Goethite Enhances Cr(VI) Reduction by S. oneidensis MR-1 under Different Conditions: Mechanistic Insights
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Culture and Medium
2.2. Material Preparation
2.3. Cr(VI) Reduction Experiments under Different Environmental Conditions
2.4. Analysis Method of Cr(VI)
2.5. Characterization Analysis Method
2.6. Statistical Analysis
3. Results and Discussion
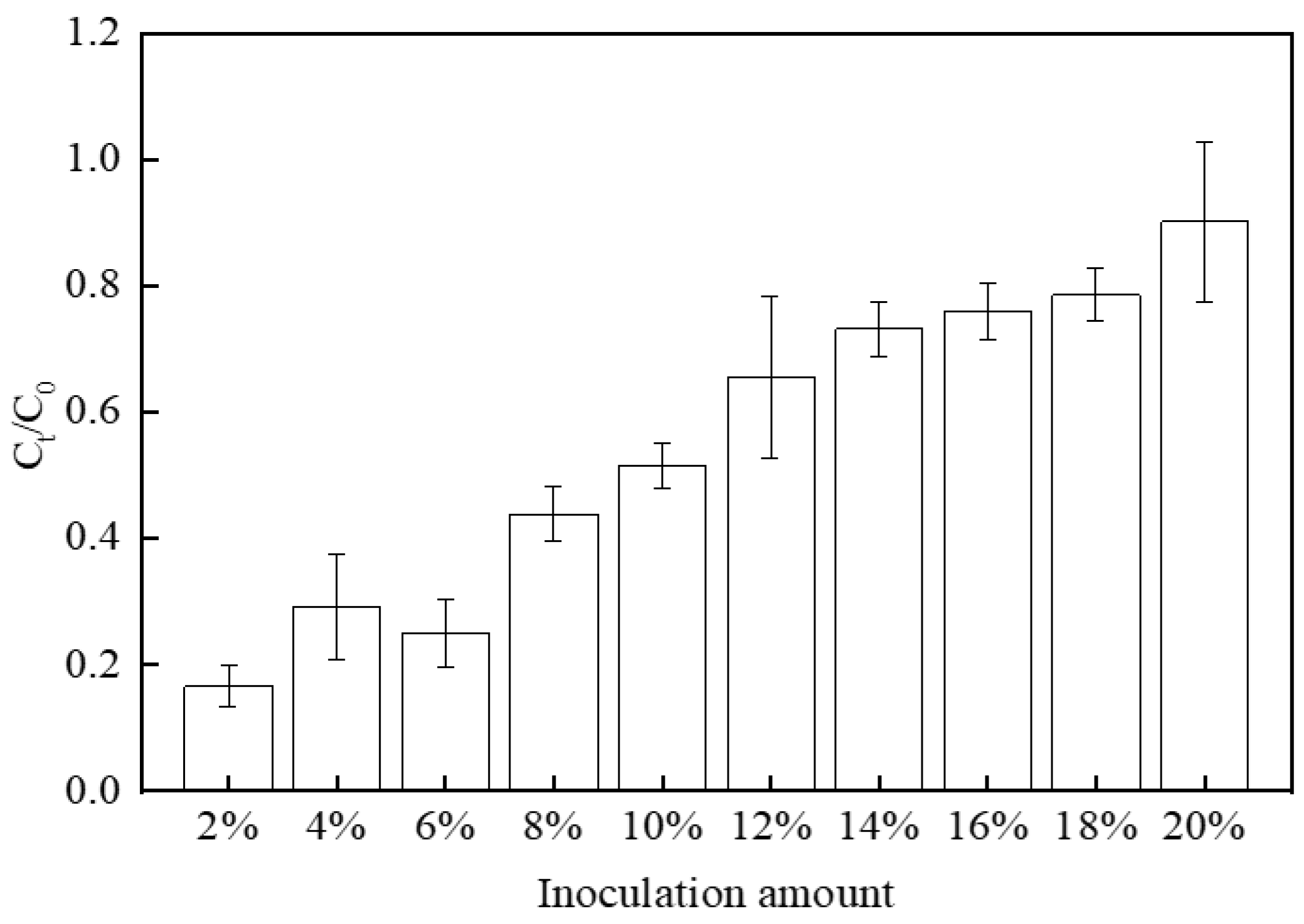
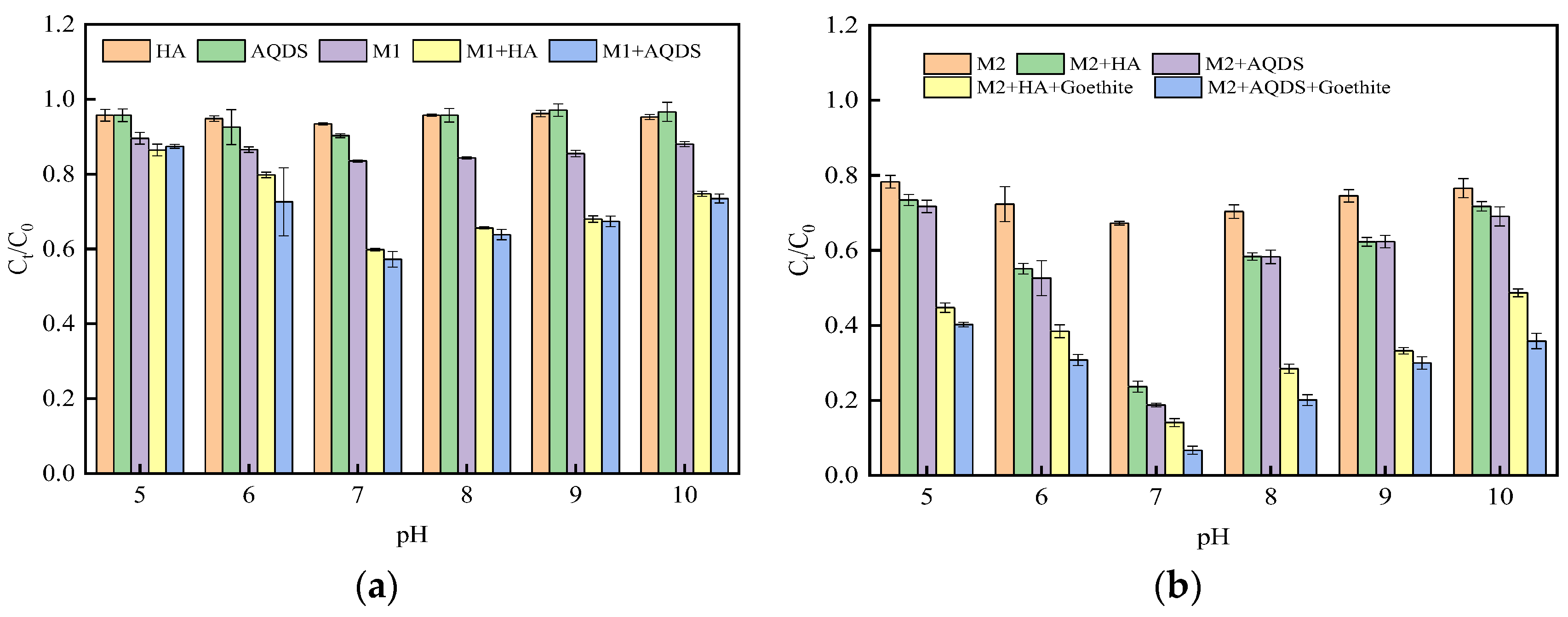
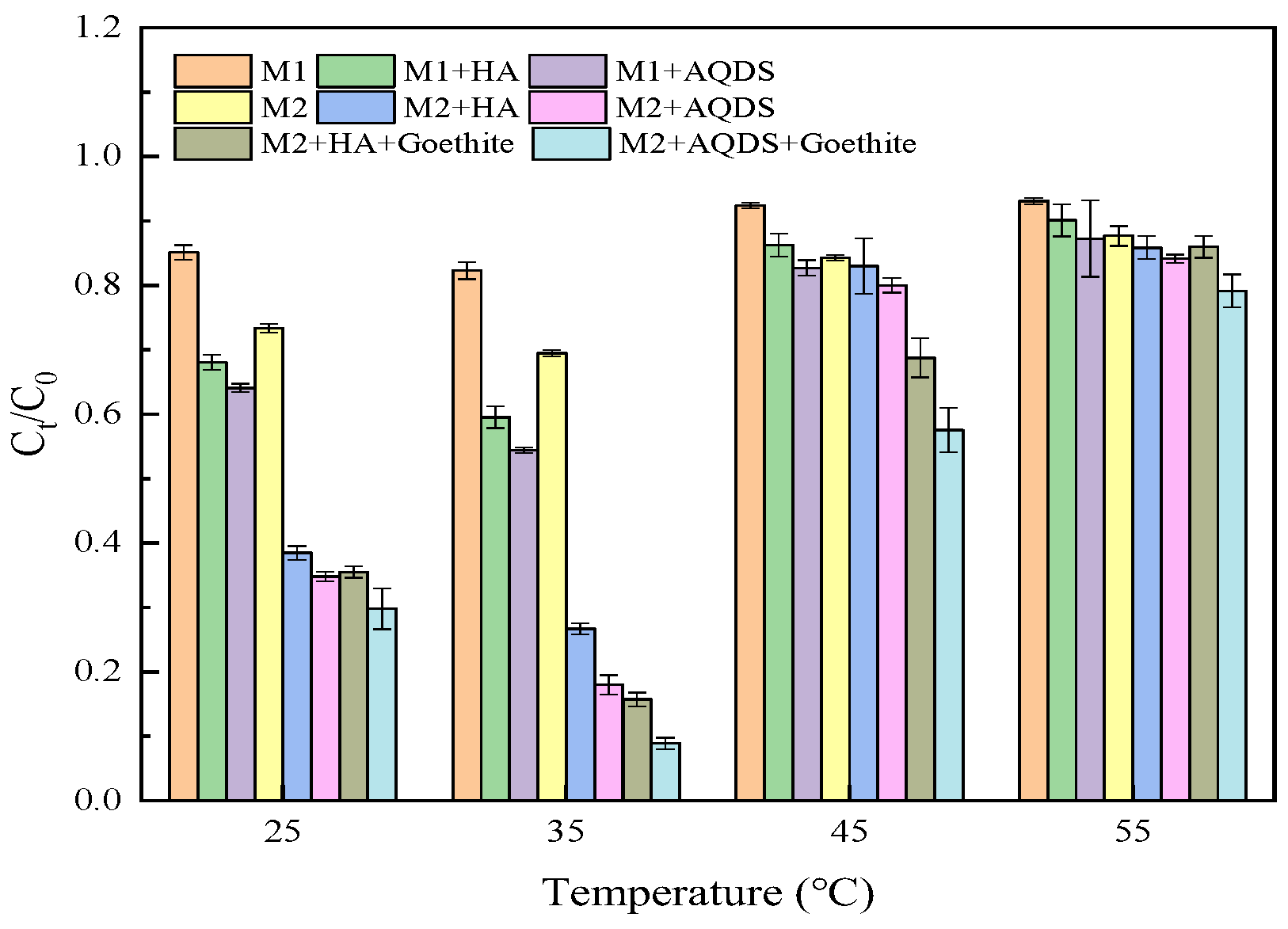
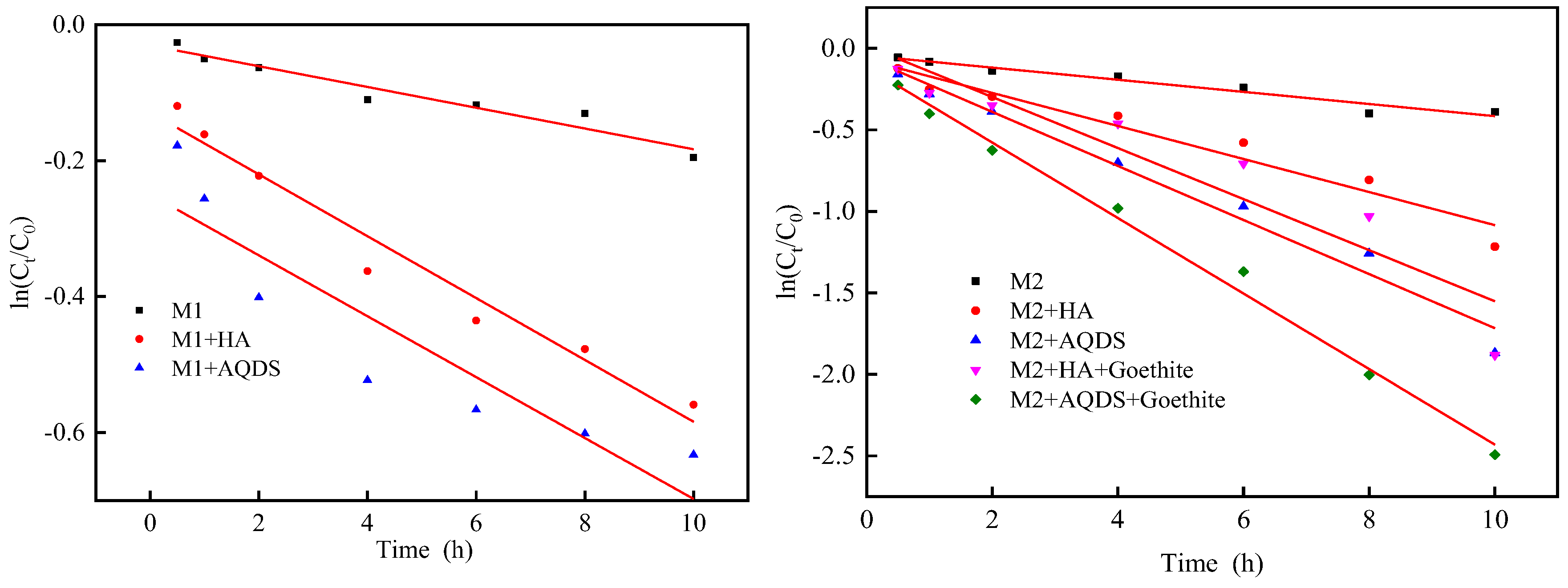
3.1. Influence of Different Environmental Factors on the Reduction Effect of Cr(VI)
3.2. Mechanism Analysis of Cr(VI) Reduction Process
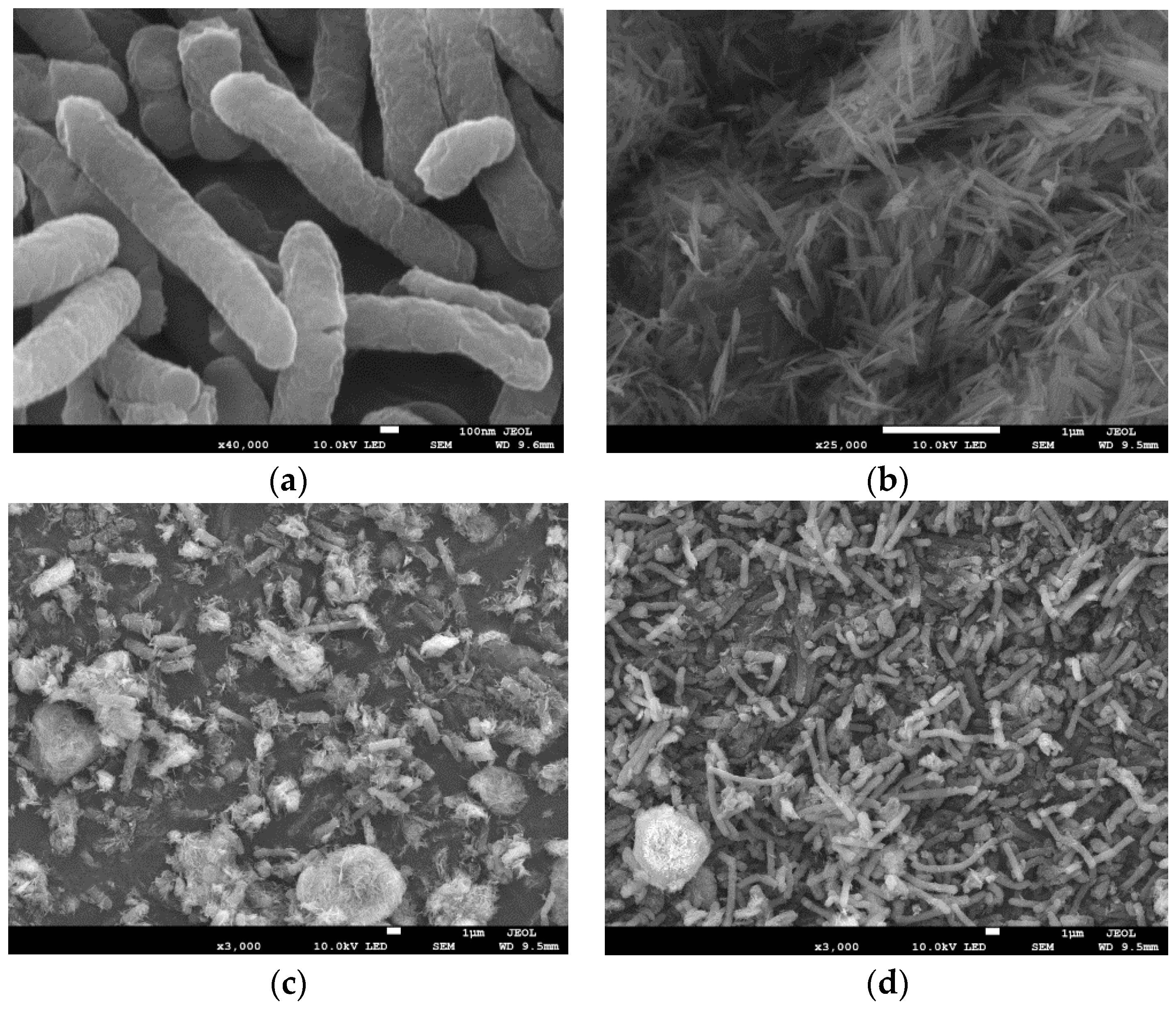
3.2.1. SEM and EDS Analysis before and after Reduction
3.2.2. XRD Analysis before and after Reduction
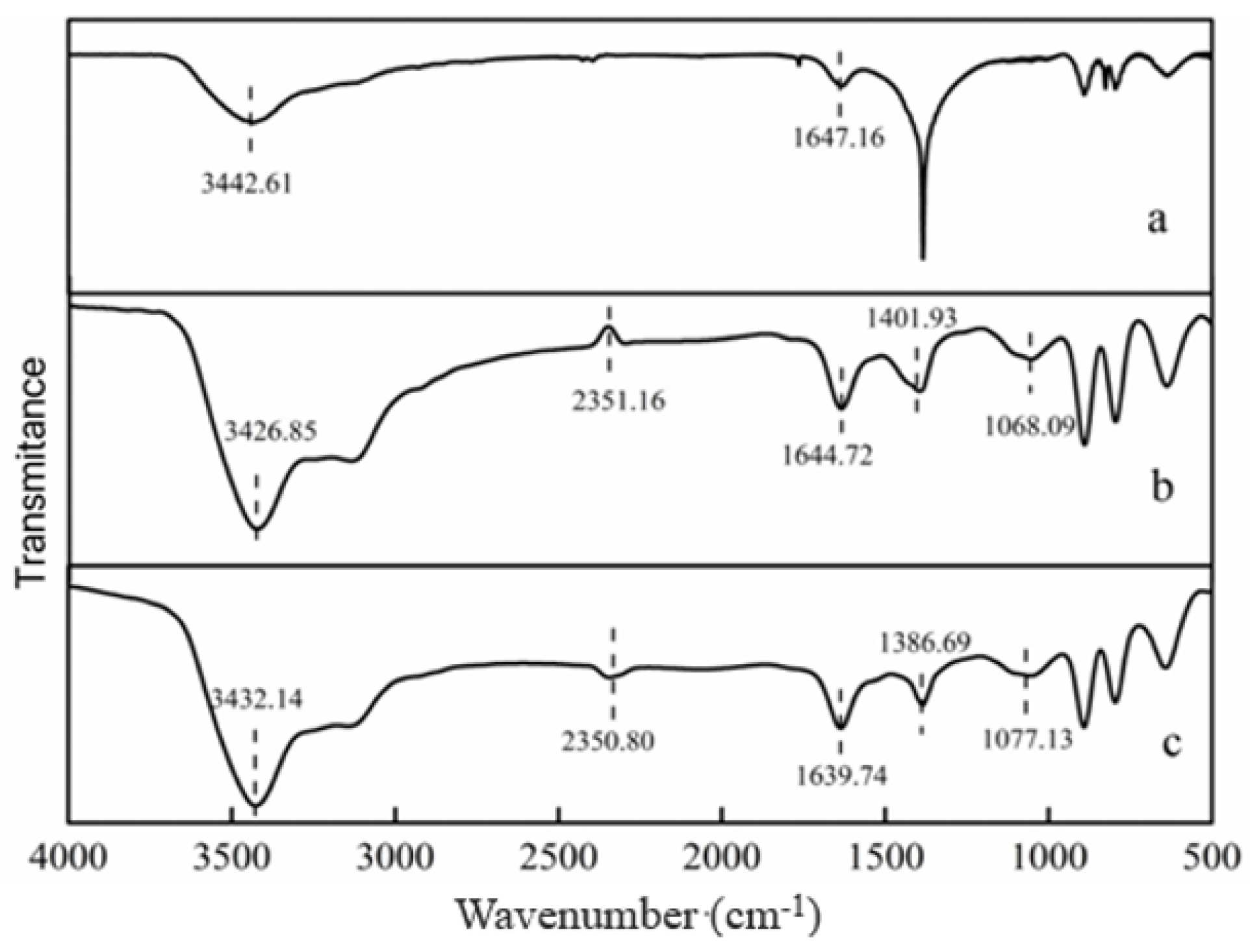
3.2.3. FTIR Analysis before and after Reduction
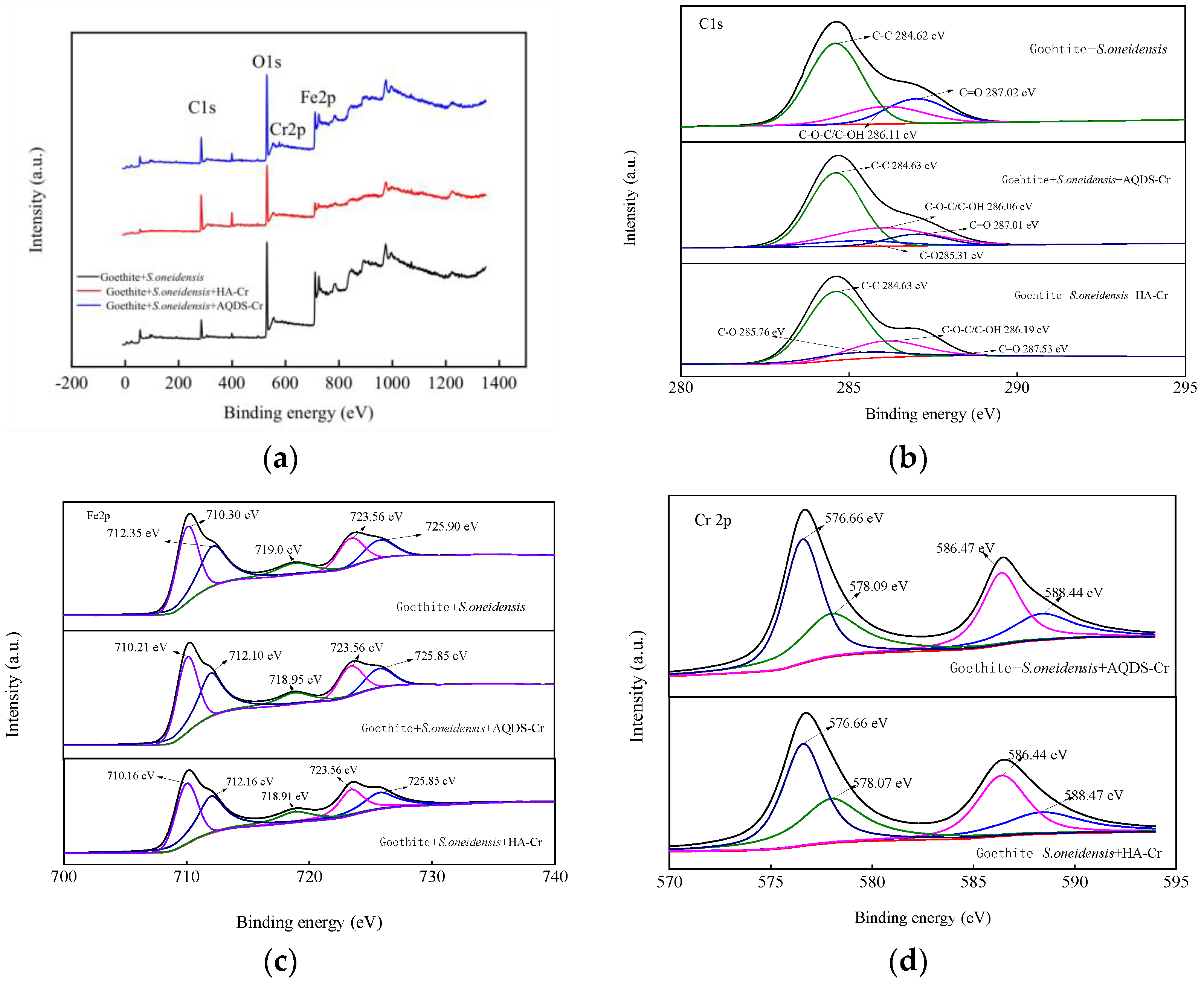
3.2.4. XPS Analysis before and after Reduction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Das, A.J.; Lal, S.; Kumar, R.; Verma, C. Bacterial Biosurfactants Can Be an Ecofriendly and Advanced Technology for Remediation of Heavy Metals and Co-Contaminated Soil. Int. J. Environ. Sci. Technol. 2017, 14, 1343–1354. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorption of Heavy Metals by Saccharomyces Cerevisiae: A Review. Biotechnol. Adv. 2006, 24, 427–451. [Google Scholar] [CrossRef]
- Kondalkar, M.; Fegade, U.; Inamuddin; Kanchi, S.; Altalhi, T.; Suryawanshi, K.E.; Patil, A.M. Adsorption of Cr(VI) on Ultrafine Al2O3-Doped MnFe2O4 Nanocomposite Surface: Experimental and Theoretical Study Using Double-Layer Modeling. J. Phys. Chem. Solids 2022, 163, 110544. [Google Scholar] [CrossRef]
