Understanding Acanthamoeba Keratitis: An In-Depth Review of a Sight-Threatening Eye Infection
Abstract
:1. Introduction
2. Incidence
3. Risk Factors
4. Classification
5. Pathogenesis
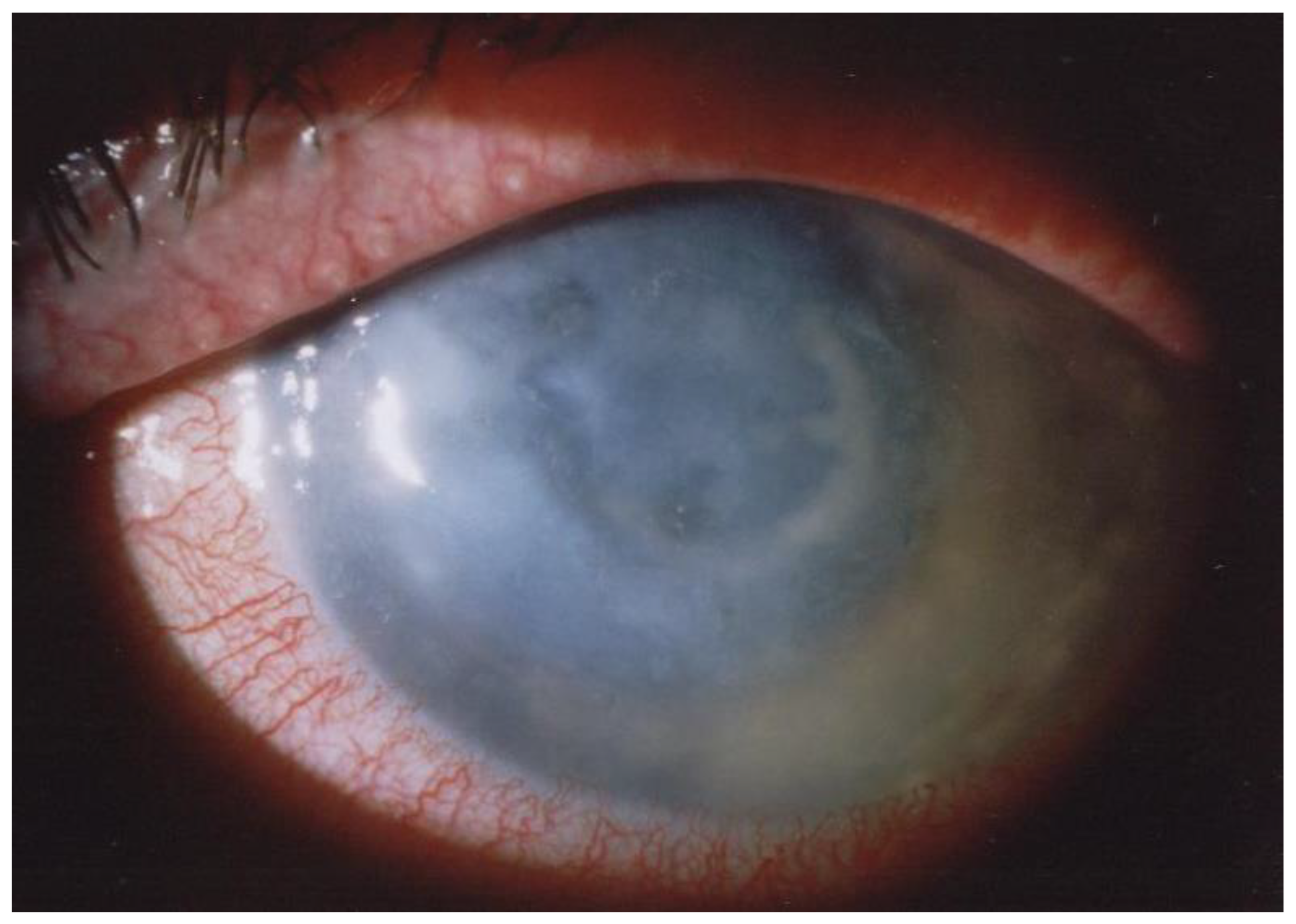
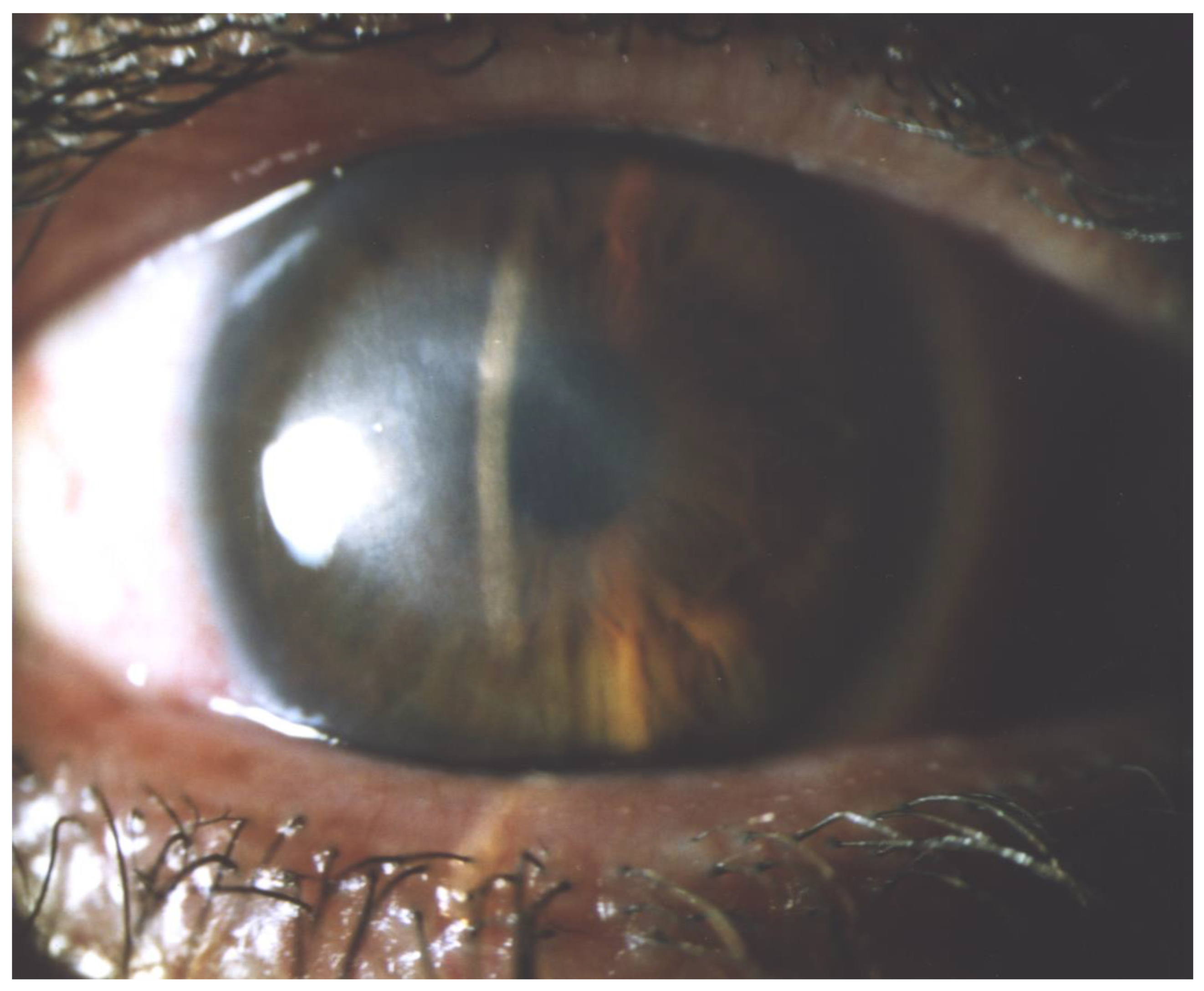
6. Clinical Symptoms
7. Diagnosis
7.1. In Vivo Confocal Microscopy (IVCM)
7.2. Corneal Scraping
7.3. PCR
7.4. Anterior Segment Optical Coherence Tomography (AS-OCT)
7.5. Impression Cytology
8. Treatment
8.1. Conservative Treatment
8.1.1. Biguanides
8.1.2. Aromatic Diamidines
8.1.3. Additional Medicaments
8.1.4. Steroids
8.1.5. Crosslinking
8.2. Surgical Treatment
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cabrera-Aguas, M.; Khoo, P.; Watson, S.L. Infectious keratitis: A review. Clin. Exp. Ophthalmol. 2022, 50, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Ho, C.S.; Deshmukh, R.; Said, D.G.; Dua, H.S. Infectious keratitis: An update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye 2021, 35, 1084–1101. [Google Scholar] [CrossRef]
- Petrillo, F.; Petrillo, A.; Sasso, F.P.; Schettino, A.; Maione, A.; Galdiero, M. Viral Infection and Antiviral Treatments in Ocular Pathologies. Microorganisms 2022, 10, 2224. [Google Scholar] [CrossRef]
- Petrillo, F.; Sinoca, M.; Fea, A.M.; Galdiero, M.; Maione, A.; Galdiero, E.; Guida, M.; Reibaldi, M. Candida Biofilm Eye Infection: Main Aspects and Advance in Novel Agents as Potential Source of Treatment. Antibiotics 2023, 12, 1277. [Google Scholar] [CrossRef] [PubMed]
- JKrachmer, H.; Mannis, M.J.; Holland, E.J. (Eds.) Surgery of the Cornea and Conjunctiva, 3rd ed.; Mosby Elsevier: Maryland Heights, MI, USA, 2011. [Google Scholar]
- Jones, B.R.; McGill, J.I.; Steele, A.D. Recurrent suppurative kerato-uveitis with loss of eye due to infection by Acanthamoeba castellani. Trans. Ophthalmol. Soc. UK 1975, 95, 210–213. [Google Scholar]
- Zhang, Y.; Xu, X.; Wei, Z.; Cao, K.; Zhang, Z.; Liang, Q. The global epidemiology and clinical diagnosis of Acanthamoeba keratitis. J. Infect. Public Health 2023, 16, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Verani, J.R.; Lorick, S.A.; Yoder, J.S.; Beach, M.J.; Braden, C.R.; Roberts, J.M.; Conover, C.S.; Chen, S.; McConnell, K.A.; Chang, D.C.; et al. National outbreak of Acanthamoeba keratitis associated with use of a contact lens solution, United States. Emerg. Infect. Dis. 2009, 15, 1236–1242. [Google Scholar] [CrossRef]
- Joslin, C.E.; Tu, E.Y.; McMahon, T.T.; Passaro, D.J.; Stayner, L.T.; Sugar, J. Epidemiological Characteristics of a Chicago-area Acanthamoeba Keratitis Outbreak. Am. J. Ophthalmol. 2006, 142, 212–217.e2. [Google Scholar] [CrossRef]
- Fraser, M.N.; Wong, Q.; Shah, L.; Holland, S.P.; Morshed, M.; Isaac-Renton, J.; Chong, M.; Kibsey, P.; Patrick, D.M. Characteristics of an Acanthamoeba Keratitis Outbreak in British Columbia between 2003 and 2007. Ophthalmology 2012, 119, 1120–1125. [Google Scholar] [CrossRef]
- Radford, C.F.; Lehmann, O.J.; Dart, J.K.G. Acanthamoeba keratitis: Multicentre survey in England 1992-6. Br. J. Ophthalmol. 1998, 82, 1387–1392. [Google Scholar] [CrossRef]
- Seal, D.V.; Kirkness, C.M.; Bennett, H.G.B.; Peterson, M. Population-based cohort study of microbial keratitis in Scotland: Incidence and features. Contact Lens Anterior Eye 1999, 22, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Radford, C.F.; Minassian, D.C.; Dart, J.K.G. Acanthamoeba keratitis in England and Wales: Incidence, outcome, and risk factors. Br. J. Ophthalmol. 2002, 86, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Bhatti, A.; Desai, R.; Barua, A. Analysis from a year of increased cases of Acanthamoeba Keratitis in a large teaching hospital in the UK. Contact Lens Anterior Eye 2019, 42, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Randag, A.C.; Van Rooij, J.; Van Goor, A.T.; Verkerk, S.; Wisse, R.P.L.; Saelens, I.E.Y.; Stoutenbeek, R.; Van Dooren, B.T.H.; Cheng, Y.Y.Y.; Eggink, C.A. The rising incidence of Acanthamoeba keratitis: A 7-year nationwide survey and clinical assessment of risk factors and functional outcomes. PLoS ONE 2019, 14, e0222092. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.E.; Ivarsen, A.; Hjortdal, J. Increasing incidence of Acanthamoeba keratitis in a large tertiary ophthalmology department from year 1994 to 2018. Acta Ophthalmol. 2020, 98, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Abell, R.G.; Mitra, B.; Ferdinands, M.; Vajpayee, R.B. Risk factors, demographics and clinical profile of Acanthamoeba keratitis in Melbourne: An 18-year retrospective study. Br. J. Ophthalmol. 