Abstract
Ramularia sphaeroidea was primarily identified based on the characteristics of its conidia and several sequences. The fungus causes severe leaf spot disease on hairy vetch (Vicia villosa var. glabrescens) in Yunnan Province in China. The growth, sporulation, fungicide efficacy, and host range of the pathogen were evaluated to aid in disease management. Different types of culture media and carbon and nitrogen sources were used to evaluate the growth of R. sphaeroidea. Oatmeal, maltose, and potassium nitrate agar had a higher amount of sporulation. Difenoconazole (10%) was the most effective fungicide against the leaf disease caused by R. sphaeroidea. In addition, foliar inoculation sprays were used to assess the host range of R. sphaeroidea in six different plant species, including alfalfa (Medicago sativa L.), sainfoin (Onobrychis viciifolia Scop.), erect milkvetch (Astragalus adsurgens Pall.), common vetch (Vicia sativa L.), red clover (Trifolium pratense L.), and white clover (Trifolium repens L.). R. sphaeroidea successfully infected these plants, indicating that it has a wider host range than hairy vetches.
1. Introduction
The legume hairy vetch (Vicia villosa Roth var. glabrescens) is a variant of Vicia villosa Roth [1,2,3] and is also known as Vicia villosa Roth var. glabrescens [4]. It is native to Europe and western Asia, and it is also the second most common vetch cultivated in the world [5]. It is primarily found in the USA, Canada, Japan, Argentina, South Korea, and Iran [6,7,8]. It was introduced to China from the state of Oregon, USA, in 1946 and has been planted in Jiangsu, Anhui, Shandong, Henan, Hubei, Yunnan, and other provinces [3,9]. In addition, it was planted as a winter cover crop in Japan and South Korea. Leguminous cover crops are preferably cultivated, owing to their ability to harbor bacteria that fix nitrogen in nodules [7]. Compared with other cover crops, planting hairy vetch leads to improved soil quality and health [10,11].
Previous studies around the world have described more than 10 diseases in species of Vicia, including chocolate spot (Botrytis fabae) that infected the leaves of V. fabae [12], root rot caused by Macrophomina phaseolina and Rhizoctonia solani [13], and Ramularia sphaeroidea Sacc. in V. villosa Roth [14]. In China, Nan and Li [15] described 10 diseases of V. villosa Roth in 1994, including Ascochyta pisi, Colletotrichum viciae, Leveillula leguminosarum Golov., Fusarium oxysporum Schlecht., and Stemphylium vesicarium.
Ramularia is a rich genus that includes important plant pathogens such as R. collo-cygni and R. beticola, which caused severe economic losses to barley and sugar beet crops, respectively [16]. Ramularia leaf spot is an important disease on wheat, turfgrass, and perennial grasses and forms a potential threat to other agriculturally important crops [17]. Leaf spot disease caused by R. collo-cygni on barley has occurred in European countries and New Zealand, which can lead to yield losses of up to 25% in winter barley [18,19]. R. collo-cygni can also lead to symptomless infection through a special stomatopodium structure and epiphytic hyphal network during the entire barley growing season when it completes its life cycle [17,20].
R. sphaeroidea is also an important pathogen in Ramularia, which was first described by Braun in 1995, based on its hyaline conidiophores and conidia [21,22]. With the advent of molecular techniques, R. sphaeroidea was first identified in the second year [14]. In 2021, leaf spot disease caused by R. sphaeroidea in hairy vetch was reported for the first time in China [23].
The availability of different nutrients has a significant influence on the progression of diseases caused by pathogens [24,25]. Fungal growth and sporulation need a lot of elements, such as carbon, nitrogen, oxygen, and hydrogen [26]. Many nutrients are required, including carbohydrates, nucleic acids, and proteins, which are involved in host–pathogen interactions [27,28,29,30]. R. sphaeroidea has difficulties in inducing conidial production, which limits further study on its biological characteristics and effective control [14,23]. Therefore, it is essential to determine the optimum nutrient conditions for the growth and sporulation of R. sphaeroidea.
In 2002, Strobilurin-based fungicides were found to be the most effective fungicide to control the disease caused by R. collo-cygni [31]. Less than ten years later, there was a rapid decline in strobilurin-based fungicides for Ramularia disease because of resistance [32]. Azoxystrobin in combination with chlorothalonil was considered to be effective [33]. The high adaptive potential of Ramularia species led to the low efficacy of the current fungicides. To our knowledge, comprehensive identification and effective fungicides are lacking, and the effects of different nutrients on R. sphaeroidea are poorly understood. Understanding the host range and fungicide efficacy of the R. sphaeroidea strain from Yunnan could guide strategies to prevent the widespread dissemination of this disease in China. Therefore, this study was conducted to investigate the host range through artificial inoculation under greenhouse conditions, and the most effective fungicide against leaf diseases caused by R. sphaeroidea was identified. Furthermore, the effects of carbon and nitrogen on the colony growth and sporulation of R. sphaeroidea were evaluated. In addition, we conducted a more accurate phylogenetic analysis of the multigene sequences.
2. Materials and Methods
2.1. Isolation of the Fungus
Twenty diseased leaves (four leaves per plant) of Vicia villosa Roth var. glabrescens were randomly collected from Malong County (103°36′10″ E, 25°31′78″ N at an elevation of 1985.8 m) in 2019 [34]. The leaves were cut into pieces, soaked in 75% ethanol for 45 s and 1% sodium hypochlorite (NaClO) for 75 s, washed four times with sterile water, dried with sterilized filter paper, and then placed on potato dextrose agar (PDA) using sterilized forceps [35]. A total of 107 isolates were identified, and the frequency was 33.64%. Three representative isolates were selected for further analysis. The diseased specimen with the number MHLZU19326 and representative isolates (YN1931401, YN1931402, and YN1931403) were deposited at the Mycological Herbarium of Lanzhou University (MHLZU). Morphological characterizations of conidiophore and conidia were observed using a light microscope and photographed with a Canon DS126391 camera (Canon, Lanzhou, China).
2.2. Multigene Sequencing
A Fungal DNA Kit (D3195) purchased from Omega-BioTek (Norcross, GA, USA) was used to extract DNA from three isolates of R. sphaeroidea. The 28S rRNA gene (LSU), internal transcribed spacer regions (ITS), calmodulin (cmdA), translation elongation factor 1-α (tef1-α), histone H3 (his3), glyceraldehyde-3-phosphate dehydrogenase (gapdh), and RNA polymerase II second largest subunit (rpb2) genes were amplified and sequenced with the primers LR5/LSU1Fd [36,37], ITS4/V9G [38,39], CAL-228F/CAL-737R [40], EF1-728F/TEF-1R [41,42], CylH3F/CylH3R [43,44], GPD1/GPD2 [45], and RPB2-5f2/RPB2-7 [46,47], respectively. PCR was performed as previously described by Videira [16].
2.3. Growth, Sporulation, and Germination of R. sphaeroidea
2.3.1. Culture Media
Five types of agars were used to test different media, including PDA, oatmeal agar (OMA), potato carrot agar (PCA), corn meal agar (CMA), and L-malic acid added to PDA [14,48]. Each medium was sterilized at 121 °C for 20 min and then cooled to room temperature. Each medium was poured into 90 mm diameter Petri dishes and solidified for 24 h in aseptic conditions.
2.3.2. Carbon and Nitrogen Media
The components of the basal medium consisted of 2 g of sodium phosphate (Na3PO4), 1 g of magnesium sulfate (MgSO4), 5 g of potassium nitrate (KNO3), and 20 g of dextrose in different types of carbon and nitrogen media [48]. The six types of carbon media included D-fructose, glucose, sucrose, lactose, starch, and maltose, and the four types of nitrogen media included potassium nitrate, ammonium nitrate, peptone, and ammonium chloride. The pathogen was cultured on PDA for 45 days. Fungal plugs that were 5 mm in diameter were excised and placed in the middle of each Petri dish. All the plates were cultured at 25 °C.
Colony diameters were recorded after 7, 14, 21, 28, and 35 days of incubation. On day 35, the daily growth rate (mm day−1) was calculated, and a hemocytometer was used to calculate the concentration of conidial suspensions in each medium. The YN1931401 strain was used in this experiment, and four replicates were designed for each treatment.
2.3.3. Effects of Temperature on Conidial Germination
Conidia were washed with distilled water from 45-day-old colonies growing on PDA, and the concentrations were adjusted to approximately 106 conidia mL−1 with a hemocytometer. Three 10 μL droplets of conidial suspension were added to the PDA plate and incubated at temperatures ranging from 5 to 35 °C at 5 °C intervals. The Petri dishes were removed from the incubators at 24, 48, 72, and 96 h, respectively. The percentage germination of each treatment was evaluated by microscopy.
2.4. Phylogenetic Analysis
All reference sequences were downloaded from GenBank and listed in Supplementary Table S1 [49,50,51,52,53], as described by Videira et al. [16]. Each sequence was aligned using MEGA 7.0.2. The sequence was used to combine the seven loci using Matrix 1.8. MrModeltest v. 2.3 was used to select the best-fit nucleotide substitution models for phylogenetic analysis and added to MrBayes v. 3.2.6. The full dataset was then run for 2,000,000 generations and sampled every 100 generations. For maximum likelihood, the test of phylogeny was the bootstrap method, and the number of bootstrap replications was 1000.
2.5. Pathogenicity and Host Range
To test Koch’s postulates, a spray inoculation experiment was conducted in September 2021 to test the pathogenicity of R. sphaeroidea. The seeds of Vicia villosa var. glabrescens were obtained from the Academy of Grassland and Animal Science (Kunming, China) in 2018. R. sphaeroidea strain YN1931401, isolated from diseased hairy vetch plants, was used in this study. The pathogen was cultured at 25 °C for 45 days. About 10 mL conidial suspension (1 × 106 conidia mL−1) containing Tween 80 (0.01%) was sprayed on the leaves of 25 plants, and an additional 25 plants were sprayed with sterile water as the control group.
