Microbial Indoles: Key Regulators of Organ Growth and Metabolic Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Bacterial Culture
2.3. Metabolic Chamber Assessment
2.4. LC-MS Targeted Metabolomic Analysis
2.5. NMR Sample Preparation, Data Acquisition, and Data Analysis
2.6. Histological Analysis
2.7. Immunohistochemistry
2.8. Quantitative Real-Time PCR
2.9. Western Blotting
3. Results
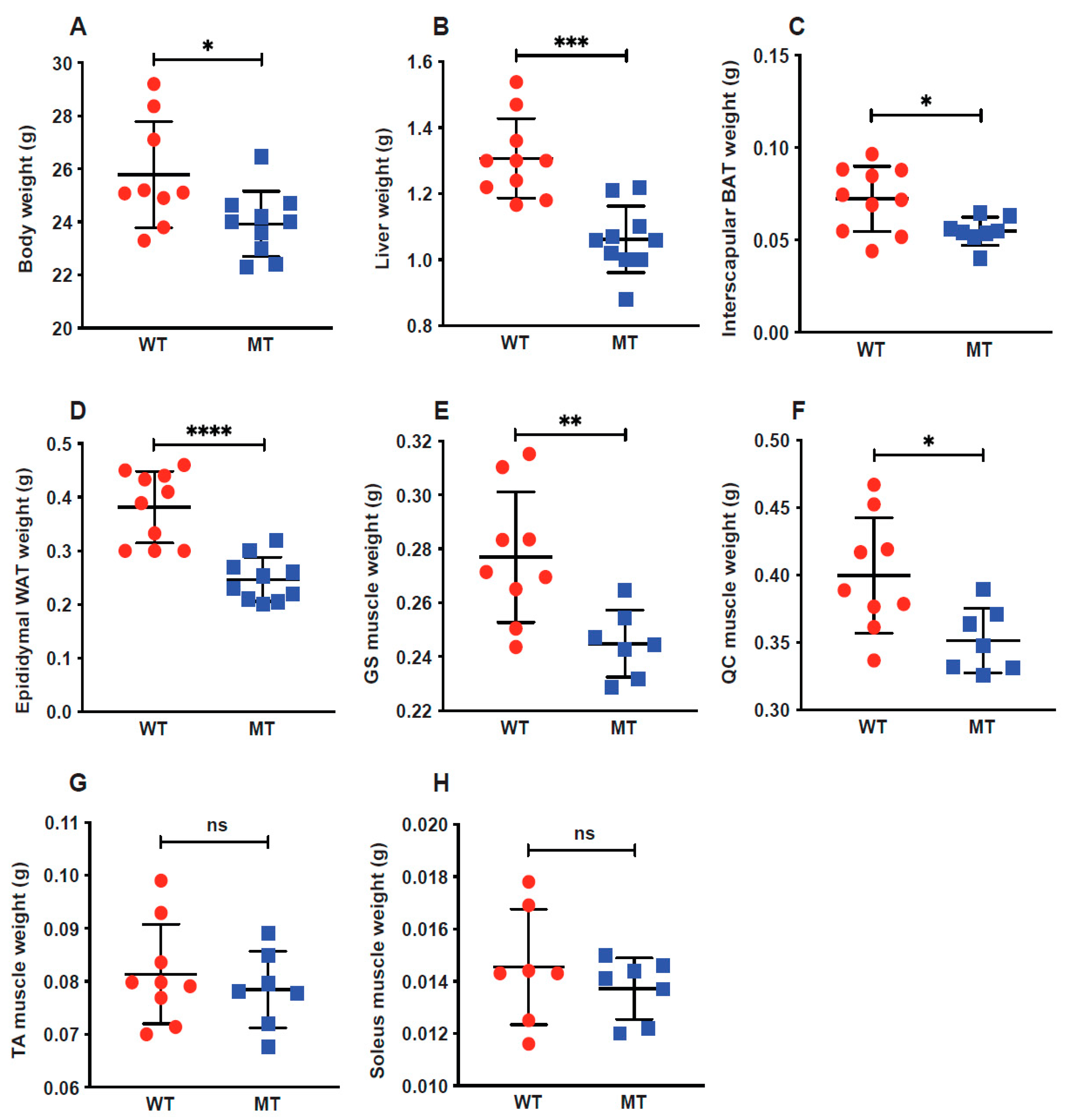
3.1. Indole Mutant Mice Display Reduced Body Weight and Weight Reduction across Multiple Organs
3.2. Indole Mutant Mice Display Alterations in Serum Metabolite Profiles
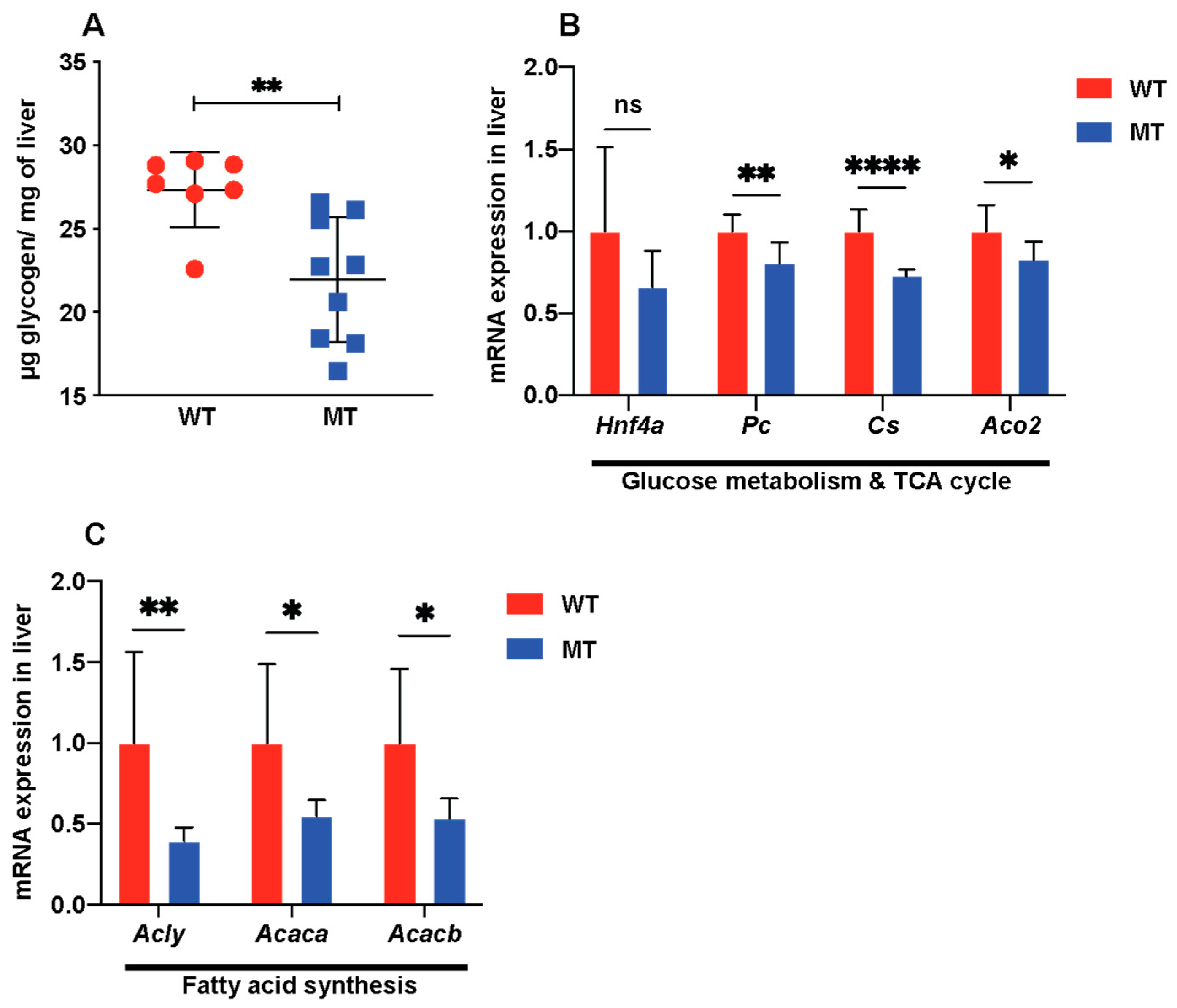
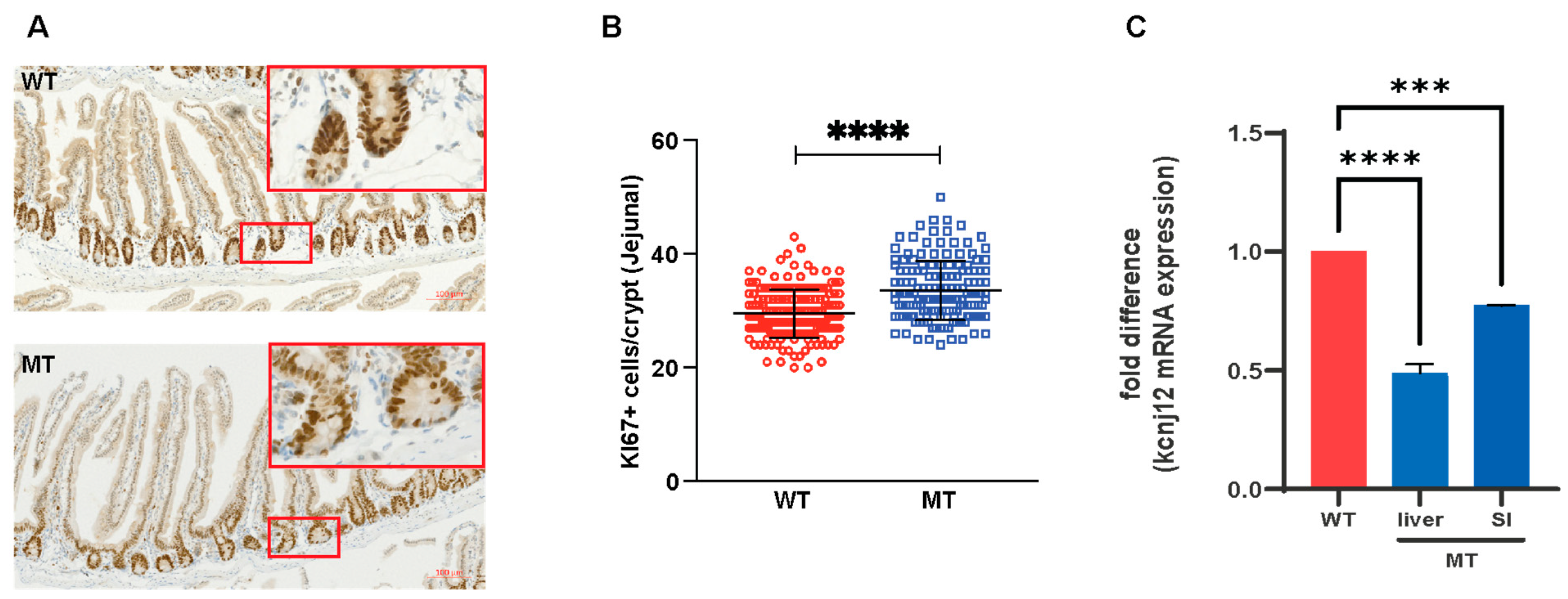
3.3. Indole Mutant Mice Display Alterations in Host Liver Metabolic Functions, Mitochondrial Functions, and Lipid Synthesis
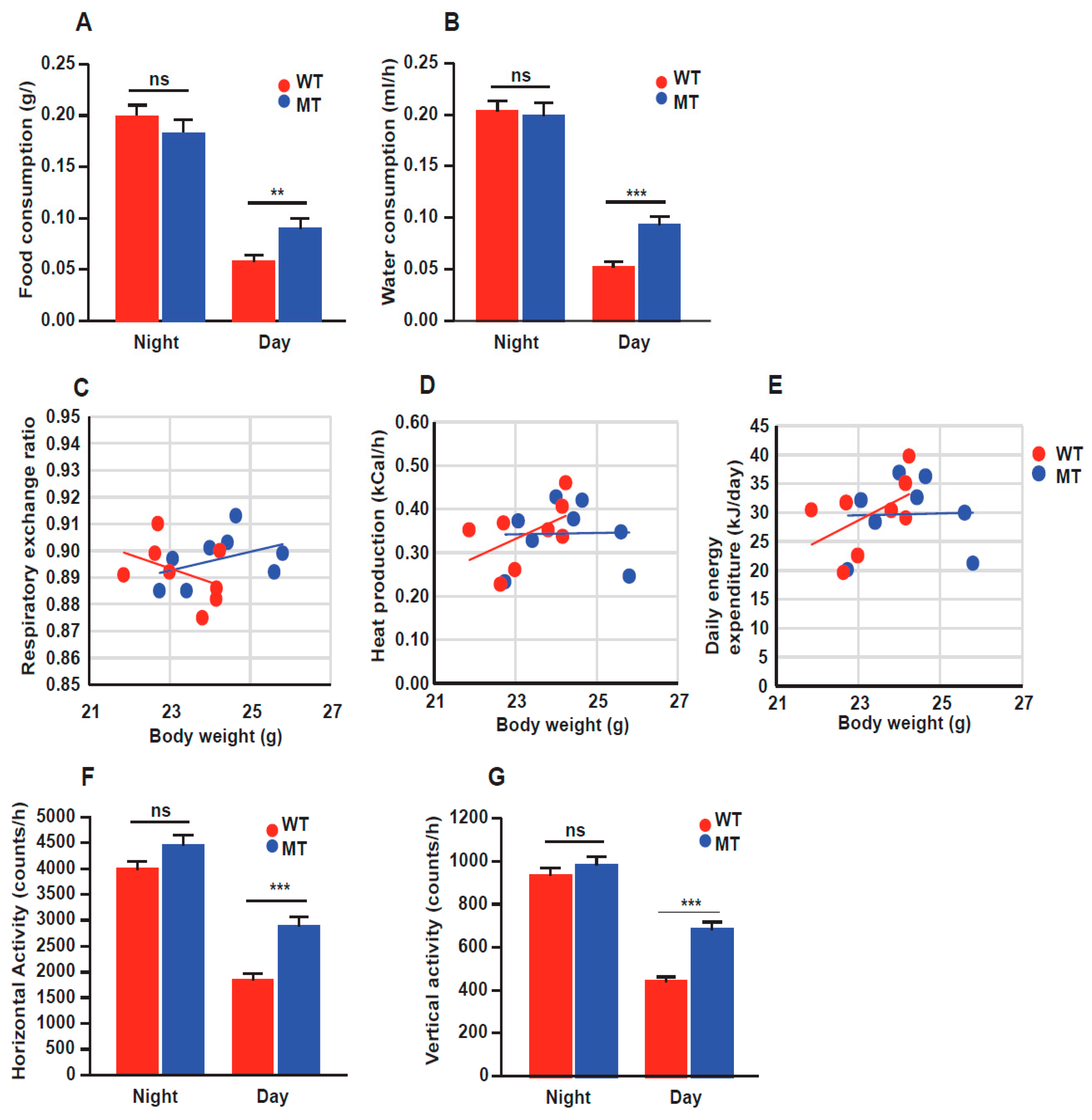
3.4. Indole Mutant (MT) Mice Show Increased Food Intake and Locomotion through Calorimetric Cage Analysis
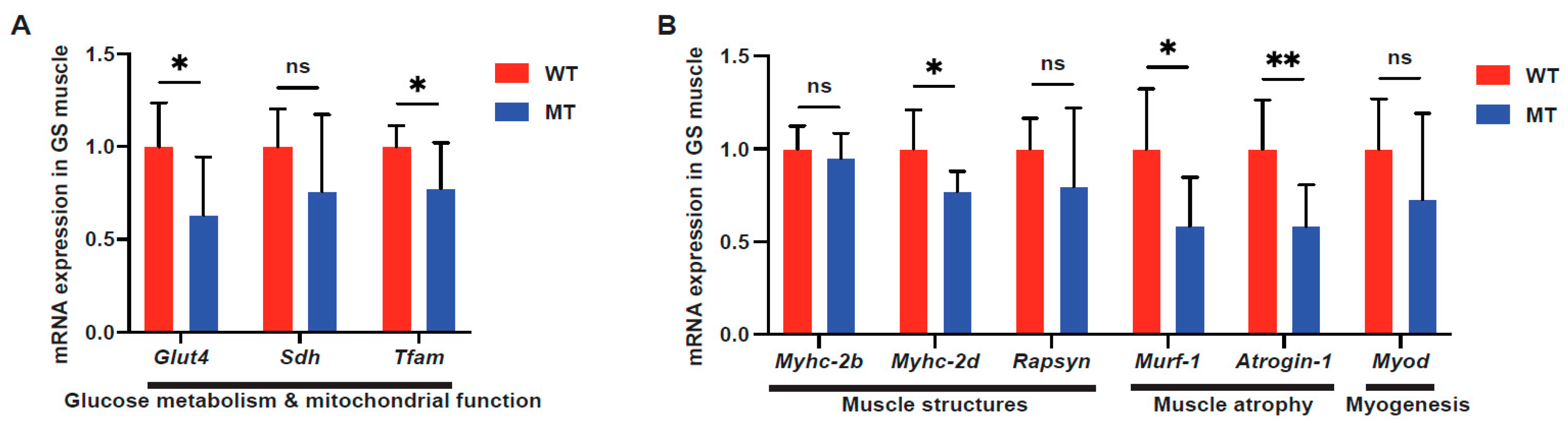
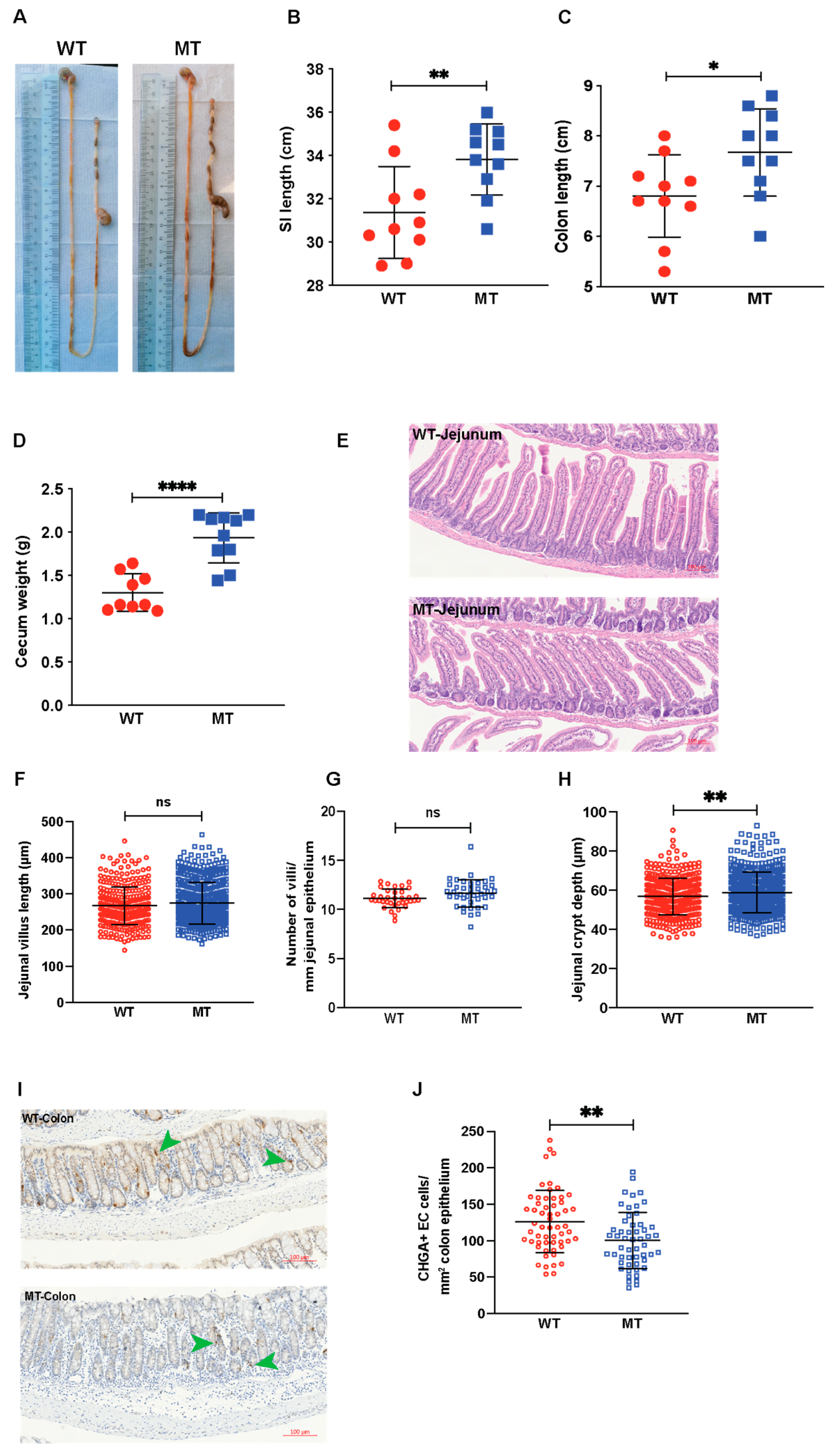
3.5. Indole Mutant (MT) Mice Have Increased Intestinal Length, Enlarged Cecum, Increased Epithelial Cell Growth, and Reduced Serotonin-Producing Colonic Enterochromaffin Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Abdul Rahim, M.B.H.; Chilloux, J.; Martinez-Gili, L.; Neves, A.L.; Myridakis, A.; Gooderham, N.; Dumas, M.E. Diet-induced metabolic changes of the human gut microbiome: Importance of short-chain fatty acids, methylamines and indoles. Acta Diabetol. 2019, 56, 493–500. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, C.; Gao, J. Extensive Summary of the Important Roles of Indole Propionic Acid, a Gut Microbial Metabolite in Host Health and Disease. Nutrients 2022, 15, 151. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [PubMed]