2018, 102, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.Y.; Chan, F.M.; Beckingsale, P. Acanthamoeba keratitis cluster: An increase in Acanthamoeba keratitis in Australia. Clin. Exp. Ophthalmol. 2009, 37, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, P.; Lin, C.C.; Srinivasan, M.; Mascarenhas, J.; Prajna, N.V.; Keenan, J.D.; McLeod, S.D.; Acharya, N.R.; Lietman, T.M.; Porco, T.C. Acanthamoeba Keratitis in South India: A Longitudinal Analysis of Epidemics. Ophthalmic Epidemiol. 2012, 19, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Por, Y.M.; Mehta, J.S.; Chua, J.L.L.; Koh, T.-H.; Khor, W.B.; Fong, A.C.Y.; Lim, J.W.K.; Heng, W.J.; Loh, R.S.K.; Lim, L.; et al. Acanthamoeba Keratitis Associated with Contact Lens Wear in Singapore. Am. J. Ophthalmol. 2009, 148, 7–12.e2. [Google Scholar] [CrossRef]
- Graffi, S.; Peretz, A.; Jabaly, H.; Koiefman, A.; Naftali, M. Acanthamoeba keratitis: Study of the 5-year incidence in Israel: Table 1. Br. J. Ophthalmol. 2013, 97, 1382–1383. [Google Scholar] [CrossRef]
- Wilhelmus, K.R.; Jones, D.B.; Matoba, A.Y.; Hamill, M.B.; Pflugfelder, S.C.; Weikert, M.P. Bilateral Acanthamoeba Keratitis. Am. J. Ophthalmol. 2008, 145, 193–197.e1. [Google Scholar] [CrossRef] [PubMed]
- Varacalli, G.; Di Zazzo, A.; Mori, T.; Dohlman, T.H.; Spelta, S.; Coassin, M.; Bonini, S. Challenges in Acanthamoeba Keratitis: A Review. J. Clin. Med. 2021, 10, 942. [Google Scholar] [CrossRef] [PubMed]
- Van Meter, W.S.; Musch, D.C.; Jacobs, D.S.; Kaufman, S.C.; Reinhart, W.J.; Udell, I.J. Safety of Overnight Orthokeratology for Myopia. Ophthalmology 2008, 115, 2301–2313.e1. [Google Scholar] [CrossRef] [PubMed]
- Illingworth, C.D.; Cook, S.D.; Karabatsas, C.H.; Easty, D.L. Acanthamoeba keratitis: Risk factors and outcome. Br. J. Ophthalmol. 1995, 79, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Yoder, J.S.; Verani, J.; Heidman, N.; Hoppe-Bauer, J.; Alfonso, E.C.; Miller, D.; Jones, D.B.; Bruckner, D.; Langston, R.; Jeng, B.H.; et al. Acanthamoeba keratitis: The Persistence of Cases Following a Multistate Outbreak. Ophthalmic Epidemiol. 2012, 19, 221–225. [Google Scholar] [CrossRef]
- Bharathi, J.; Srinivasan, M.; Ramakrishnan, R.; Meenakshi, R.; Padmavathy, S.; Lalitha, P. A study of the spectrum of Acanthamoeba keratitis: A three-year study at a tertiary eye care referral center in South India. Indian J. Ophthalmol. 2007, 55, 37. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Sun, X.; Wang, Z.; Zhang, Y. Acanthamoeba Keratitis: Clinical Characteristics and Management. Ocul. Surf. 2015, 13, 164–168. [Google Scholar] [CrossRef]
- Houang, E.; Lam, D.; Fan, D.; Seal, D. Microbial keratitis in Hong Kong: Relationship to climate, environment and contact-lens disinfection. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 361–367. [Google Scholar] [CrossRef]
- Voyatzis, G.; McElvanney, A. Bilateral Acanthamoeba Keratitis in an Experienced Two-Weekly Disposable Contact Lens Wearer. Eye Contact Lens Sci. Clin. Pract. 2007, 33, 201–202. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. The biology of Acanthamoeba keratitis. Exp. Eye Res. 2021, 202, 108365. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef]
- Desai, N.; Green, D.A.; Kristan, J.; Cimic, A.; Baskota, S.U. Acanthamoeba keratitis. Diagn. Cytopathol. 2023, 51, 98–100. [Google Scholar] [CrossRef]
- Reyes-Batlle, M.; Sifaoui, I.; Rodríguez-Expósito, R.L.; Piñero, J.E.; Lorenzo-Morales, J. New Insights in Acanthamoeba. Pathogens 2022, 11, 609. [Google Scholar] [CrossRef]
- Diehl, M.L.N.; Paes, J.; Rott, M.B. Genotype distribution of Acanthamoeba in keratitis: A systematic review. Parasitol. Res. 2021, 120, 3051–3063. [Google Scholar] [CrossRef] [PubMed]
- Derda, M.; Wojtkowiak-Giera, A.; Kolasa-Wołosiuk, A.; Kosik-Bogacka, D.; Hadaś, E.; Jagodziński, P.P.; Wandurska-Nowak, E. Acanthamoeba infection in lungs of mice expressed by toll-like receptors (TLR2 and TLR4). Exp. Parasitol. 2016, 165, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, P.A.; Booton, G.C.; Crary, M. Phylogenetic Analysis and the Evolution of the 18S rRNA Gene Typing System of Acanthamoeba. J. Eukaryot. Microbiol. 2015, 62, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Castrillón, J.C.; Orozco, L.P. Acanthamoeba spp. como parásitos patógenos y oportunistas. Rev. Chil. Infectol. 2013, 30, 147–155. [Google Scholar] [CrossRef]
- Khan, N.A. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef]
