Investigation of Yellow Fever Virus at the Human–Animal Interface after a Zika Virus Outbreak in Midwest Brazil
Abstract
1. Introduction
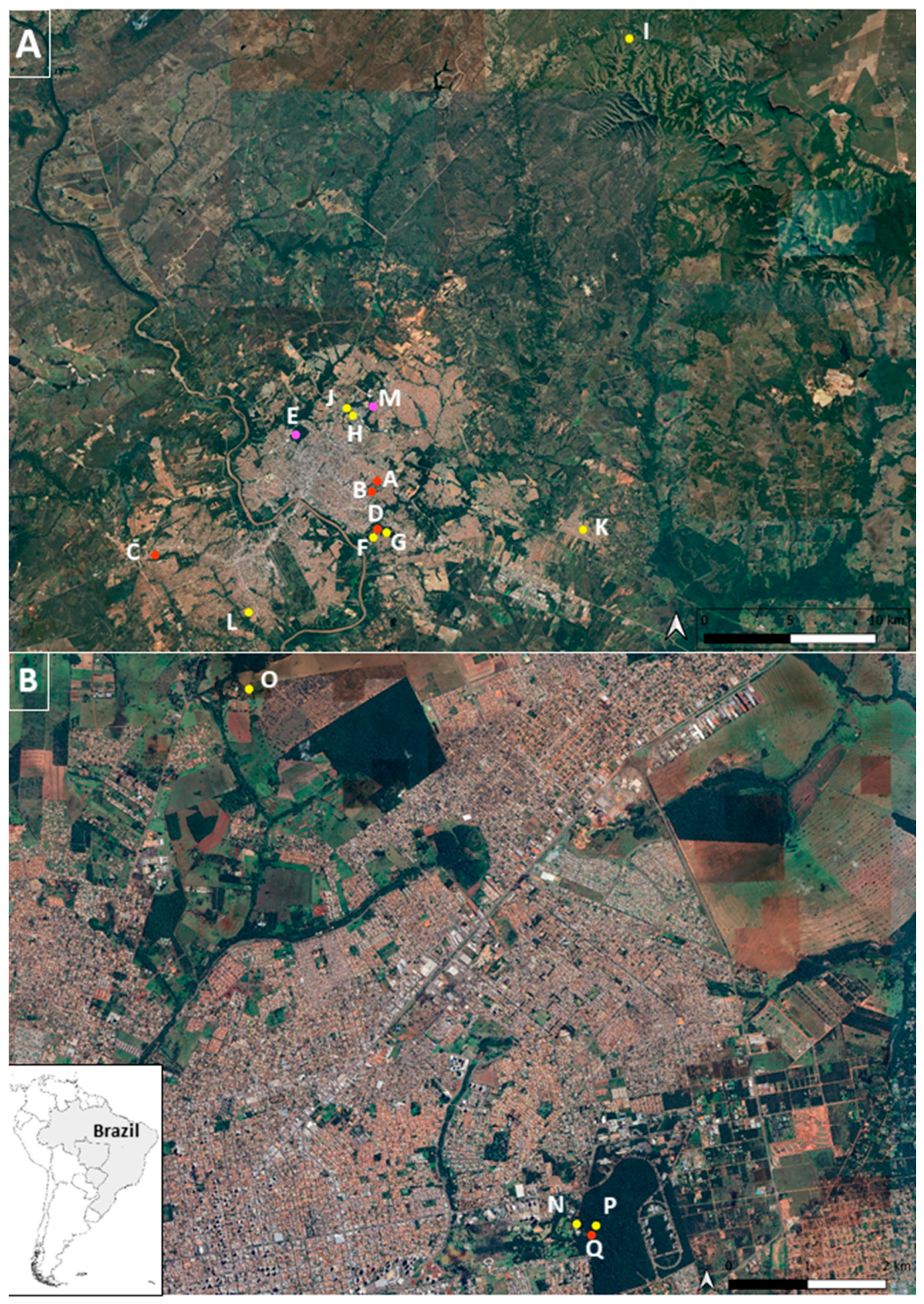
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kramer, L.D. Complexity of virus–vector interactions. Curr. Opin. Virol. 2016, 21, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.F.d.C. Febre amarela. Rev. Soc. Bras. Med. Trop. 2003, 36, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.A.; Errante, P.R. Aspectos clínicos, prevenção e epidemiologia da febre Amarela no Brasil. UNILUS Ensino Pesqui. 2017, 14, 169. Available online: http://revista.unilus.edu.br/index.php/ruep/article/view/796/u2017v14n34e796 (accessed on 25 July 2021).