- Mytych, P.; Cieśla, P.; Stasicka, Z. Photoredox Processes in the Cr(VI)–Cr(III)–Oxalate System and Their Environmental Relevance. Appl. Catal. B Environ. 2005, 59, 161–170. [Google Scholar] [CrossRef]
- Lewicki, S.; Zdanowski, R.; Krzyżowska, M.; Lewicka, A.; Dębski, B.; Niemcewicz, M.; Goniewicz, M. The Role of Chromium III in the Organism and Its Possible Use in Diabetes and Obesity Treatment. Ann. Agric. Environ. Med. 2014, 21, 331–335. [Google Scholar] [CrossRef]
- Singh, P.N.; Tiwary, D.; Sinha, I. Starch-Functionalized Magnetite Nanoparticles for Hexavalent Chromium Removal from Aqueous Solutions. Desalin. Water Treat. 2016, 57, 12608–12619. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Yanful, E.K. Arsenic and Chromium Removal by Mixed Magnetite–Maghemite Nanoparticles and the Effect of Phosphate on Removal. J. Environ. Manag. 2010, 91, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, Y.; Wang, J.; Qiao, W.; Long, D.; Ling, L. Effective Removal of Hexavalent Chromium from Aqueous Solutions by Adsorption on Mesoporous Carbon Microspheres. J. Colloid Interface Sci. 2016, 462, 200–207. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium Speciation, Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System: A Review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- Wang, X.-H.; Liu, F.-F.; Lu, L.; Yang, S.; Zhao, Y.; Sun, L.-B.; Wang, S.-G. Individual and Competitive Adsorption of Cr(VI) and Phosphate onto Synthetic Fe–Al Hydroxides. Colloids Surf. Physicochem. Eng. Asp. 2013, 423, 42–49. [Google Scholar] [CrossRef]
- Xu, S.; Wei, J.; Feng, S.; Wang, J.; Li, X. A Study in the Adsorption Behaviors of Cr (VI) on Crosslinked Cationic Starches. J. Polym. Res. 2004, 11, 211–215. [Google Scholar] [CrossRef]