- Taher, E.E.; Méabed, E.M.H.; Abdallah, I.; Abdel Wahed, W.Y. Acanthamoeba keratitis in noncompliant soft contact lenses users: Genotyping and risk factors, a study from Cairo, Egypt. J. Infect. Public Health 2018, 11, 377–383. [Google Scholar] [CrossRef]
- Badenoch, P.R. Pathogenicity of Acanthamoeba and a Corynebacterium in the Rat Cornea. Arch. Ophthalmol. 1990, 108, 107. [Google Scholar] [CrossRef]
- Alizadeh, H.; Neelam, S.; Hurt, M.; Niederkorn, J.Y. Role of Contact Lens Wear, Bacterial Flora, and Mannose-Induced Pathogenic Protease in the Pathogenesis of Amoebic Keratitis. Infect. Immun. 2005, 73, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Iovieno, A.; Ledee, D.R.; Miller, D.; Alfonso, E.C. Detection of Bacterial Endosymbionts in Clinical Acanthamoeba Isolates. Ophthalmology 2010, 117, 445–452.e3. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, T.R.; Sobek, D.; Gautom, R.K. Enhancement of in vitro cytopathogenicity by Acanthamoeba spp. following acquisition of bacterial endosymbionts. FEMS Microbiol. Lett. 1998, 166, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Heffler, K.F.; Eckhardt, T.J.; Reboli, A.C.; Stieritz, D. Acanthamoeba Endophthalmitis in Acquired Immunodeficiency Syndrome. Am. J. Ophthalmol. 1996, 122, 584–586. [Google Scholar] [CrossRef]
- Jones, D.B.; Visvesvara, G.S.; Robinson, N.M. Acanthamoeba polyphaga keratitis and Acenthamoeba uveitis associated with fatal meningoencephalitis. Trans. Ophthalmol. Soc. UK 1975, 95, 221–232. [Google Scholar] [PubMed]
- Moshari, A. Chorioretinitis after keratitis caused by Acanthamoeba Case report and review of the literature. Ophthalmology 2001, 108, 2232–2236. [Google Scholar] [CrossRef] [PubMed]
- Marciano-Cabral, F.; Toney, D.M. The Interaction of Acanthamoeba spp. with Activated Macrophages and with Macrophage Cell Lines. J Eukaryot. Microbiol. 1998, 45, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.L.; Kim, I.; Shupe, K.; Alizadeh, H.; Silvany, R.; McCulley, J.P.; Niederkorn, J.Y. Chemotactic response of macrophages to Acanthamoeba castellanii antigen and antibody-dependent macrophage-mediated killing of the parasite. J. Parasitol. 1992, 78, 849–855. [Google Scholar] [CrossRef]
- Hurt, M.; Proy, V.; Niederkorn, J.Y.; Alizadeh, H. The Interaction of Acanthamoeba Castellanii Cysts with Macrophages and Neutrophils. J. Parasitol. 2003, 89, 565–572. [Google Scholar] [CrossRef]
- Ferrante, A.; Rowan-Kelly, B. Activation of the alternative pathway of complement by Acanthamoeba culbertsoni. Clin. Exp. Immunol. 1983, 54, 477–485. [Google Scholar]
- Toney, D.M.; Marciano-Cabral, F. Resistance of Acanthamoeba species to complement lysis. J. Parasitol. 1998, 84, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, H.; Apte, S.; El-Agha, M.-S.H.; Li, L.; Hurt, M.; Howard, K.; Cavanagh, H.D.; McCulley, J.P.; Niederkorn, J.Y. Tear IgA and Serum IgG Antibodies Against Acanthamoeba in Patients with Acanthamoeba Keratitis. Cornea 2001, 20, 622–627. [Google Scholar] [CrossRef]
- Cursons, R.T.; Brown, T.J.; Keys, E.A.; Moriarty, K.M.; Till, D. Immunity to pathogenic free-living amoebae: Role of humoral antibody. Infect. Immun. 1980, 29, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Suguri, S.; Harada, M.; Hayabara, T.; Suzumori, R.; Ohta, N. Acanthamoeba-specific human T-cell clones isolated from healthy individuals. Parasitol. Res. 1994, 80, 549–553. [Google Scholar] [CrossRef]
- McClellan, K.; Howard, K.; Niederkorn, J.Y.; Alizadeh, H. Effect of steroids on Acanthamoeba cysts and trophozoites. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2885–2893. [Google Scholar]
- Carnt, N.; Robaei, D.; Watson, S.L.; Minassian, D.C.; Dart, J.K.G. The Impact of Topical Corticosteroids Used in Conjunction with Antiamoebic Therapy on the Outcome of Acanthamoeba Keratitis. Ophthalmology 2016, 123, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Daas, L.; Szentmáry, N.; Eppig, T.; Langenbucher, A.; Hasenfus, A.; Roth, M.; Saeger, M.; Nölle, B.; Lippmann, B.; Böhringer, D.; et al. Das Deutsche Akanthamöbenkeratitis-Register: Erste Ergebnisse einer multizentrischen Erhebung. Ophthalmologe 2015, 112, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Goodall, K.; Brahma, A.; Ridgway, A. Acanthamoeba keratitis: Masquerading as adenoviral keratitis. Eye 1996, 10, 643–644. [Google Scholar] [CrossRef]
- Tabin, G.; Taylor, H.; Snibson, G.; Murchison, A.; Gushchin, A.; Rogers, S. Atypical Presentation of Acanthamoeba Keratitis. Cornea 2001, 20, 757–759. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Martín-Navarro, C.M.; López-Arencibia, A.; Arnalich-Montiel, F.; Piñero, J.E.; Valladares, B. Acanthamoeba keratitis: An emerging disease gathering importance worldwide? Trends Parasitol. 2013, 29, 181–187. [Google Scholar] [CrossRef]
