Evaluation of Cellular Responses by Chlamydomonas reinhardtii in Media Containing Dairy-Processing Residues Derived from Cheese as Nutrients by Analyzing Cell Growth Activity and Comprehensive Gene Transcription Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culturing and Growth—Evaluation of Green Alga C. reinhardtii
2.2. Evaluation of Composition and Concentration of Lipids in Cells with Gas Chromatography
2.3. Measurement of Levels of Gene Transcription
3. Results
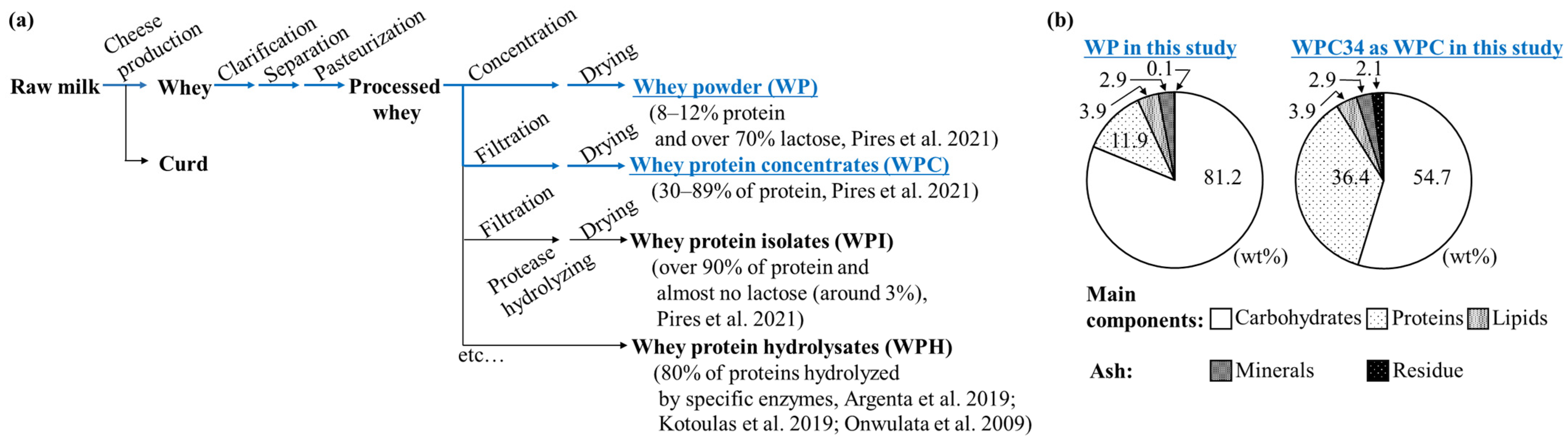
3.1. Compositions of Powders of WPC34 and WP
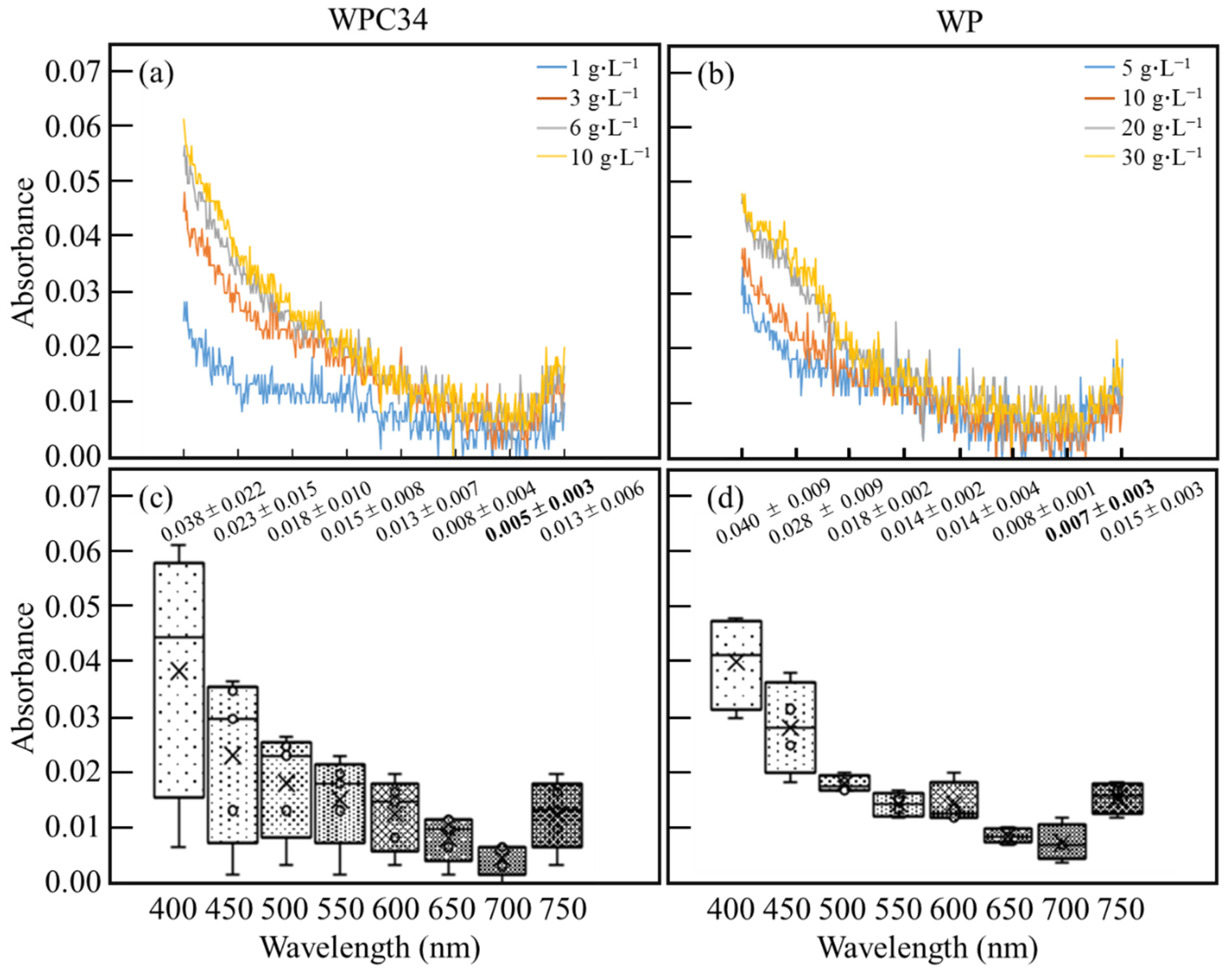
3.2. Evaluation of Photo-Absorbances Derived from WPC34 and WP in Medium
3.3. Evaluation of Growth of C. reinhardtii in TAP Medium Supplied with WPC34 and WP as Nutrients
3.4. Contents and Compositions of Lipid in C. reinhardtii Cells in Media Containing WPC34 and WP
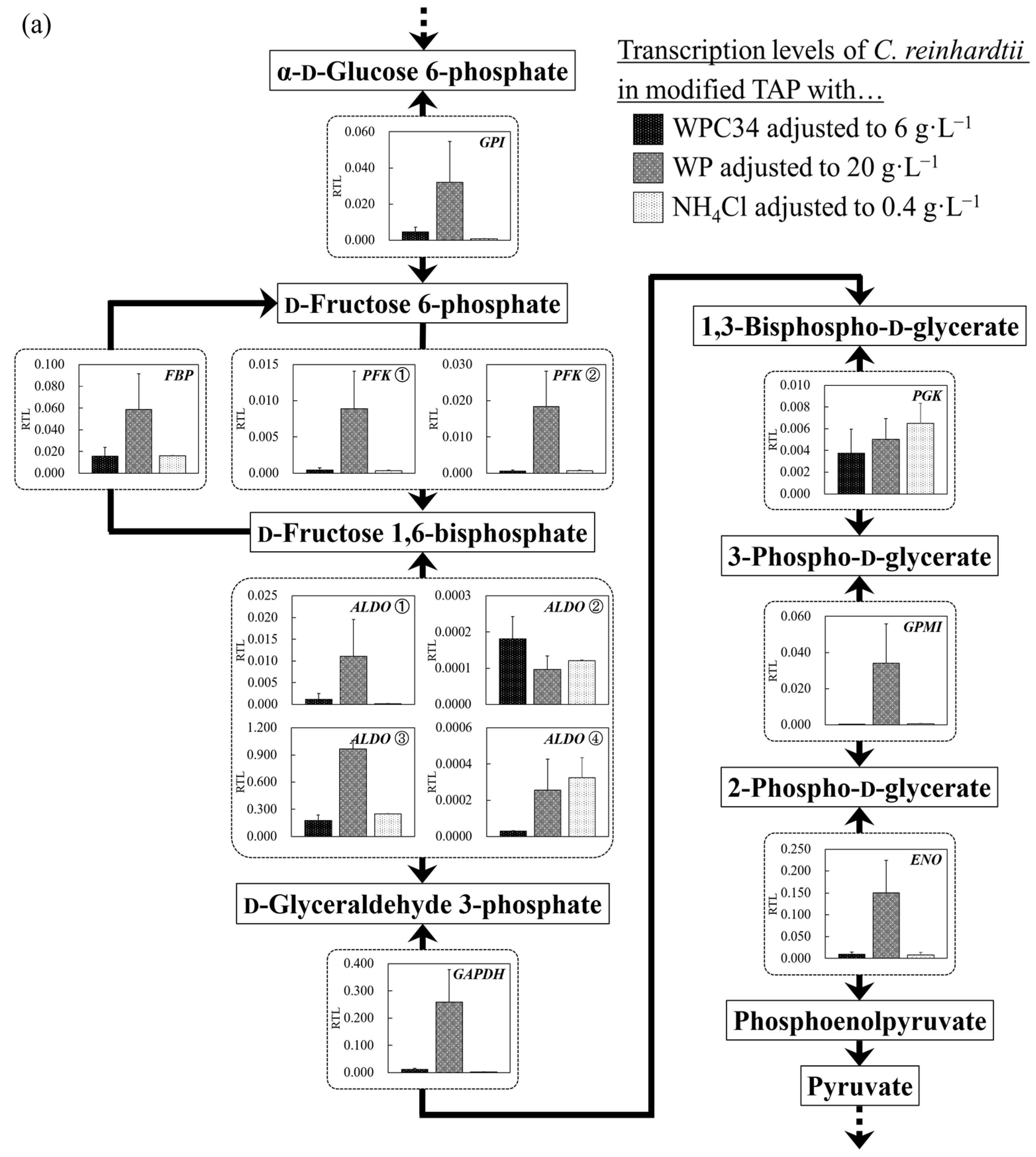
3.5. Evaluation of Cell Responses of C. reinhardtii by Analyzing Gene Transcription Levels in Each Broth Containing WPC34 and WP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Venetsaneas, N.; Antonopoulou, G.; Stamatelatou, K.; Kornaros, M.; Lyberatos, G. Using cheese whey for hydrogen and methane generation in a two-stage continuous process with alternative pH controlling approaches. Bioresour. Technol. 2009, 100, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445–446, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Lappa, I.K.; Papadaki, A.; Kachrimanidou, V.; Terpou, A.