Abstract
A cross-sectional study was conducted to assess the prevalence, molecular detection, and antimicrobial resistance of Salmonella isolates within 162 poultry farms in selected urban and peri-urban areas of central Ethiopia. A total of 1515 samples, including cloacal swabs (n = 763), fresh fecal droppings (n = 188), litter (n = 188), feed (n = 188), and water (n = 188), were bacteriologically tested. The molecular detection of some culture-positive isolates was performed via polymerase chain reaction (PCR) by targeting spy and sdfl genes for Salmonella Typhimurium and Salmonella Enteritidis, respectively. Risk factors for the occurrence of the bacterial isolates were assessed. Antimicrobial susceptibility testing of PCR-confirmed Salmonella isolates was conducted using 12 antibiotics. In this study, it was observed that 50.6% of the farms were positive for Salmonella. The overall sample-level prevalence of Salmonella was 14.4%. Among the analyzed risk factors, the type of production, breed, and sample type demonstrated a statistically significant association (p < 0.05) with the bacteriological prevalence of Salmonella. The PCR test disclosed that 45.5% (15/33) and 23.3% (10/43) of the isolates were positive for genes of Salmonella Typhimurium and Salmonella Enteritidis, respectively. The antimicrobial susceptibility test disclosed multi-drug resistance to ten of the tested antibiotics that belong to different classes. Substantial isolation of Salmonella Typhimurium and Salmonella Enteritidis in poultry and on poultry farms, along with the existence of multi-drug resistant isolates, poses an alarming risk of zoonotic and food safety issues. Hence, routine flock testing, farm surveillance, biosecurity intervention, stringent antimicrobial use regulations, and policy support for the sector are highly needed.
1. Introduction
The poultry sector in Ethiopia is undergoing significant changes due to the growing human population, particularly in and around major cities and towns [1]. Poultry production plays a crucial role as a livelihood source, ensuring food security, promoting nutrition, and contributing to the economic development of the country. The sector is deeply integrated in Ethiopian society, with poultry farming being practiced by nearly every household in both rural and urban areas [2,3,4].
The considerable productivity potential of the poultry sector in Ethiopia faces several constraints, including reliance on traditional technologies, a high prevalence of diseases, insufficient availability of inputs (such as good quality feed and veterinary drugs), the limited genetic potential of breeds, and suboptimal management practices [5,6]. In Ethiopia, infectious diseases such as Newcastle disease, Salmonellosis, fowl cholera, coccidiosis, and fowl pox were identified as predominant contributors to high morbidity and mortality across various scales of poultry production [7,8,9,10].
Salmonellosis is one of the most important bacterial diseases in the poultry industry and other avian species, causing heavy economic loss due to lowered productivity and also causing a public health hazard by virtue of zoonoses, which is associated with high medication costs [9,11,12]. Avian Salmonellosis occurs in chickens by host-specific Salmonella serovars, such as Salmonella Pullorum and Salmonella Gallinarum, which cause a typhoid-like systemic disease, or a wide range of non-typhoidal Salmonella, mainly Salmonella Enteritidis and Salmonella Typhimurium, together with serovars such as S. Newport, S. Heidelberg, S. Kentucky, S. Infantis, S. Concord, S. Javiana, etc. [13,14,15,16]. Non-typhoidal Salmonellosis is responsible for undetected illness at the farm level, and following the consumption of poultry meat and eggs, humans acquire infection at the plate end. In particular, the non-typhoidal species Salmonella Typhimurium and Salmonella Enteritidis are responsible for subclinical Salmonellosis in chickens that can induce human infections [17,18,19,20]. Accordingly, it was estimated that non-typhoidal Salmonellosis causes about 93 million enteric infections and 155,000 fatalities worldwide on an annual basis [21,22]. The non-host specificity of the pathogen, its route of transmission, and the existence of multiple antimicrobial resistances are the main reasons contributing to the majority of non-typhoidal Salmonellosis infections [23]. In developing countries of Africa with poor hygiene, weak biosecurity measures, and no or few food safety regulations, prevailing non-typhoidal Salmonellosis remains a serious public health problem [24,25,26], with an occurrence of 10–100/100,000 new cases per year [27]. In Ethiopia, human Salmonellosis is one of the major diseases. For instance, a pooled prevalence of 57.9% was recorded for non-typhoidal Salmonella [25]. Previous studies in Ethiopia also indicated that different Salmonella serovars have been detected in various regions of the country and have been isolated from humans, animals, food of animal origin, and their environment [10,28,29,30]. In Ethiopia, as well as Sub-Saharan African countries, the problem of Salmonellosis in poultry, as well as humans, is exacerbated by little or no national epidemiological surveillance, a lack of legislation, and an absence of strict enforcement of regulations and intervention measures to address farm biosecurity practices, public hygiene, and regular screening of individuals handling foodstuffs for public consumption [25,31].
In the past, as well as in the present, poultry Salmonellosis has been prevented and controlled by the use of various types of antimicrobials. Unfortunately, there is an increasing trend in the utilization of antimicrobial drugs for animal production to meet the rising demands for animal-derived products by the human population. For instance, the quantity of antimicrobials utilized is anticipated to double in the BRICS countries, encompassing Brazil, Russia, India, China, and South Africa [32]. Quantitatively, the number of antimicrobials used in the livestock sector worldwide was predicted to be 63,151 tons in 2010, and it is estimated to increase by 67% by 2030, attaining nearly 105,500 tons [33].
The emergence of antimicrobial resistance (AMR) can be attributed to an irrational use of first-line drugs and extensive use of antimicrobial drugs coupled with increased consumption of animal products. Previous studies have underlined the potential horizontal dissemination of AMR bacteria and genes between poultry flocks and farms, as well as the extent of zoonotic transmission through the food value chain [34,35,36,37]. Consequently, in the developed world, such as America and Europe, the majority of Salmonella isolates from poultry farms and poultry products were found to be resistant to several antimicrobials [38,39,40,41]. However, in Ethiopia, such consolidated findings are lacking, and thus, further studies are needed.