- Dart, J.K.G.; Saw, V.P.J.; Kilvington, S. Acanthamoeba Keratitis: Diagnosis and Treatment Update 2009. Am. J. Ophthalmol. 2009, 148, 487–499.e2. [Google Scholar] [CrossRef]
- Alkharashi, M.; Lindsley, K.; Law, H.A.; Sikder, S. Medical interventions for acanthamoeba keratitis. Cochrane Database Syst. Rev. 2015, 2015, CD010792. [Google Scholar] [CrossRef] [PubMed]
- Szentmáry, N.; Goebels, S.; Matoula, P.; Schirra, F.; Seitz, B. Die Akanthamöbenkeratitis—Ein seltenes und oft spät diagnostiziertes Chamäleon. Klin. Monatsbl Augenheilkd. 2012, 229, 521–528. [Google Scholar] [CrossRef]
- Claerhout, I.; Goegebuer, A.; Van Den Broecke, C.; Kestelyn, P.H. Delay in diagnosis and outcome of Acanthamoeba keratitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 242, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Awwad, S.T.; Petroll, W.M.; McCulley, J.P.; Cavanagh, H.D. Updates in Acanthamoeba Keratitis. Eye Contact Lens Sci. Clin. Pract. 2007, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-H.; Wang, Y.-C.; Yen, C.-Y.; Lin, C.-C.; Chen, C.-C. Case Series: Unusual Presentation of Acanthamoeba Coinfection in the Cornea. Optom. Vis. Sci. 2022, 99, 605–611. [Google Scholar] [CrossRef]
- Tu, E.Y.; Joslin, C.E.; Sugar, J.; Shoff, M.E.; Booton, G.C. Prognostic Factors Affecting Visual Outcome in Acanthamoeba Keratitis. Ophthalmology 2008, 115, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Feist, R.M. Radial Keratoneuritis in Pseudomonas Keratitis. Arch. Ophthalmol. 1991, 109, 774. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.D.; Donnenfeld, E.D.; Foulks, G.N.; Moadel, K.; Kanellopoulos, A.J. Decreased Corneal Sensation as an Initial Feature of Acanthamoeba Keratitis. Ophthalmology 1995, 102, 1565–1568. [Google Scholar] [CrossRef] [PubMed]
- Kelley, P.S.; Dossey, A.P.; Patel, D.; Whitson, J.T.; Hogan, R.N.; Cavanagh, H.D. Secondary Glaucoma Associated with Advanced Acanthamoeba Keratitis. Eye Contact Lens Sci. Clin. Pract. 2006, 32, 178–182. [Google Scholar] [CrossRef]
- Papathanassiou, M.; Gartry, D. Sterile corneal ulcer with ring infiltrate and hypopyon after recurrent erosions. Eye 2007, 21, 124–126. [Google Scholar] [CrossRef]
- Thomas, K.E.; Purcell, T.L.; Tanzer, D.J.; Schanzlin, D.J. Delayed diagnosis of microsporidial stromal keratitis: Unusual Wessely ring presentation and partial treatment with medications against Acanthamoeba. Case Rep. 2011, 2011, bcr0820103233. [Google Scholar] [CrossRef]
- Awwad, S.T.; Heilman, M.; Hogan, R.N.; Parmar, D.N.; Petroll, W.M.; McCulley, J.P.; Cavanagh, H.D. Severe Reactive Ischemic Posterior Segment Inflammation in Acanthamoeba Keratitis. Ophthalmology 2007, 114, 313–320. [Google Scholar] [CrossRef]
- Herz, N.L.; Matoba, A.Y.; Wilhelmus, K.R. Rapidly Progressive Cataract and Iris Atrophy during Treatment of Acanthamoeba Keratitis. Ophthalmology 2008, 115, 866–869. [Google Scholar] [CrossRef]
- Thebpatiphat, N.; Hammersmith, K.M.; Rocha, F.N.; Rapuano, C.J.; Ayres, B.D.; Laibson, P.R.; Eagle, R.C.; Cohen, E.J. Acanthamoeba Keratitis: A Parasite on the Rise. Cornea 2007, 26, 701–706. [Google Scholar] [CrossRef]
- Cohen, E.J.; Buchanan, H.W.; Laughrea, P.A.; Adams, C.P.; Galentine, P.G.; Visvesvara, G.S.; Folberg, R.; Arentsen, J.J.; Laibson, P.R. Diagnosis and Management of Acanthamoeba Keratitis. Am. J. Ophthalmol. 1985, 100, 389–395. [Google Scholar] [CrossRef]
- Hamburg, A.; De Jonckheere, J.F. Amoebic Keratitis. Ophthalmologica 1980, 181, 74–80. [Google Scholar] [CrossRef]
- Kaufman, S.C.; Musch, D.C.; Belin, M.W.; Cohen, E.J.; Meisler, D.M.; Reinhart, W.J.; Udell, I.J.; Van Meter, W.S. Confocal microscopy. Ophthalmology 2004, 111, 396–406. [Google Scholar] [CrossRef]
- Yera, H.; Ok, V.; Lee Koy Kuet, F.; Dahane, N.; Ariey, F.; Hasseine, L.; Delaunay, P.; Martiano, D.; Marty, P.; Bourges, J.L. PCR and culture for diagnosis of Acanthamoeba keratitis. Br. J. Ophthalmol. 2021, 105, 1302–1306. [Google Scholar] [CrossRef]
- De Craene, S.; Knoeri, J.; Georgeon, C.; Kestelyn, P.; Borderie, V.M. Assessment of Confocal Microscopy for the Diagnosis of Polymerase Chain Reaction–Positive Acanthamoeba Keratitis. Ophthalmology 2018, 125, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Villani, E.; Baudouin, C.; Efron, N.; Hamrah, P.; Kojima, T.; Patel, S.V.; Pflugfelder, S.C.; Zhivov, A.; Dogru, M. In Vivo Confocal Microscopy of the Ocular Surface: From Bench to Bedside. Curr. Eye Res. 2014, 39, 213–231. [Google Scholar] [CrossRef]
