Pathological Responses in Asian House Shrews (Suncus murinus) to the Naturally Acquired Orientia tsutsugamushi Infection
Abstract
1. Introduction
2. Methods
2.1. Trapping and Identification of Shrews and Tissue Sample Collection for Histopathology
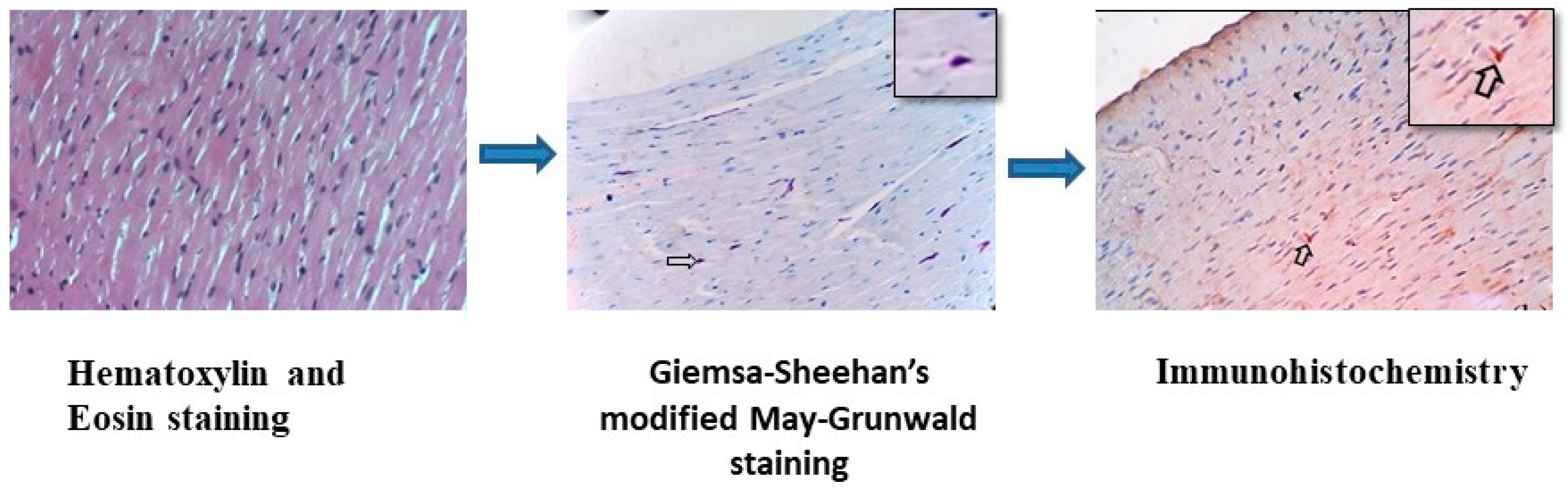
2.2. Methods Followed for Histology and Special Staining Techniques
2.3. Immunohistochemistry (IHC) for Localization of O. tsutsugamushi
Procedure for Immunohistochemistry
2.4. Screening O. tsutsugamushi in Blood and Tissues of IHC-Positive Samples by Real-Time PCR and Molecular Typing by Nested PCR
3. Results
3.1. Histochemical Staining for Rickettsial Organisms in Various Tissues of S. murinus
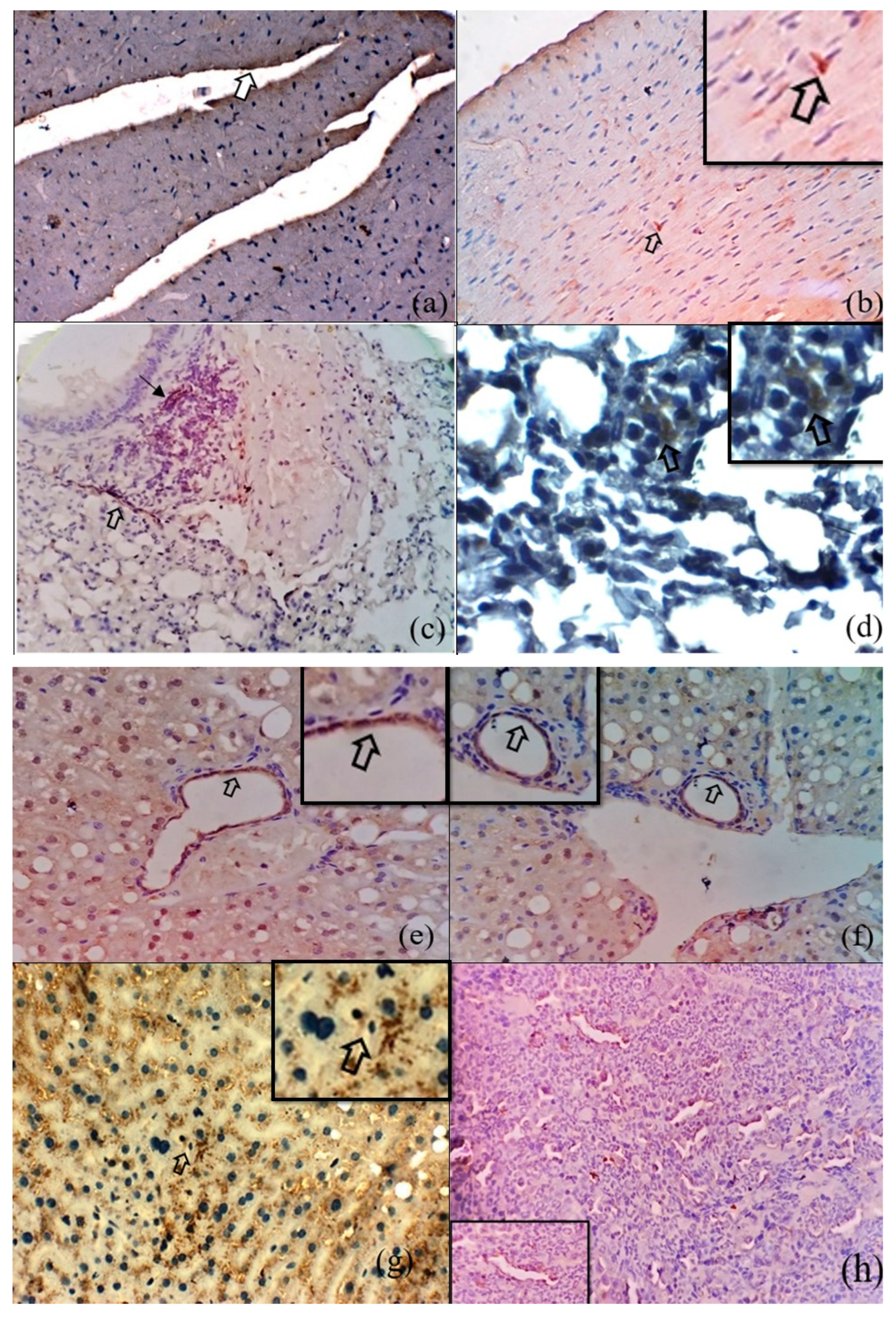
3.2. Immunohistochemical Detection of O. tsutsugamushi in the Tissues of S. murinus
3.3. Microscopic Features of Orientia-Antigen-Positive Tissues
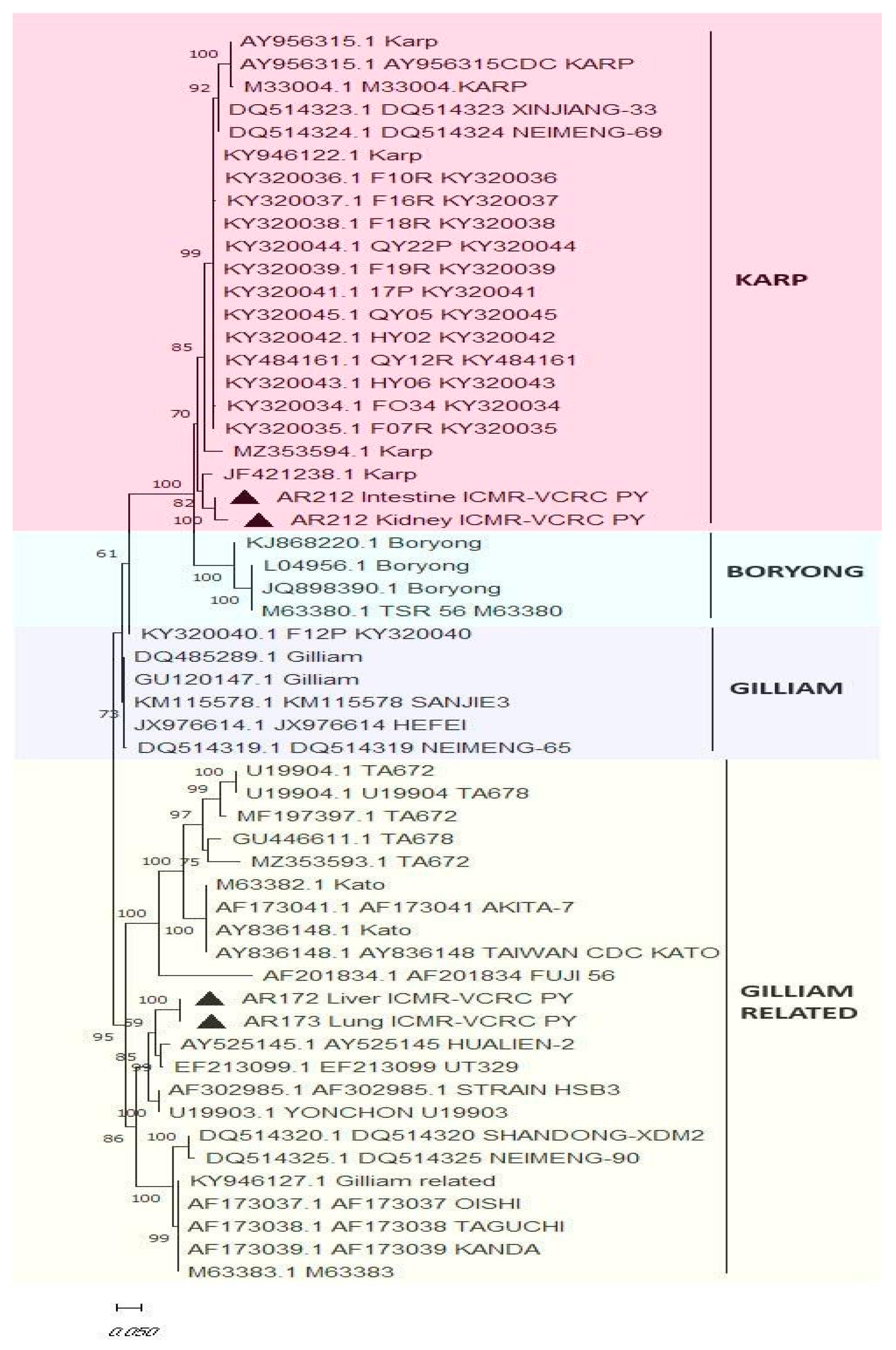
Molecular Detection of O. tsutsugamushi in Blood and Tissue Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, G.; Walker, D.H.; Jupiter, D.; Melby, P.C.; Arcari, C.M. A review of the global epidemiology of scrub typhus. PLoS Negl. Trop. Dis. 2017, 11, e0006062. [Google Scholar] [CrossRef] [PubMed]
- Candasamy, S.; Ayyanar, E.; Paily, K.; Karthikeyan, P.A.; Sundararajan, A.; Purushothaman, J. Abundance & distribution of trombiculid mites and Orientia tsutsugamushi, the vectors & pathogen of scrub typhus in rodents and shrews collected from Puducherry and Tamil Nadu India. Indian J. Med. Res. 2016, 144, 893–900. [Google Scholar] [PubMed]