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Cheese whey processing: Integrated biorefinery concepts and emerging food applications. Foods 2019, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J. An integrated approach for the valorization of cheese whey. Foods 2021, 10, 564. [Google Scholar] [CrossRef]
- Baldasso, C.; Barros, T.C.; Tessaro, I.C. Concentration and purification of whey proteins by ultrafiltration. Desalination 2011, 278, 381–386. [Google Scholar] [CrossRef]
- Chen, G.Q.; Qu, Y.; Gras, S.L.; Kentish, S.E. Separation technologies for whey protein fractionation. Food Eng. Rev. 2023, 15, 438–465. [Google Scholar] [CrossRef]
- Tejayadi, S.; Cheryan, M. Lactic acid from cheese whey permeate: Productivity and economics of a continuous membrane bioreactor. Appl. Microbiol. Biotechnol. 1995, 43, 242–248. [Google Scholar] [CrossRef]
- Guimarães, P.M.R.; Teixeira, J.A.; Domingues, L. Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnol. Adv. 2010, 28, 375–384. [Google Scholar] [CrossRef]
- Comino, E.; Riggio, V.A.; Rosso, M. Biogas production by anaerobic co-digestion of cattle slurry and cheese whey. Bioresour. Technol. 2012, 114, 46–53. [Google Scholar] [CrossRef]
- Lyons, T.P.; Cunningham, J.D. Fuel alcohol from whey. Am. Dairy Rev. 1980, 42, 11. Available online: https://www.osti.gov/biblio/5140196 (accessed on 7 March 2024).