- Padhi, T.R.; Das, S.; Sharma, S.; Rath, S.; Rath, S.; Tripathy, D.; Panda, K.G.; Basu, S.; Besirli, C.G. Ocular parasitoses: A comprehensive review. Surv. Ophthalmol. 2017, 62, 161–189. [Google Scholar] [CrossRef]
- Garg, P.; Kalra, P.; Joseph, J. Non-contact lens related Acanthamoeba keratitis. Indian. J. Ophthalmol. 2017, 65, 1079–1086. [Google Scholar] [CrossRef]
- Hammersmith, K.M. Diagnosis and management of Acanthamoeba keratitis. Curr. Opin. Ophthalmol. 2006, 17, 327–331. [Google Scholar] [CrossRef]
- Clarke, D.W.; Alizadeh, H.; Niederkorn, J.Y. Failure of Acanthamoeba castellanii to Produce Intraocular Infections. Invest. Ophthalmol. Vis. Sci. 2005, 46, 2472. [Google Scholar] [CrossRef]
- Shigeyasu, C.; Shimazaki, J. Ocular Surface Reconstruction after Exposure to High Concentrations of Antiseptic Solutions. Cornea 2012, 31, 59–65. [Google Scholar] [CrossRef]
- Pfister, D.R.; Cameron, J.D.; Krachmer, J.H.; Holland, E.J. Confocal Microscopy Findings of Acanthamoeba Keratitis. Am. J. Ophthalmol. 1996, 121, 119–128. [Google Scholar] [CrossRef]
- Alomar, T.; Matthew, M.; Donald, F.; Maharajan, S.; Dua, H.S. In vivo confocal microscopy in the diagnosis and management of acanthamoeba keratitis showing new cystic forms. Clin. Exp. Ophthalmol. 2009, 37, 737–739. [Google Scholar] [CrossRef]
- Kobayashi, A.; Yokogawa, H.; Yamazaki, N.; Ishibashi, Y.; Oikawa, Y.; Tokoro, M.; Sugiyama, K. In Vivo Laser Confocal Microscopy Findings of Radial Keratoneuritis in Patients with Early Stage Acanthamoeba Keratitis. Ophthalmology 2013, 120, 1348–1353. [Google Scholar] [CrossRef]
- Chidambaram, J.D.; Prajna, N.V.; Palepu, S.; Lanjewar, S.; Shah, M.; Elakkiya, S.; Macleod, D.; Lalitha, P.; Burton, M.J. In Vivo Confocal Microscopy Cellular Features of Host and Organism in Bacterial, Fungal, and A canthamoeba Keratitis. Am. J. Ophthalmol. 2018, 190, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, H.D.; Petroll, W.M.; Alizadeh, H.; He, Y.-G.; McCulley, J.P.; Jester, J.V. Clinical and Diagnostic Use of In Vivo Confocal Microscopy in Patients with Corneal Disease. Ophthalmology 1993, 100, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, H.; Kobayashi, A.; Yamazaki, N.; Ishibashi, Y.; Oikawa, Y.; Tokoro, M.; Sugiyama, K. Bowman’s layer encystment in cases of persistent Acanthamoeba keratitis. Clin. Ophthalmol. 2012, 6, 1245–1251. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Gopal, B.P.; Deshmukh, R.; Seitzman, G.D.; Said, D.G.; Dua, H.S. Diagnostic armamentarium of infectious keratitis: A comprehensive review. Ocul. Surf. 2022, 23, 27–39. [Google Scholar] [CrossRef]
- Marangon, F.B.; Miller, D.; Alfonso, E.C. Impact of Prior Therapy on the Recovery and Frequency of Corneal Pathogens. Cornea 2004, 23, 158–164. [Google Scholar] [CrossRef]
- Heaselgrave, W.; Hamad, A.; Coles, S.; Hau, S. In Vitro Evaluation of the Inhibitory Effect of Topical Ophthalmic Agents on Acanthamoeba Viability. Trans. Vis. Sci. Technol. 2019, 8, 17. [Google Scholar] [CrossRef]
- Tu, E.Y.; Shoff, M.E.; Gao, W.; Joslin, C.E. Effect of Low Concentrations of Benzalkonium Chloride on Acanthamoebal Survival and Its Potential Impact on Empirical Therapy of Infectious Keratitis. JAMA Ophthalmol. 2013, 131, 595. [Google Scholar] [CrossRef]
- Fanselow, N.; Sirajuddin, N.; Yin, X.-T.; Huang, A.J.W.; Stuart, P.M. Acanthamoeba Keratitis, Pathology, Diagnosis and Treatment. Pathogens 2021, 10, 323. [Google Scholar] [CrossRef]
- Singh, A.; Sahu, S.K.; Sharma, S.; Das, S. Acanthamoeba Keratitis Versus Mixed Acanthamoeba and Bacterial Keratitis: Comparison of Clinical and Microbiological Profiles. Cornea 2020, 39, 1112–1116. [Google Scholar] [CrossRef]
- Raghavan, A.; Baidwal, S.; Venkatapathy, N.; Rammohan, R. The Acanthamoeba–Fungal Keratitis Study. Am. J. Ophthalmol. 2019, 201, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Sagerfors, S.; Ejdervik-Lindblad, B.; Söderquist, B. Does the sampling instrument influence corneal culture outcome in patients with infectious keratitis? A retrospective study comparing cotton tipped applicator with knife blade. BMJ Open Ophthalmol. 2020, 5, e000363. [Google Scholar] [CrossRef] [PubMed]
- Muiño, L.; Rodrigo, D.; Villegas, R.; Romero, P.; Peredo, D.E.; Vargas, R.A.; Liempi, D.; Osuna, A.; Jercic, M.I. Effectiveness of sampling methods employed for Acanthamoeba keratitis diagnosis by culture. Int. Ophthalmol. 2019, 39, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Schuster, F.L. Cultivation of Pathogenic and Opportunistic Free-Living Amebas. Clin. Microbiol. Rev. 2002, 15, 342–354. [Google Scholar] [CrossRef]