- Devaraju, P.; Arumugam, B.; Mohan, I.; Paraman, M.; Ashokkumar, M.; Kasinathan, G.; Purushothaman, J. Evidence of natural infection of Orientia tsutsugamushi in vectors and animal hosts—Risk of scrub typhus transmission to humans in Puducherry, South India. Indian J. Public Health 2020, 64, 27–31. [Google Scholar] [CrossRef]
- Walsh, D.S.; Delacruz, E.C.; Abalos, R.M.; Tan, E.V.; Jiang, J.; Richards, A.L.; Eamsila, C.; Rodkvantook, W.; Myint, K.S.A. Clinical and histological features of inoculation site skin lesions in cynomolgus monkeys experimentally infected with Orientia tsutsugamushi. Vector Borne Zoonotic Dis. 2007, 7, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.A.; Hauptmann, M.; Kolbaum, J.; Gharaibeh, M.; Neumann, M.; Glatzel, M.; Fleischer, B. Dissemination of Orientia tsutsugamushi and inflammatory responses in a murine model of scrub typhus. PLoS Negl. Trop. Dis. 2014, 8, e3064. [Google Scholar] [CrossRef] [PubMed]
- Soong, L.; Mendell, N.L.; Olano, J.P.; Rockx-Brouwer, D.; Xu, G.; Goez-Rivillas, Y.; Drom, C.; Shelite, T.R.; Valbuena, G.; Walker, D.H.; et al. An intradermal inoculation mouse model for immunological investigations of acute scrub typhus and persistent infection. PLoS Negl. Trop. Dis. 2016, 10, e0004884. [Google Scholar] [CrossRef] [PubMed]
- Mendell, N.L.; Bouyer, D.H.; Walker, D.H. Murine models of scrub typhus associated with host control of Orientia tsutsugamushi infection. PLoS Negl. Trop. Dis. 2017, 11, e0005453. [Google Scholar] [CrossRef] [PubMed]