Six locally grown perennial legumes, including sainfoin (Onobrychis viciaefolia Scop.), alfalfa (Medicago sativa L.), white clover (Trifolium repens L.), common vetch (V. sativa L.), erect milkvetch (Astragalus adsurgens Pall.), and red clover (T. pratense L.), were used to access the host range of the YN1931401 strain of R. sphaeroidea isolated from hairy vetch plants. In total, 120 plants were used in this experiment. Twenty seeds of each plant species were selected and surface-sterilized with 75% ethanol for 50 s and 1% sodium hypochlorite (NaClO) for 90 s, followed by rinsing three times with sterile water. Approximately 10 mL of a conidial suspension of R. sphaeroidea (1 × 106 conidia mL−1) was sprayed on ten plants, and the same amount of sterile water was sprayed on the other ten control plants.
Polyethylene bags were used to cover all plants for 48 h to maintain humidity above 95%. After this, all the plants were placed randomly on a rack in a greenhouse under an 18 h/6 h (light/dark) regime with a temperature of 22 °C during the day and 18 °C at night. The incidence of infected leaves was evaluated 14 days after inoculation, and the pathogen was re-isolated from the infected lesions to confirm its identity using morphological approaches.
2.6. Fungicide Sensitivity Experiments
To determine the efficacy of four fungicides, including chlorothalonil (a.i. 75%, Sichuan, China), mancozeb (a.i. 80%, Shandong, China), difenoconazole (a.i. 10%, Zhejiang, China), and pentazole alcohol (a.i. 50%, Zhejiang, China), were used against R. sphaeroidea. Different concentrations of chlorothalonil (187.5, 375, 750, 1500, and 3000 mg/L), mancozeb (200, 400, 800, 1600, and 3200 mg/L), difenoconazole (200, 400, 800, 1600, and 3200 mg/L), and pentazole alcohol (200, 400, 800, 1600, and 3200 mg/L) were added to sterilized PDA media before solidifying. PDA without fungicide was used as a control. Fungal mycelium plugs with a 5 mm diameter were transferred to the center of a Petri dish containing various concentrations of fungicides. The colony diameter was measured using a ruler after two weeks [54] at a temperature of 22 °C. The mean was calculated based on four replicates, and regression analysis was conducted using percent inhibition values obtained from mycelium growth tests and logarithmic values of the fungicide doses. Subsequently, EC50 values were calculated [55].
3. Results
3.1. Phylogenetic Analysis
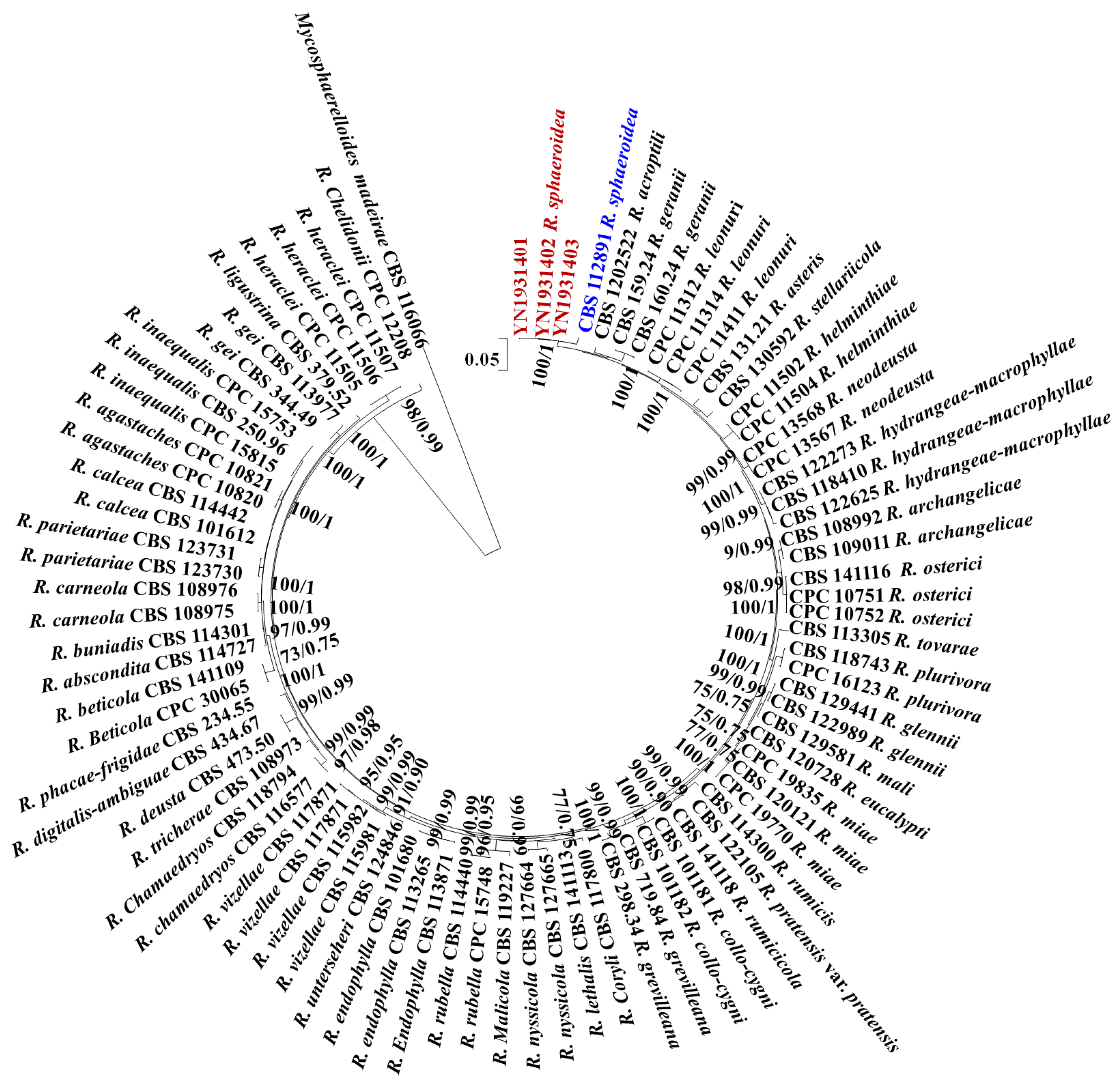
All sequences of the reference Ramularia species were downloaded from GenBank, and Mycosphaerelloides madeirae was used as the outgroup. The sequence contained 3155 characters (442 for ITS, 659 for LSU, 177 for cmdA, 578 for gapdh, 346 for his3, 574 for rpb2, and 379 for tef1-α). The following models were selected by MrModeltest 2.3 for MrBayes analysis: GTR+G for ITS, GTR+G for LSU, GTR+G for cmdA, GTR+I+G for gapdh, GTR+G for his3, GTR+G for rpb2, and SYM +G for tef1-α. The combined multigene tree constructed using ITS, LSU, cmdA, gapdh, his3, rpb2, and tef1-α indicated that the isolates YN1931401, YN1931402, and YN1931403 were R. sphaeroidea (Figure 1).
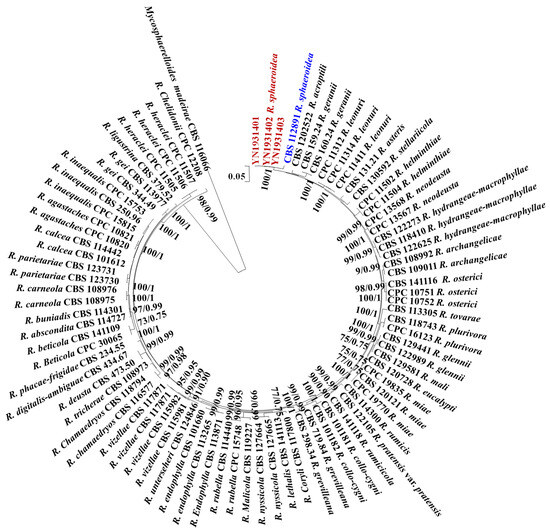
Figure 1.
Maximum likelihood phylogenetic tree of concatenated partial sequences of ITS, LSU, cmdA, gapdh, his3, rpb2, and tef1-α gene alignments of Ramularia species. Bootstrap values for the maximum likelihood and Bayesian posterior probabilities are shown above the branches. Mycosphaerelloides madeirae (CBS 116066) is used as an outgroup. The isolates YN1931401, YN1931402, and YN1931403 in this study were marked in red and the R. sphaeroidea that reported in 2015 was marked in blue.
3.2. Symptoms, Pathogen Isolation, and Conidia Germination
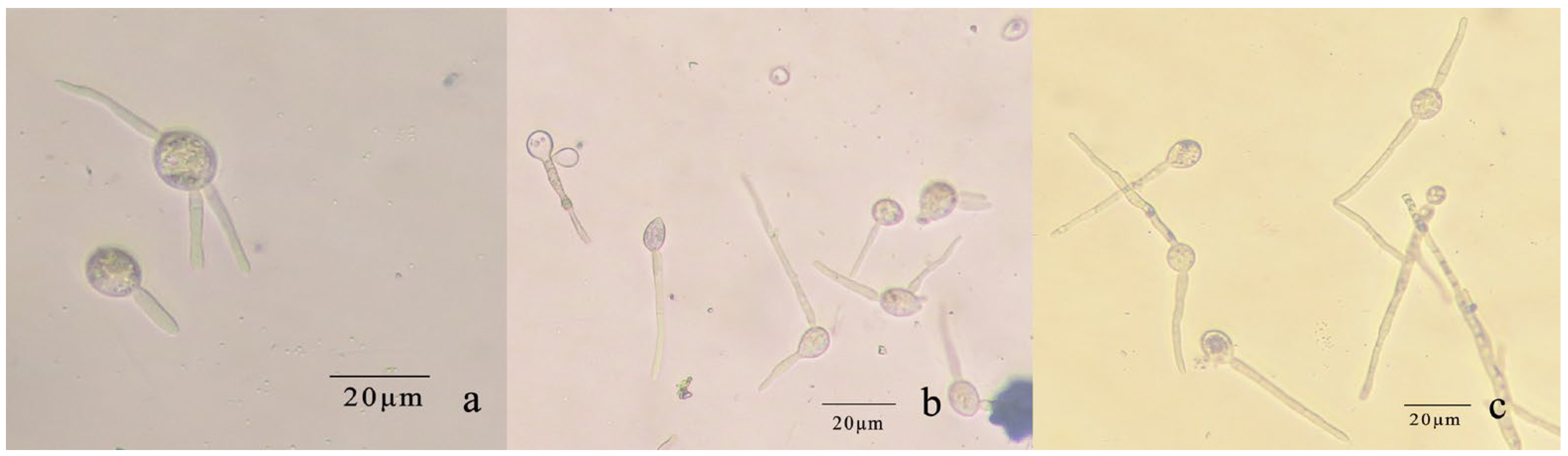
From 2019 to 2021, leaf spot disease in hairy vetch was observed between November and December in Malong County, Qujing City, Yunnan Province, China. The disease generally occurred in the older leaves. The initial symptoms were small, irregular, brown to dark brown spots (Figure 2a), which eventually developed into a white mass of mycelia in the middle portion of the lesions, with conidia that were found directly in the leaves (Figure 2b,c). The leaf lesions were initially dark and then fused with the surrounding black spots.