- Marines, H.M.; Osato, M.S.; Font, R.L. The Value of Calcofluor White in the Diagnosis of Mycotic and Acanthamoeba Infections of the Eye and Ocular Adnexa. Ophthalmology 1987, 94, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, H.E.; Waring, G.O.; Akor, C.; Castellano-Sanchez, A.A.; Bennett, K. Evaluation of hematoxylin and eosin and special stains for the detection of acanthamoeba keratitis in penetrating keratoplasties. Am. J. Ophthalmol. 2003, 136, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Elhardt, C.; Schweikert, R.; Hartmann, L.M.; Vounotrypidis, E.; Kilani, A.; Wolf, A.; Wertheimer, C.M. The role of the calcofluor white staining in the diagnosis of Acanthamoeba keratitis. J. Ophthal. Inflamm. Infect. 2023, 13, 23. [Google Scholar] [CrossRef]
- Bharathi, M.J. Microbiological diagnosis of infective keratitis: Comparative evaluation of direct microscopy and culture results. Br. J. Ophthalmol. 2006, 90, 1271–1276. [Google Scholar] [CrossRef]
- Liu, H.Y.; Hopping, G.C.; Vaidyanathan, U.; Ronquillo, Y.C.; Hoopes, P.C.; Moshirfar, M. Polymerase Chain Reaction and Its Application in the Diagnosis of Infectious Keratitis. Med. Hypothesis Discov. Innov. Ophthalmol. 2019, 8, 152–155. [Google Scholar]
- Ung, L.; Bispo, P.J.M.; Shanbhag, S.S.; Gilmore, M.S.; Chodosh, J. The persistent dilemma of microbial keratitis: Global burden, diagnosis, and antimicrobial resistance. Surv. Ophthalmol. 2019, 64, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.W.Y.; Harrison, R.; Hau, S.; Alexander, C.L.; Tole, D.M.; Avadhanam, V.S. Comparison of In Vivo Confocal Microscopy, PCR and Culture of Corneal Scrapes in the Diagnosis of Acanthamoeba Keratitis. Cornea 2018, 37, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Schaefer, J.L.; Jaber, R.; Paterson, J.; Liu, W.; Gonzalez-Fernandez, F. The Value of Cytology Smears for Acanthamoeba Keratitis. Case Rep. Ophthalmol. Med. 2016, 2016, 4148968. [Google Scholar] [CrossRef]
- Booton, G.C.; Kelly, D.J.; Chu, Y.-W.; Seal, D.V.; Houang, E.; Lam, D.S.C.; Byers, T.J.; Fuerst, P.A. 18S Ribosomal DNA Typing and Tracking of Acanthamoeba Species Isolates from Corneal Scrape Specimens, Contact Lenses, Lens Cases, and Home Water Supplies of Acanthamoeba Keratitis Patients in Hong Kong. J. Clin. Microbiol. 2002, 40, 1621–1625. [Google Scholar] [CrossRef]
- Schroeder, J.M.; Booton, G.C.; Hay, J.; Niszl, I.A.; Seal, D.V.; Markus, M.B.; Fuerst, P.A.; Byers, T.J. Use of Subgenic 18S Ribosomal DNA PCR and Sequencing for Genus and Genotype Identification of Acanthamoebae from Humans with Keratitis and from Sewage Sludge. J. Clin. Microbiol. 2001, 39, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Stothard, D.R.; Schroeder-Diedrich, J.M.; Awwad, M.H.; Gast, R.J.; Ledee, D.R.; Rodriguez-Zaragoza, S.; Dean, C.L.; Fuerst, P.A.; Byers, T.J. The Evolutionary History of the Genus Acanthamoeba and the Identification of Eight New 18S rRNA Gene Sequence Types. J Eukaryot. Microbiol. 1998, 45, 45–54. [Google Scholar] [CrossRef]
- Yera, H.; Zamfir, O.; Bourcier, T.; Ancelle, T.; Batellier, L.; Dupouy-Camet, J.; Chaumeil, C. Comparison of PCR, microscopic examination and culture for the early diagnosis and characterization of Acanthamoeba isolates from ocular infections. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Asokan, R.; Therese, K.L.; Lakshmipathy, M. Bilateral Acanthamoeba keratitis with radial keratoneuritis—Utility of AS-OCT in management and treatment. Clin. Exp. Optom. 2021, 104, 871–873. [Google Scholar] [CrossRef]
- Lloreda Martin, L.; Burgos-Blasco, B.; Matilla Rodero, M. AS-OCT and anterior segment negative image in the study of acanthamoeba keratitis. Arch. Soc. Esp. Oftalmol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Park, Y.M.; Lee, J.S.; Yoo, J.-M.; Park, J.M.; Seo, S.-W.; Chung, I.-Y.; Kim, S.J. Comparison of anterior segment optical coherence tomography findings in acanthamoeba keratitis and herpetic epithelial keratitis. Int. J. Ophthalmol. 2018, 11, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, N.; Kobayashi, A.; Yokogawa, H.; Ishibashi, Y.; Oikawa, Y.; Tokoro, M.; Sugiyama, K. In Vivo Imaging of Radial Keratoneuritis in Patients with Acanthamoeba keratitis by Anterior-Segment Optical Coherence Tomography. Ophthalmology 2014, 121, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Yuan, C.; Huang, A.J.W. Impression cytology in the diagnosis of acanthamoeba keratitis with surface involvement. Am. J. Ophthalmol. 2004, 137, 323–328. [Google Scholar] [CrossRef]