Despite the contribution of the poultry sector to the national economy of Ethiopia, inadequate and fragmented information is available about the true prevalence, distribution, economics, public health significance, and antimicrobial resistance profiles of the zoonotic Salmonella serovars Salmonella Enteritidis and Salmonella Typhimurium in the poultry sector. Having adequate data will contribute to instituting Salmonella control programs that ensure poultry health, combatting the risk of foodborne zoonotic infections, and minimizing the escalating risk of antimicrobial-resistant Salmonella in poultry farming. Therefore, the present study aimed at monitoring the Salmonella Enteritidis and Salmonella Typhimurium status in poultry in central Ethiopia, a poultry-dense region characterized by a high variety of production systems. More specifically, the aim of this research was to evaluate the prevalence and AMR profiles of Salmonella Enteritidis and Salmonella Typhimurium in chickens and environmental samples collected at poultry farms in four selected areas of central Ethiopia.
2. Materials and Methods
2.1. Study Area
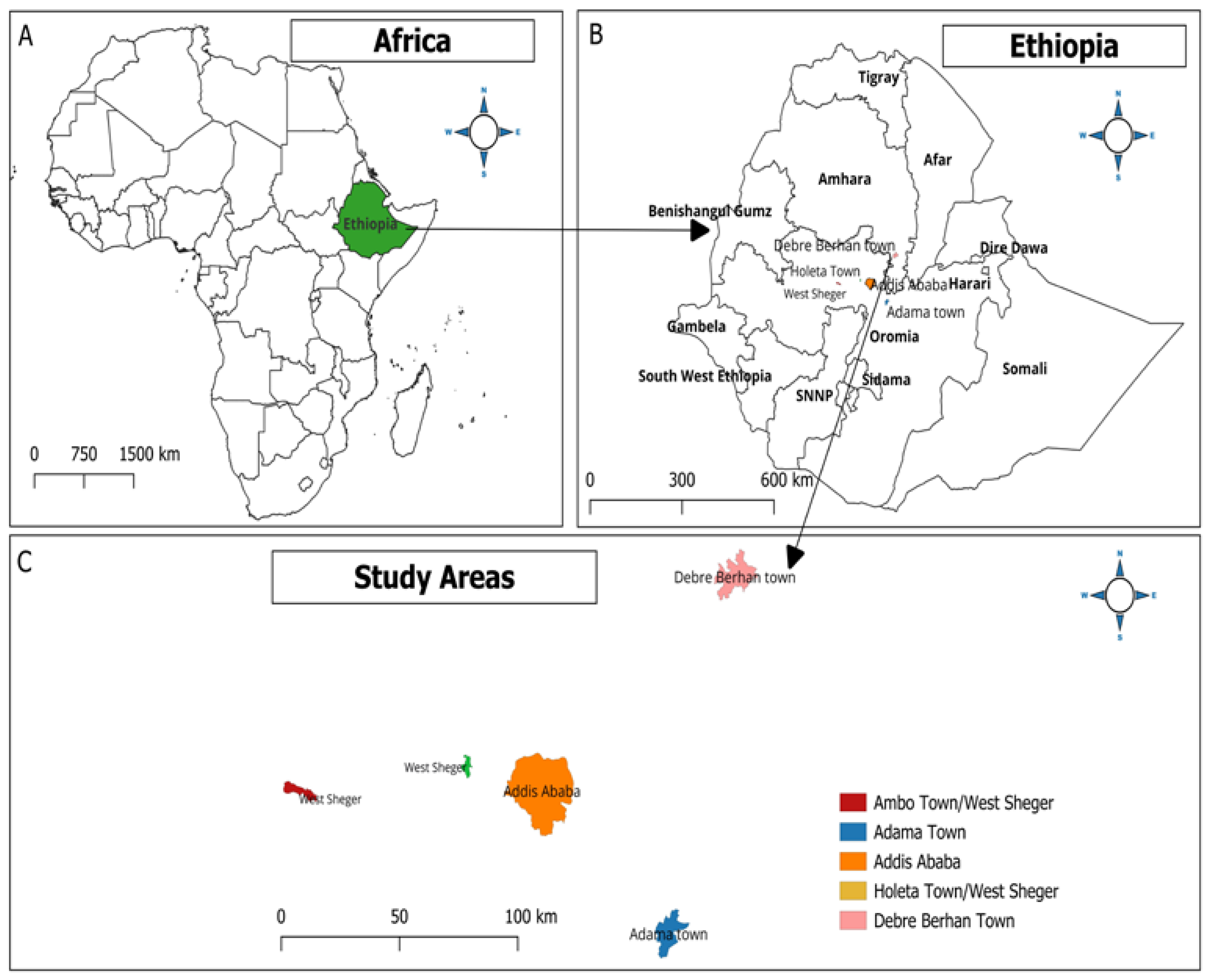
The present study was conducted in four selected areas of central Ethiopia, namely Adama, Addis Ababa, Debre Birhan, and West of Shaggar City, located within a 130 km of the capital city, Addis Ababa (Figure 1). In all the study areas, climate conditions have a bimodal rainfall trend comprising a long rainy season (June to September), a short rainy season (February to May), and a prolonged dry season from late November to February [42].

Figure 1.
Map showing the study areas using QGIS Ver. 3.14 (QGIS Development Team, 2009. QGIS Geographic Information System. Open-Source Geospatial Foundation. http://qgis.org (accessed on 19 February 2024).