Figure 2.
Leaf spot symptoms on hairy vetch plants: (a) field symptoms of leaf spot, (b) leaf symptoms of blackening and obvious white mold on the leaves, and (c) subglobose conidia directly found in leaves. Scale bar = 20 μm.
3.3. Growth, Sporulation, and Germination of R. sphaeroidea
3.3.1. Culture Media
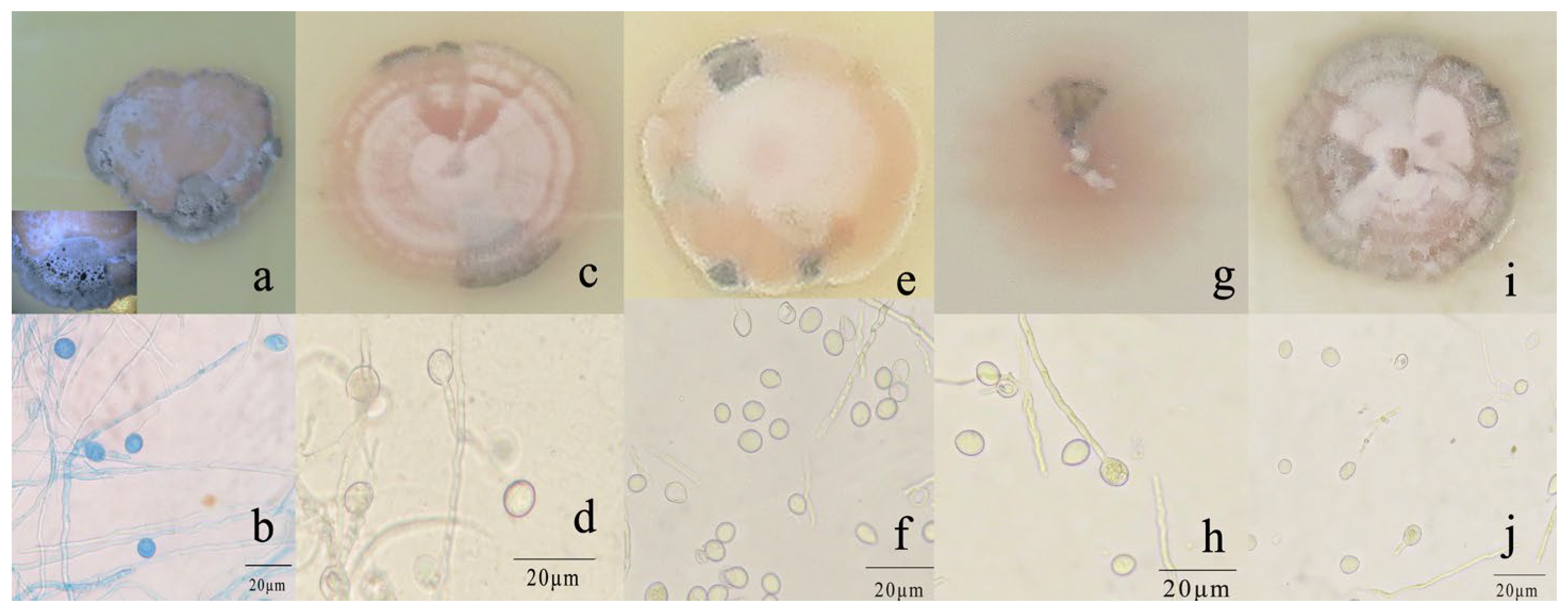
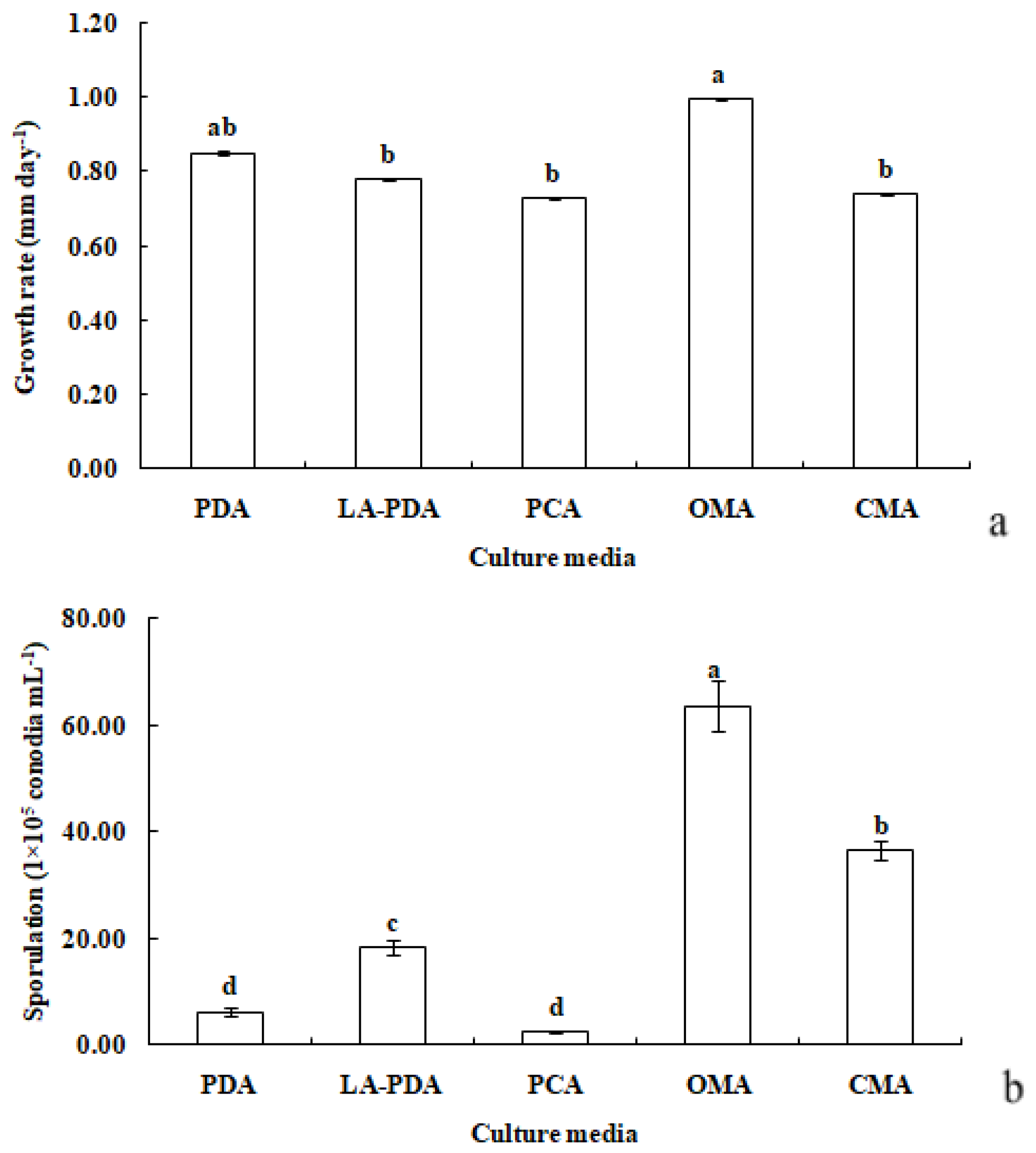
R. sphaeroidea grew and produced spores on all media tested (Figure 3). The conidia were aseptate on all media, and their shapes were ellipsoid to spherical (Table 1). Among the growth media tested, cultures grown on OMA grew the fastest at a rate of 1.0 mm per day followed by PDA with an average daily growth rate of 0.85 mm. Moreover, the colonies on both L-malic acid PDA and CMA grew an average of 0.78 and 0.74 day−1, respectively. The fungi cultured on PCA grew 0.73 mm day−1 on average, which was the lowest rate of growth compared with those on the other media (Figure 4a). The results from this study showed that OMA promoted the highest number of conidia with a suspension of 63.50 ×105 conidia mL−1 followed by the numbers produced on CMA. Simultaneously, the lowest amount of sporulation was recorded on PCA followed by PDA (Figure 4b).
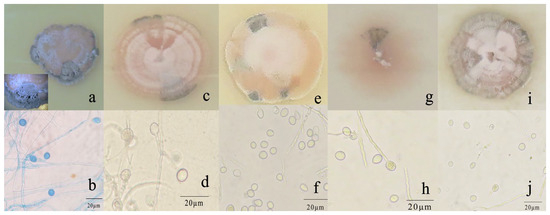
Figure 3.
Morphological and cultural characteristics of R. sphaeroidea. (a,c,e,g,i) Colony morphology on PDA, OMA, LA-PDA, PCA, and CMA, respectively. (b,d,f,h,j) Subglobose to globose conidia on PDA, OMA, LA-PDA, PCA, and CMA, respectively. Scale bar = 20 μm.

Table 1.
Morphological characteristics of different media and carbon and nitrogen sources on R. sphaeroidea.
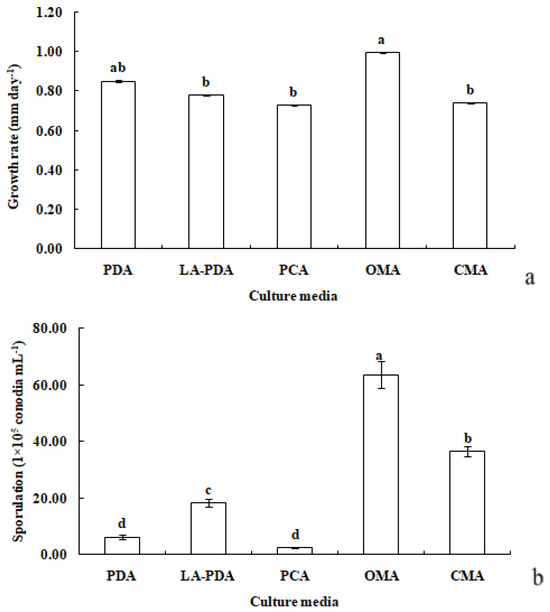
Figure 4.