- Darkoh, C.; Chappell, C.; Gonzales, C.; Okhuysen, P. A rapid and specific method for the detection of indole in complex biological samples. Appl. Environ. Microbiol. 2015, 81, 8093–8097. [Google Scholar] [CrossRef]
- Dong, F.; Hao, F.; Murray, I.A.; Smith, P.B.; Koo, I.; Tindall, A.M.; Kris-Etherton, P.M.; Gowda, K.; Amin, S.G.; Patterson, A.D.; et al. Intestinal microbiota-derived tryptophan metabolites are predictive of Ah receptor activity. Gut Microbes 2020, 12, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sonowal, R.; Swimm, A.; Sahoo, A.; Luo, L.; Matsunaga, Y.; Wu, Z.; Bhingarde, J.A.; Ejzak, E.A.; Ranawade, A.; Qadota, H.; et al. Indoles from commensal bacteria extend healthspan. Proc. Natl. Acad. Sci. USA 2017, 114, E7506–E7515. [Google Scholar] [CrossRef]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef]
- Smith, S.A.; Elliott, K.R.F.; Pogson, C.I. Inhibition of hepatic gluconeogenesis by tryptophan metabolites in rats and guinea pigs. Biochem. Pharmacol. 1979, 28, 2145–2148. [Google Scholar] [CrossRef]
- Virtue, A.T.; McCright, S.J.; Wright, J.M.; Jimenez, M.T.; Mowel, W.K.; Kotzin, J.J.; Joannas, L.; Basavappa, M.G.; Spencer, S.P.; Clark, M.L.; et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci. Transl. Med. 2019, 11, eaav1892. [Google Scholar] [CrossRef]
- Ma, L.; Li, H.; Hu, J.; Zheng, J.; Zhou, J.; Botchlett, R.; Matthews, D.; Zeng, T.; Chen, L.; Xiao, X.; et al. Indole Alleviates Diet-induced Hepatic Steatosis and Inflammation in a Manner Involving Myeloid Cell PFKFB3. Hepatology 2020, 72, 1191–1203. [Google Scholar] [CrossRef]