Livestock production in the study areas was characterized by mixed farming systems, with poultry, dairy, and small ruminant farming being integrated with certain industrial manufacturing activities as the main source of income. These selected areas were representative of commercial poultry production practices in typical highly populated urban areas and surrounding peri-urban communities. The present study areas were considered purposively due to the availability of a high number of commercial poultry farms as well as high demand for poultry and poultry products.
2.2. Study Design
A cross-sectional study was conducted from January 2021 to June 2022 in four selected areas of central Ethiopia. Exotic breed chickens reared for the purpose of egg production (Bovans Brown) and meat production (Cobb-500) and, to some extent, mixed types (Saso) maintained under an intensive management system with a deep litter housing system were considered as the study population. With regard to the Saso breed, in some study areas, they are kept as layers while in others as broilers. About ten percent of the poultry farms had a history of Salmonella vaccination and were thus purposively excluded from this study. The study targeted only medium-sized farms, with between 1000 and 5000 chickens per farm.
2.3. Sample Size Determination and Sampling Strategies
A total of 1515 samples were collected, including cloacal swabs, fresh fecal droppings, litter samples, and feed and drinking water. The repartition of the different samples collected in the respective study areas is illustrated in Table 1.

where

Table 1.
Distribution of sample types and number.
Table 1.
Distribution of sample types and number.
| Study Areas | Sample Type and Number | * p (Expected Prevalence) | |||||
|---|---|---|---|---|---|---|---|
| Cloacal Swabs | Fresh Fecal Droppings | Litter | Feed | Water | Total | ||
| Adama | 260 | 30 | 30 | 30 | 30 | 380 | 28.8% [30] |
| Addis Ababa | 167 | 56 | 56 | 56 | 56 | 391 | 16.5% [28] |
| Debre Birhan | 176 | 52 | 52 | 52 | 52 | 384 | 50% (No previous work) |
| West of Shaggar City | 160 | 50 | 50 | 50 | 50 | 360 | 19% [43] |
| Total | 763 | 188 | 188 | 188 | 188 | 1515 | |
* For all the study areas, the sample size was calculated taking into account expected prevalence (as shown in Table 1), 95% confidence interval, and 5% absolute precision as per the formula given by Thrusfield [44]. However, in order to increase precision, additional samples were added.
N = (Zα/2)2 × P(1 − P)
d2
d2
N = sample size required;
d = absolute precision (0.05);
P = expected prevalence.
2.4. Sampling
Samples were collected according to the recommendations of OIE [45]. A total of 162 poultry farms from the four selected study sites were considered. More specifically, per poultry house, one sample of 25 g pooled fresh fecal droppings (at least from three droppings taken randomly in different locations in the house) was collected with a sterile spatula into the sterile polypropylene tube. Pooled cloacal swabs (of at least three chickens per house) were collected from randomly selected birds using sterile cotton-tipped swabs. Cotton swabs were moistened in buffered peptone water solution before being inserted in the cloaca by gentle rotation in the cloaca of the birds. Immediately following collection, the cloacal swabs were pooled in 10 mL of sterile buffered peptone water, properly plugged and shaken within test tubes. About 25 g of pooled feed and 100 mL of pooled drinking water samples were collected, respectively, from randomly selected chicken feeders and drinkers throughout the poultry house. Furthermore, pooled five-litter samples weighing 5 g each from different sides on the floor of a poultry house were collected using sterile gloves. Collected samples were transported and stored at 4 °C and were either immediately processed upon arrival at the laboratory or the day after.
2.5. Isolation and Identification of Salmonella
Salmonella was isolated and identified according to standardized protocols described by the International Organization for Standardization for Salmonella detection in food and animal feedstuffs ISO 6579 and OIE [45,46]. All sample types were pre-enriched in 225 mL of Buffered Peptone Water (BPW) (1:9) and homogenized by vortexing for two minutes. Then, all pre-enriched samples were incubated at 37 °C for 18–24 h [47,48]. Subsequently, for selective enrichment, 0.1 mL of well-vortexed pre-enrichment sample was inoculated in 10 mL of Rappaport Vassiliadis Soya Peptone (RVS) broth and incubated at 37 °C for 18–24 h [45].
Afterward, incubation of selective/differential culture was performed by streaking a loopful of suspension from the edge of the turbid growth zone onto Xylose Lysine Deoxycholate (XLD) agar and incubating at 37 °C for 24 to 48 h. After incubation, presumptive Salmonella colonies were purified on XLD agar. However, agar plates were incubated for a further 24 h and reexamined if typical Salmonella colonies were not present. Salmonella colonies on XLD were morphologically identified as red colonies with black centers. Typical Salmonella colonies were confirmed through six biochemical tests, more specifically Triple Sugar Iron Agar (TSI), Citrate Utilization, Indole, Methyl-Red and Voges–Proskauer (MR-VP) and Lysine Decarboxylation tests [49,50,51,52,53]. These Salmonella broth cultures were sub-cultured at two-week intervals adhering to similar procedures until molecular analysis was performed [53].
2.6. Molecular Detection of Salmonella Enteritidis and Typhimurium
The biochemically confirmed Salmonella isolates were further characterized as Salmonella Enteritidis or Salmonella Typhimurium by molecular detection of the sdfI gene [54] or spy gene [55], respectively. Nearly 50% of randomly selected biochemically positive samples from Adama and Debre Birhan areas were subjected to molecular tests. However, molecular confirmation was only performed on Salmonella isolates of Adama and Debre Birhan regions.
2.6.1. DNA Extraction
Extraction of DNA was carried out from Tryptic Soy Broth (Becton Dickinson GmbH, Heidelberg, Germany) and TSB sub-cultured Salmonella using a DNeasy Blood and Tissue extraction kit (Qiagen, Dusseldorf, Germany) as per the instructions provided by the manufacturer.