- Abreu, F.V.S.; Delatorre, E.; dos Santos, A.A.C.; Ferreira-de-Brito, A.; de Castro, M.G.; Ribeiro, I.P.; Meneguete, P.; Bonaldo, M.C.; Bello, G.; Lourenço-de-Oliveira, R. Combination of surveillance tools reveals that Yellow Fever virus can remain in the same Atlantic Forest area at least for three transmission seasons. Mem. Inst. Oswaldo Cruz. 2019, 114, e190076. [Google Scholar] [CrossRef] [PubMed]
- Couto-Lima, D.; Madec, Y.; Bersot, M.I.; Campos, S.S.; Motta, M.d.A.; Santos, F.B.d.; Vazeille, M.; da Costa Vasconcelos, P.F.; Lourenço-de-Oliveira, R.; Failloux, A.-B. Potential risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Sci. Rep. 2017, 7, 4848. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.S.; da Costa, A.C.; de Azevedo Fernandes, N.C.C.; Guerra, J.M.; dos Santos, F.C.P.; Nogueira, J.S.; D’Agostino, L.G.; Komninakis, S.V.; Witkin, S.S.; Ressio, R.A.; et al. Epizootics due to Yellow Fever Virus in São Paulo State, Brazil: Viral dissemination to new areas (2016–2017). Sci. Rep. 2019, 9, 5474. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.S.O.; Romanos, M.T.V.; Wigg, M.D. Virologia Humana, 3rd ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Silva, N.I.O.; Sacchetto, L.; de Rezende, I.M.; Trindade, G.d.S.; LaBeaud, A.D.; de Thoisy, B.; Drumond, B.P. Recent sylvatic yellow fever virus transmission in Brazil: The news from an old disease. Virol. J. 2020, 17, 9. [Google Scholar] [CrossRef]
- Araujo, F.A.A.; Ramos, D.G.; Santos, A.L.; Passos, P.H.d.O.; Elkhoury, A.N.S.M.; Costa, Z.G.A.; Leal, S.G.; Romano, A.P.M. Epizootias em primatas não humanos durante reemergência do vírus da febre amarela no Brasil, 2007 a 2009. Epidemiol. Serviços Saúde 2011, 20, 527–536. [Google Scholar] [CrossRef]
- Almeida, M.A.; Cardoso, J.d.C.; Dos Santos, E.; da Fonseca, D.F.; Cruz, L.L.; Faraco, F.J.; Bercini, M.A.; Vettorello, K.C.; Porto, M.A.; Mohrdieck, R.; et al. Surveillance for yellow Fever virus in non-human primates in southern Brazil, 2001–2011: A tool for prioritizing human populations for vaccination. PLoS Negl. Trop. Dis. 2014, 8, e2741. [Google Scholar] [CrossRef]
- Pauvolid-Corrêa, A.; Dias, H.G.; Maia, L.M.S.; Porfírio, G.; Morgado, T.O.; Sabino-Santos, G.; Rita, P.H.S.; Barreto, W.T.G.; de Macedo, G.C.; Torres, J.M.; et al. Zika Virus Surveillance at the Human–Animal Interface in West-Central Brazil, 2017–2018. Viruses 2019, 11, 1164. [Google Scholar] [CrossRef]
- Lane, J. Neotropical Culicidae; Editora da Universidade de São Paulo: São Paulo, Brazil, 1953; Volume 2, p. 1660. [Google Scholar]
- Forattini, O.P. Culicidologia Médica: Identificação, Biologia, Epidemiologia; Editora da Universidade de São Paulo: São Paulo, Brazil, 2002; p. 860. [Google Scholar]
- Domingo, C.; Patel, P.; Yillah, J.; Weidmann, M.; Méndez, J.A.; Nakouné, E.R.; Niedrig, M. Advanced Yellow Fever Virus Genome Detection in Point-of-Care Facilities and Reference Laboratories. J. Clin. Microbiol. 2012, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Vasconcelos, P.F.C. Yellow fever. J. Clin. Virol. 2015, 64, 160. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde (BR); Secretaria de Vigilância em Saúde; Departamento de Vigilância das Doenças Transmissíveis. Guia de Vigilância de Epizootias em Primatas não Humanos e Entomologia Aplicada à Vigilância da Febre Amarela, 2nd ed.; Ministério da Saúde: Brasília, Brazil, 2014; 100p.