- Jennis, M.; Cavanaugh, C.R.; Leo, G.C.; Mabus, J.R.; Lenhard, J.; Hornby, P.J. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol. Motil. 2018, 30. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Jia, Y.; Shi, J.; Tong, Z.; Fang, D.; Yang, B.; Su, C.; Li, R.; Xiao, X.; et al. Gut microbiome alterations in high-fat-diet-fed mice are associated with antibiotic tolerance. Nat. Microbiol. 2021, 6, 874–884. [Google Scholar] [CrossRef]
- Hidalgo-Romano, B.; Gollihar, J.; Brown, S.A.; Whiteley, M.; Valenzuela, E.; Kaplan, H.B.; Wood, T.K.; McLean, R.J.C. Indole inhibition of N-acylated homoserine lactone-mediated quorum signalling is widespread in Gram-negative bacteria. Microbiology 2014, 160, 2464–2473. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef] [PubMed]
- Whiley, L.; Nye, L.C.; Grant, I.; Andreas, N.; Chappell, K.E.; Sarafian, M.H.; Misra, R.; Plumb, R.S.; Lewis, M.R.; Nicholson, J.K.; et al. Ultrahigh-performance liquid chromatography tandem mass spec-trometry with electrospray ionization quantification of tryptophan metabolites andmarkers of gut health in serum and plasma-application to clinical and epidemiologycohorts. Anal. Chem. 2019, 91, 5207–5216. [Google Scholar] [CrossRef]
- Dona, A.C.; Jiménez, B.; Schäfer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Steinhauser, M.L.; Olenchock, B.A.; O’Keefe, J.; Lun, M.; Pierce, K.A.; Lee, H.; Pantano, L.; Klibanski, A.; Shulman, G.I.; Clish, C.B.; et al. The circulating metabolome of human starvation. JCI Insight 2018, 3, e121434. [Google Scholar] [CrossRef]
- Hanson, R.W.; Reshef, L. Glyceroneogenesis revisited. Biochimie 2003, 85, 1199–1205. [Google Scholar] [CrossRef]