2.6.2. Polymerase Chain Reaction (PCR)
The Polymerase Chain Reaction (PCR) was conducted using a thermal cycler (Applied Bio-systems; Genetic Systems Company, Watsonville, CA, USA) for amplification of the Salmonella Typhimurium specific gene (spy) with an amplicon size of 401 bp and the Salmonella Enteritidis specific gene (sdfl) with an amplicon size of 304 bp. The forward and reverse primers set for the spy gene were 3′- TTA TTC ACT TTT TAC CCC TGA A- 5′ and 5′- CCC TGA CAG CCG TTA GAT ATT- 3′, respectively. Similarly, for the sdfl gene, the primer sets were 3′- TGTGTTTTATCTGATGCAAGAGG- 5′forward and 5′- TGAACTACGTTCGTTCTTCTGG- 3′ reverse. The PCR reaction was standardized in a final volume of 20 µL containing nuclease-free water (3 µL), 5 pmol/µL each forward and reverse primers (2 µL for each), I Q TM supper mix (10 µL) (Bio-Rad Laboratories, Marnes-la-Coquette, France) containing (Taq DNA polymerase, dNTPs, MgCl2 and PCR buffer) and DNA template (3 µL). Likewise, positive control (Salmonella positive), extraction control (devoid of template DNA), and negative control (nuclease-free water) were also prepared.
DNA amplification was conducted according to the following reaction conditions: an initial denaturation step at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 52 °C for 40 s, and extension at 72 °C for 30 s with 7 min final extension at 72 °C and holding temperature at 4 °C until gel electrophoresis is performed.
2.6.3. Gel Electrophoresis DNA Band Visualization
After amplification, the PCR fragments were checked using agarose gel electrophoresis and visualized using UV light. Before gel electrophoresis, agarose gel (1.5%), loading dyes, and molecular markers (100-bp) were prepared based on the manufacturer’s recommendation. The total volume of 10 µL mixture of PCR products and loading dye was loaded on 1.5% agarose gel wells, which were prepared from 1% TAE Buffer (Tris-acetate-EDTA) and agarose powder. Similarly, samples, positive and negative control (10 µL each), and molecular ladders (10 µL, 100 bp) were gently loaded on the separate agarose gel wells (lane). Consequently, the amplified DNA product was electrophoresed at 120 volts for one hour. The migration of DNA bands from the agarose gel was visualized using a UV gel documentation apparatus. The amplicons (bands) size of around 401 bp of the target gene (spy) for Salmonella Typhimurium and 304 bp of the target gene (sdfI) for Salmonella Enteritidis were visualized and captured on a UV transluminator. The presence of visible bands at or around the expected size was considered positive, whereas the absence of bands at the expected size was considered negative.
2.7. Antimicrobial Susceptibility Test
The disc-diffusion method was employed for antimicrobial susceptibility testing of 10 and 15 PCR-confirmed Salmonella Typhimurium and Salmonella Enteritidis isolates obtained from Adama and Debre Birhan areas, respectively. Based on the recommendations of the International Committee for Clinical Laboratory Standards [56]. This test was performed on Muller Hinton agar medium. Antimicrobial susceptibility was tested for twelve different antimicrobials, namely ampicillin (AMP: 10 µg), azithromycin (AZM: 15 µg), ceftazidime (CAZ: 30 µg), ciprofloxacin (CIP: 5 µg), chloramphenicol (CHL: 30 µg), erythromycin (ERT), gentamycin (GET: 10 µg), kanamycin (KAN: 30 µg), nalidixic acid (NAL), oxytetracycline (OXT: 30 µg), sulfamethoxazole/trimethoprim (SXT: 25 µg) and tetracycline (TET: 30 µg). Antimicrobials were chosen based on their widespread use for the treatment and/or prevention of Salmonella infection in livestock production and human health, as well as their accessibility in local markets [28,57,58]. Of each isolate, four to five well-isolated colonies were transferred with the sterile loop into tubes containing 5 mL of Tryptone soya broth (OXOID, CM129, Oxoid Limited, Hampshire, UK). Then, the broth culture was incubated at 37 °C for 6 h and adjusted to attain a turbidity of 0.5 McFarland standards.
Subsequently, a sterile cotton swab was immersed into the suspension, and the bacteria were swabbed evenly over the surface of the Muller Hinton agar plate. Antibiotic disks from each selected antibiotic were placed on the Muller Hinton Agar plate at least 15 mm apart using sterile forceps to avoid overlapping of the inhibition zone. The plates were incubated at 37 °C for 24 h. The diameter of the clear zone of inhibition was measured using a caliper. The results of the antimicrobial sensitivity test were interpreted as sensitive, intermediate, or resistant, according to the interpretation cut-off points for the susceptibility status of bacterial isolates [56].
2.8. Data Management and Analysis
All data collected were entered and saved into a Microsoft Excel spreadsheet and then transferred to STATA Version 12 (Stata Corp., College Station, TX, USA) [59] for statistical analysis. The prevalence of Salmonella isolates was calculated using descriptive statistics, in which the number of positives was divided by the total number of samples and multiplied by 100. Pearson’s Chi-square was utilized to assess the statistical significance of various risk factors with the result of the bacteriological and PCR tests. Fisher’s exact test was used for risk factors with few observations. With the ultimate aim of quantifying the crude and adjusted odds ratio (OR), univariate and multivariable logistic regression analyses were conducted, respectively. Statistical significance was declared whenever a p-value of less than 5% (p < 0.05) was attained. With regard to determining the effect of various risk factors on the basis of an OR 95% confidence interval, the significance of the statistical test was assumed whenever the confidence interval excluded one of its values.
3. Results
3.1. Isolation and Identification of Salmonella Species
A cross-sectional study carried out in poultry farms in urban and peri-urban areas of central Ethiopia disclosed an overall farm-level Salmonella species prevalence of 50.6% (82/162). However, Adama (70%) and Debre Birhan (73.1%) scored higher prevalences, and there was no statistically significant difference (χ2 6.3 and p > 0.05) between the farm level prevalence and studied areas (Table 2).