Growth rate (a) and sporulation (b) of R. sphaeroidea in five types of culture media. The same lowercase letter indicates no significant difference between treatments at p ≤ 0.05, according to Tukey’s HSD.
3.3.2. Carbon Sources
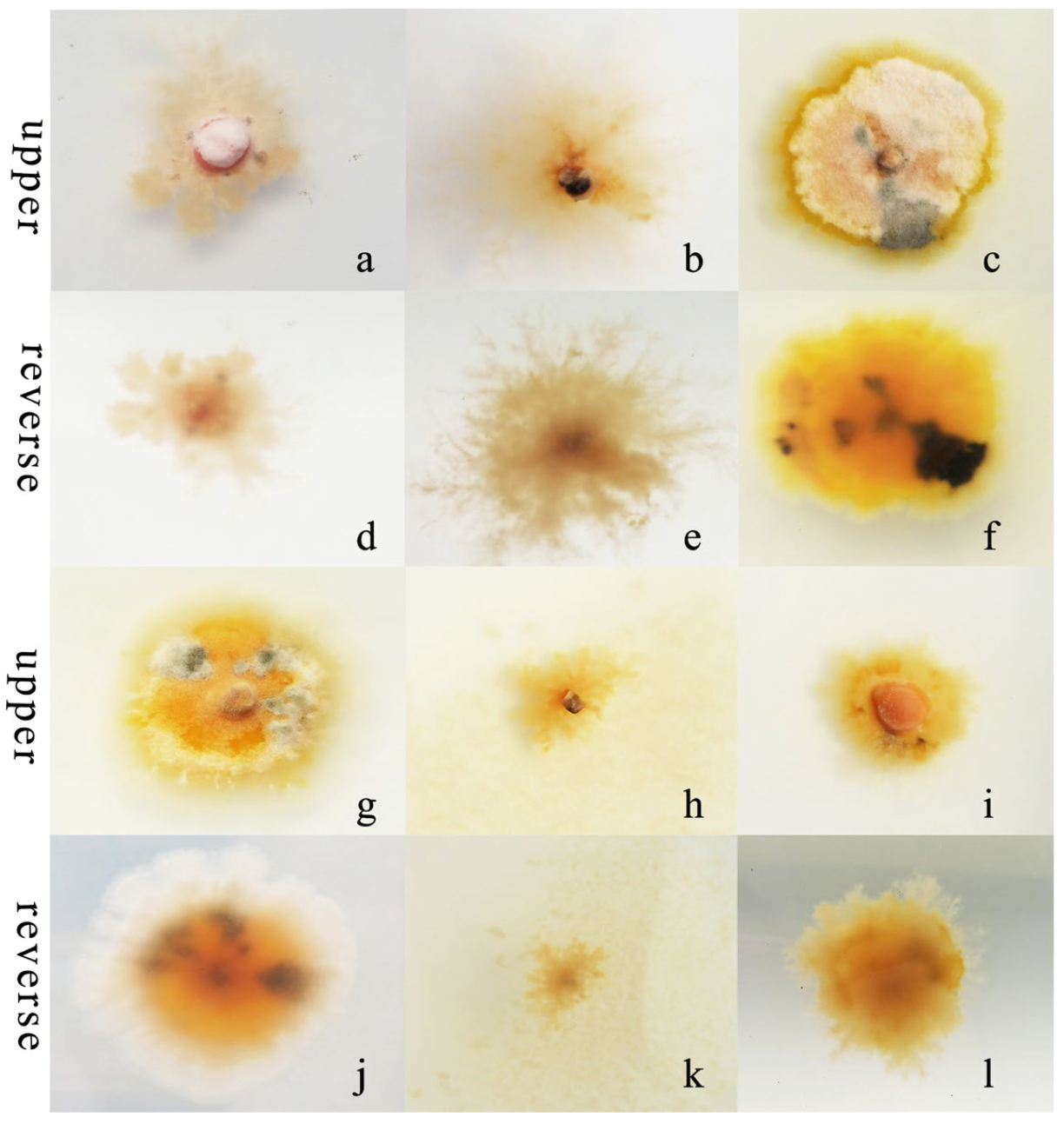
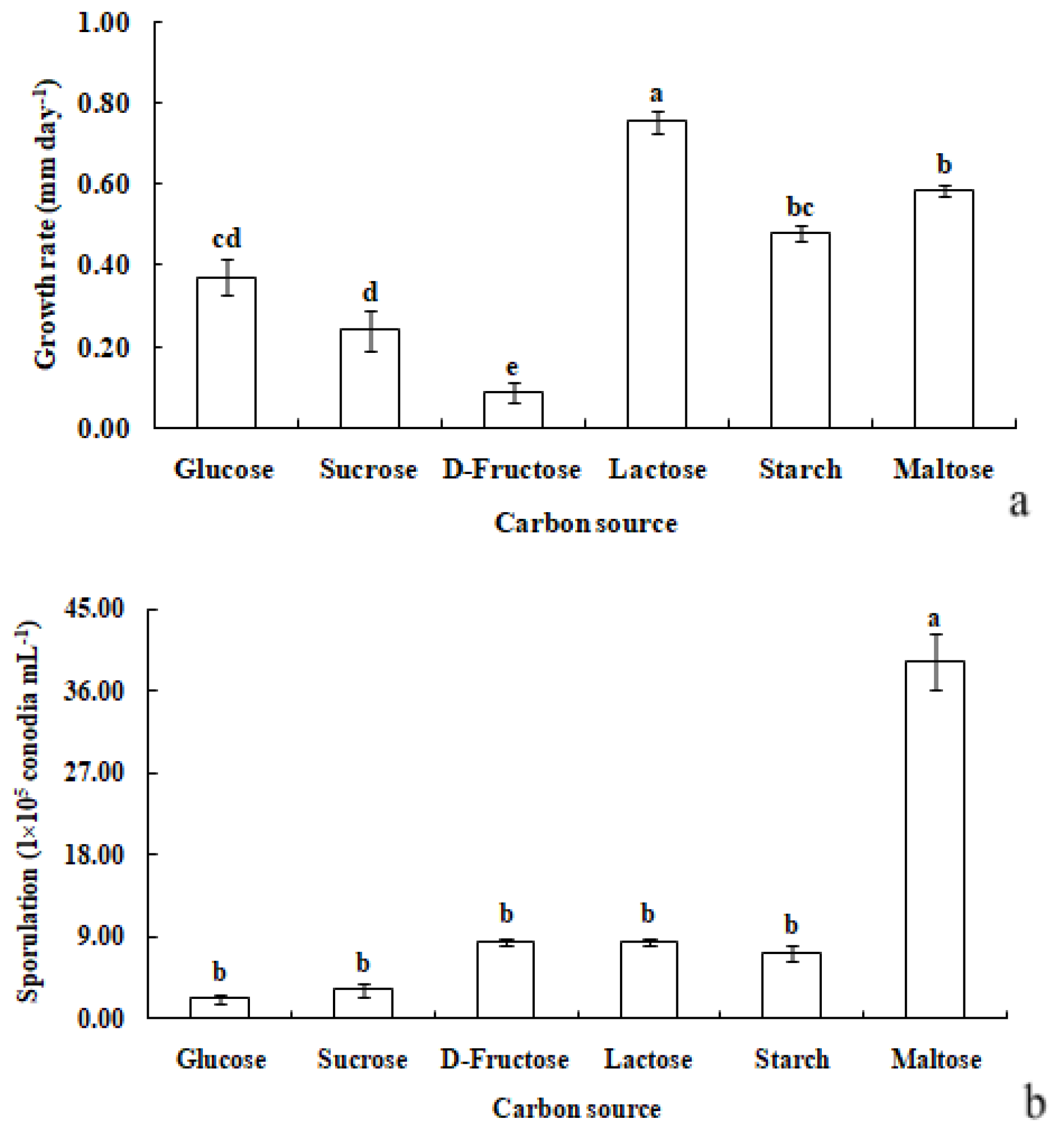
R. sphaeroidea grew and produced spores in all the carbon media (Figure 5). It grew better on lactose and maltose with an average daily growth rate of 0.75 and 0.59 mm, respectively. The average growth rate on starch was 0.48 mm day−1 (Figure 6a). Moreover, the average growth on glucose was 0.37 mm day−1. It had the lowest growth rate on D-fructose of 0.09 mm day−1. No significant difference was observed in its growth on starch, maltose, glucose, and sucrose (Figure 6a). The pathogen sporulated prolifically on maltose, which was followed by lactose, D-fructose, and starch. However, the amount of sporulation was significantly reduced on glucose and sucrose (Figure 6b).
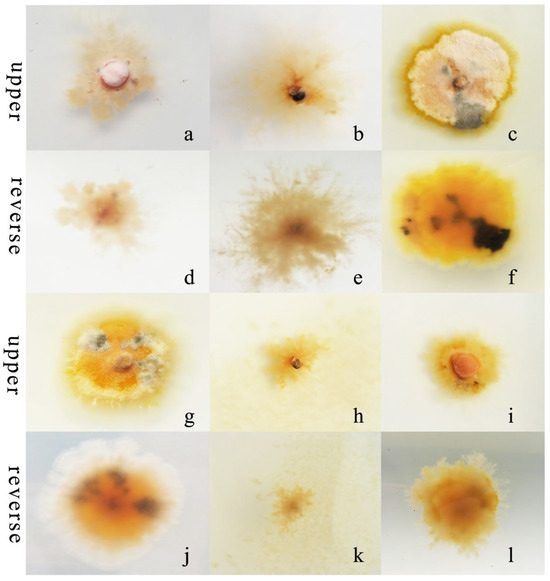
Figure 5.