- Kendig, D.M.; Grider, J.R. Serotonin and colonic motility. Neurogastroenterol. Motil. 2015, 27, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Leithner, K.; Triebl, A.; Trötzmüller, M.; Hinteregger, B.; Leko, P.; Wieser, B.I.; Grasmann, G.; Bertsch, A.L.; Züllig, T.; Stacher, E.; et al. The glycerol backbone of phospholipids derives from noncarbohydrate precursors in starved lung cancer cells. Proc. Natl. Acad. Sci. USA 2018, 115, 6225–6230. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Gehart, H.; Clevers, H. Tales from the crypt: New insights into intestinal stem cells. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Chinni, S.R.; Li, Y.; Upadhyay, S.; Koppolu, P.K.; Sarkar, F.H. Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene 2001, 20, 2927–2936. [Google Scholar] [CrossRef]
- Obata, Y.; Castaño, Á.; Boeing, S.; Bon-Frauches, A.C.; Fung, C.; Fallesen, T.; de Agüero, M.G.; Yilmaz, B.; Lopes, R.; Huseynova, A.; et al. Neuronal programming by microbiota regulates intestinal physiology. Nature 2020, 578, 284–289. [Google Scholar] [CrossRef]
- Lee, I.; Park, C.; Kang, W.K. Knockdown of inwardly rectifying potassium channel Kir2.2 suppresses tumorigenesis by inducing reactive oxygen species-mediated cellular senescence. Mol. Cancer Ther. 2010, 9, 2951–2959. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, B.; Shah, K.; Wincent, E. AHR in the intestinal microenvironment: Safeguarding barrier function. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 559–570. [Google Scholar] [CrossRef]
- Wostmann, B.S. The germfree animal in nutritional studies. Annu. Rev. Nutr. 1981, 1, 257–279. [Google Scholar] [CrossRef]
- Ge, X.; Ding, C.; Zhao, W.; Xu, L.; Tian, H.; Gong, J.; Zhu, M.; Li, J.; Li, N. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J. Transl. Med. 2017, 15, 13. [Google Scholar] [CrossRef]
