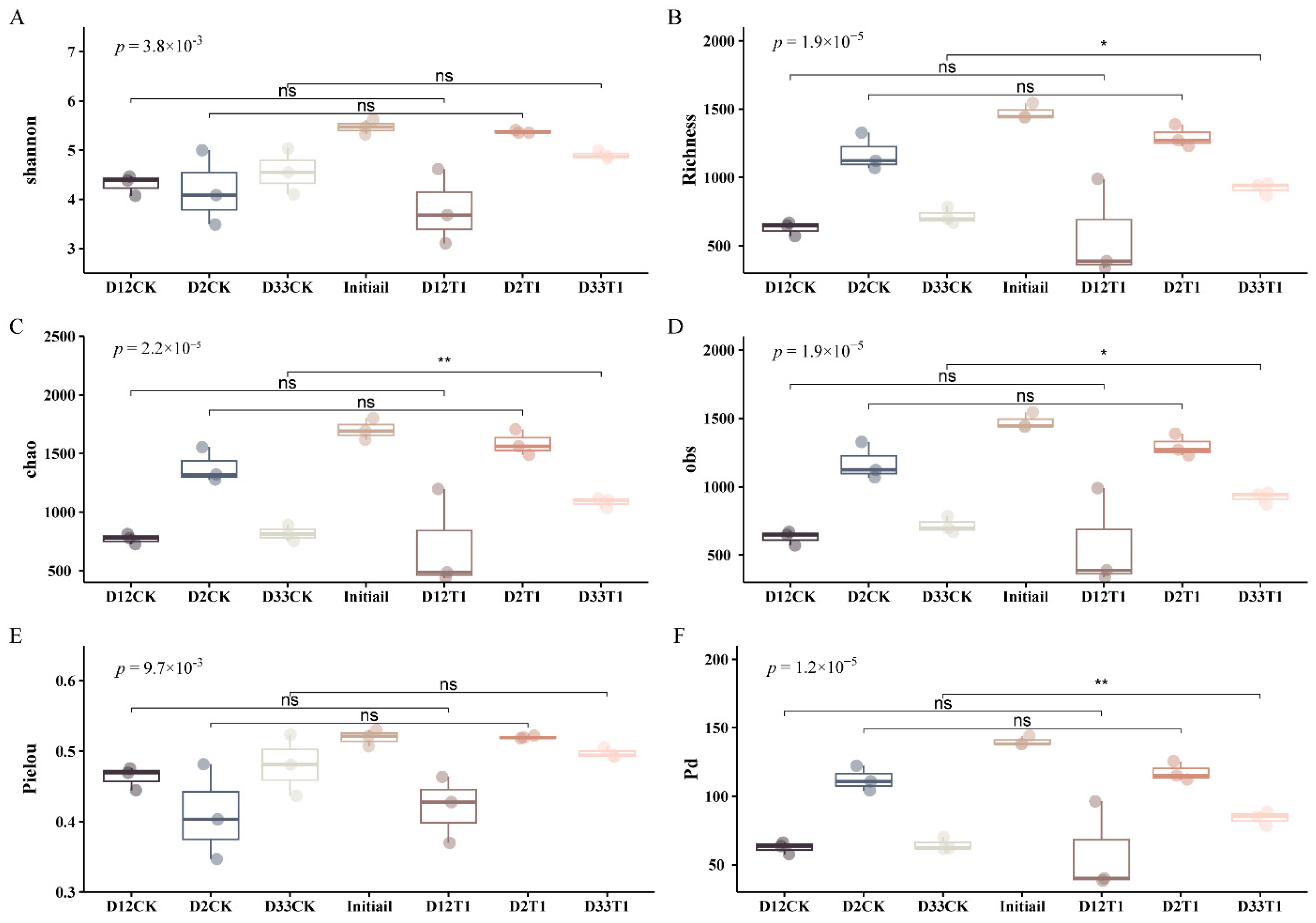
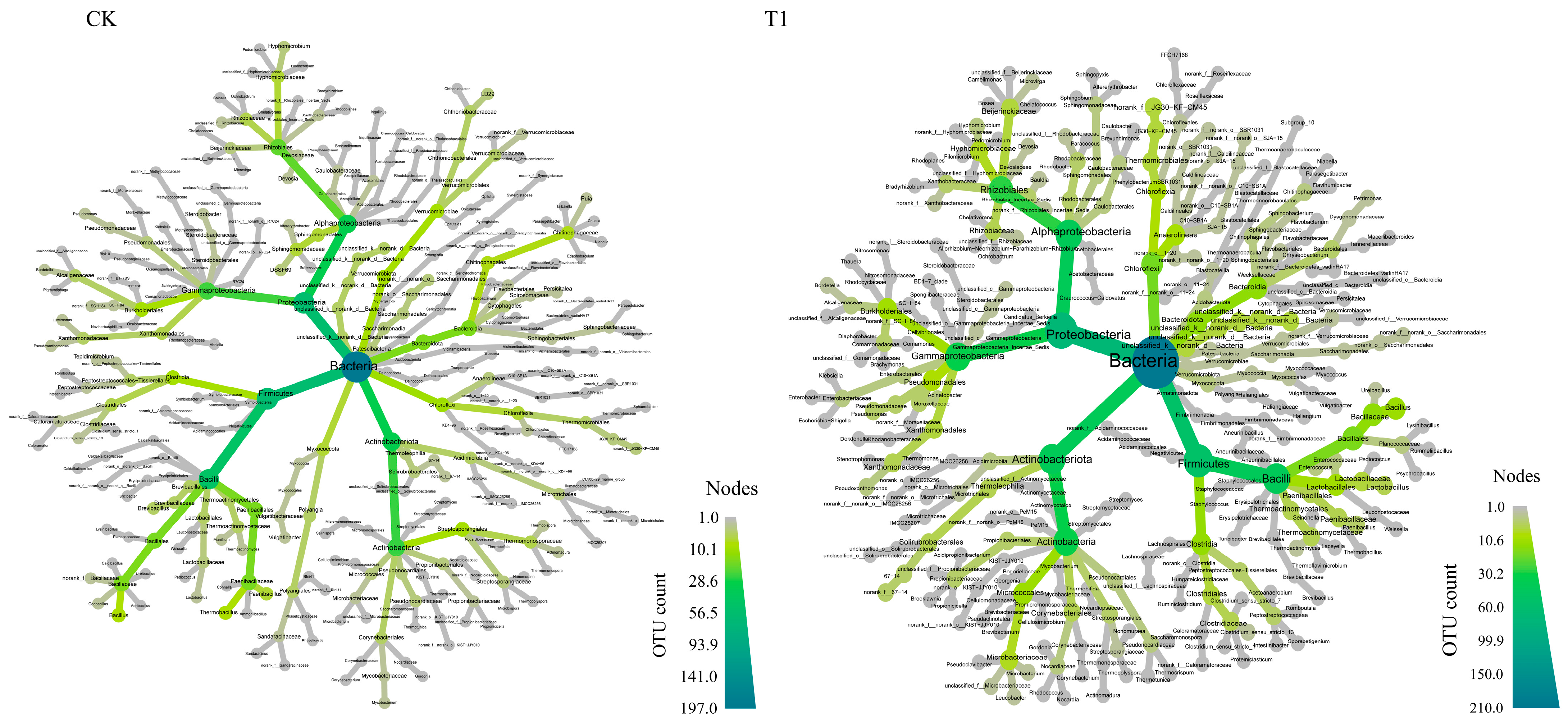
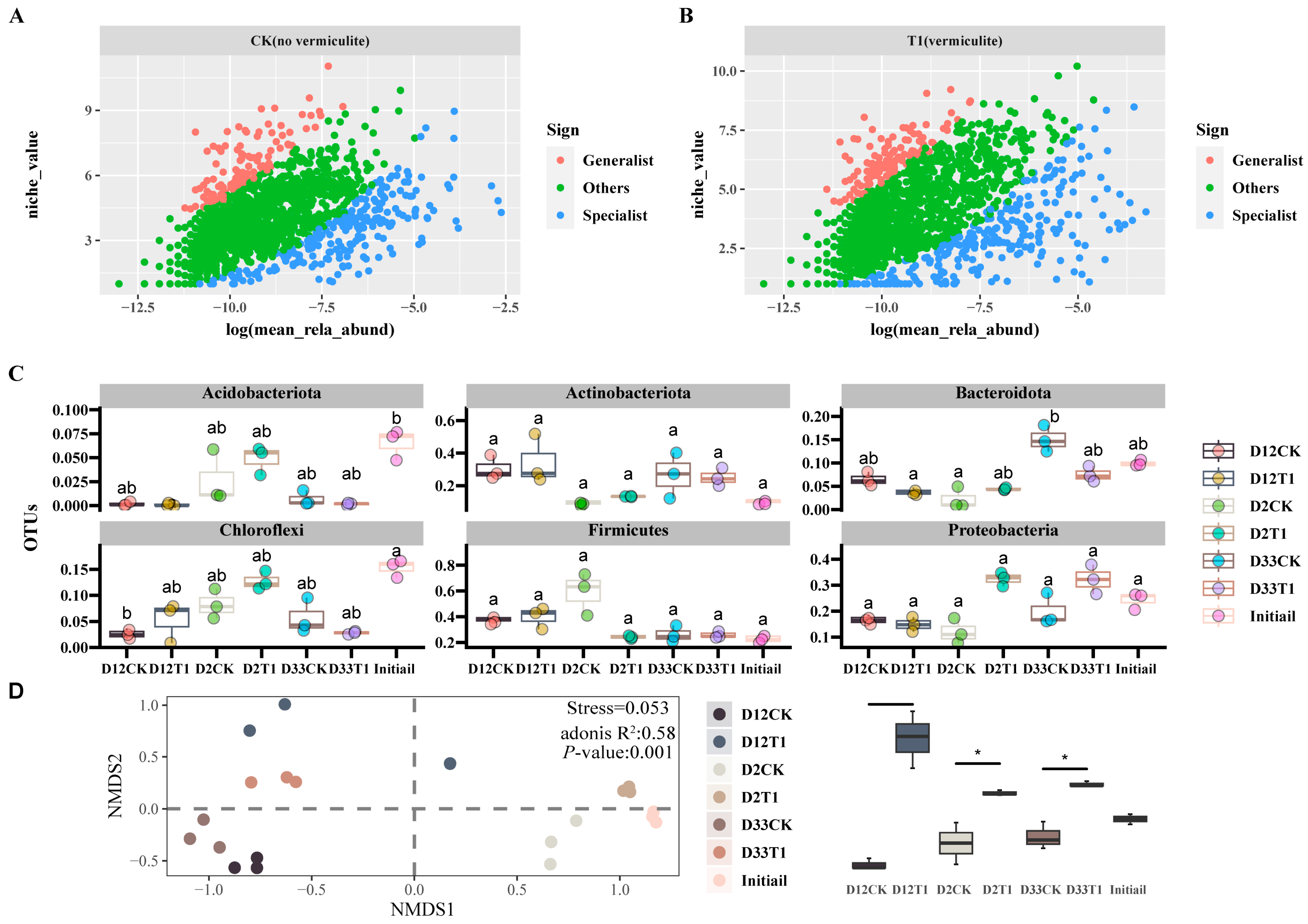
3.1. α and β Diversities
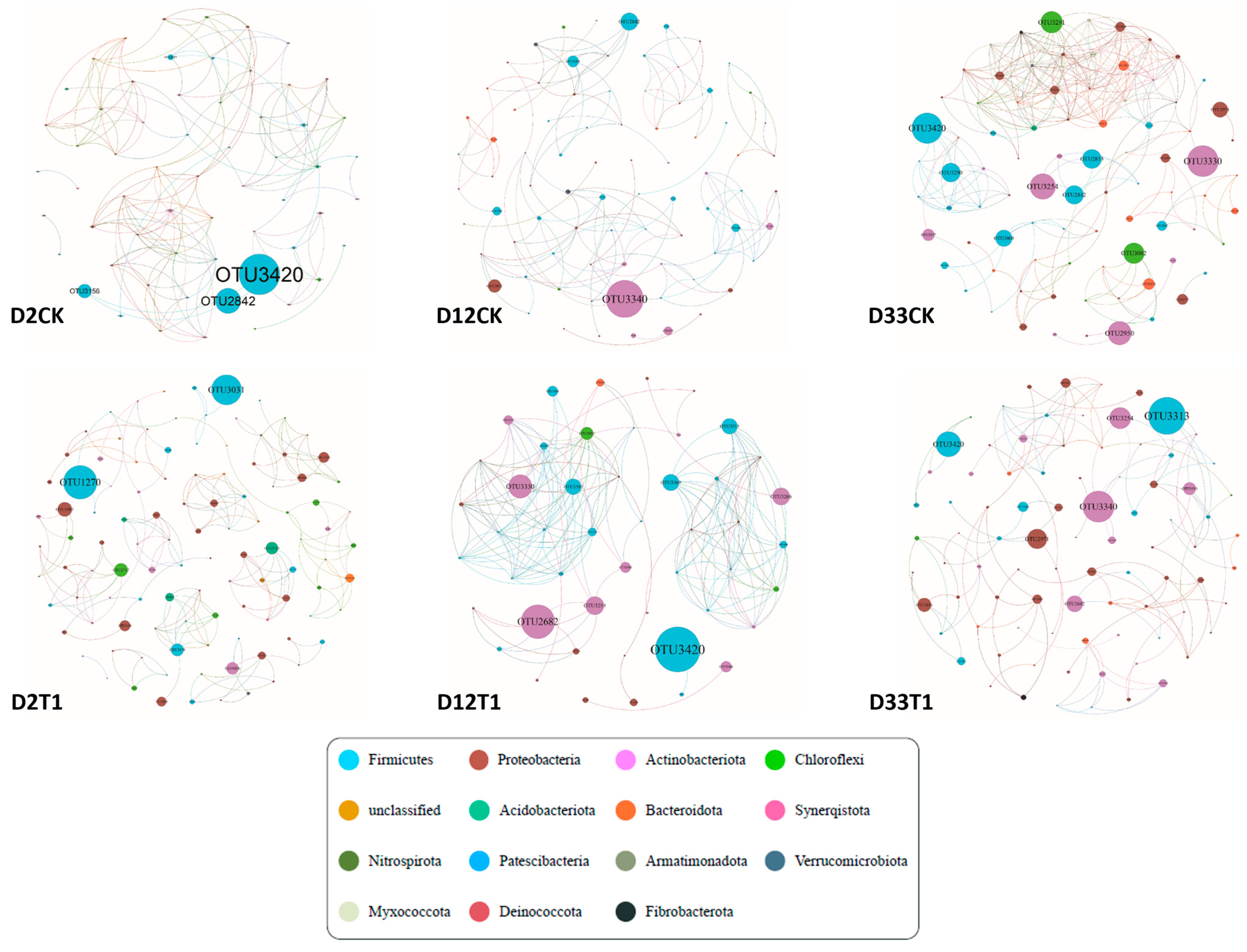
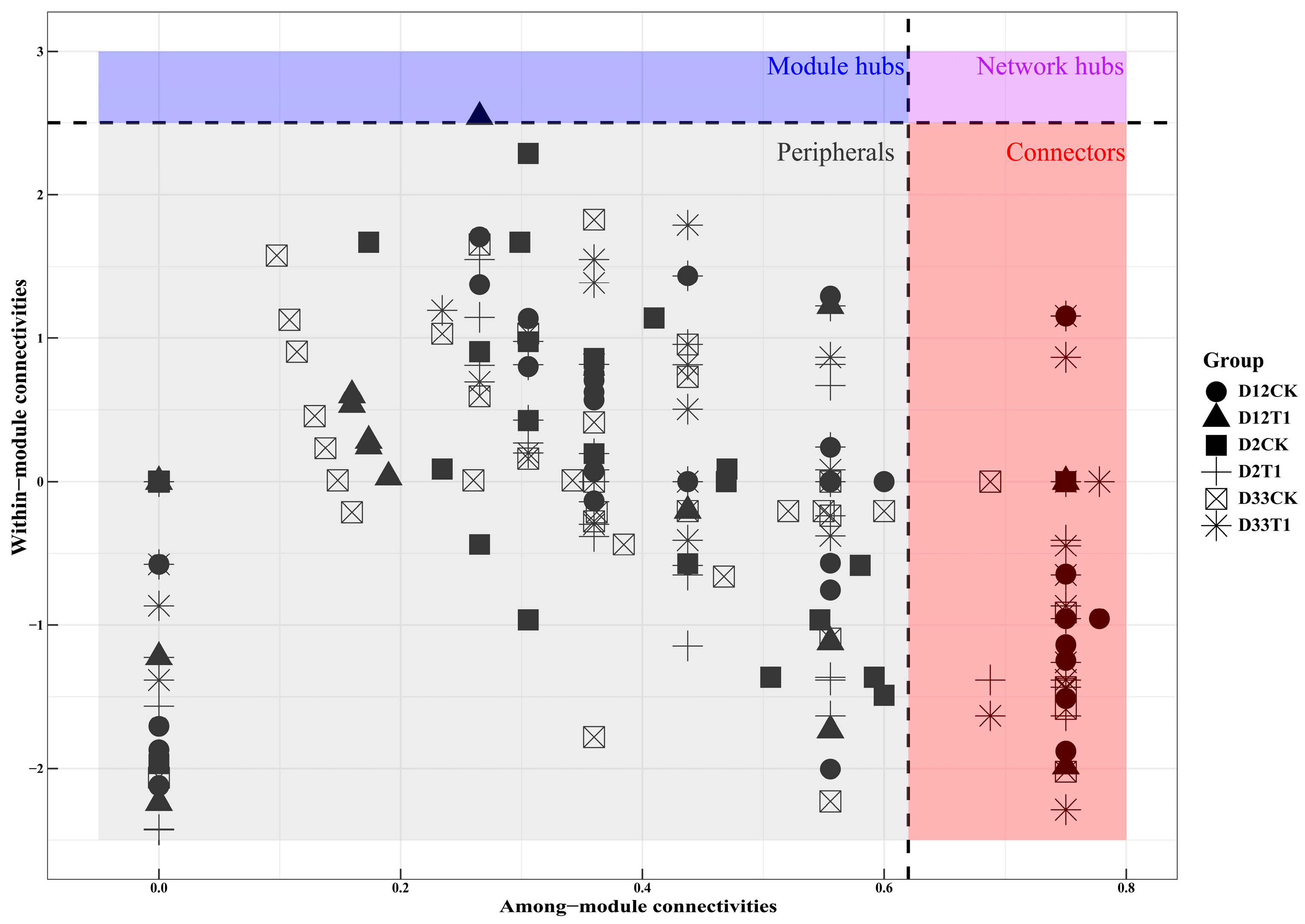
3.2. Analysis of Co-Network and Modular Topology
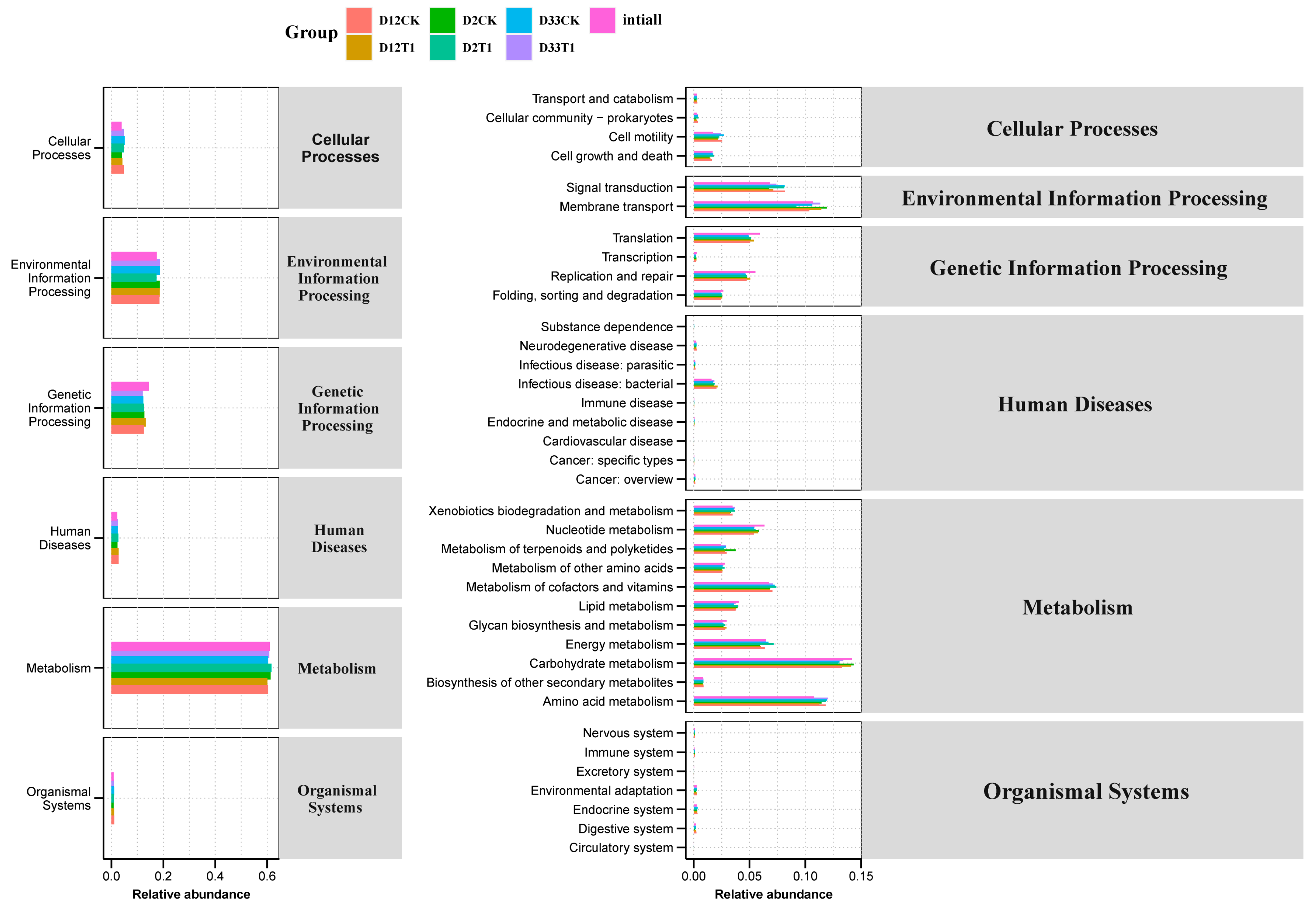
3.3. Function Analysis of Metabolism Networks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Awasthi, M.K.; Awasthi, S.K.; Wang, Q.; Wang, Z.; Lahori, A.H.; Ren, X.N.; Chen, H.Y.; Wang, M.J.; Zhao, J.C.; Zhang, Z.Q. Influence of biochar on volatile fatty acids accumulation and microbial community succession during biosolids composting. Bioresour. Technol. 2018, 251, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.W.; Xiao, R.; Klammsteiner, T.; Kong, X.L.; Yan, B.H.; Mihai, F.C.; Liu, T.; Zhang, Z.Q.; Awasthi, M.K. Recent trends and advances in composting and vermicomposting technologies: A review. Bioresour. Technol. 2022, 360, 127591. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Hu, Q.X.; Bai, X.; Zhang, W.J.; Guo, H.W. Increasing the value of Phragmites australis straw in a sustainable integrated agriculture model (SIAM) comprising edible mushroom cultivation and spent mushroom substrate compost. Sci. Total Environ. 2023, 869, 161807. [Google Scholar] [CrossRef] [PubMed]