- Christopherson, A.T.; Zottola, E.A. The use of whey permeates as starter media in cheese production. J. Dairy Sci. 1989, 72, 2862–2868. [Google Scholar] [CrossRef]
- de Divitiis, M.; Ami, D.; Pessina, A.; Palmioli, A.; Sciandrone, B.; Airoldi, C.; Regonesi, M.E.; Brambilla, L.; Lotti, M.; Natalello, A.; et al. Cheese-whey permeate improves the fitness of Escherichia coli cells during recombinant protein production. Biotechnol. Biofuels Bioprod. 2023, 16, 30. [Google Scholar] [CrossRef]
- Rivera Flores, V.K.; DeMarsh, T.A.; Fan, X.; Alcaine, S.D. Cheese whey permeate as a precursor of lactose-free, galactose-rich bioproducts: An approach for optimization and application. Food Bioproc. Technol. 2023, 1–21. [Google Scholar] [CrossRef]
- Hasunuma, T.; Takaki, A.; Matsuda, M.; Kato, Y.; Vavricka, C.J.; Kondo, A. Single-stage astaxanthin production enhances the nonmevalonate pathway and photosynthetic central metabolism in Synechococcus sp. PCC 7002. ACS Synth. Biol. 2019, 8, 2701–2709. [Google Scholar] [CrossRef]
- Dashko, S.; Zhou, N.; Compagno, C.; Piškur, J. Why, when, and how did yeast evolve alcoholic fermentation? FEMS Yeast Res. 2014, 14, 826–832. [Google Scholar] [CrossRef]
- Gietz, R.D.; Woods, R.A. Genetic transformation of yeast. Biotechniques 2001, 30, 816–831. [Google Scholar] [CrossRef]
- Yang, X.; Lee, S.J.; Yoo, H.Y.; Choi, H.S.; Park, C.; Kim, S.W. Biorefinery of instant noodle waste to biofuels. Bioresour. Technol. 2014, 159, 17–23. [Google Scholar] [CrossRef]
- Khawla, B.J.; Sameh, M.; Imen, G.; Donyes, F.; Dhouha, G.; Raoudha, E.G.; Oumèma, N.E. Potato peel as feedstock for bioethanol production: A comparison of acidic and enzymatic hydrolysis. Ind. Crops Prod. 2014, 52, 144–149. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Ayala-Moreno, V.G.; Mejia-Melara, C.A.; Rodríguez-Rodríguez, J.; Cuellar-Bermudez, S.P.; González-González, R.B.; Coronado-Apodaca, K.G.; Farfan-Cabrera, L.I.; González-Meza, G.M.; Iqbal, H.M.N.; et al. Growth behavior, biomass composition and fatty acid methyl esters (FAMEs) production potential of Chlamydomonas reinhardtii, and Chlorella vulgaris cultures. Mar. Drugs 2023, 21, 450. [Google Scholar] [CrossRef]
- Wu, K.; Fang, Y.; Hong, B.; Cai, Y.; Xie, H.; Wang, Y.; Cui, X.; Yu, Z.; Liu, Y.; Ruan, R.; et al. Enhancement of carbon conversion and value-added compound production in heterotrophic Chlorella vulgaris using sweet sorghum extract. Foods 2022, 11, 2579. [Google Scholar] [CrossRef]
- Chen, Y.; Du, H.; Liang, H.; Hong, T.; Li, T. Enhanced carotenoid production in Chlamydomonas reinhardtii by overexpression of endogenousand exogenous beta-carotene ketolase (BKT) genes. Int. J. Mol. Sci. 2023, 24, 11382. [Google Scholar] [CrossRef]