- Soenen, S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. The ageing gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Pakarinen, M.P.; Miettinen, T.A.; Lauronen, J.; Halttunen, J. Does increased crypt cell proliferation impair cholesterol absorption after proximal gut resection? Scand. J. Gastroenterol. 2000, 35, 719–725. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Chevalier, C.; Stojanović, O.; Colin, D.J.; Suarez-Zamorano, N.; Tarallo, V.; Veyrat-Durebex, C.; Rigo, D.; Fabbiano, S.; Stevanović, A.; Hagemann, S.; et al. Gut Microbiota Orchestrates Energy Homeostasis during Cold. Cell 2015, 163, 1360–1374. [Google Scholar] [CrossRef]
- Mandić, A.D.; Woting, A.; Jaenicke, T.; Sander, A.; Sabrowski, W.; Rolle-Kampcyk, U.; von Bergen, M.; Blaut, M. Clostridium ramosum regulates enterochromaffin cell development and serotonin release. Sci. Rep. 2019, 9, 1177. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.D.; Murray, I.A.; Perdew, G.H. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab. Dispos. 2015, 43, 1522–1535. [Google Scholar] [CrossRef] [PubMed]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Scaini, G.; Santos, P.M.; Benedet, J.; Rochi, N.; Gomes, L.M.; Borges, L.S.; Rezin, G.T.; Pezente, D.P.; Quevedo, J.; Streck, E.L. Evaluation of Krebs cycle enzymes in the brain of rats after chronic administration of antidepressants. Brain Res. Bull. 2010, 82, 224–227. [Google Scholar] [CrossRef]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin′s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [PubMed]
- Veliova, M.; Ferreira, C.M.; Benador, I.Y.; Jones, A.E.; Mahdaviani, K.; Brownstein, A.J.; Desousa, B.R.; Acín-Pérez, R.; Petcherski, A.; Assali, E.A.; et al. Blocking mitochondrial pyruvate import in brown adipocytes induces energy wasting via lipid cycling. EMBO Rep. 2020, 21, e49634. [Google Scholar] [CrossRef] [PubMed]