- Shinzato, M.C.; Wu, L.F.; Mariano, T.O.; Freitas Juliana, G.; Martins, T.S. Mineral sorbents for ammonium recycling from industry to agriculture. Environ. Sci. Pollut. Res. 2020, 27, 13599–13616. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.Q.; Zhang, L. Vermiculite and humic acid improve the quality of green waste compost as a growth medium for Centaurea cyanus L. Environ. Technol. Innov. 2021, 24, 101945. [Google Scholar] [CrossRef]
- Liao, P.H.; Jones, L.; Lau, A.K.; Walkemeyer, S.; Egan, B.; Holbek, N. Composting of fish wastes in a full-scale in vessel system. Bioresour. Technol. 1997, 59, 163–168. [Google Scholar] [CrossRef]
- Seo, J.Y.; Heo, J.S.; Kim, T.H.; Joo, W.H.; Crohn, D.M. Effect of vermiculite addition on compost produced from Korean food wastes. Waste Manag. 2004, 24, 981–987. [Google Scholar] [CrossRef]
- Tang, J.C.; Katayama, A. Relating quinone profile to the aerobic biodegradation in thermophilic composting processes of cattle manure with various bulking agents. World J. Microbiol. Biotechnol. 2005, 21, 1249–1254. [Google Scholar] [CrossRef]
- Turan, N.G.; Akdemir, A.; Ergun, O.N. Removal of volatile organic compounds by natural materials during composting of poultry litter. Bioresour. Technol. 2009, 100, 798–803. [Google Scholar] [CrossRef]
- Tang, J.C.; Zhou, Q.X.; Katayama, A. Effects of raw materials and bulking agents on the thermophilic composting process. J. Microbiol. Biotechnol. 2010, 20, 925–934. [Google Scholar] [CrossRef]
- Zhang, L.C.; Luo, L.; Zhang, S.Z. Integrated investigations on the adsorption mechanisms of fulvic and humic acids on three clay minerals. Colloids Surf. A Physicochem. Eng. Asp. 2012, 406, 84–90. [Google Scholar] [CrossRef]