- Young, E.B.; Reed, L.; Berges, J.A. Growth parameters and responses of green algae across a gradient of phototrophic, mixotrophic and heterotrophic conditions. PeerJ 2022, 10, e13776. [Google Scholar] [CrossRef]
- Murbach, T.S.; Glávits, R.; Endres, J.R.; Hirka, G.; Vértesi, A.; Béres, E.; Szakonyiné, I.P. A toxicological evaluation of Chlamydomonas reinhardtii, a green algae. Int. J. Toxicol. 2018, 37, 53–62. [Google Scholar] [CrossRef]
- Zhang, M.P.; Wang, M.; Wang, C. Nuclear transformation of Chlamydomonas reinhardtii: A review. Biochimie 2021, 181, 1–11. [Google Scholar] [CrossRef]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Maréchal-Drouard, L.; et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef]
- Nakanishi, A.; Mori, M.; Yamamoto, N.; Nemoto, S.; Kanamaru, N.; Yomogita, M.; Omino, N.; Matsumoto, R. Evaluation of cell responses of Saccharomyces cerevisiae under cultivation using wheat bran as a nutrient resource by analyses of growth activities and comprehensive gene transcription levels. Microorganisms 2023, 11, 2674. [Google Scholar] [CrossRef]
- Nakanishi, A.; Zhang, K.; Matsumoto, R.; Yamamoto, N. Estimation of carbon metabolism in Saccharomyces cerevisiae acclimatized to glycerol assimilation with quantitative PCR. Microorganisms 2022, 10, 1173. [Google Scholar] [CrossRef]
- Berges, J.A.; Franklin, D.J.; Harrison, P.J. Evolution of an artificial seawater medium: Improvements in enriched seawater, artificial water over the last two decades. J. Phycol. 2001, 37, 1138–1145. [Google Scholar] [CrossRef]
- Gorman, D.S.; Levine, R.P. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1965, 54, 1665–1669. [Google Scholar] [CrossRef]
- Ho, S.H.; Nakanishi, A.; Ye, X.; Chang, J.S.; Chen, C.Y.; Hasunuma, T.; Kondo, A. Dynamic metabolic profiling of the marine microalga Chlamydomonas sp. JSC4 and enhancing its oil production by optimizing light intensity. Biotechnol. Biofuels 2015, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy by-products: A review on the valorization of whey and second cheese whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Argenta, A.B.; Scheer, A.D.P. Membrane separation processes applied to whey: A review. Food Rev. Int. 2019, 36, 499–528. [Google Scholar] [CrossRef]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.E.; Tatoulis, T.I.; Akratos, C.S.; Tekerlekopoulou, A.G.; Vayenas, D.V. Second Cheese Whey Treatment Using Zeolite under Continuous Flow Mode and Its Application on Wheat Growth. Water 2019, 11, 928. [Google Scholar] [CrossRef]