- Wang, Z.; Li, R.; Zhang, C.; Luo, S.; Sun, X.; Jin, X. Morphological characteristics in corneal smear of acanthamoeba keratitis. Zhonghua Yan Ke Za Zhi 2010, 46, 432–436. [Google Scholar] [PubMed]
- Papa, V.; Van Der Meulen, I.; Rottey, S.; Sallet, G.; Overweel, J.; Asero, N.; Minassian, D.C.; Dart, J.K.G. Safety and tolerability of topical polyhexamethylene biguanide: A randomised clinical trial in healthy adult volunteers. Br. J. Ophthalmol. 2022, 106, 190–196. [Google Scholar] [CrossRef]
- Lim, N.; Goh, D.; Bunce, C.; Xing, W.; Fraenkel, G.; Poole, T.R.G.; Ficker, L. Comparison of Polyhexamethylene Biguanide and Chlorhexidine as Monotherapy Agents in the Treatment of Acanthamoeba Keratitis. Am. J. Ophthalmol. 2008, 145, 130–135. [Google Scholar] [CrossRef]
- Lindquist, T.D. Treatment of Acanthamoeba Keratitist. Cornea 1998, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Maycock, N.J.R.; Jayaswal, R. Update on Acanthamoeba Keratitis: Diagnosis, Treatment, and Outcomes. Cornea 2016, 35, 713–720. [Google Scholar] [CrossRef] [PubMed]
- McKelvie, J.; Alshiakhi, M.; Ziaei, M.; Patel, D.V.; McGhee, C.N. The rising tide of Acanthamoeba keratitis in Auckland, New Zealand: A 7-year review of presentation, diagnosis and outcomes (2009–2016). Clin. Exp. Ophthalmol. 2018, 46, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Hargrave, S.L.; McCulley, J.P.; Husseini, Z. Results of a trial of combined propamidine isethionate and neomycin therapy for acanthamoeba keratitis. Ophthalmology 1999, 106, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Illingworth, C.D.; Cook, S.D. Acanthamoeba Keratitis. Surv. Ophthalmol. 1998, 42, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Larkin, D.F.P.; Kilvington, S.; Dart, J.K.G. Treatment of Acanthamoeba Keratitis with Polyhexamethylene Biguanide. Ophthalmology 1992, 99, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Seal, D.V.; Hay, J. Acanthamoeba keratitis: Early diagnosis, rational drug intervention and prevention. Hong Kong J. Ophthalmol. 1997, 1, 46–52. [Google Scholar]
- Dart, J.K.G.; Papa, V.; Rama, P.; Knutsson, K.A.; Ahmad, S.; Hau, S.; Sanchez, S.; Franch, A.; Birattari, F.; Leon, P.; et al. The Orphan Drug for Acanthamoeba Keratitis (ODAK) Trial. Ophthalmology 2023, 131, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, A.; Kheirkhah, A.; Abud, T.B.; Goyal, S.; Dana, R. Management of high-risk corneal transplantation. Surv. Ophthalmol. 2017, 62, 816–827. [Google Scholar] [CrossRef]
- Cohen, E.J.; Parlato, C.J.; Arentsen, J.J.; Genvert, G.I.; Eagle, R.C.; Wieland, M.R.; Laibson, P.R. Medical and Surgical Treatment of Acanthamoeba Keratitis. Am. J. Ophthalmol. 1987, 103, 615–625. [Google Scholar] [CrossRef]
- Hay, J.; Kirkness, C.M.; Seal, D.V.; Wright, P. Drug resistance and Acanthamoeba Keratitis: The quest for alternative antiprotozoal chemotherapy. Eye 1994, 8, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.H.; Wolf, T.C.; Jensen, H.G.; Parmley, V.C.; Rowsey, J.J. Combined Treatment of Acanthamoeba Keratitis with Propamidine, Neomycin, and Polyhexamethylene Biguanide. Am. J. Ophthalmol. 1993, 115, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.T.; Mondino, B.J.; Hoft, R.H.; Donzis, P.B.; Holland, G.N.; Farley, M.K.; Levenson, J.E. Successful Medical Management of Acanthamoeba Keratitis. Am. J. Ophthalmol. 1990, 110, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Padzik, M.; Baltaza, W.; Conn, D.; Szaflik, J.; Chomicz, L. Effect of povidone iodine, chlorhexidine digluconate and toyocamycin on amphizoic amoebic strains, infectious agents of Acanthamoeba keratitis—A growing threat to human health worldwide. Ann. Agric. Env. Med. 2018, 25, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Iovieno, A.; Miller, D.; Ledee, D.R.; Alfonso, E.C. Cysticidal activity of antifungals against different genotypes of Acanthamoeba. Antimicrob. Agents Chemother. 2014, 58, 5626–5628. [Google Scholar] [CrossRef] [PubMed]
- Alsoudi, A.F.; Golen, J.R.; Seitzman, G.D.; Lietman, T.M.; Keenan, J.D. Comparison of two confocal microscopes for diagnosis of acanthamoeba keratitis. Eye 2021, 35, 2061–2063. [Google Scholar] [CrossRef] [PubMed]
- Hadaś, E.; Derda, M.; Cholewiński, M. Evaluation of the effectiveness of tea tree oil in treatment of Acanthamoeba infection. Parasitol. Res. 2017, 116, 997–1001. [Google Scholar] [CrossRef]
- Somani, S.N.; Ronquillo, Y.; Moshirfar, M. Acanthamoeba Keratitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024; Available online: http://www.ncbi.nlm.nih.gov/books/NBK549863/ (accessed on 9 March 2024).