- He, Z.H.; Lin, H.; Hao, J.W.; Kong, X.S.; Tian, K.; Bei, Z.L.; Tian, X.Q. Impact of vermiculite on ammonia emissions and organic matter decomposition of food waste during composting. Bioresour. Technol. 2018, 263, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Pisa, C.; Wuta, M.; Muchaonyerwa, P. Effects of incorporation of vermiculite on carbon and nitrogen retention and concentration of other nutrients during composting of cattle manure. Bioresour. Technol. Rep. 2020, 9, 100383. [Google Scholar] [CrossRef]
- Liu, C.; Ai, C.; Liao, H.; Wen, C.; Gao, T.; Yang, Q.; Zhou, S. Distinctive community assembly enhances the adaptation to extreme environments during hyperthermophilic composting. Waste Manag. 2023, 157, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.M.; Awasthi, M.K.; Wu, H.H.; Yang, J.F.; Li, Z.L.; Ni, X.H.; Zhang, J.T.; Zhang, Z.Q.; Li, H.K. Biochar regulates bacterial-fungal diversity and associated enzymatic activity during sheep manure composting. Bioresour. Technol. 2022, 346, 12667. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Bao, J.; Li, S.; Liu, D.; Dai, D.; Qv, M.; Zhu, L. Biochar amendment of aerobic composting for the effective biodegradation of heavy oil and succession of bacterial community. Bioresour. Technol. 2022, 362, 127820. [Google Scholar] [CrossRef]
- Martins, G.L.; de Souza, A.J.; Mendes, L.W.; Gontijo, J.B.; Rodrigues, M.M.; Coscione, A.R.; Oliveira, F.C.; Regitano, J.B. Physicochemical and bacterial changes during composting of vegetable and animal-derived agro-industrial wastes. Bioresour. Technol. 2023, 376, 128842. [Google Scholar] [CrossRef]