- Onwulata, C.; Huth, P. Whey Processing, Functionality and Health Benefits; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 227–284. ISBN 978-0-813-80384-5. [Google Scholar]
- Lemaire, C.; Wollman, F.A. The chloroplast ATP synthase in Chlamydomonas reinhardtii. J. Biol. Chem. 1989, 264, 10228–10234. [Google Scholar] [CrossRef] [PubMed]
- May, R. The Arp2/3 complex: A central regulator of the actin cytoskeleton. Cell. Mol. Life Sci. 2001, 58, 1607–1626. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xu, L.; Huang, R.; Wang, Q. Improved biohydrogen production with an expression of codon-optimized hemH and lba genes in the chloroplast of Chlamydomonas reinhardtii. Bioresour. Technol. 2011, 102, 2610–2616. [Google Scholar] [CrossRef] [PubMed]
- Palatnik, D.R.; Ostermann Porcel, M.V.; González, U.; Zaritzky, N.; Campderrós, M.E. Recovery of caprine whey protein and its application in a food protein formulation. LWT-Food Sci. Technol. 2015, 63, 331–338. [Google Scholar] [CrossRef]
- Macedo, A.; Azedo, D.; Duarte, E.; Pereira, C. Valorization of goat cheese whey through an integrated process of ultrafiltration and nanofiltration. Membranes 2021, 11, 477. [Google Scholar] [CrossRef]
- Reig, M.; Vecino, X.; Cortina, J.L. Use of membrane technologies in dairy industry: An overview. Foods 2021, 10, 2768. [Google Scholar] [CrossRef]
- Lin, M.-J.; Grandison, A.S.; Lewis, M.J. Partitioning of calcium and magnesium (total divalent cations) during membrane filtration of milk. J. Food Eng. 2015, 149, 153–158. [Google Scholar] [CrossRef]
- Chioccioli, M.; Hankamer, B.; Ross, I.L. Flow cytometry pulse width data enables rapid and sensitive estimation of biomass dry weight in the microalgae Chlamydomonas reinhardtii and Chlorella vulgaris. PLoS ONE 2014, 9, e97269. [Google Scholar] [CrossRef] [PubMed]
- Gorban, A.N.; Pokidysheva, L.I.; Smirnova, E.V.; Tyukina, T.A. Law of the minimum paradoxes. Bull. Math. Biol. 2011, 73, 2013–2044. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.P.; LaCroix, D.E.; McDonough, F.E. Minerals in whey and whey fractions. J. Dairy Sci. 1978, 61, 1700–1703. [Google Scholar] [CrossRef] [PubMed]
- Vegarud, G.E.; Langsrud, T.; Svenning, C. Mineral-binding milk proteins and peptides; occurrence, biochemical and technological characteristics. Br. J. Nutr. 2000, 84, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Ren, L.J.; Zhao, Q.Y.; Ji, X.J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, T.; Angun, P.; Demiray, Y.E.; Ozkan, A.D.; Elibol, Z.; Tekinay, T. Differential effects of nitrogen and sulfur deprivation