- Huang, R.; Ma, X.; Li, X.; Guo, L.; Xie, X.; Zhang, M.; Li, J. A Novel Ion-Imprinted Polymer Based on Graphene Oxide-Mesoporous Silica Nanosheet for Fast and Efficient Removal of Chromium (VI) from Aqueous Solution. J. Colloid Interface Sci. 2018, 514, 544–553. [Google Scholar] [CrossRef]
- Zhongqi Ren, D.K.; Keyuan, W. Preparation and Adsorption Characteristics of an Imprinted Polymer for Selective Removal of Cr(vi) Ions from Aqueous Solutions. J. Mater. Chem. Mater. Energy Sustain. 2014, 2, 17952–17961. [Google Scholar]
- Hirata, S.; Kozaki, D.; Sakanishi, K.; Nakagoshi, N.; Tanaka, K. Simultaneous Determinations of Cr(VI) and Cr(III) by Ion-Exclusion/Cation-Exchange Chromatography with an Unmodified Silica-Gel Column. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2010, 26, 387–390. [Google Scholar] [CrossRef]
- Alvarado, L.; Torres, I.R.; Chen, A. Integration of Ion Exchange and Electrodeionization as a New Approach for the Continuous Treatment of Hexavalent Chromium Wastewater. Sep. Purif. Technol. 2013, 105, 55–62. [Google Scholar] [CrossRef]
- Zang, Y.; Yue, Q.; Kan, Y.; Zhang, L.; Gao, B. Research on Adsorption of Cr(Ⅵ) by Poly-Epichlorohydrin-Dimethylamine (EPIDMA) Modified Weakly Basic Anion Exchange Resin D301. Ecotoxicol. Environ. Saf. 2018, 161, 467–473. [Google Scholar] [CrossRef]
- Mıhçıokur, H.; Peker, I. Batch Study and Kinetics of Hexavalent Chromium Removal from Aqueous Solutions by Anion Exchange Resin (Dowex 21 KCl). Desalin. Water Treat. 2013, 51, 2116–2120. [Google Scholar] [CrossRef]
- Peng, C.; Meng, H.; Song, S.; Lu, S.; Lopez-Valdivieso, A. Elimination of Cr(VI) from Electroplating Wastewater by Electrodialysis Following Chemical Precipitation. Sep. Sci. Technol. 2005, 39, 1501–1517. [Google Scholar] [CrossRef]
- Xie, B.; Shan, C.; Xu, Z.; Li, X.; Zhang, X.; Chen, J.; Pan, B. One-Step Removal of Cr(VI) at Alkaline pH by UV/Sulfite Process: Reduction to Cr(III) and in Situ Cr(III) Precipitation. Chem. Eng. J. 2017, 308, 791–797. [Google Scholar] [CrossRef]