- Shelite, T.R.; Saito, T.B.; Mendell, N.L.; Gong, B.; Xu, G.; Soong, L.; Valbuena, G.; Bouyer, D.H.; Walker, D.H. A hematogenously disseminated Orientia tsutsugamushi-infected murine model of scrub typhus. PLoS Negl. Trop. Dis. 2014, 8, e2966. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.C. Taxonomic Studies on Indian Muridae and Hystricidae (Mammalia: Rodentia); Zoological Survey of India: Kolkata, India, 2000.
- Luna, L.G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology; Mc Graw Hill: New York, NY, USA, 1968. [Google Scholar]
- Sheehan, D.C.; Hrapchak, B.B. Theory and Practice of Histotechnology; Battelle: Columbus, OH, USA, 1980; pp. 190–192. [Google Scholar]
- Tantibhedhyangkul, W.; Wongsawat, E.; Silpasakorn, S.; Waywa, D.; Saenyasiri, N.; Suesuay, J.; Thipmontree, W.; Suputtamongkol, Y. Use of multiplex real-time PCR to diagnose scrub typhus. J. Clin. Microbiol. 2017, 55, 1377–1387. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, D.-M.; Cho, Y.S.; Yoon, S.H.; Shim, S.K. Usefulness of eschar PCR for diagnosis of scrub typhus. J. Clin. Microbiol. 2006, 44, 1169–1171. [Google Scholar] [CrossRef]
- Stephen, S.; Sangeetha, B.; Ambroise, S.; Sarangapani, K.; Gunasekaran, D.; Hanifah, M.; Somasundaram, S. Outbreak of scrub typhus in Puducherry & Tamil Nadu during cooler months. Indian J. Med. Res. 2015, 142, 591. [Google Scholar] [PubMed]
- Kundin, W.D.; Liu, C.; Harmon, P.; Rodina, P. Pathogenesis of scrub typhus infection (Rickettsia tsutsugamushi) as studied by immunofluorescence. J. Immunol. 1964, 93, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Varghese, G.M.; Abraham, O.C.; Mathai, D.; Thomas, K.; Aaron, R.; Kavitha, M.L.; Mathai, E. Scrub typhus among hospitalized patients with febrile illness in South India: Magnitude and clinical predictors. J. Inf. 2006, 52, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Kamala, T. Hock immunization: A humane alternative to mouse footpad injections. J. Immunol. Methods. 