on growth and biodiesel feedstock production of Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2012, 109, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Y.; Meng, H.; Li, S.; Wang, S.; Xiao, Z.; Chang, P.; Zhang, X.; Li, Q.; Guo, L.; et al. Characterization of fatty acid exporters involved in fatty acid transport for oil accumulation in the green alga Chlamydomonas reinhardtii. Biotechnol. Biofuels 2019, 12, 14. [Google Scholar] [CrossRef]
- Hounslow, E.; Evans, C.A.; Pandhal, J.; Sydney, T.; Couto, N.; Pham, T.K.; Gilmour, D.J.; Wright, P.C. Quantitative proteomic comparison of salt stress in Chlamydomonas reinhardtii and the snow alga Chlamydomonas nivalis reveals mechanisms for salt-triggered fatty acid accumulation via reallocation of carbon resources. Biotechnol. Biofuels 2021, 14, 121. [Google Scholar] [CrossRef]
- Wolberger, C. How structural biology transformed studies of transcription regulation. J. Biol. Chem. 2021, 296, 100741. [Google Scholar] [CrossRef]
- Johnson, X.; Alric, J. Central carbon metabolism and electron transport in Chlamydomonas reinhardtii: Metabolic constraints for carbon partitioning between oil and starch. Eukaryot. Cell 2013, 12, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Pivato, M.; Ballottari, M. Chlamydomonas reinhardtii cellular compartments and their contribution to intracellular calcium signaling. J. Exp. Bot. 2021, 72, 5312–5335. [Google Scholar] [CrossRef] [PubMed]
- Klein, U. Compartmentation of glycolysis and of the oxidative pentose-phosphate pathway in Chlamydomonas reinhardtii. Planta 1986, 167, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Koksharova, O.; Schubert, M.; Shestakov, S.; Cerff, R. Genetic and biochemical evidence for distinct key functions of two highly divergent GAPDH genes in catabolic and anabolic carbon flow of the cyanobacterium Synechocystis sp. PCC 6803. Plant Mol. Biol. 1998, 36, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Trost, P.; Fermani, S.; Marri, L.; Zaffagnini, M.; Falini, G.; Scagliarini, S.; Pupillo, P.; Sparla, F. Thioredoxin-dependent regulation of photosynthetic glyceraldehyde-3-phosphate dehydrogenase: Autonomous vs. CP12-dependent mechanisms. Photosynth. Res. 2006, 89, 263–275. [Google Scholar] [CrossRef]
- Zaffagnini, M.; Fermani, S.; Costa, A.; Lemaire, S.D.; Trost, P. Plant cytoplasmic GAPDH: Redox post-translational modifications and moonlighting properties. Front. Plant Sci. 2013, 4, 450. [Google Scholar] [CrossRef]