Table 2.
The isolation of Salmonella species on the basis of poultry farms examined in selected areas of central Ethiopia.
The present study revealed the farm-level prevalence of Salmonella species on the basis of types of production, breed, and age of animals. Accordingly, broiler farms scored 56.5% (26/46)—a relatively higher prevalence as compared to layers, which was 48.3% (56/116). In terms of breed, the higher prevalence was documented in Cobb at 64.7% (22/34) followed by Saso at 50.0% (19/38) and Bovans Brown at 45.6% (41/90). The farm-level Salmonella species prevalence indicated that chickens aged 2–5 months had 55.6% (15/27). However, there were no statistically significant differences (p > 0.05) between the farm-level prevalence and purpose of production, breed, and age (Table 2).
Among the types of samples examined, the highest prevalence was recorded from fresh fecal droppings (20.2%), followed by litter (19.7%), cloacal swabs (14.5%), and 8.5% for both feed and water. The difference in the overall sample level prevalence across the different samples examined was statistically significant (p < 0.05) (Table 3).

Table 3.
Association of different sample types with Salmonella prevalence in different regions in central Ethiopia based on routine bacteriological tests.
In general, the prevalence was higher in fresh fecal droppings and litter samples of Adama accounting for 50% (15/30) and 43% (13/30), respectively. Relatively, the prevalence was lower in water samples examined from Addis Ababa (1.8%), Debre Birhan (5.8%) and West of Shaggar City (10%). There was a statistically significant difference in the prevalence of Salmonella species on the basis of type of sample in both Adama (χ2 test 12.5 and p < 0.05) and Debre Birhan areas (χ2 test 10.6 and p < 0.05). However, considering the overall prevalence with respect to the type of sample in central Ethiopia, the difference was statistically significant (χ2 test 15.1 and p < 0.05) (Table 3).
A total of 1515 samples were obtained from cloacal swabs, fresh fecal droppings, litter, feed, and water samples from 162 poultry farms in four selected areas of central Ethiopia. Accordingly, the overall sample level prevalence of Salmonella species was 14.4% (218/1515). The sample level bacteriological prevalence in the specific study areas including Adama, Addis Ababa, Debre Birhan, and West of Shaggar City were 88 (23.2%), 23 (5.9%), 55 (14.3%), and 52 (14.4%), respectively. There was a statistically significant difference in the sample level prevalence of Salmonella species and study areas (χ2 test 46.7 and p < 0.001). Types of production and breed were risk factors with a statistically significant difference in the bacteriological prevalence of Salmonella species (Table 4).

Table 4.
Association of different factors with Salmonella prevalence in different regions in central Ethiopia based on routine bacteriological tests.
3.2. Molecular Detection of Salmonella Typhimurium and Salmonella Enteritidis
Molecular detection of Salmonella Typhimurium and Salmonella Enteritidis was conducted on a total of 76 randomly selected cultures, after biochemical confirmation (43 from Adama and 33 from Debre Birhan), which represents a selection of nearly 50% of the bacteriologically confirmed isolates from both study areas. The findings showed the highest molecular detection of Salmonella Typhimurium among isolates originated from fresh fecal droppings at 58.3% (7 out of 12), followed by litter at 50% (3 out of 6), and none for water samples. Similarly, the PCR test disclosed that 23.3% (10/43) of the isolates were positive for the Salmonella Enteritidis specific gene (SdfI gene) (Figures S1 and S2 in the Supplementary File) and Table 5). The findings indicated that the highest molecular detection of Salmonella Enteritidis was among isolates that originated from fresh fecal droppings at 37.5% (3/8), followed by cloacal swabs at 24% (6/25) and litter at 25% (1/4). However, both water and feed samples were negative for Salmonella Enteritidis (Table 5).

Table 5.
Molecular detection of Salmonella Typhimurium and Salmonella Enteritidis on the basis of the type of samples examined from Debre Birhan and Adama, central Ethiopia.
In the multivariable logistic regression analysis, age of chickens (2–5 and >6 months) and sample type (fresh fecal droppings and litter) were statistically significantly associated with the bacteriological isolation and molecular detection of Salmonella at a p-value of <0.05. Accordingly, multivariable logistic regression analysis revealed 4.8 times higher likelihood of bacteriologically isolating Salmonella species in chickens with an age of >6 months (p < 0.05). Similarly, the odds of isolating Salmonella species was 2.3 times (p < 0.05) among chickens with ages of 2–5 months (Table 6). Moreover, fresh fecal droppings had higher bacteriological isolation of Salmonella species than cloacal swab samples [OR for fresh fecal droppings vs. cloacal swab = 1.87; 95% CI for OR = 1.22–2.88; p < 0.05]. Similarly, litter had higher bacteriological isolation of Salmonella species than cloacal swab samples [OR for litter vs. cloacal swab = 1.81; 95% CI for OR = 1.17–2.79; p < 0.05]. Likewise, molecular detection of Salmonella Typhimurium and Salmonella Enteritidis was 1.51 and 1.27 times higher (p < 0.05) in fresh fecal droppings and litter sample types, respectively (Table 6).

Table 6.
Multivariable logistic regression analysis of Salmonella with various risk factors in central Ethiopia.