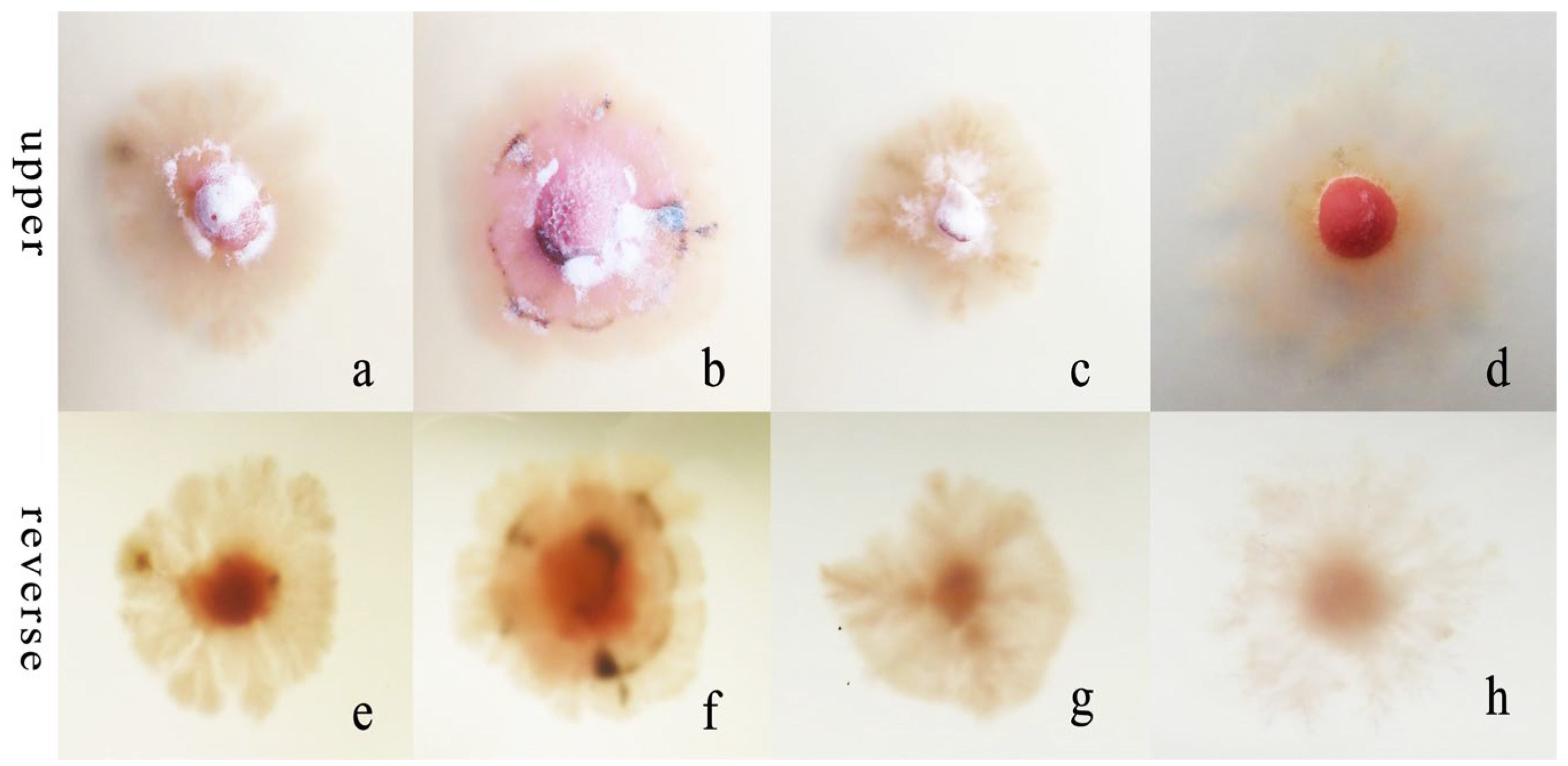
Morphological and cultural traits of R. sphaeroidea. (a–c,g–i) Colony morphology on the upper side of glucose, sucrose, lactose, D-fructose, starch, and maltose, respectively. (d–f,j–l) Colony morphology on the reverse side of glucose, sucrose, lactose, D-fructose, starch, and maltose, respectively.
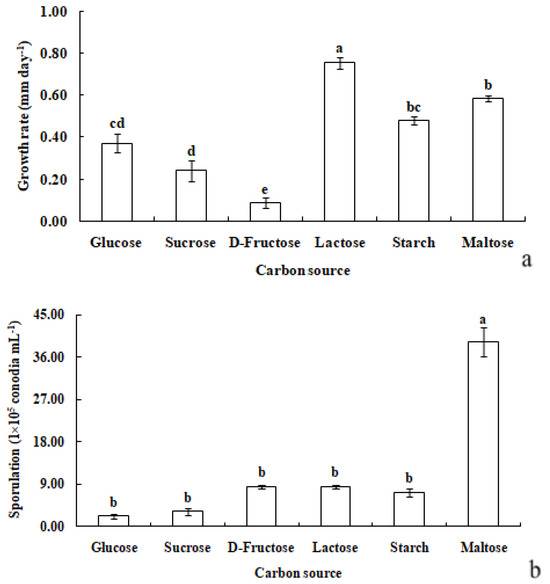
Figure 6.
Growth rate (a) and sporulation (b) of R. sphaeroidea on six types of carbon media. The same lowercase letter indicates no significant difference between treatments at p ≤ 0.05 according to Tukey’s HSD.
3.3.3. Nitrogen Sources
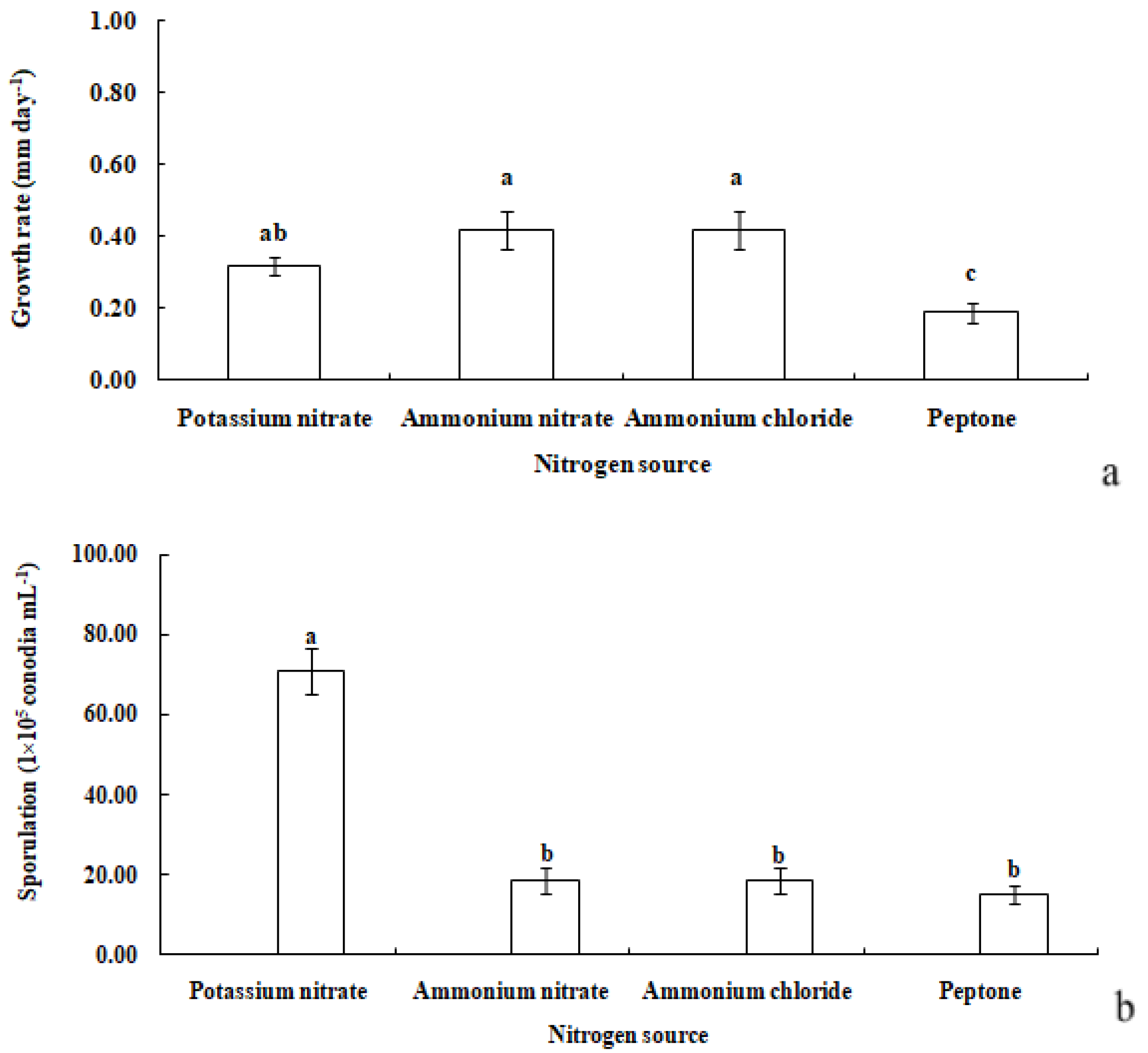
R. sphaeroidea grew and produced spores on four types of nitrogen media (Figure 7). It grew the fastest on ammonium nitrate and ammonium chloride with an average growth rate of 0.42 mm day−1. The pathogen grew the most slowly on peptone with an average of 0.19 mm day−1 (Figure 8a). The colonies sporulated the most heavily on media that contained potassium nitrate followed by ammonium nitrate and ammonium chloride, and the least amount of sporulation was observed on peptone (Figure 8b).
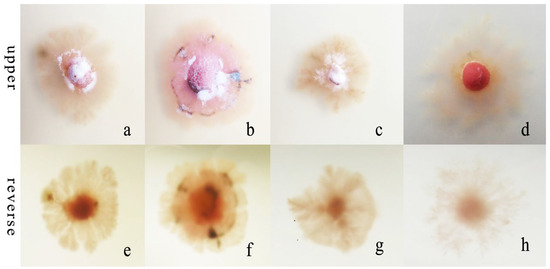
Figure 7.