- Rímoli, J.; Milagres, A.P. Avaliação do Risco de Extinção de Mico Melanurus (É. Geoffroy em Humboldt, 1812) no Brasil. Processo de Avaliação do Risco de Extinção da Fauna Brasileira. ICMBio. 2015. Available online: http://www.icmbio.gov.br/portal/biodiversidade/fauna-brasileira/estado-de-conservacao/7231-mamiferos-mico-melanurus-sagui-marrom.html (accessed on 2 March 2022).
- Sauer, A.C.L.; Barroso, W.A.; Santos, U.F.; Portela, M.J.L.; Machado, A.F.; Camera, B.F.; Canale, G.R. Efeito da sazonalidade sobre o padrão comportamental de um grupo de saguis-do-rabo-preto (Mico melanurus) em um fragmento florestal urbano. In A Primatologia No Brasil; Silva, V.L., Ferreira, R.G., Oliveira, M.A.B., Eds.; Editora UFPE: Recife, Brazil, 2017; pp. 266–276. [Google Scholar]
- Roehrig, J.T.; Hombach, J.; Barrett, A.D.T. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol. 2008, 21, 123–132. [Google Scholar] [CrossRef]
- Pauvolid-Corrêa, A.; Campos, Z.; Juliano, R.; Velez., J.; Nogueira, R.M.; Komar, N. Serological evidence of widespread circulation of West Nile virus and other flaviviruses in equines of the Pantanal, Brazil. PLoS Negl. Trop. Dis. 2014, 8, e2706. [Google Scholar] [CrossRef]
- Miyashiro, G. O Atual surto de Febre Amarela Pode ter Relação Com o Desequilíbrio Ecológico Produzido Pelo Desmatamento, Aumento da Fronteira Agrícola e Desastres Ambientais. Escola Politécnica de Saúde Joaquim Venâncio (EPSJV/Fiocruz). 10 February 2017. Available online: http://www.epsjv.fiocruz.br/noticias/entrevista/o-atual-surto-de-febre-amarela-pode-ter-relacao-com-o-desequilibrio-ecologico (accessed on 12 January 2022).