2007, 328, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Long, K.M.; Heise, M. Safe and effective mouse footpad inoculation. Mouse Models Innate Immun. 2013, 1031, 97–100. [Google Scholar]
- Paddock, C.D.; Sanders, J.H.; Denison, A.M.; Muehlenbachs, A.; Zaki, S.R. Routine argyrophil techniques detect Rickettsia rickettsii in tissues of patients with fatal Rocky Mountain spotted fever. J. Histotechnol. 2016, 39, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-C.; Shu, P.-Y.; Mu, J.-J.; Wang, H.-C. High prevalence of Rickettsia spp. infections in small mammals in Taiwan. Vector Borne Zoonotic Dis. 2015, 15, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, F.; Liao, Y.; Shen, J.-J.; Xu, J.-M.; Chen, Y.-Z.; Li, J.-H.; Holmes, E.C.; Zhang, Y.-Z. Epidemiology and Diversity of Rickettsiales Bacteria in Humans and Animals in Jiangsu and Jiangxi provinces, China. Sci. Rep. 2019, 9, 13176. [Google Scholar] [CrossRef] [PubMed]
- Moron, C.G.; Popov, V.L.; Feng, H.-M.; Wear, D.; Walker, D.H. Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus. Mod. Pathol. 2001, 14, 752–759. [Google Scholar] [CrossRef]
- Tseng, B.; Yang, H.; Liou, J.; Chen, L.; Hsu, Y. Immunohistochemical study of scrub typhus: A report of two cases. Kaohsiung J. Med. Sci. 2008, 24, 92–98. [Google Scholar] [CrossRef]