- Anderson ST and Paschos, G.K. The role for the microbiome in the regulation of the circadian clock and metabolism. In Translational Epigenetics, Nutritional Epigenomics; Academic Press: Cambridge, MA, USA, 2019; Volume 14, pp. 231–248. [Google Scholar]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Matsuda, M.; Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M. Increased oxidative stress in obesity and its impact on metabolic syndrome Find the latest version: Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Rivest, R.W.; Aubert, M.L.; Lang, U.; Sizonenko, P.C. Puberty in the rat: Modulation by melatonin and light. J. Neural Transm. Suppl. 1986, 21, 81–108. [Google Scholar] [PubMed]
- Waldhauser, F.; Boepple, P.A.; Schemper, M.; Mansfield, M.J.; Crowley, W.F., Jr. Serum melatonin in central precocious puberty is lower than in age-matched prepubertal children. J. Clin. Endocrinol. Metab. 1991, 73, 793–796. [Google Scholar] [CrossRef] [PubMed]
- DE Almeida-Neto, P.F.; Bulhões-Correia, A.; DE Matos, D.G.; DE Alcântara Varela, P.W.; Pinto, V.C.M.; Dantas, P.M.S.; Aidar, F.J.; DE Araújo Tinoco Cabral, B.G. Relationship of Biological Maturation with Muscle Power in Young Female Athletes. Int. J. Exerc. Sci. 2021, 14, 696–706. [Google Scholar] [PubMed]
- Zhang, Y.; Zhang, M.; Zhu, W.; Yu, J.; Wang, Q.; Zhang, J.; Cui, Y.; Pan, X.; Gao, X.; Sun, H. Succinate accumulation induces mitochondrial reactive oxygen species generation and promotes status epilepticus in the kainic acid rat model. Redox Biol. 2020, 28, 101365. [Google Scholar] [CrossRef] [PubMed]