- Bertolotti, R.; Armbruster-Hilbert, L.; Okayama, H. Liver fructose-1,6-bisphosphatase cDNA: Trans-complementation of fission yeast and characterization of two human transcripts. Differentiation 1995, 59, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Abbriano, R.; Vardar, N.; Yee, D.; Hildebrand, M. Manipulation of a glycolytic regulator alters growth and carbon partitioning in the marine diatom Thalassiosira pseudonana. Algal Res. 2018, 32, 250–258. [Google Scholar] [CrossRef]
- Graña, X.; Pérez de la Ossa, P.; Broceño, C.; Stöcker, M.; Garriga, J.; Puigdomènech, P.; Climent, F. 2,3-Bisphosphoglycerate-independent phosphoglycerate mutase is conserved among different phylogenic kingdoms. Comp. Biochem. Physiol. B Biochem. Molecul. Biol. 1995, 112, 287–293. [Google Scholar] [CrossRef]
- Polle, J.E.W.; Neofotis, P.; Huang, A.; Chang, W.; Sury, K.; Wiech, E.M. Carbon partitioning in green algae (Chlorophyta) and the enolase enzyme. Metabolites 2014, 4, 612–628. [Google Scholar] [CrossRef]
- Mitchell, B.F.; Pedersen, L.B.; Feely, M.; Rosenbaum, J.L.; Mitchell, D.R. ATP production in Chlamydomonas reinhardtii flagella by glycolytic enzymes. Mol. Biol. Cell 2005, 16, 4509–4518. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Kleczkowski, L.A. The glycerate and phosphorylated pathways of serine synthesis in plants: The branches of plant glycolysis linking carbon and nitrogen metabolism. Front. Plant Sci. 2018, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Mallén-Ponce, M.J.; Pérez-Pérez, M.E.; Crespo, J.L. Photosynthetic assimilation of CO2 regulates TOR activity. Proc. Natl. Acad. Sci. USA 2022, 119, e2115261119. [Google Scholar] [CrossRef] [PubMed]
- Huergo, L.F.; Dixon, R. The emergence of 2-oxoglutarate as a master regulator metabolite. Microbiol. Mol. Biol. Rev. 2015, 79, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Scherlinger, M.; Tsokos, G.C. Shortage of aspartate in mitochondria fuels arthritis. Nat. Immunol. 2021, 22, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.D.; Cai, J.J.; Fei, X.W. Involvement of phosphatidate phosphatase in the biosynthesis of triacylglycerols in Chlamydomonas reinhardtii. J. Zhejiang Univ. Sci. B 2013, 14, 1121–1131. [Google Scholar] [CrossRef]
- Jia, B.; Yin, J.; Li, X.; Li, Y.; Yang, X.; Lan, C.; Huang, Y. Increased lipids in Chlamydomonas reinhardtii by multiple regulations of DOF, LACS2, and CIS1. Int. J. Mol. Sci. 2022, 23, 10176. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Achard, D.; Jang, S.; Legéret, B.; Kamisuki, S.; Ko, D.; Schulz-Raffelt, M.; Kim, Y.; Song, W.Y.; Nishida, I.; et al. Identification of a Chlamydomonas plastidial 2-lysophosphatidic acid acyltransferase and its use to engineer microalgae with increased oil content. Plant Biotechnol. J. 2016, 14, 2158–2167. [Google Scholar] [CrossRef]