- Kim, I.S.; Seong, S.Y.; Woo, S.G.; Choi, M.S.; Kang, J.S.; Chang, W.H. Rapid diagnosis of scrub typhus by a passive hemagglutination assay using recombinant 56-kilodalton polypeptides. J. Clin. Microbiol. 1993, 31, 2057–2060. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Seong, S.Y.; Woo, S.G.; Choi, M.S.; Chang, W.H. High-level expression of a 56-kilodalton protein gene (bor56) of Rickettsia tsutsugamushi Boryong and its application to enzyme-linked immunosorbent assays. J. Clin. Microbiol. 1993, 31, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Ritu, G.P.; Arif, W.; Sihag, K.K.; Chakravarthi, A.; Anthony, T.N.; Srinivasan, L.; Balakrishnan, V.; Kumar, A.; Ayanar, E.; Devaraju, P. Comparative Evaluation of Different Tissues and Molecular Techniques for the Zoonotic Surveillance of Scrub Typhus. Vector-Borne Zoonotic Dis. 2024. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Thiriot, J.; Liang, Y.; Fisher, J.; Walker, D.H.; Soong, L. Host transcriptomic profiling of CD-1 outbred mice with severe clinical outcomes following infection with Orientia tsutsugamushi. PLoS Negl. Trop. Dis. 2022, 16, e0010459. [Google Scholar] [CrossRef]
- Jiang, L.; Morris, E.K.; Aguilera-Olvera, R.; Zhang, Z.; Chan, T.C.; Shashikumar, S.; Chao, C.C.; Casares, S.A.; Ching, W.M. Dissemination of Orientia tsutsugamushi, a Causative Agent of Scrub Typhus, and Immunological Responses in the Humanized DRAGA Mouse. Front. Immunol. 2018, 9, 816. [Google Scholar] [CrossRef]



| S.no. | Organs | Features | Frequency of Lesions |
|---|---|---|---|
| 1 | Heart | Normal histology | 13 |
| Vacuolar degeneration | 1 | ||
| Focal necrosis | 1 | ||
| Neutrophil infiltration | 1 | ||
| Linear hemorrhage | 1 | ||
| Fibrosis | 1 | ||
| 2 | Lung | Normal histology | 3 |
| Congestion | 9 | ||
| Hemorrhage | 5 | ||