- Sumi, K.; Hatanaka, Y.; Takahashi, R.; Wada, N.; Ono, C.; Sakamoto, Y.; Sone, H.; Iida, K. Citrate Synthase Insufficiency Leads to Specific Metabolic Adaptations in the Heart and Skeletal Muscles Upon Low-Carbohydrate Diet Feeding in Mice. Front. Nutr. 2022, 9, 925908. [Google Scholar] [CrossRef]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int. J. Mol. Sci. 2022, 23, 1264. [Google Scholar] [CrossRef]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 2019, 11, 2062. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Fukuwatari, T. Large amounts of picolinic acid are lethal but small amounts increase the conversion of tryptophan-nicotinamide in rats. J. Nutr. Sci. Vitaminol. 2014, 60, 334–339. [Google Scholar] [CrossRef] [PubMed]


| Metabolic Pathways/ Physiological Roles | Molecules | Serum Levels in MT Mice Compared with WT Mice | Statistical Significance | p Values |
|---|---|---|---|---|
| Essential amino acid | Tryptophan | - | ns | 0.7537 |
| Host tryptophan metabolites—kynurenine-producing pathway | Kynurenine | ↑ | * | 0.0405 |
| Kynurenic acid | - | ns | 0.4083 | |
| 3OH-Kyn | - | ns | 0.1140 | |
| Xanthurenic acid | - | ns | 0.7954 | |
| 3HAA | - | ns | 0.5626 | |
| Picolinic acid | ↑ | * | 0.0398 | |
| Quinolinic acid | - | ns | 0.4603 | |
| NAD+ | - | ns | 0.0970 | |
| Nicotinic acid metabolites | Nicotinic acid | ↓ | *** | 0.0007 |
| NMN | - | ns | 0.1363 | |
| NaR | - | ns | 0.6148 | |
| Host tryptophan metabolites—serotonin-producing pathway | Serotonin | ↓ | * | 0.0238 |
| 5-HIAA | - | ns | 0.0751 | |
| Melatonin | ↑ | **** | <0.0001 | |
| Microbial tryptophan metabolites | Tryptamine | - | ns | 0.2705 |
| ILA | - | ns | 0.1909 | |
| IAA | ↓ | ** | 0.0055 | |
| TCA cycle intermediates | Succinate | ↓ | * | 0.0262 |
| Citrate | ↓ | * | 0.0381 | |
| Lipids | Triglycerides | ↓ | * | 0.0139 |
| Cholesterol | ↓ | * | 0.0348 | |
| Lipid digestion | Bile acids | ↓ | * | 0.0426 |
| Short-chain fatty acids | Formate | ↑ | ** | 0.0073 |