- Consoli, R.A.G.B.; Lourenço-de-Oliveira, R. Principais Mosquitos de Importância Sanitária no Brasil; Editora FIOCRUZ: Rio de Janeiro, Brazil, 1994; 228p. [Google Scholar]
- Lourenço-de-Oliveira, R.; Vazeille, M.; de Filippis, A.M.B.; Failloux, A.B. Aedes aegypti in Brazil: Genetically differentiated populations with high susceptibility to dengue and yellow fever viruses. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.d.C.; Torres, M.A.N.; Paula, M.B.d.; Fernandes, A.; Marassá, A.M.; Consales, C.A.; Fonseca, D.F. Ecologia de Haemagogus e Sabethes (Diptera: Culicidae) em áreas epizoóticas do vírus da febre amarela, Rio Grande do Sul, Brasil. Epidemiol. Serviços Saúde 2010, 19, 101–113. [Google Scholar] [CrossRef]


| Non-Human Primate Species | N | Common Name | Conservation Status |
|---|---|---|---|
| Sapajus cay | 13 | Azara’s Capuchin | Vulnerable |
| Alouatta caraya | 5 | Howler Monkey | Threatened |
| Aotus lemurinus | 2 | Colombian Night Monkey | Vulnerable |
| Ateles marginatus | 2 | White-cheeked Spider Monkey | Threatened |
| Callithrix jacchus | 4 | Common Marmoset | Least Concern |
| Callithrix penicillata | 1 | Black-penciled Marmoset | Least Concern |
| Mico melanurus | 48 | Black-tailed Marmoset | Threatened |
| Sapajus apella | 5 | Black-capped Capuchin | Least Concern |
| TOTAL | 80 |
| Trap Type | City | Aedes (n) | Culex (n) | Haemagogus (n) | Sabethes (n) | Mansonia (n) | Psorophora (n) | Total (n) |
|---|---|---|---|---|---|---|---|---|
| Handheld aspirator | Cuiabá | 103 | 821 | 33 | 1 | 0 | 5 | |
| Campo Grande | 0 | 0 | 48 | 7 | 0 | 0 | ||
| Total | 103 | 821 | 81 | 8 | 0 | 5 | 1018 | |
| BG | Cuiabá | 0 | 433 | 0 | 0 | 0 | 0 | |
| Campo Grande | 1 | 4 | 0 | 6 | 1 | 0 | ||
| Total | 1 | 437 | 0 | 6 | 1 | 0 | 445 | |
| CDC light | Cuiabá | 10 | 65 | 4 | 0 | 0 | 1 | |
| Campo Grande | 10 | 4 | 0 | 0 | 0 | 13 | ||
| Total | 20 | 69 | 4 | 0 | 0 | 14 | 107 | |
| CDC with CO2 | Cuiabá | 6 | 224 | 2 | 0 | 0 | 0 | |
| Campo Grande | 7 | 40 | 0 | 2 | 0 | 193 | ||
| Total | 13 | 264 | 2 | 2 | 0 | 193 | 473 | |
| Resting | Cuiabá | 0 | 213 | 0 | 0 | 0 | 0 | |
| Campo Grande | 1 | 1 | 0 | 0 | 0 | 0 | ||
| Total | 1 | 214 | 0 | 0 | 0 | 0 | 215 | |
| Total | 138 | 1805 | 87 | 16 | 1 | 212 | 2258 | |
| Trap Type | City, State | Aedes | Haemagogus | Sabethes | Total |
|---|---|---|---|---|---|
| Handheld aspirator | Cuiabá, MT | 27 | 4 | 0 | |
| Campo Grande, MS | 2 | 4 | 0 | ||
| Total | 29 | 8 | 0 | 37 | |
| BG | Cuiabá, MT | 4 | 0 | 0 | |
| Campo Grande, MS | 2 | 0 | 0 | ||
| Total | 6 | 0 | 0 | 6 | |
| CDC light | Cuiabá, MT | 1 | 0 | 0 | |
| Campo Grande, MS | 1 | 0 | 0 | ||
| Total | 2 | 0 | 0 | 2 | |
| CDC with CO2 | Cuiabá, MT | 0 | 0 | 0 | |
| Campo Grande, MS | 5 | 0 | 1 | ||
| Total | 5 | 0 | 1 | 6 | |
| Resting | Cuiabá, MT | 1 | 0 | 0 | |
| Campo Grande, MS | 0 | 0 | 0 | ||
| Total | 1 | 0 | 0 | 1 | |
| No location | Total | 22 | 0 | 0 | 22 |
| Total | 65 | 8 | 1 | 74 |