| Emphysema | 4 | ||
| Neutrophil infiltration | 6 | ||
| Mononuclear infiltration | 1 | ||
| Pyogranuloma | 1 | ||
| Focal necrosis | 1 | ||
| Pigmentation | 1 | ||
| 3 | Liver | Cell swelling | 12 |
| Vacuolar degeneration | 2 | ||
| Fatty change | 13 | ||
| Karyomegaly | 1 | ||
| Neutrophil infiltration | 3 | ||
| Mononuclear infiltration | 3 | ||
| Mixed cell infiltration | 1 | ||
| Congestion | 2 | ||
| Fibrosis | 2 | ||
| Extramedullary hematopoiesis | 1 | ||
| 4 | Spleen | Normal histology | 6 |
| Hyperplasia of white pulp | 3 | ||
| Hyperplasia of red pulp | 3 | ||
| Atrophy of white pulp | 1 | ||
| Pigmentation | 1 | ||
| Neutrophil infiltration | 1 | ||
| 5 | Intestine | Normal histology | 12 |
| Hyperplasia of intestinal epithelium | 3 | ||
| Parasitic enteritis | 3 | ||
| 6 | Kidney | Normal histology | 2 |
| S.no. | Features | No. |
|---|---|---|
| 1 | Number of tissues immunopositive for O. tsutsugamushi | 81 |
| 2 | Number of O. tsutsugamushi immunopositive tissues with normal histological features | 36 |
| 3 | Number of O. tsutsugamushi immunopositive tissues with degenerative/inflammatory lesions | 35 |
| 4 | Number of O. tsutsugamushi immunopositive tissues with other histopathological changes (vascular changes, pigmentation, growth disturbances) | 10 |
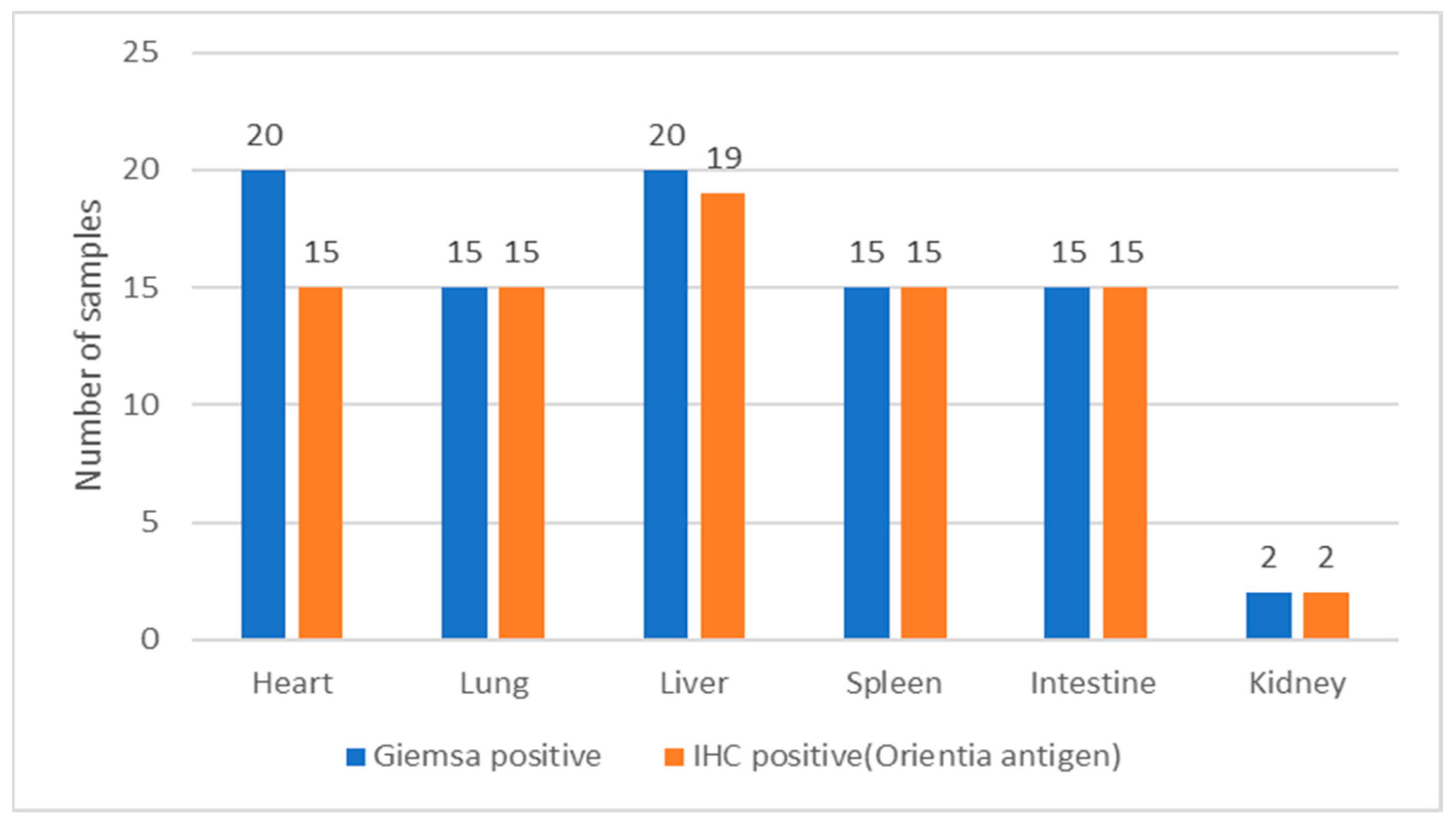
| Sl.No | Tissues Tested Positive for O. tsutsugamushi by PCR | Total | |||||
|---|---|---|---|---|---|---|---|
| Heart | Lung | Liver | Spleen | Intestine | Kidney | ||
| No. of samples tested positive for O. tsutsugamushi by IHC | 19 | 15 | 15 | 15 | 15 | 2 | 81 |