- Jafari, A.J.; Golbaz, S.; Kalantary, R.R. Treatment of Hexavalent Chromium by Using a Combined Fenton and Chemical Precipitation Process. J. Water Reuse Desalin. 2013, 3, 373–380. [Google Scholar] [CrossRef]
- Reyes-Serrano, A.; López-Alejo, J.E.; Hernández-Cortázar, M.A.; Elizalde, I. Removing Contaminants from Tannery Wastewater by Chemical Precipitation Using CaO and Ca(OH)2. Chin. J. Chem. Eng. 2020, 28, 1107–1111. [Google Scholar] [CrossRef]
- Velez, P.A.; Talano, M.A.; Paisio, C.E.; Agostini, E.; González, P.S. Synergistic Effect of Chickpea Plants and Mesorhizobium as a Natural System for Chromium Phytoremediation. Environ. Technol. 2017, 38, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.; Pakshirajan, K.; Chaturvedi, R. Chromium Tolerance, Bioaccumulation and Localization in Plants: An Overview. J. Environ. Manag. 2018, 206, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Ton, S.-S.; Lee, M.-W.; Yang, Y.-H.; Hoi, S.-K.; Cheng, W.-C.; Wang, K.-S.; Chang, H.-H.; Chang, S.-H. Effects of Reductants on Phytoextraction of Chromium (VI) by Ipomoea aquatica. Int. J. Phytoremed. 2015, 17, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Desjardin, V.; Bayard, R.; Huck, N.; Manceau, A.; Gourdon, R. Effect of Microbial Activity on the Mobility of Chromium in Soils. Waste Manag. 2002, 22, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Filali, B.K.; Taoufik, J.; Zeroual, Y.; Dzairi, F.Z.; Talbi, M.; Blaghen, M. Waste Water Bacterial Isolates Resistant to Heavy Metals and Antibiotics. Curr. Microbiol. 2000, 41, 151–156. [Google Scholar] [CrossRef]
- Kale, R.A.; Lokhande, V.H.; Ade, A.B. Investigation of Chromium Phytoremediation and Tolerance Capacity of a Weed, Portulaca oleracea L. in a Hydroponic System. Water Environ. J. 2015, 29, 236–242. [Google Scholar] [CrossRef]