Morphological and cultural characteristics of R. sphaeroidea. (a–d) Colony morphology on the upper side of media that contained potassium nitrate, ammonium nitrate, ammonium chloride, and peptone, respectively. (e–h) Colony morphology on the reverse side.
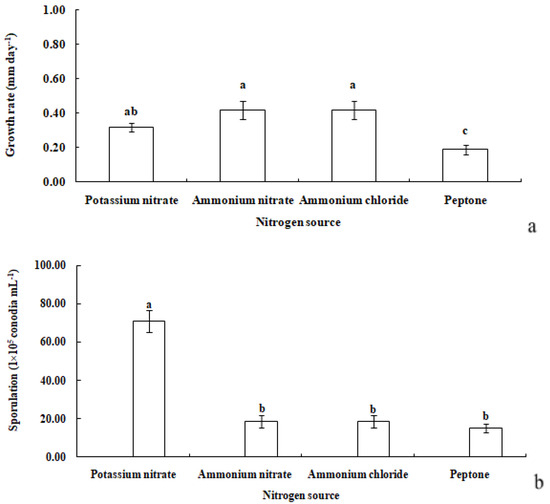
Figure 8.
Growth rate (a) and sporulation (b) of R. sphaeroidea on four types of nitrogen media. The same lowercase letter indicates no significant difference between treatments at p ≤ 0.05 according to Tukey’s HSD.
3.3.4. Effects of Temperature on Conidial Germination
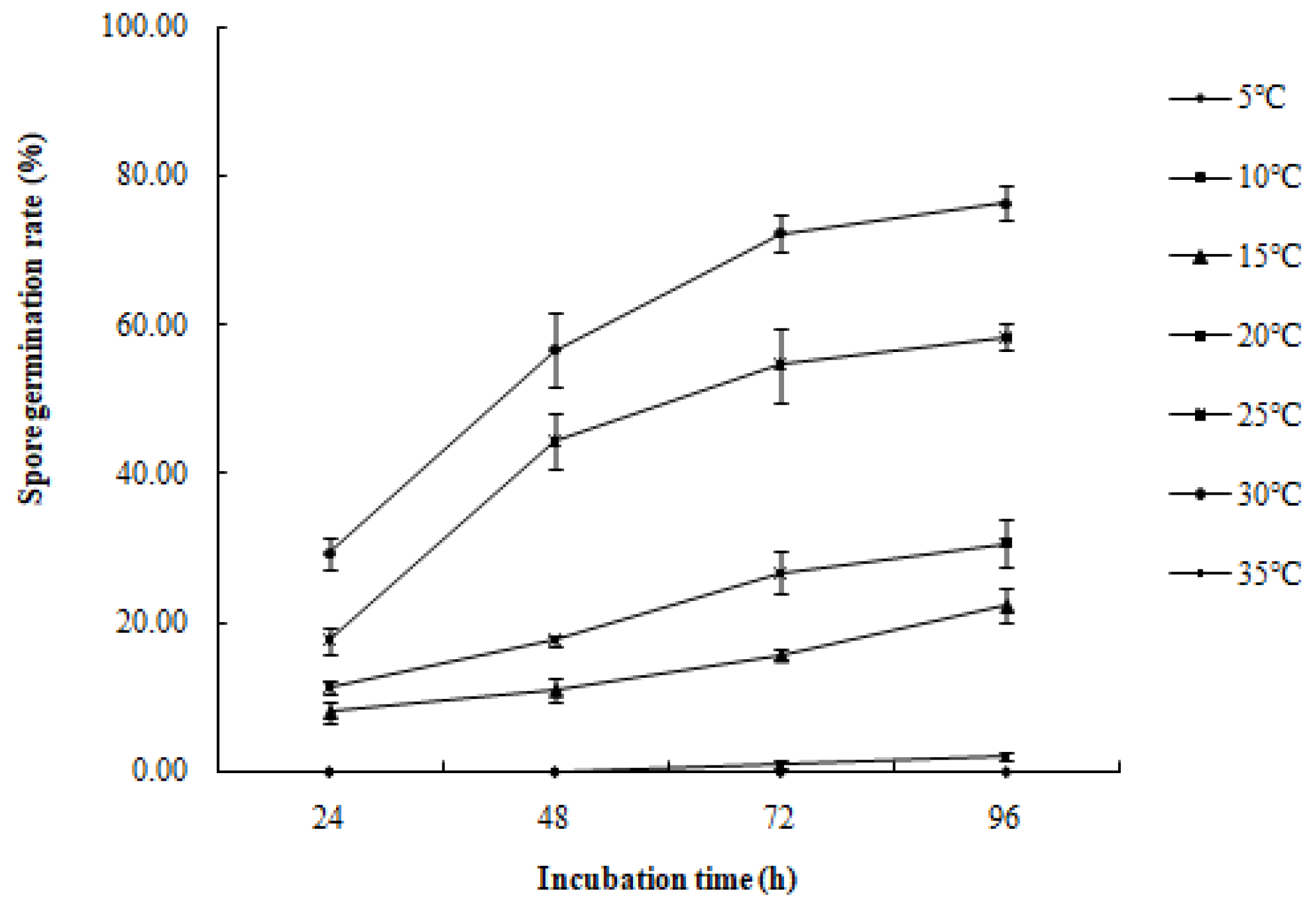
The conidia of R. sphaeroidea cultured on PDA medium germinated in temperatures from 15 to 30 °C (Figure 9). Spore germination occurred at 24 h at 15–30 °C. The spores produce one to three germ tubes after imbibition (Figure 10a). At 48 h, the bud tube was gradually elongated (Figure 10b). After 72 h, the elongated bud tube formed hyphae with one or more septa (Figure 10c). The optimal temperature for conidial germination was 30 °C. At 96 h after incubation, 30 °C resulted in the highest germination percentage (76.33%). Only a few conidia germinated at 10 °C (2.10%), and no conidia germinated at 5 °C and 35 °C (Figure 9).
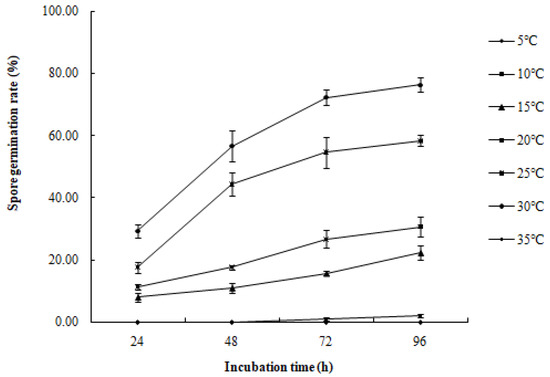
Figure 9.
Effect of temperature on the spore germination rate of R. sphaeroidea.
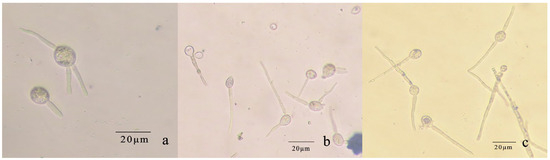
Figure 10.
Conidial germination of R. sphaeroidea. (a) Spores produce 1–3 germ tubes. (b,c) The elongated bud tube formed hyphae with one or more septa.
3.4. Pathogenicity and Host Ranges
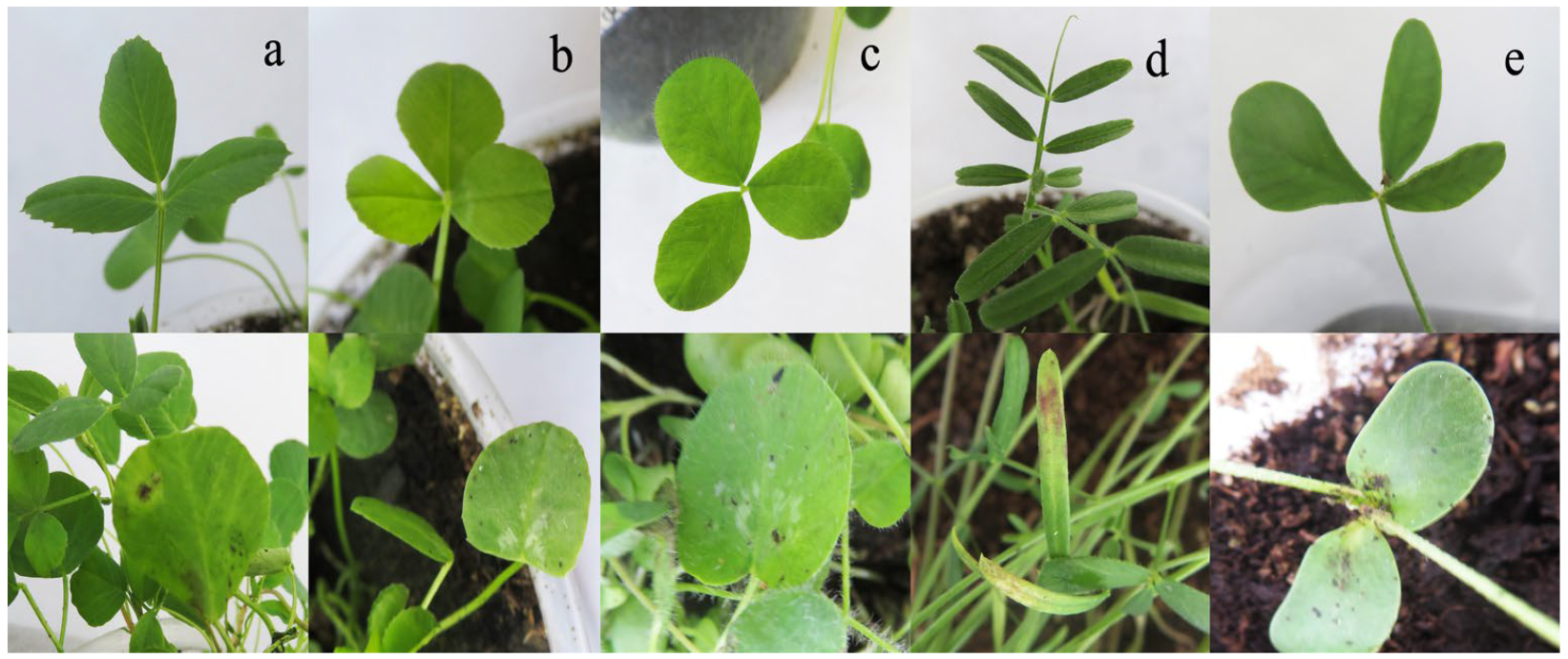
After inoculation with YN1931401, brown to dark brown lesions were observed at the edge of the leaves (Figure 11). Moreover, no symptoms appeared on the control plants. R. sphaeroidea was re-isolated from the inoculated plants. Two weeks after inoculation with R. sphaeroidea, the five perennial legumes, including sainfoin, alfalfa, white clover, red clover, and common vetch, exhibited irregular, brown to dark brown spots on the leaves (Figure 12). Erect milkvetch plants had no apparent symptoms. R. sphaeroidea was successfully reisolated from the chlorotic leaves of sainfoin, alfalfa, white clover, red clover, and common vetch plants, and the control plants remained healthy. R. sphaeroidea was not isolated from the control.