3.3. Antimicrobial Susceptibility Profile of Salmonella Typhimurium and Salmonella Enteritidis
Antimicrobial susceptibility testing performed on a total of 15 PCR-positive Salmonella Typhimurium isolates obtained from Debre Birhan, and 10 PCR-positive Salmonella Enteritidis isolates obtained from Adama, central Ethiopia, indicated that all were resistant or intermediately resistant to two or more of the antimicrobials. The level and extent of resistance of Salmonella Typhimurium isolates were the highest for ampicillin (93.3%), followed by oxytetracycline (86.7%) and sulfamethoxazole/trimethoprim (46.7%). Similarly, the Salmonella Typhimurium isolates showed 40% resistance each for tetracycline, kanamycin, and erythromycin. On the other hand, Salmonella Typhimurium isolates exhibited 100% susceptibility to only two of the twelve antibiotics tested, namely ceftazidime and ciprofloxacin. The majority of Salmonella Typhimurium isolates were relatively susceptible to azithromycin (86.7%) and gentamycin (73.3%). The Salmonella Enteritidis isolates revealed the highest resistance for ampicillin (90%), followed by 80% resistance against oxytetracycline and tetracycline and nalidixic acid (70%). On the contrary, Salmonella Enteritidis isolates showed 100% susceptibility to ceftazidime and ciprofloxacin. The majority of Salmonella Enteritidis isolates were relatively susceptible to azithromycin (90%) and chloramphenicol and gentamycin (80%) (Table 7).

Table 7.
Antimicrobial susceptibility profile of Salmonella Typhimurium and Salmonella Enteritidis, central Ethiopia.
In this study, multi-drug resistance patterns were clearly demonstrated in the different Salmonella Typhimurium and Salmonella Enteritidis isolates. Out of 15 tested Salmonella Typhimurium isolates, 26.7%, 20%, and 20.0% exhibited resistance to two, three, and four antibiotics, respectively. However, even two isolates show resistance against eight different antimicrobials (Table 8).

Table 8.
Multiple antimicrobial resistance patterns of Salmonella Typhimurium isolates (n = 15).
Similarly, the current study showed a multi-drug resistant profile of PCR positive Salmonella Enteritidis isolates, exhibiting nine different resistant patterns. Accordingly, out of nine multi-drug resistant isolates, 11.1%, 33.3%, 33.3%, 11.1%, and 11.1% revealed resistance to three, four, five, six and nine antibiotics, respectively (Table 9).

Table 9.
Multiple antimicrobial resistance patterns of Salmonella Enteritidis isolates (n = 9).
4. Discussion
The findings of the present cross-sectional study, for the first time, indicated a higher overall farm-level prevalence of Salmonella species, accounting for 50.6% (82/162) in poultry farms situated in urban and peri-urban areas of central Ethiopia. The present finding of 50.6% of farm-level Salmonella prevalence was higher than a report from Iran at 36.4% [60], Algeria at 34.37% [48] and Uganda at 20.7% [61]. Low levels of farm prevalence of Salmonella were recorded from developed parts of the world, e.g., Denmark at 1.8% [62], Poland at 1.57% [63], and France at 8.6% [64]. On the other hand, the current farm-level prevalence of Salmonella was in agreement with Nigeria at 47.9% [65] and Vietnam at 46.3% [66]. The occurrence of low levels of Salmonella from European and developed countries can be linked to the application of specific control programs [67], which are deficient and irregular in developing countries like Ethiopia. The high Salmonella prevalence in the present research was attributed to the most likely contributing factor that all the poultry farms were medium-scale carrying thousands of chickens and the husbandry practices associated with intensification permit easy propagation of the bacteria within the farm. In addition, the level of biosecurity implementation in poultry farms in urban and peri-urban parts of central Ethiopia was highly compromised, favoring the occurrence of various diseases, including Salmonella [26,68].
The findings of the current study showed no statistically significant differences between the farm-level prevalence of Salmonella and the examined risk factors (study area, purpose of production, breed, and age). The widespread occurrence of the bacteria, as well as the relaxation of biosecurity practices in central Ethiopia, contributes to almost equal exposure to the pathogen [26,69,70]. In addition, all the studied farms had uniformity in terms of farm size being medium scale and the production system involving intensive management with a deep litter housing system. This might have resulted in narrow differences in the prevalence of Salmonella in the above-mentioned risk factors.
Based on the traditional culture method, Salmonella species was identified in 14.4% of the 1515 samples collected from the different selected areas of central Ethiopia. This finding was relatively consistent with earlier reports from Ethiopia at around 15% [68,71,72]. On the contrary, our findings were lower than previous studies carried out in the USA at 38.8% [73], in India at 55% [74], Bangladesh at 31.25% [75], and Uganda at 20.7% [61]. This finding is greater than previous studies [76,77], reporting 2.98% and 9.27% in Jimma and Kefa of southwestern Ethiopia, respectively, and 9.84% in Morocco [78]. However, the lowest level of occurrence of Salmonella was observed in European countries, accounting for 2.34% [79,80]. The observed variations in the sample and farm level prevalence of Salmonella species could be attributed to factors such as poultry management practices, the housing system of chicken, discrepancies in the biosecurity status, absence of strict disease control programs, scale of farms and hygienic conditions, and intermittent shedding of Salmonellosis [81]. In connection to this, all farms investigated in the present study had deep litter housing systems, and the disregard for sanitary settings might favor widespread Salmonella infection [82,83].
The findings of the present study disclosed three risk factors (namely age, breed, and sample type) having a statistically significant association with bacteriological isolation of Salmonella species. Consequently, the odds of isolation of Salmonella species were 4.98 times and 2.39 times (p < 0.05) among chickens with ages of >6 and 2–5 months, respectively. This implies that the chance of acquiring Salmonella from the environment increases with age. This is in agreement with earlier studies from Bangladesh [84]. On the contrary, a higher prevalence of Salmonella in young chicks was reported from Iran [60]. The observed discrepancies might be attributed to disparities in the production process, isolation technique, and variation in the level of biosecurity practices.
Although differences in the sample prevalence of Salmonella species were observed in the different breeds (21.1% for Cobb 500; 12.7% for Saso and 12% for Bovans Brown), it is difficult to establish if susceptibility is linked to genetic variations. More importantly, the farm management system, production types, and level of biosecurity practices could potentially contribute to the differences.