| No. of samples tested positive for O. tsutsugamushi by real-time PCR targeting the 47-kDa gene | 5 | 9 | 3 | 1 | 3 | 1 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balasubramanian, T.; Sambath, U.; Radja, R.D.; Thangaraj, G.; Devaraju, P.; Srinivasan, L.; Srinivasan, P.; Nair, M.G.; Raja, K.; Lakkawar, A.W.; et al. Pathological Responses in Asian House Shrews (Suncus murinus) to the Naturally Acquired Orientia tsutsugamushi Infection. Microorganisms 2024, 12, 748. https://doi.org/10.3390/microorganisms12040748
Balasubramanian T, Sambath U, Radja RD, Thangaraj G, Devaraju P, Srinivasan L, Srinivasan P, Nair MG, Raja K, Lakkawar AW, et al. Pathological Responses in Asian House Shrews (Suncus murinus) to the Naturally Acquired Orientia tsutsugamushi Infection. Microorganisms. 2024; 12(4):748. https://doi.org/10.3390/microorganisms12040748
Chicago/Turabian StyleBalasubramanian, Tharani, Uma Sambath, Ranjana Devi Radja, Gowdham Thangaraj, Panneer Devaraju, Lakshmy Srinivasan, Pushpa Srinivasan, Madhavan Gopalakrishnan Nair, Kumar Raja, Avinash Warundeo Lakkawar, and et al. 2024. "Pathological Responses in Asian House Shrews (Suncus murinus) to the Naturally Acquired Orientia tsutsugamushi Infection" Microorganisms 12, no. 4: 748. https://doi.org/10.3390/microorganisms12040748
APA StyleBalasubramanian, T., Sambath, U., Radja, R. D., Thangaraj, G., Devaraju, P., Srinivasan, L., Srinivasan, P., Nair, M. G., Raja, K., Lakkawar, A. W., & Soong, L. (2024). Pathological Responses in Asian House Shrews (Suncus murinus) to the Naturally Acquired Orientia tsutsugamushi Infection. Microorganisms, 12(4), 748. https://doi.org/10.3390/microorganisms12040748