- Bencheikh-Latmani, R.; Williams, S.M.; Haucke, L.; Criddle, C.S.; Wu, L.; Zhou, J.; Tebo, B.M. Global Transcriptional Profiling of Shewanella oneidensis MR-1 during Cr(VI) and U(VI) Reduction. Appl. Environ. Microbiol. 2005, 71, 7453–7460. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xiao, X.; Xu, C.; Cao, D.; Du, D. Decolorization and Detoxification of a Sulfonated Triphenylmethane Dye Aniline Blue by Shewanella oneidensis MR-1 under Anaerobic Conditions. Appl. Microbiol. Biotechnol. 2013, 97, 7439–7446. [Google Scholar] [CrossRef]
- Mao, F.; Liu, X.; Wu, K.; Zhou, C.; Si, Y. Biodegradation of Sulfonamides by Shewanella oneidensis MR-1 and Shewanella sp. Strain MR-4. Biodegradation 2018, 29, 129–140. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, Y.; Xu, M.; Cui, H.; Tan, L.; Feng, N.; Liu, X.; Qiu, G.; Dong, H.; Xie, J. Microbial Synthesis of Pd–Pt Alloy Nanoparticles Using Shewanella oneidensis MR-1 with Enhanced Catalytic Activity for Nitrophenol and Azo Dyes Reduction. Nanotechnology 2018, 30, 065607. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, Y.; Shen, Q.; Zheng, X.; Chen, Y. Enhancement of Hexavalent Chromium Reduction by Shewanella oneidensis MR-1 in Presence of Copper Nanoparticles via Stimulating Bacterial Extracellular Electron Transfer and Environmental Adaptability. Bioresour. Technol. 2022, 361, 127686. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Du, Y.; Chen, S.; Zhang, F.; Zhan, W.; Du, D.; Zhang, T.C. Nanoscale Zero-Valent Iron Coupling with Shewanella oneidensis MR-1 for Enhanced Reduction/Removal of Aqueous Cr(VI). Sep. Purif. Technol. 2021, 277, 119488. [Google Scholar] [CrossRef]