Figure 11.
Hairy vetch leaves inoculated with R. sphaeroidea after 14 days. (a) Control and (b) hairy vetch inoculated with conidia.

Figure 12.
Reaction of five perennial legume plants to R. sphaeroidea with irregular brown to dark brown spots 2 weeks after inoculation. (a) An alfalfa seedling on the lower panel is inoculated with R. sphaeroidea, and the seedling on the upper panel is the control. (b) A white clover seedling with and without inoculation. (c) A red clover seedling with and without inoculation. (d) A common vetch seedling with and without inoculation. (e) A sainfoin seedling with and without inoculation.
3.5. Fungicide Sensitivity Test
Difenoconazole (10%) was the most effective fungicide with an EC50 value of 319.54 mg L−1, and there was a significant difference when compared with the other three fungicides. Furthermore, the EC50 values of chlorothalonil, mancozeb, and difenoconazole were 2743.42, 624.72, and 330.95 mg/L, respectively (Table 2).

Table 2.
Inhibitory effects of different fungicides on R. sphaeroidea.
4. Discussion
This article extends the knowledge of the growth, sporulation, fungicide efficacy, and host range of R. sphaeroidea, which had been previously described on V. fabae and V. sativa in the 19th century [21]. Purple vetch (V. benhalensis) and “Lana” woolly pod vetch (V. villosa sp. varia) in the Salinas Valley (CA, USA) showed a leaf spot disease that was identified as R. sphaeroidea [14]. The identification was based largely on morphological characteristics, such as the distinctive shape of the conidia and colonies. The morphological features of R. sphaeroidea, such as the conidial shape, also clearly differed from those of the other species of Ramularia. The spores of R. sphaeroidea are hyaline, spherical, smooth, aseptate, and 2.13 to 3.67 × 4.56 to 5.77 mm (n = 50). The spores of R. stellenboschensis, R. abscondita, R. trollii, and R. unterseheri are hyaline, thin-walled, smooth, erect, septate, cylindrical-oblong, and unbranched; in contrast, the spores of R. sphaeroidea are spherical [14]. Moreover, the spores of R. sphaeroidea were smaller than those described in the USA, possibly owing to climatic differences. The pathogenicity on hairy vetch was also confirmed, and white colonies were often observed on the lower parts of plants at unfavorable temperatures or relative humidity.
The current identification of Ramularia is based on the nucleotide sequences of specific gene regions [16]. Phylogenetic analyses conducted based on the combination of seven loci, including ITS, LSU, cmdA, gapdh, his3, rpb2, and tef1-α sequences and morphological characteristics, clearly distinguished R. sphaeroidea (YN1931401, YN1931402, and YN1931403) from the other closely related species of Ramularia.
This study describes that R. sphaeroidea grew the fastest on OMA, whereas other studies showed that L-malic acid PDA was the optimal media for the mycelial growth of R. sphaeroidea isolated from vetches [14]. The most obvious colony characteristic of R. sphaeroidea was an irregular bulge with transparent exudates on all media. In the field, after the occurrence of the disease caused by R. sphaeroidea, the leaves turned yellow and fell off prematurely. Studies have shown that pathogens such as Fusarium graminearum produce the mycotoxin deoxynivalenol (DON), which promotes its expansion during infection of its plant host, wheat [56]. Some compounds can even harm livestock. For example, the pathogen Alternaria gansuense causes a systemic disease in a legume and produces swainsonine, an indolizidine alkaloid known as a glycosidase inhibitor. This compound causes an irreversible lysosomal storage disease known as locoism in Western and Northern America, Canada, and China [57]. Further studies should be conducted to determine whether diseased hairy vetch and pure cultures of R. sphaeroidea contain toxic substances that lead to this phenomenon.
Vicia villosa var. glabrescens is a green manure plant that harbors bacteria capable of fixing nitrogen in nodules and spreading over a large area in China [3,9]. Previously, Vicia villosa var. glabrescens, V. benhalensis, V. villosa sp. varia, V. fabae, and V. sativa were recognized as hosts for R. sphaeroidea [14,23]. This study showed that R. sphaeroidea was pathogenic to sainfoin, common vetch, red clover, white clover, and alfalfa when artificially inoculated. These observations indicate that R. sphaeroidea can potentially damage other leguminous plants, emphasizing the importance of incorporating it into field management strategies. Further experiments are required to study the infection cycle of R. sphaeroidea.
Studies have shown that chemical control is one of the most effective and rapid control methods [58]. Difenoconazole is a triazole fungicide with proven bioefficacy against grapevine powdery mildew disease [59]. In this study, difenoconazole (10%) was found to be the most effective fungicide against leaf diseases caused by R. sphaeroidea. The terminal residues of difenoconazole in whole bananas and pulp were 0.45~0.84 mg/kg and 0.19~0.37 mg/kg, respectively, which were lower than the maximum residue level established in China [60].
5. Conclusions
In summary, R. sphaeroidea grew and produced spores on all the media tested in this study and grew best on OMA, lactose, maltose, ammonium nitrate, and ammonium chloride media. R. sphaeroidea can infect other leguminous plants, such as sainfoin, common vetch, red clover, white clover, and alfalfa. The association between R. sphaeroidea and a decline in hairy vetch yield and potential control measures to reduce the disease merit further study. Difenoconazole (10%) was the most effective fungicide to control the leaf spot disease caused by R. sphaeroidea. It can be widely used to control other Ramularia leaf spot diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12040766/s1, Table S1: Collection details and GenBank accession numbers in this study. References [49,50,51,52,53] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, M.S. and Y.-Z.L.; methodology, M.S.; software, M.S.; validation, M.S. and Y.-Z.L.; formal analysis, M.S.; investigation, M.S.; resources, M.S.; data curation, M.S.; writing—original draft preparation, M.S.; writing—review and editing, M.S.; visualization, Y.-Z.L.; supervision, Y.-Z.L.; project administration, Y.-Z.L.; funding acquisition, Y.-Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key R & D Program of China (2022YFD1401103), the National Natural Science Foundation of China (No. 32061123004), the National Forestry and Grassland Administration (20220104), and the Earmarked Fund for CARS (CARS-34).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available in GenBank (https://www.ncbi.nlm.nih.gov/ accessed on 9 March 2022).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, G.X.; He, P.; Ao, X.C.; Liu, Q. Biological characters of Liangshan shiny leaf purple flower vetch (Vicia villosa) and analysis of affecting factors for its seed and forage production. Pratacultural Sci. 2006, 23, 30–34. [Google Scholar] [CrossRef]
- Kissing Kucek, L.; Riday, H.; Rufener, B.P.; Burke, A.N.; Wiering, N.P. Pod Dehiscence in hairy vetch (Vicia villosa Roth). Front. Plant Sci. 2020, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.L.; Tang, L.L.; Xiong, J.L.; Han, Y.; Zhang, Y.R.; Long, M.; Yang, Q.G.; Jao, Y.X. The study on the nutrition and feeding value of smooth vetch. Pratacultural Sci. 2005, 22, 52–56. [Google Scholar]
- Liu, P.; Wang, Y.R.; Liu, Z.P. Karyotypes analysis of 43 Vicia accessions. Pratacultural Sci. 2015, 32, 908–926. [Google Scholar]
- Renzi, J.P.; Chantre, G.R.; Smykal, P.; Presotto, A.D.; Zubiaga, L.; Garayalde, A.F.; Cantamutto, M.A. Diversity of naturalized hairy vetch (Vicia villosa Roth) populations in central argentina as a source of potential adaptive traits for breeding. Front. Plant Sci. 2020, 11, 189. [Google Scholar] [CrossRef]
- Pouryousef, M.; Alizadeh, K. Forage yield improvement at proper ratio and seed density of smooth vetch and barley mix cropping under cold rain-fed conditions. Legume Res. 2014, 37, 402. [Google Scholar] [CrossRef]
- Pramanik, P.; Haque, M.M.; Kim, P.J. Effect of nodule formation in roots of hairy vetch (Vicia villosa) on methane and nitrous oxide emissions during succeeding rice cultivation. Agric. Ecosyst. Environ. 2013, 178, 51–56. [Google Scholar] [CrossRef]
- Wang, T.J.; Lu, Z.Z.; Chen, G. Determination on growth and grazing tolerance of the new strain of Vicia villosa Roth var. glabrescens cv. Liangshan in winter. China Herbiv. Sci. 2013, 33, 34–36. [Google Scholar]
- Gu, L.J.; Duan, T.Y. Current status of research on Vicia villosa var. glabresens as indicated by a bibliometric analysis using the China National Knowledge Infrastructure (CNKI) database. Acta Prataculturae Sin. 2021, 30, 221–228. [Google Scholar]
- Abawi, G.S.; Ludwig, J.W. Host efficiency of 16 cover crops to the lesion nematode. Phytopathology 1995, 1, 1554–1555. [Google Scholar]
- Abawi, G.S.; Widmer, T.L. Impact of soil health management practices on soilborne pathogens, nematodes and root diseases of vegetable crops. Appl. Soil Ecol. 2000, 15, 37–47. [Google Scholar] [CrossRef]