- Johnston, S.P.; Sriram, R.; Qvarnstrom, Y.; Roy, S.; Verani, J.; Yoder, J.; Lorick, S.; Roberts, J.; Beach, M.J.; Visvesvara, G. Resistance of Acanthamoeba Cysts to Disinfection in Multiple Contact Lens Solutions. J. Clin. Microbiol. 2009, 47, 2040–2045. [Google Scholar] [CrossRef]
- Khan, N.A. Pathogenicity, morphology, and differentiation of Acanthamoeba. Curr. Microbiol. 2001, 43, 391–395. [Google Scholar] [CrossRef]
- del Buey, M.A.; Cristóbal, J.A.; Casas, P.; Goñi, P.; Clavel, A.; Mínguez, E.; Lanchares, E.; García, A.; Calvo, B. Evaluation of in vitro efficacy of combined riboflavin and ultraviolet a for Acanthamoeba isolates. Am. J. Ophthalmol. 2012, 153, 399–404. [Google Scholar] [CrossRef]
- Garduño-Vieyra, L.; Gonzalez-Sanchez, C.R.; Hernandez-Da Mota, S.E. Ultraviolet-A Light and Riboflavin Therapy for Acanthamoeba Keratitis: A Case Report. Case Rep. Ophthalmol. 2011, 2, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.A.; Kashiwabuchi, R.T.; Martins, S.A.; Castro-Combs, J.M.; Kalyani, S.; Stanley, P.; Flikier, D.; Behrens, A. Riboflavin and Ultraviolet Light A Therapy as an Adjuvant Treatment for Medically Refractive Acanthamoeba Keratitis. Ophthalmology 2011, 118, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Henein, C.; Said, D.G.; Dua, H.S. Photoactivated chromophore for infectious keratitis—Corneal cross-linking (PACK-CXL): A systematic review and meta-analysis. Ocul. Surf. 2019, 17, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Prajna, N.V.; Radhakrishnan, N.; Lalitha, P.; Austin, A.; Ray, K.J.; Keenan, J.D.; Porco, T.C.; Lietman, T.M.; Rose-Nussbaumer, J. Cross-Linking-Assisted Infection Reduction: A Randomized Clinical Trial Evaluating the Effect of Adjuvant Cross-Linking on Outcomes in Fungal Keratitis. Ophthalmology 2020, 127, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Sarnicola, E.; Sarnicola, C.; Sabatino, F.; Tosi, G.M.; Perri, P.; Sarnicola, V. Early Deep Anterior Lamellar Keratoplasty (DALK) for Acanthamoeba Keratitis Poorly Responsive to Medical Treatment. Cornea 2016, 35, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Szentmáry, N.; Daas, L.; Shi, L.; Laurik, K.L.; Lepper, S.; Milioti, G.; Seitz, B. Acanthamoeba keratitis—Clinical signs, differential diagnosis and treatment. J. Curr. Ophthalmol. 2019, 31, 16–23. [Google Scholar] [CrossRef]
- Roozbahani, M.; Hammersmith, K.M.; Rapuano, C.J.; Nagra, P.K.; Zhang, Q. Therapeutic penetrating keratoplasty for acanthamoeba keratitis: A review of cases, complications and predictive factors. Int. Ophthalmol. 2019, 39, 2889–2896. [Google Scholar] [CrossRef]


| Diagnostic Tool | Advantages | Disadvantages |
|---|---|---|
| IVCM | Primary approach. High specificity and sensitivity, non-invasive and rapid. It can be used during all the stages of the disease. | Not widespread. It is operator-dependent and it requires a significant learning curve. It analyzes only a restricted area of the cornea per scan. It has difficulty in detecting trophozoite forms and it can mask Acanthamoeba cysts by stromal corneal inflammation. |
| COLTURE | High specificity and good sensitivity. | Acanthamoebae typically infiltrate deeply in the cornea and are not commonly found on its surface. Misdiagnosis in case of co-infection with another microbes. |
| PCR | Quick and very sensitive. | Positive results can be obtained even in cases where non-viable Acanthamoeba genomes are present. |
| AS-OCT | Non-invasive and useful for evaluating the radial keratoneuritis and the differential diagnosis. | It cannot detect directly the Acanthamoeba cyst or trophozoites. |
| IMPRESSION CITOLOGY | High specificity and relatively non-invasive. | It requires the use of specialized stains and expertise in cytopathology. It is not able to detect the presence of the cysts in the deep corneal layers. |
| Treatment | Type of Treatment | When to Use It |
|---|---|---|
| Medical treatment | Biguanides (PHMB, chlorhexidine) | At a diluted concentration of 0.02% is the first line of treatment (alone or in combination with diamidines) |
| Aromatic diamidines (propamidine, hexamidine) | Alongside biguanides | |
| Neomycin | Only with PHMB and propamidine | |
| Antifungical agents (voriconazole, posaconazole) | Efficacy against only the cystic phase | |
| Miltefosine | Effective, but expensive and not easily accessible | |
| Extract from tea tree | Only in laboratory settings | |
| Steroids | No role | |
| NSAIDs | With severe corneal inflammation, limbitis, or scleritis | |
| Parasurgical treatment | Cross-linking of corneal collagen | Non-universal consensus on amoebicidal impact of riboflavin + UVA |
| Surgical treatment | DALK | Cases resistant to medical therapy (uncertain role and timing) |
| PK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrillo, F.; Tortori, A.; Vallino, V.; Galdiero, M.; Fea, A.M.; De Sanctis, U.; Reibaldi, M. Understanding Acanthamoeba Keratitis: An In-Depth Review of a Sight-Threatening Eye Infection. Microorganisms 2024, 12, 758. https://doi.org/10.3390/microorganisms12040758
Petrillo F, Tortori A, Vallino V, Galdiero M, Fea AM, De Sanctis U, Reibaldi M. Understanding Acanthamoeba Keratitis: An In-Depth Review of a Sight-Threatening Eye Infection. Microorganisms. 2024; 12(4):758. https://doi.org/10.3390/microorganisms12040758
Chicago/Turabian StylePetrillo, Francesco, Antonia Tortori, Veronica Vallino, Marilena Galdiero, Antonio M. Fea, Ugo De Sanctis, and Michele Reibaldi. 2024. "Understanding Acanthamoeba Keratitis: An In-Depth Review of a Sight-Threatening Eye Infection" Microorganisms 12, no. 4: 758. https://doi.org/10.3390/microorganisms12040758
APA StylePetrillo, F., Tortori, A., Vallino, V., Galdiero, M., Fea, A. M., De Sanctis, U., & Reibaldi, M. (2024). Understanding Acanthamoeba Keratitis: An In-Depth Review of a Sight-Threatening Eye Infection. Microorganisms, 12(4), 758. https://doi.org/10.3390/microorganisms12040758