- Wielinga, B.; Mizuba, M.M.; Hansel, C.M.; Fendorf, S. Iron Promoted Reduction of Chromate by Dissimilatory Iron-Reducing Bacteria. Environ. Sci. Technol. 2001, 35, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yuan, Q.; Luan, F. Thermodynamic Considerations on the Combined Effect of Electron Shuttles and Iron(III)-Bearing Clay Mineral on Cr(VI) Reduction by Shewanella oneidensis MR-1. J. Hazard. Mater. 2023, 459, 132144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Nan, S.; Hongyang, H.; Yun, C. Evaluating Decolorization Capacity about Alginate Encapsulation System of Shewanella oneidensis MR-1 Mingled with Conductive Materials. Environ. Technol. Innov. 2020, 21, 101344. [Google Scholar]
- Ri, C.; Tang, J.; Liu, F.; Lyu, H.; Li, F. Enhanced Microbial Reduction of Aqueous Hexavalent Chromium by Shewanella oneidensis MR-1 with Biochar as Electron Shuttle. J. Environ. Sci. 2022, 113, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, G.; Jin, R.; Zhou, J. Goethite-Humic Acid Coprecipitate Mediated Fenton-like Degradation of Sulfanilamide: The Role of Coprecipitated Humic Acid in Accelerating Fe(III)/Fe(II) Cycle and Degradation Efficiency. J. Hazard. Mater. 2021, 403, 124026. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Han, X.; Wang, W.; Zhou, X.; Lin, J. Effects of Humic Acid Concentration on the Microbially-Mediated Reductive Solubilization of Pu(IV) Polymers. J. Hazard. Mater. 2017, 339, 347–353. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Z.; Wang, Y.; Yang, Y.; Chen, M. Synergy between Indigenous Bacteria and Extracellular Electron Shuttles Enhances Transformation and Mobilization of Fe(III)/As(V). Sci. Total Environ. 2021, 783, 147002. [Google Scholar] [CrossRef]