| Acetate | ↓ | * | 0.0100 | |
| Amino acids | Glutamate | ↓ | * | 0.0218 |
| Aspartate | ↓ | * | 0.0229 | |
| Phenylalanine | ↓ | * | 0.0135 | |
| Tyrosine | ↓ | * | 0.0218 | |
| Dopamine/dopamine metabolites | Dopamine | ↓ | * | 0.0138 |
| Dopamine-O-sulfate | ↓ | * | 0.0219 | |
| Dopamine-B-glucuronide | ↓ | *** | 0.0005 | |
| GTP catabolite | Neopterin | - | ns | 0.0761 |
| Urea cycle intermediate | Citrulline | - | ns | 0.4277 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, P.Y.; Agrawal, R.; Jayaraman, A.; Martin, K.A.; Zhang, G.W.; Ngu, E.L.; Faylon, L.E.; Kjelleberg, S.; Rice, S.A.; Wang, Y.; et al. Microbial Indoles: Key Regulators of Organ Growth and Metabolic Function. Microorganisms 2024, 12, 719. https://doi.org/10.3390/microorganisms12040719
Xing PY, Agrawal R, Jayaraman A, Martin KA, Zhang GW, Ngu EL, Faylon LE, Kjelleberg S, Rice SA, Wang Y, et al. Microbial Indoles: Key Regulators of Organ Growth and Metabolic Function. Microorganisms. 2024; 12(4):719. https://doi.org/10.3390/microorganisms12040719
Chicago/Turabian StyleXing, Peter Yuli, Ruchi Agrawal, Anusha Jayaraman, Katherine Ann Martin, George Wei Zhang, Ee Ling Ngu, Llanto Elma Faylon, Staffan Kjelleberg, Scott A. Rice, Yulan Wang, and et al. 2024. "Microbial Indoles: Key Regulators of Organ Growth and Metabolic Function" Microorganisms 12, no. 4: 719. https://doi.org/10.3390/microorganisms12040719
APA StyleXing, P. Y., Agrawal, R., Jayaraman, A., Martin, K. A., Zhang, G. W., Ngu, E. L., Faylon, L. E., Kjelleberg, S., Rice, S. A., Wang, Y., Bello, A. T., Holmes, E., Nicholson, J. K., Whiley, L., & Pettersson, S. (2024). Microbial Indoles: Key Regulators of Organ Growth and Metabolic Function. Microorganisms, 12(4), 719. https://doi.org/10.3390/microorganisms12040719