Regarding the isolation and identification of Salmonella species on the basis of sample type, the prevalence showed variability being higher in fresh fecal droppings (20.2%) followed by litter (19.7%) and cloacal swabs (14.5%). However, consistent findings were noted in the case of feed (8.5%) and water (8.5%) samples. The results of the present study were higher than earlier reports from Modjo, central Ethiopia, where the isolation from cloacal swabs, feces, litter, and feed accounted for 0.3%, 5.5%, 3.4%, and 0% [30] and 15.2% from feces from six countries of Latin America [85]. On the other hand, this finding is in line with previous studies conducted in southern Ethiopia, which revealed 14.8% in cloacal swabs [71], and Nigeria, which revealed 23% in feces and 20.3% in litter [86]. Isolation of Salmonella isolates from fresh fecal droppings (20.2%) and litter (19.7%) in the present study is much lower than the 59.1% reported from poultry litter samples in Nigeria [87] and fresh fecal droppings (92%) from Spain (46.09%) [88]. Similarly, reports from Bangladesh showed higher findings with respect to cloacal swabs (46.09%), feed (18.75%) and water (17.19%) [75]. Likewise, a previous report from Egypt disclosed that 55% of cloacal swabs were positive for Salmonella species [89]. The higher isolation of Salmonella species in fecal droppings might be due to the fact that the gastrointestinal system is thought to be a potential source of contamination during the intermittent shedding of the pathogen with feces from carrier chickens [90,91]. The detection of Salmonella in water and feed, despite lower concentrations, signals a risk of bacterial contamination due to inadequate hygiene management at the farm level, as well as within the feed supply chain. This underscores the importance of implementing robust hygiene practices in poultry operations. Water sources, particularly well water or surface water, can become contaminated right from the supply or through bacterial transmission from the chickens themselves [92].
In alignment with the findings of the current study, globally, Salmonella Typhimurium and Salmonella Enteritidis are the most predominant isolates responsible for foodborne infections resulting from the consumption of contaminated poultry products in the past couple of decades [17,55,93,94]. Salmonella Typhimurium is one of the most threatening serotypes of public health importance and is commonly associated with antibiotic resistance [95]. The 50% detection of Salmonella Typhimurium by PCR test in the present study was found to be slightly comparable to 48.9% from Vietnam [96], 46.4% from South Africa [95], 43.35% from Morocco [97], and 40% from Greece [98]. On the contrary, this finding is a lot higher than that of Iran [99], Turkey [100], Singapore [101] and Egypt [102], who reported 1.6%, 9.4%, 18.1%, and 33%, respectively. The results from this study revealed that the existence of Salmonella in poultry farms was affected by numerous risk factors and that deep litter systems favor the persistence of Salmonella and higher chances of infection [65,103]. The level of application of biosecurity practices could also significantly contribute to the observed variation in different countries [26,104]. Salmonella Typhimurium, being the most prevalent isolate in poultry, is well known for its capacity to infect a wide range of animals and for its ability to survive in the environment for long periods, making it one of the most common causes of Salmonellosis [105,106].
The gene sdfI was reported to be found only in Salmonella Enteritidis and designed as a strong marker for these Salmonella serovars [107]. The level of molecular detection of Salmonella Enteritidis from the present study was 23.8%. This finding is consistent with the previous reports from Pakistan at 23.3% [10] and Turkey at 21.9% [100], whereas the current finding is much greater than the earlier reports of 7.1% from Ethiopia [108] and 13% from South Africa [109]. The high isolation of Salmonella Enteritidis may be because it is more invasive than other serotypes. However, no statistically significant association was observed between the level of molecular detection of Salmonella Typhimurium and Salmonella Enteritidis and all the risk factors considered in the current study. This might be due to the small number of samples subjected to PCR tests.
The findings of the antimicrobial susceptibility test disclosed that all PCR-confirmed Salmonella Typhimurium and Salmonella Enteritidis isolates were 100% susceptible to Ceftazidime and Ciprofloxacin. On the contrary, all Salmonella Typhimurium and Salmonella Enteritidis were noted to express over 80% resistance to ampicillin, oxytetracycline, and tetracycline among the tested antimicrobials. This finding coincides with previous studies carried out in Ethiopia, which reported resistance of Salmonella isolates to ampicillin of 97.8% [71] and 100% [110,111]. Interestingly, there has been a practice of extensive antimicrobial usage in poultry farms in central Ethiopia [58]. A recent study carried out in Ethiopia revealed tetracyclines, aminoglycosides, and trimethoprim-sulfonamides were frequently used classes of antibiotics [112].
In contrast to the present study, previous reports from Uganda [61] and Bangladesh [113] revealed that 50.0% and 100% of the Salmonella isolates were resistant to ciprofloxacin, respectively. The high level of resistance observed to nine tested antimicrobials is alarming, suggesting that critical antibiotic classes are becoming less effective. This poses challenges in selecting suitable drugs for treating bacterial diseases in poultry. This finding sheds light on the potential consequences of indiscriminate antimicrobial use in poultry farming. The current scenario of antimicrobial resistance is increasingly recognized as a global issue affecting both human and animal health. A key contributing factor to bacterial resistance is the extensive use of antimicrobials in both animal farming and human medicine, coupled with insufficient advocacy and monitoring of antimicrobial utilization [58,112,113,114,115,116]. The use of antimicrobials without prescription and improper dispensing might favor selection pressure that increases the maintenance of resistance genes in bacteria [117]. The present study revealed a multi-drug resistant profile of PCR-confirmed Salmonella Typhimurium and Salmonella Enteritidis isolates. Accordingly, nine of the eleven tested antimicrobials demonstrated 12 different resistance patterns. These isolates exhibited resistance to two to nine different antibiotics. Such widespread and high degrees of multi-drug resistance have also been demonstrated in other developing countries, such as Egypt [118], Ghana [119], Nigeria [120], Uganda [61], and Senegal [121].