- He, J.; Zhu, N.M.; Xu, Y.S.; Wang, L.; Zheng, J.Q.; Li, X. The microbial mechanisms of enhanced humification by inoculation with Phanerochaete chrysosporium and Trichoderma longibrachiatum during biogas residues composting. Bioresour. Technol. 2022, 351, 126973. [Google Scholar] [CrossRef]
- Yu, Z.J.; Wang, B.; Jiang, X.Y.; Zeng, W.M.; Yu, R.L.; Wu, X.Y.; Shen, L.; Wu, X.L.; Li, J.K.; Liu, Y.D. Behavior and biochemical mechanism of high iron attapulgite dosages affecting sewage sludge composting. Sustainability 2023, 15, 12527. [Google Scholar] [CrossRef]
- Wu, X.Y.; Wang, J.S.; Yu, Z.J.; Amanze, C.; Shen, L.; Wu, X.L.; Li, J.K.; Yu, R.L.; Liu, Y.D.; Zeng, W.M. Impact of bamboo sphere amendment on composting performance and microbial community succession in food waste composting. J. Environ. Manag. 2022, 303, 114144. [Google Scholar] [CrossRef]
- Wen, T.; Niu, G.Q.; Chen, T.; Shen, Q.R.; Yuan, J.; Liu, Y.X. The best practice for microbiome analysis using R. Protein Cell 2023, 14, 713–725. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Ding, Y.Z.; Ren, X.N.; Xie, J.W.; Kumar, S.; Zhang, Z.Q.; Wang, Q. Effect of micronutrient selenium on greenhouse gas emissions and related functional genes during goat manure composting. Bioresour. Technol. 2022, 349, 126805. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; An, X.J.; An, W.J.; Xiao, X.S.; Li, H.G.; Xia, X.; Zhang, Q.H. Effect of bioaugmentation on lignocellulose degradation and antibiotic resistance genes removal during biogas residues composting. Bioresour. Technol. 2021, 340, 125742. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Awasthi, M.K.; Bao, H.Y.; Bie, J.Y.; Lei, S.; Lv, J.L. Exploring the microbial mechanisms of organic matter transformation during pig manure composting amended with bean dregs and biochar. Bioresour. Technol. 2020, 313, 123647. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Chen, W.J.; Liu, D.; Tao, Y.Y.; Ma, H.T.; Feng, Z.W.; Li, S.R.; Zhou, K.Y.; Wu, J.; Li, J.; et al. Effect of superphosphate addition on heavy metals speciation and microbial communities during composting. Bioresour. Technol. 2022, 359, 127478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Yang, H.K.; Wang, B.; Chen, C.; Zou, X.S.; Cheng, T.; Li, J. Aerobic co-composting of mature compost with cattle manure: Organic matter conversion and microbial community characterization. Bioresour. Technol. 2023, 382, 129187. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Tian, L.; Li, Y.X.; Zhong, C.; Tian, C.J. Effects of exogenous cellulose-degrading bacteria on humus formation and bacterial community stability during composting. Bioresour. Technol. 2022, 359, 127458. [Google Scholar] [CrossRef] [PubMed]
- Li, F.S.; Ghanizadeh, H.; Cui, G.L.; Liu, J.Y.; Miao, S.; Liu, C.; Song, W.W.; Chen, X.L.; Cheng, M.Z.; Wang, P.W.; et al. Microbiome-based agents can optimize composting of agricultural wastes by modifying microbial communities. Bioresour. Technol. 2023, 374, 128765. [Google Scholar] [CrossRef] [PubMed]