- Sahile, S.; Ahmed, S.; Fininsa, C.; Abang, M.M.; Sakhuja, P.K. Survey of chocolate spot (Botrytis fabae) disease of faba bean (Vicia faba L.) and assessment of factors influencing disease epidemics in Northern Ethiopia. Crop J. 2008, 27, 1457–1463. [Google Scholar] [CrossRef]
- Devi, S.I.; Talukdar, N.C.; Sharma, K.C.; Jeyaram, K.; Rohinikumar, M. Screening of Rhizobacteria for their plant growth promotion ability and antagonism againstdamping off and root rot diseases of broad bean (Vicia faba L.). Indian J. Microbiol. 2011, 51, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Koike, S.T.; Smith, R.F.; Crous, P.W.; Groenewald, J.Z. Leaf and stem spot caused by Ramularia sphaeroidea on purple and lana woollypod vetch (Vicia spp.) cover crops in California. Plant Dis. 2004, 88, 221. [Google Scholar] [CrossRef] [PubMed]
- Nan, Z.B.; Li, C.J. List of forage fungal diseases in China. Lanzhou. Pratacultural Sci. 1994, 11, 36–37. [Google Scholar]
- Videira, S.; Groenewald, J.Z.; Braun, U.; Shin, H.D.; Crous, P.W. All that glitters is not Ramularia. Study Mycol. 2016, 83, 49–163. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Piotrowska, M.J.; Fountaine, J.M.; Gorniak, K.; Grann, G.R.D.; Armstrong, A.; Havis, N.D. Infection strategy of Ramularia collo-cygni and development of ramularia leaf spot on barley and alternative graminaceous hosts. Plant Pathol. 2017, 66, 45–55. [Google Scholar] [CrossRef]
- Walters, D.R.; Havis, N.D.; Oxley, S.J.P. Ramularia collo-cygni: The biology of an emerging pathogen of barley. FEMS Microbiol. Lett. 2008, 279, 1–7. [Google Scholar] [CrossRef]
- Cromey, M.G.; Harvey, I.C.; Sheridan, J.E.; Grbavac, N. Occurrence, importance and control of Ramularia collo-cygni in New Zealand. In Proceedings of the Second International Workshop Barley Leaf Blights, Aleppo, Syria, 7–11 April 2002; pp. 7–11. [Google Scholar]
- Nyman, M.; Havis, N.D.; Oxley, S.J.P. Importance of seed-borne infection of Ramularia Collo-Cygni. Asp. Appl. Biol. 2009, 92, 91–96. [Google Scholar]
- Braun, U. A monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes). Cryptogam. Mycol. 1995, 21, 131–132. [Google Scholar] [CrossRef]
- Zhang, Z.X. Flora Fungorum Sinicorum; China Press: Beijing, China, 2006. [Google Scholar]
- Shi, M.; Li, Y.Z. First report of leaf spot caused by Ramularia sphaeroidea on Vicia villosa var. glabrescens in China. Plant Dis. 2021, 105, 4159. [Google Scholar] [CrossRef] [PubMed]
- Adour, L.; Couriol, C.; Amrane, A.; Prigent, Y. Carbon and nitrogen yields during batch cultures of Geotrichum candidum and Penicillium camembertii. Process Biochem. 2004, 39, 1449–1454. [Google Scholar] [CrossRef]
- Oritsejafor, J.J. Carbon and nitrogen nutrition in relation to growth and sporulation of Fusarium oxysporum f.sp. elaeidis. Trans. Br. Mycol. Soc. 1986, 87, 519–524. [Google Scholar] [CrossRef]
- Li, D.P.; Holdom, D.G. Effects of nutrients on colony formation, growth, and sporulation of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes). J. Invertebr. Pathol. 1995, 65, 253–260. [Google Scholar] [CrossRef]
- Engelkes, C.A.; Nuclo, R.L.; Fravel, D.R. Effect of carbon, nitrogen, and C:N ratio on growth, sporulation, and biocontrol efficacy of Talaromyces flavus. Phytopathology 1997, 87, 500. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, S.I.; Morrison, S.M.; Boyd, W.L. Nutritional requirements for growth and sporulation of Clostridium perfringens. J. Appl. Bacteriol. 2010, 38, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Safavi, S.A.; Shah, F.A.; Pakdel, A.K.; Reza, R.G.; Bandani, A.R.; Butt, T.M. Effect of nutrition on growth andvirulence of the entomopathogenic fungus Beauveria bassiana. FEMS Microbiol. Lett. 2010, 270, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Wang, C.S.; Butt, T.M. Nutrition influences growth and virulence of the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol. Lett. 2010, 2, 259–266. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. Former. Pestic. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef]
- Fountaine, J.M.; Fraaije, B.A. Development of QoI resistant alleles in populations of Ramularia collo-cygni. Asp. Appl. Biol. 2009, 92, 123–126. [Google Scholar]
- Pereyra, S.A.; Viera, J.P.; Havis, N.D. Managing Ramularia leaf spot of barley in Uruguay. Phytopathology 2014, 104, 91. [Google Scholar]
- Li, Y.Z.; Nan, Z.B. The Methods of Diagnose, Investigation and Loss Evaluation for Forage Diseases; Jiangsu Phoenix Science and Technology Press: Nan Jing, China, 2015. [Google Scholar]
- Li, Y.Z. Studies of Yellow Stunt and Root Rot of Standing Milk-Vetch (Astragalus adsuegens Pall.); Lanzhou University: Lanzhou, China, 2007. [Google Scholar]
- Crous, P.; Summerell, B.; Carnegie, A.; Wingfield, M.; Groenewald, J. Novel species of Mycosphaerellaceae and Teratosphaeriaceae. Persoonia-Mol. Phylogeny Evol. Fungi 2009, 23, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; van den Ende, A.H.G.G. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 1998, 41, 183–189. [Google Scholar] [CrossRef] [PubMed]
- White, T.J. A Guide to Methods and Applications. PCR Protoc. 1990, 1, 315. [Google Scholar]
- Carbone, L.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Videira, S.; Groenewald, J.Z.; Kolecka, A.; Haren, L.V.; Boekhout, T.; Crous, P.W. Elucidating the Ramularia eucalypti species complex. Persoonia 2015, 34, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Wingfield, M.J.; Mansilla, J.P.; Alfenas, A.C.; Groenewald, J.Z. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Study Mycol. 2006, 55, 99–131. [Google Scholar] [CrossRef]
- Crous, P.W.; Schoch, C.L.; Hyde, K.D.; Wood, A.R.; Gueidan, C.; Hoog, G.D.; Groenewald, J.Z. Phylogenetic lineages in the Capnodiales. Study Mycol. 2009, 64, 17–47. [Google Scholar] [CrossRef]
- Berbee, M.L.; Hubbard, M.P. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 1999, 91, 964–977. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. Psychiatr. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.H.; Sung, J.M.; Jones, H.; Spatafora, J.W. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenetics Evol. 2007, 44, 1204–1223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Nan, Z.B. Nutritional study on Embellisia astragali, a fungal pathogen of milk vetch (Astragalus adsurgens). Antonie Van Leeuwenhoek 2009, 95, 275–284. [Google Scholar] [CrossRef]
- Videira, S.I.R.; Groenewald, J.Z.; Verkley, G.J.M.; Braun, U.; Crous, P.W. The rise of Ramularia from the Mycosphaerella labyrinth. Fungal Biol. 2015, 119, 823–843. [Google Scholar] [CrossRef] [PubMed]
- Verkley, G.J.; Crous, P.W.; Groenewald, J.E.; Braun, U.; Aptroot, A. Mycosphaerella punctiformis revisited: Morphology, phylogeny, and epitypification of the type species of the genus Mycosphaerella (Dothideales, Ascomycota). Mycol. Res. 2004, 108, 1271–1282. [Google Scholar] [CrossRef]
- Simon, U.K.; Groenewald, J.Z.; Crous, P.W. Cymadothea trifolii, an obligate biotrophic leaf parasite of Trifolium, belongs to Mycosphaerellaceae as shown by nuclear ribosomal DNA analyses. Persoonia-Mol. Phylogeny Evol. Fungi 2009, 22, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Verkley, G.J.M.; Quaedvlieg, W.; Shin, H.D.; Crous, P.W. A new approach to species delimitation in Septoria. Stud. Mycol. 2013, 75, 213–305. [Google Scholar] [CrossRef]
- Batzer, J.C.; Gleason, M.L.; Harrington, T.C.; Tiffany, L.H. Expansion of the sooty blotch and flyspeck complex on apples based on analysis of ribosomal DNA gene sequences and morphology. Mycologia 2005, 97, 1268–1286. [Google Scholar] [CrossRef]
- Wei, W.T. Indoor toxicity measurement of five fungicides on gummy stem blight from netted malon. Guizhou Agric. Sci. 2013, 41, 88–90. [Google Scholar]
- Esra, G.Ü.L.; Karatas, Z.; Karakaya, A. Evaluation of the fungicide resistance of gray mold (Botrytis cinerea) in tomatoes to boscalid and pyraclostrobin in greenhouse areas of Turkey. J. Agric. Sci. 2021, 31, 487–493. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, L.; Wang, M.; Li, Y.; Jian, Y.; Wu, L.; Kistler, H.C.; Ma, Z.; Yin, Y. Plant defense compound triggers mycotoxin synthesis by regulating H2B ub1 and H3K4 me2/3 deposition. New Phytol. 2021, 232, 2106–2123. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Li, Y.Z. Alternaria gansuense, a plant systematic fungal pathogen producing swainsonine in vivo and in vitro. Curr. Microbiol. 2023, 80, 232. [Google Scholar] [CrossRef] [PubMed]
- Saeed, E.E.; Sham, A.; El-Tarabily, K.; Abu Elsamen, F.; Iratni, R.; AbuQamar, S.F. Chemical control of black scorch disease on date palm caused by the fungal pathogen Thielaviopsis punctulata in United Arab Emirates. Plant Dis. 2016, 100, 2370–2376. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Oulkar, D.P.; Patil, S.H.; Dasgupta, S.; Adsule, P.G. Degradation kinetics and safety evaluation of tetraconazole and difenoconazole residues in grape. Pest Manag. Sci. 2008, 64, 283–289. [Google Scholar] [CrossRef]
- Zheng, Q.; Qin, D.; Yang, L.; Liu, B.; Lin, S.; Ma, Q.; Zhang, Z. Dissipation and distribution of difenoconazole in bananas and a risk assessment of dietary intake. Environ. Sci. Pollut. Res. 2020, 27, 15365–15374. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).