| Culicidae Species | N | % |
|---|---|---|
| Aedes aegypti | 105 | 51.7 |
| Aedes albopictus | 56 | 27.6 |
| Aedes augustivittatus | 2 | 1.0 |
| Aedes fulvithorax | 4 | 2.0 |
| Aedes spp. | 36 | 17.7 |
| Total | 203 | 100 |
| Culex spp. | 1805 | 100 |
| Total | 1805 | 100 |
| Haemagogus janthinomys | 58 | 61.0 |
| Haemagogus leucocelaenus | 24 | 25.3 |
| Haemagogus spp. | 13 | 13.7 |
| Total | 95 | 100 |
| Psorophora albigenu | 1 | 0.5 |
| Psorophora cilipes | 37 | 17.5 |
| Psorophora cingulata | 31 | 14.7 |
| Psorophora dimidiata | 108 | 51.2 |
| Psorophora lanei | 22 | 10.4 |
| Psorophora spp. | 12 | 5.7 |
| Total | 211 | 100 |
| Mansonia sp. | 1 | 100 |
| Total | 1 | 100 |
| Sabethes glaucodaemon | 3 | 17.6 |
| Sabethes purpureus | 2 | 11.8 |
| Sabethes spp. | 12 | 70.6 |
| Total | 17 | 100 |
| Total | 2332 | 100 |
| Campo Grande, MS | Cuiabá, MT | ||||||
|---|---|---|---|---|---|---|---|
| NHP | Popular Name | N | % | N | % | PRNT90 Titer | Total |
| Alouatta caraya | Black-and-Gold Howler Monkey | 4 | 20 | 1 | 33.3 | <10 | 5 |
| Ateles marginatus | White-whiskered Spider Monkey | 1 | 5 | 0 | 0 | <10 | 1 |
| Callithrix jacchus | Common Marmoset | 3 | 15 | 0 | 0 | <10 | 3 |
| Callithrix penicillata | Black-tufted-ear Marmoset | 0 | 0 | 1 | 33.3 | <10 | 1 |
| Mico melanurus | Black-tailed Marmoset | 1 | 5 | 0 | 0 | <10 | 1 |
| Sapajus cay | Hooded Capuchin | 11 | 55 | 1 | 33.3 | <10 | 12 |
| Total | 20 | 100% | 3 | 100% | 23 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranowski, L.A.; Dias, H.G.; Familiar-Macedo, D.; Sabino-Santos, G.; Herrera, H.M.; Slhessarenko, R.D.; dos Santos, F.B.; Pauvolid-Corrêa, A. Investigation of Yellow Fever Virus at the Human–Animal Interface after a Zika Virus Outbreak in Midwest Brazil. Microorganisms 2024, 12, 594. https://doi.org/10.3390/microorganisms12030594
Baranowski LA, Dias HG, Familiar-Macedo D, Sabino-Santos G, Herrera HM, Slhessarenko RD, dos Santos FB, Pauvolid-Corrêa A. Investigation of Yellow Fever Virus at the Human–Animal Interface after a Zika Virus Outbreak in Midwest Brazil. Microorganisms. 2024; 12(3):594. https://doi.org/10.3390/microorganisms12030594
Chicago/Turabian StyleBaranowski, Lis Alvarez, Helver Gonçalves Dias, Débora Familiar-Macedo, Gilberto Sabino-Santos, Heitor Miraglia Herrera, Renata Dezengrini Slhessarenko, Flávia Barreto dos Santos, and Alex Pauvolid-Corrêa. 2024. "Investigation of Yellow Fever Virus at the Human–Animal Interface after a Zika Virus Outbreak in Midwest Brazil" Microorganisms 12, no. 3: 594. https://doi.org/10.3390/microorganisms12030594
APA StyleBaranowski, L. A., Dias, H. G., Familiar-Macedo, D., Sabino-Santos, G., Herrera, H. M., Slhessarenko, R. D., dos Santos, F. B., & Pauvolid-Corrêa, A. (2024). Investigation of Yellow Fever Virus at the Human–Animal Interface after a Zika Virus Outbreak in Midwest Brazil. Microorganisms, 12(3), 594. https://doi.org/10.3390/microorganisms12030594