- Sriswasdi, S.; Yang, C.; Iwasaki, W. Generalist species drive microbial dispersion and evolution. Nat. Commun. 2017, 8, 1162. [Google Scholar] [CrossRef]
- Nagelkerke, C.J.; Menken, S.B.J. Coexistence of Habitat Specialists and Generalists in Metapopulation Models of Multiple-Habitat Landscapes. Acta Biotheor. 2013, 61, 467–480. [Google Scholar] [CrossRef]
- Li, G.L.; Zhou, C.H.; Fiore, S.; Yu, W.H. Interactions between microorganisms and clay minerals: New insights and broader applications. Appl. Clay Sci. 2019, 177, 91–113. [Google Scholar] [CrossRef]
- Xun, W.B.; Liu, Y.P.; Li, W.; Ren, Y.; Xiong, W.; Xu, Z.H.; Zhang, N.; Miao, Y.Z.; Shen, Q.R.; Zhang, R.F. Specialized metabolic functions of keystone taxa sustain soil microbiome stability. Microbiome 2021, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.F.; Lv, Y.F.; Qv, M.X.; Li, Z.; Li, T.R.; Li, S.X.; Zhu, L.D. Evaluation of key microbial community succession and enzyme activities of nitrogen transformation in pig manure composting process through multi-angle analysis. Bioresour. Technol. 2022, 362, 127797. [Google Scholar] [CrossRef]
- Chi, C.P.; Chu, S.H.; Wang, B.; Zhang, D.; Zhi, Y.; Yang, X.J.; Zhou, P. Dynamic bacterial assembly driven by Streptomyces griseorubens JSD-1 inoculants correspond to composting performance in swine manure and rice straw co-composting. Bioresour. Technol. 2020, 313, 123692. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, T.; Zhou, L.; Lou, W.; Zeng, W.A.; Liu, T.B.; Yin, H.Q.; Liu, H.W.; Liu, X.D.; Mathivanan, K.; et al. Soil microbial community assembly model in response to heavy metal pollution. Environ. Res. 2022, 213, 113576. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef]
- Yin, Y.N.; Gu, J.; Wang, X.J.; Zhang, Y.J.; Zheng, W.; Chen, R.; Wang, X.C. Effects of rhamnolipid and Tween-80 on cellulase activities and metabolic functions of the bacterial community during chicken manure composting. Bioresour. Technol. 2019, 288, 121507. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, L.; Chen, W.Y.; Sun, J.J.; Hou, S.W.; Kuang, T.X.; Wang, W.X.; Huang, X.D. Stochastic determination of the spatial variation of potentially pathogenic bacteria communities in a large subtropical river. Environ. Pollut. 2020, 264, 114683. [Google Scholar] [CrossRef] [PubMed]
- Partanen, P.; Hultman, J.; Paulin, L.; Auvinen, P.; Romantschunk, M. Bacterial diversity at different stages of the composting process. BMC Microbiol. 2010, 10, 94. Available online: https://www.biomedcentral.com/1471-2180/10/94 (accessed on 2 February 2024). [CrossRef] [PubMed]
- Biyada, S.; Merzouki, M.; Demecenko, T.; Vasilliauskiene, D.; Ivanec-Goranina, R.; Urbonavicius, J.; Marciulaitiene, E.; Vasarevicius, S.; Benlemlih, M. Microbial community dynamics in the mesophilic and thermophilic phase of textile waste composting identified through next-generation sequencing. Sci. Req. 2021, 11, 23624. [Google Scholar] [CrossRef]