- Lu, H.; Zhou, Y.; Fu, Z.; Wang, X.; Zhou, J.; Guo, W. Mutual Interaction between the Secreted Flavins and Immobilized Quinone in Anaerobic Removal of High-Polarity Aromatic Compounds Containing Nitrogen by Shewanella Sp. RQs-106. J. Hazard. Mater. 2022, 431, 128595. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Yu, L.; Fang, Y.; Ashry, N.; Riahi, Y.; Uddin, I.; Dai, K.; Huang, Q. Iron Mineral-Humic Acid Complex Enhanced Cr(VI) Reduction by Shewanella oneidensis MR-1. Chemosphere 2020, 247, 125902. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhao, Z.; Burgos, W.D.; Li, Y.; Zhang, B.; Wang, Y.; Liu, W.; Sun, L.; Lin, L.; Luan, F. Iron(III) Minerals and Anthraquinone-2,6-Disulfonate (AQDS) Synergistically Enhance Bioreduction of Hexavalent Chromium by Shewanella oneidensis MR-1. Sci. Total Environ. 2018, 640–641, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Szymcewsk, D.; Karczewsk, J.; Chrzan, A.; Jasinski, P. Three Electrode Configuration Measurements of Electrolyte-Diffusion Barrier-Cathode Interface. J. Ceram. Soc. Jpn. 2015, 123, 268–273. [Google Scholar] [CrossRef]
- Zhong, J.; Luo, L.; Chen, B.; Sha, S.; Qing, Q.; Tam, N.F.Y.; Zhang, Y.; Luan, T. Degradation Pathways of 1-Methylphenanthrene in Bacterial Sphingobium Sp. MP9-4 Isolated from Petroleum-Contaminated Soil. Mar. Pollut. Bull. 2017, 114, 926–933. [Google Scholar] [CrossRef]
- Russel, J.D.; Paarfitt, R.L.; Fraser, A.R.; Farmer, V.C. Surface Structures of Gibbsite Goethite and Phosphated Goethite. Nature 1974, 248, 220–221. [Google Scholar] [CrossRef]
- Kar, S.; Equeenuddin, S.M. Adsorption of Chromium (VI) onto Natural Mesoporous Goethite: Effect of Calcination Temperature. Groundw. Sustain. Dev. 2019, 9, 100250. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, H.; Wang, Q.; Zhu, C.; He, A. Complexation Behaviour and Removal of Organic-Cr(III) Complexes from the Environment: A Review. Ecotoxicol. Environ. Saf. 2022, 240, 113676. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Li, X.; Ai, L.; Jiang, J. Nanoscale Zerovalent Iron Decorated on Graphene Nanosheets for Cr(VI) Removal from Aqueous Solution: Surface Corrosion Retard Induced the Enhanced Performance. Chem. Eng. J. 2016, 288, 789–797. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Zhang, Y.; Wu, S.; Xin, J. Enhancement of Cr(VI) Removal by Mechanically Activated Micron-Scale Zero-Valent Aluminum (MA-mZVAl): Performance and Mechanism Especially at near-Neutral pH. Chem. Eng. J. 2018, 353, 760–768. [Google Scholar] [CrossRef]
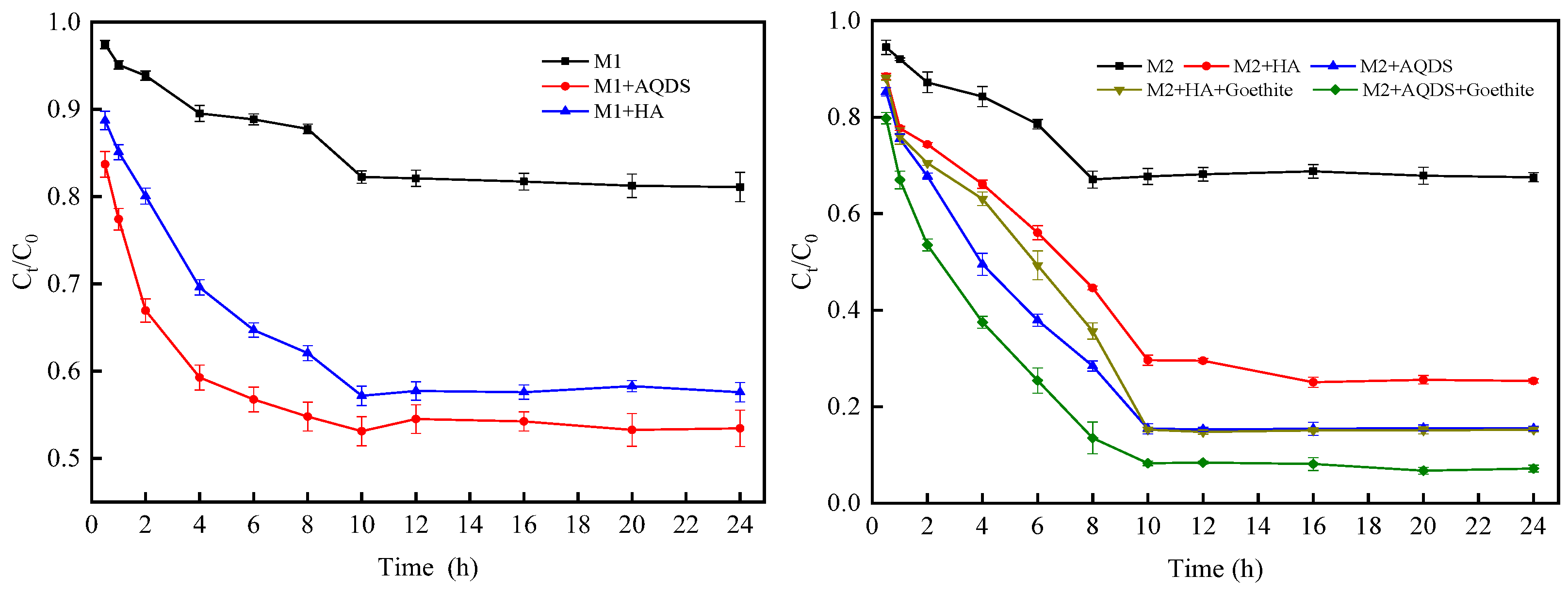



| System of Reaction | K/h−1 | R2 | EF |
|---|---|---|---|
| M1 | 0.0153 ± 0.0017 | 0.9280 | / |
| M1 and HA | 0.0402 ± 0.0038 | 0.9589 | 2.63 |
| M1 and AQDS | 0.0455 ± 0.0086 | 0.9183 | 2.97 |
| System of Reaction | K/h−1 | R2 | EF |
|---|---|---|---|
| M2 | 0.0372 ± 0.0013 | 0.9429 | / |
| M2 and HA | 0.1014 ± 0.0105 | 0.9389 | 2.72 |
| M2 and AQDS | 0.1659 ± 0.0012 | 0.9731 | 4.43 |
| M2, HA, and goethite | 0.1566 ± 0.0249 | 0.8656 | 4.21 |
| M2, AQDS, and goethite | 0.2317 ± 0.0090 | 0.9911 | 6.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Li, Y.; Wang, Y.; Zhu, Z.; Tang, S.; Zhang, J.; Pan, Q.; Hu, T. Goethite Enhances Cr(VI) Reduction by S. oneidensis MR-1 under Different Conditions: Mechanistic Insights. Microorganisms 2024, 12, 754. https://doi.org/10.3390/microorganisms12040754
Hou Y, Li Y, Wang Y, Zhu Z, Tang S, Zhang J, Pan Q, Hu T. Goethite Enhances Cr(VI) Reduction by S. oneidensis MR-1 under Different Conditions: Mechanistic Insights. Microorganisms. 2024; 12(4):754. https://doi.org/10.3390/microorganisms12040754
Chicago/Turabian StyleHou, Yu, Yanhong Li, Yaru Wang, Zongqiang Zhu, Shen Tang, Jie Zhang, Qiaodong Pan, and Ting Hu. 2024. "Goethite Enhances Cr(VI) Reduction by S. oneidensis MR-1 under Different Conditions: Mechanistic Insights" Microorganisms 12, no. 4: 754. https://doi.org/10.3390/microorganisms12040754