- Yao, H.; Dahal, S.; Yang, L. Novel context-specific genome-scale modelling explores the potential of triacylglycerol production by Chlamydomonas reinhardtii. Microb. Cell Fact. 2023, 22, 13. [Google Scholar] [CrossRef]

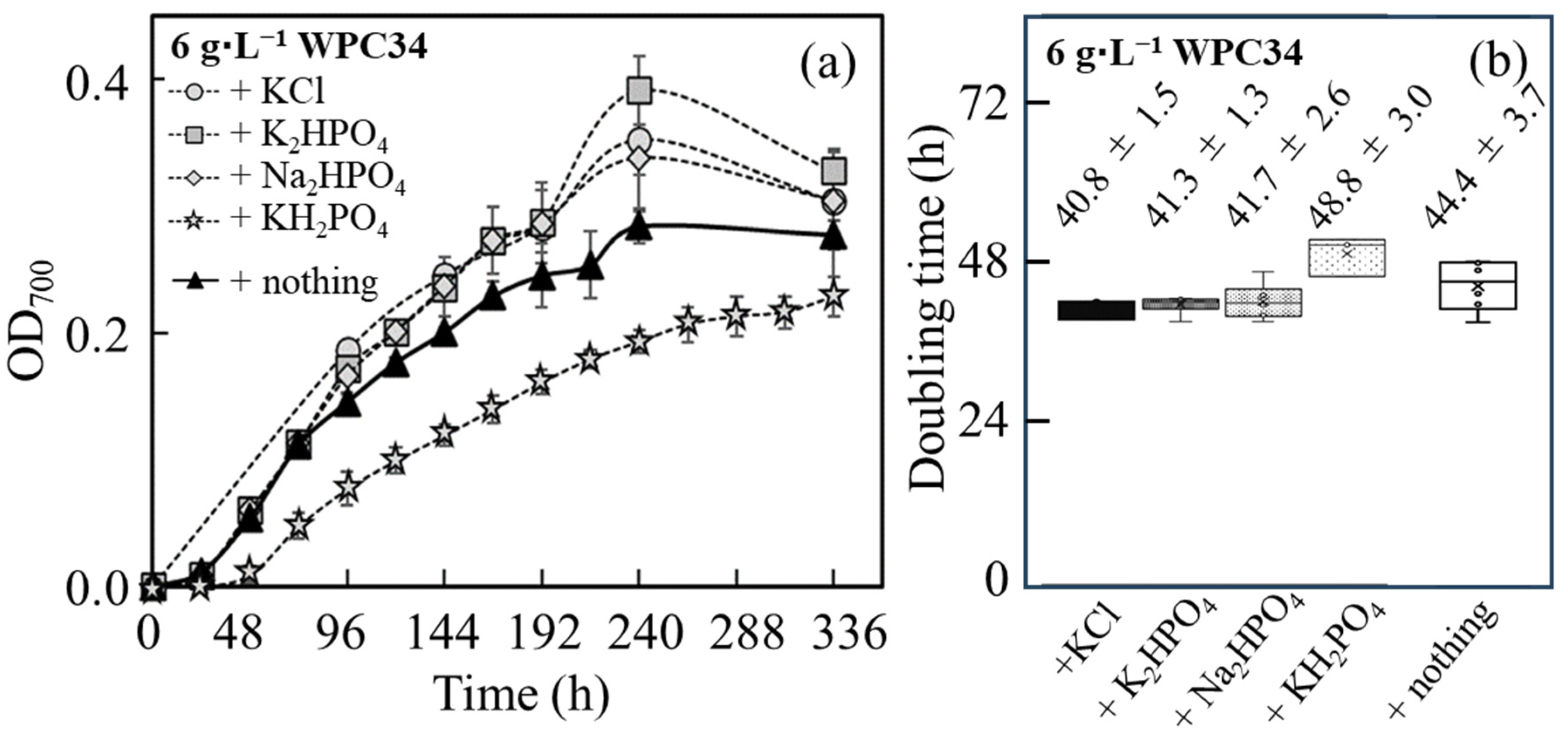

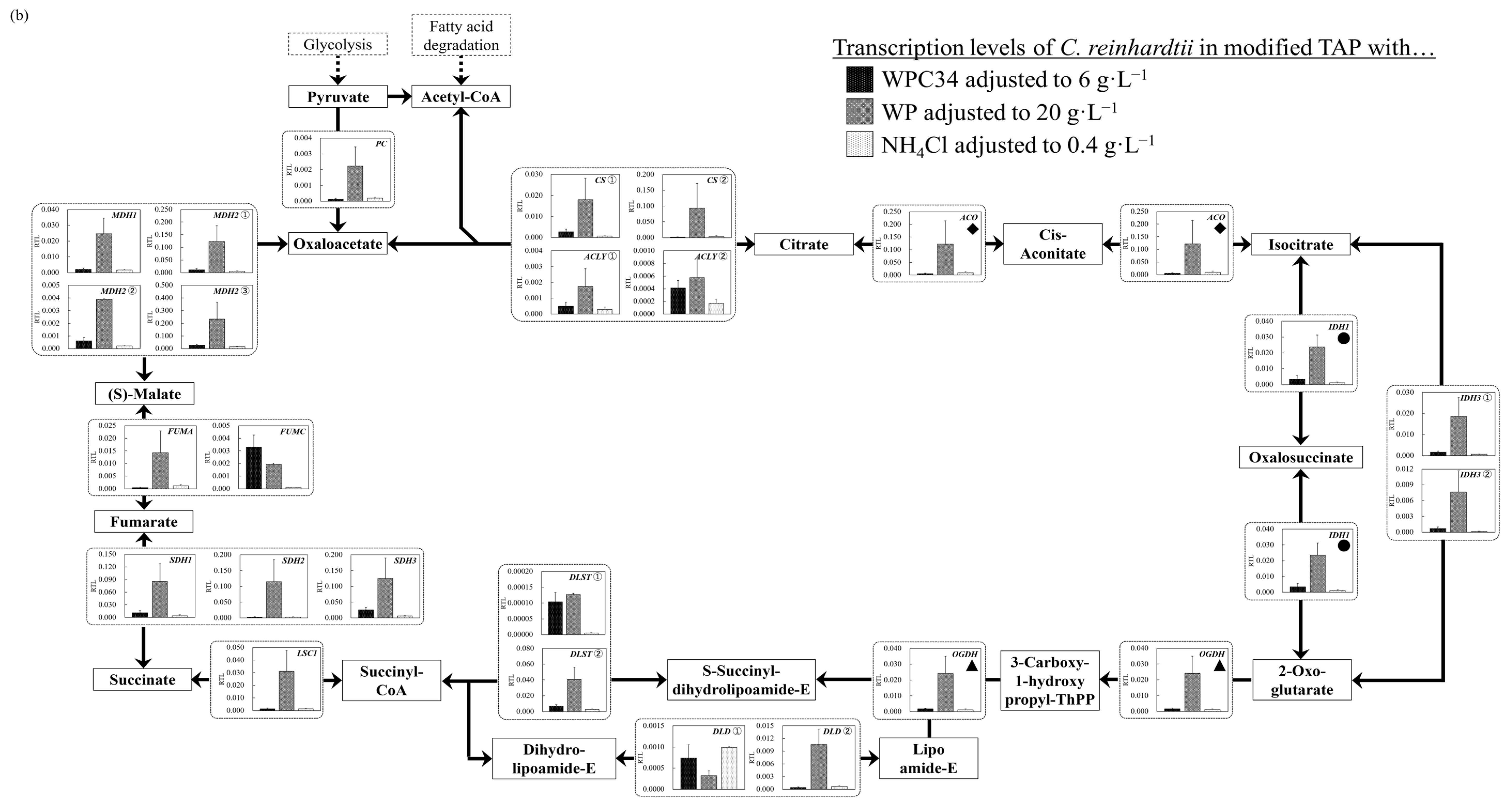
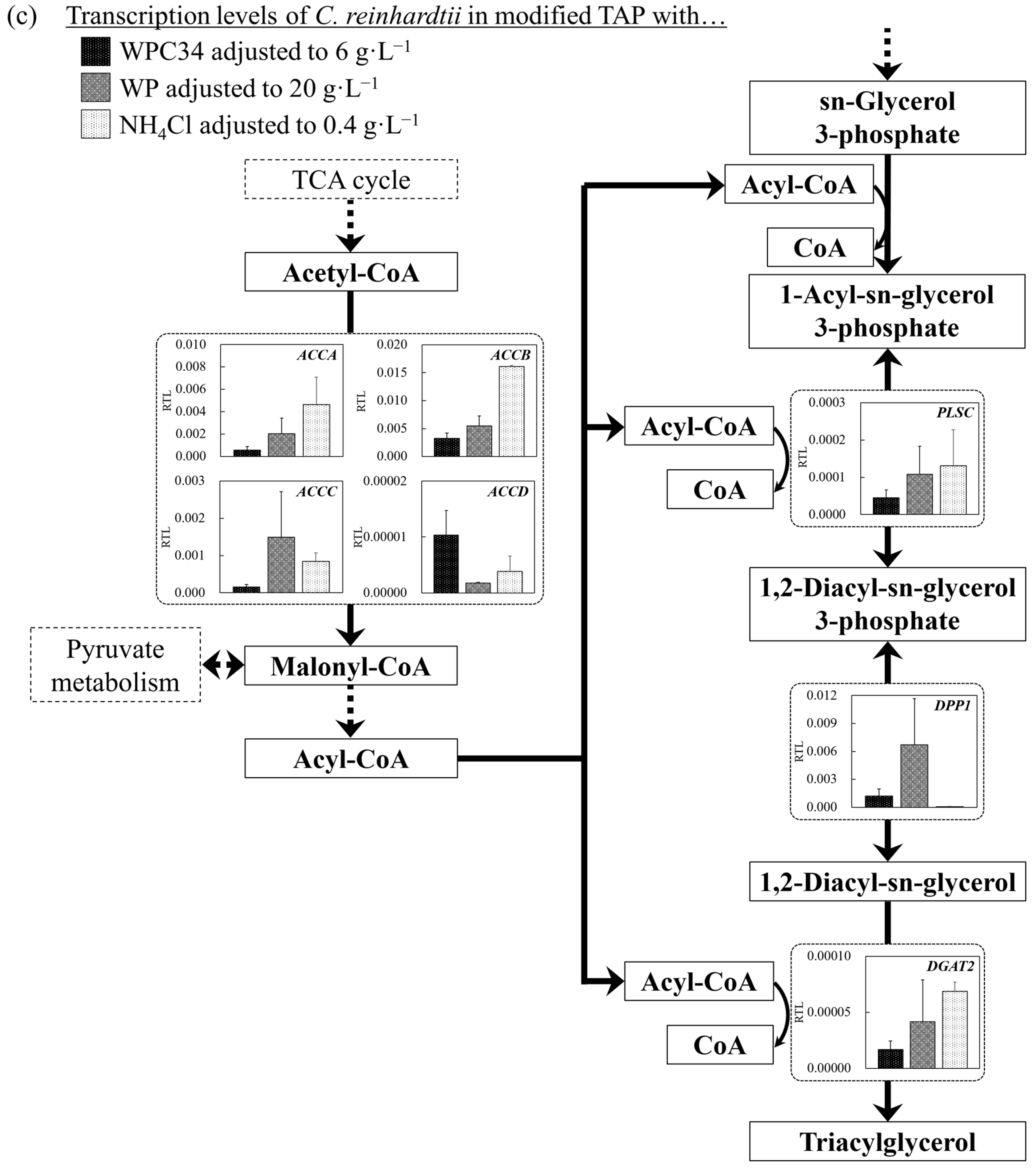
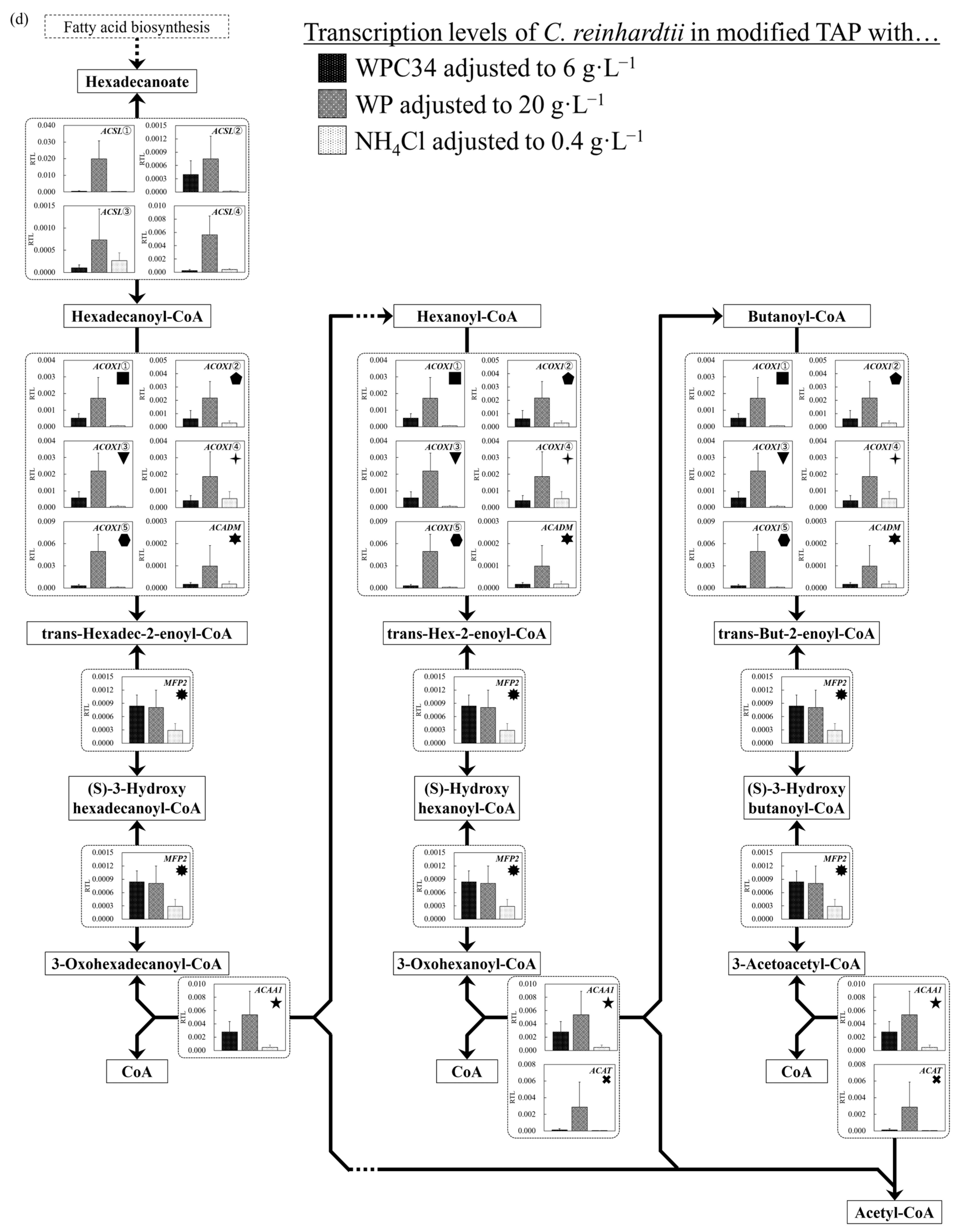
| Resource | Concentration (g·L−1) | Time Range (h) | Maximum Specific Growth Rate (µmax, h−1) | Doubling Time (h) | Maximum OD700 | Corresponding Figure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | 0 | Not detected | Not calculated | Not calculated | Not detected | Figure 3a | ||||||||
| WPC34 | 1 | 48 | ~ | 72 | 0.009 | ± | 0.001 | 74.80 | ± | 7.40 | 0.05 | ± | 0.00 | Figure 3a |
| 3 | 72 | ~ | 96 | 0.012 | ± | 0.002 | 57.68 | ± | 14.48 | 0.12 | ± | 0.00 | Figure 3a | |
| 6 | 24 | ~ | 48 | 0.065 | ± | 0.011 | 10.77 | ± | 1.73 | 0.28 | ± | 0.01 | Figure 3a | |
| 10 | 24 | ~ | 48 | 0.144 | ± | 0.072 | 5.46 | ± | 2.70 | 0.43 | ± | 0.03 | Figure 3a | |
| WP | 5 | 48 | ~ | 72 | 0.019 | ± | 0.000 | 35.28 | ± | 1.51 | 0.33 | ± | 0.00 | Figure 3d |
| 10 | 48 | ~ | 72 | 0.026 | ± | 0.002 | 26.67 | ± | 1.64 | 0.63 | ± | 0.03 | Figure 3d | |
| 20 | 72 | ~ | 96 | 0.038 | ± | 0.009 | 22.74 | ± | 4.13 | 0.68 | ± | 0.05 | Figure 3d | |
| 30 | 48 | ~ | 72 | 0.058 | ± | 0.004 | 11.93 | ± | 0.89 | 0.71 | ± | 0.01 | Figure 3d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanishi, A.; Yomogita, M.; Horimoto, T. Evaluation of Cellular Responses by Chlamydomonas reinhardtii in Media Containing Dairy-Processing Residues Derived from Cheese as Nutrients by Analyzing Cell Growth Activity and Comprehensive Gene Transcription Levels. Microorganisms 2024, 12, 715. https://doi.org/10.3390/microorganisms12040715
Nakanishi A, Yomogita M, Horimoto T. Evaluation of Cellular Responses by Chlamydomonas reinhardtii in Media Containing Dairy-Processing Residues Derived from Cheese as Nutrients by Analyzing Cell Growth Activity and Comprehensive Gene Transcription Levels. Microorganisms. 2024; 12(4):715. https://doi.org/10.3390/microorganisms12040715
Chicago/Turabian StyleNakanishi, Akihito, Misaki Yomogita, and Tomohito Horimoto. 2024. "Evaluation of Cellular Responses by Chlamydomonas reinhardtii in Media Containing Dairy-Processing Residues Derived from Cheese as Nutrients by Analyzing Cell Growth Activity and Comprehensive Gene Transcription Levels" Microorganisms 12, no. 4: 715. https://doi.org/10.3390/microorganisms12040715