- Araujo, A.S.; de Pereira, A.P.; Antunes, J.E.; Oliveira, L.M.; de Melo, W.J.; Rocha, S.M.; do Amorim, M.R.; Araujo, F.F.; Melo, V.M.; Mendes, L.W. Dynamics of bacterial and archaeal communities along the composting of tannery sludge. Environ. Sci. Pollut. Res. 2021, 28, 64295–64306. [Google Scholar] [CrossRef] [PubMed]
- Sansupa, C.; Wahdan, S.F.M.; Hossen, S.; Disayathanoowat, T.; Wubet, T.; Purahong, W. Can we use functional Annotation of Prokaryotic Taxa to Assign the ecological function of soil bacteria? Appl. Sci. 2021, 11, 688. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Wang, B.; Wu, X.; Yu, R.; Shen, L.; Wu, X.; Li, J.; Liu, Y.; Zeng, W. Exploration of the Vermiculite-Induced Bacterial Community and Co-Network Successions during Sludge–Waste Mushroom Co-Composting. Microorganisms 2024, 12, 585. https://doi.org/10.3390/microorganisms12030585
Yu Z, Wang B, Wu X, Yu R, Shen L, Wu X, Li J, Liu Y, Zeng W. Exploration of the Vermiculite-Induced Bacterial Community and Co-Network Successions during Sludge–Waste Mushroom Co-Composting. Microorganisms. 2024; 12(3):585. https://doi.org/10.3390/microorganisms12030585
Chicago/Turabian StyleYu, Zhaojing, Bin Wang, Xiaoyan Wu, Runlan Yu, Li Shen, Xueling Wu, Jiaokun Li, Yuandong Liu, and Weimin Zeng. 2024. "Exploration of the Vermiculite-Induced Bacterial Community and Co-Network Successions during Sludge–Waste Mushroom Co-Composting" Microorganisms 12, no. 3: 585. https://doi.org/10.3390/microorganisms12030585