Among other factors, the unregulated access to antimicrobials and the prophylactic use of these drugs starting from day-old chicks can contribute to the selection pressure for resistant isolates. In most instances, broad-spectrum antibiotics are widely used for the treatment of infectious diseases, including Salmonellosis. Such frequent and long-term use of broad-spectrum antibiotics is noted to favor the development of antimicrobial resistance in Ethiopia [30,71], Nigeria [122], and China [123]. In most parts of Africa, including Ethiopia, farmers are absolutely free to use antimicrobials for treatment as well as prophylactic purposes to their perceived benefit [124,125,126]. The development of antimicrobial resistance in Ethiopia is further exacerbated by the practices of accessibility of antibiotics without a valid prescription and without performing the required diagnostic tests. Such indiscriminate use of antimicrobials along with the absence of rational drug use policy and strict regulations greatly contribute to the emergence of resistance [112,127].
5. Conclusions
The significant isolation and identification of Salmonella Typhimurium and Salmonella Enteritidis in poultry and on poultry farms in selected areas of central Ethiopia, coupled with the emergence of multi-drug-resistant profiles as revealed by our study, underscore the urgent need for interventions and public engagement initiatives within the sector. This is particularly critical given the zoonotic potential of these Salmonella species. To effectively manage poultry Salmonellosis, it is imperative to implement practical control strategies that enhance biosecurity measures at various production stages. Additionally, ongoing efforts to raise awareness and provide training for farmers and farm workers on the risks of zoonotic diseases and the transmission of antimicrobial-resistant isolates are highly recommended. Establishing stringent and judicious drug use policies, along with interventions to curb the indiscriminate use of antimicrobials, is essential.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12040767/s1, Figure S1: Electrophoresis of Spy gene for Salmonella Typhimurium isolates in 1.5 % agarose gel; Figure S2: Conventional PCR for Salmonella Enteritidis.
Author Contributions
Conceptualization, H.W., K.A., H.A. and G.A.; investigation, H.W., Y.A. (Yonas Ayele), Y.A. (Yamlaksira Ayalkibet), T.T., S.A., T.A. and G.D.; methodology, H.W., K.A., H.A. and G.A.; supervision, H.W., B.M.B., T.E., K.A., H.A. and G.A.; writing—original draft, H.W., Y.A. (Yonas Ayele), Y.A. (Yamlaksira Ayalkibet), T.M., T.E., S.A. and B.M.B.; writing—review and editing, H.W., B.M.B., T.A., Z.A., K.A., H.A. and G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Addis Ababa University, Vice President for Research and Technology Transfer through a thematic research fund (AAU/VPRTT/LT-016/2021). G. Antonissen was supported by the Chair of Poultry Health Sciences (Ghent University-Vetworks bvba-Poulpharm bvba).
Institutional Review Board Statement
Ethical clearance for this research work was obtained from the Animal Research Ethical Review Committee of the College of Veterinary Medicine and Agriculture of Addis Ababa University (Reference number: VM/ERC/04/13/021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data in the present study can be shared upon legitimate request of the corresponding author.
Acknowledgments
Poultry farmers, owners, and attendants in central Ethiopia who allowed us to perform the research are highly appreciated.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shapiro, B.I.; Gebru, G.; Desta, S.; Negassa, A.; Nigussie, K.; Aboset, G.; Mechal, H. Ethiopia livestock master plan. In ILRI Project Report; International Livestock Research Institute (ILRI): Nairobi, Kenya, 2015. [Google Scholar]
- Tadelle, D.; Nigusie, D.; Alemu, Y.; Peters, K.J. The feed resource base and its potentials for increased poultry production in Ethiopia. World’s Poult. Sci. J. 2002, 58, 77–87. [Google Scholar] [CrossRef]
- Milkias, M. Chicken meat production, consumption and constraints in Ethiopia. Food Sci. Qual. Manag. 2016, 54, 1–12. [Google Scholar]
- Tolasa, B. Current status of indigenous and highly productive chicken breeds in Ethiopia. Adv. Agric. 2021, 2021, 8848388. [Google Scholar] [CrossRef]
- Habte, T.; Amare, A.; Bettridge, J.; Collins, M.; Christley, R.; Wigley, P. Guide to chicken health and management in Ethiopia. In ILRI Manual 25; International Livestock Research Institute (ILRI): Nairobi, Kenya, 2017. [Google Scholar]
- Ebsa, Y.A.; Harpal, S.; Negia, G.G. Challenges and chicken production status of poultry producers in Bishoftu, Ethiopia. Poult. Sci. 2019, 98, 5452–5455. [Google Scholar] [CrossRef]
- Chanie, M.; Negash, T.; Tilahun, S.B. Occurrence of concurrent infectious diseases in broiler chickens is a threat to commercial poultry farms in Central Ethiopia. Trop. Anim. Health Prod. 2009, 41, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Endris, M.; Taddesse, F.; Geloye, M.; Degefa, T.; Jibat, T. Sero and media culture prevalence of Salmonellosis in local and exotic chicken, Debre Zeit, Ethiopia. Afr. J. Microbiol. Res. 2013, 7, 1041–1044. [Google Scholar]
- Wubet, W.; Bitew, M.; Mamo, G.; Gelaye, E.; Tesfaw, L.; Sori, H.; Abayneh, T. Evaluation of inactivated vaccine against fowl cholera developed from local isolates of Pasteurella multocida in Ethiopia. Afr. J. Microbiol. Res. 2019, 13, 500–509. [Google Scholar]
- Asfaw, Y.T.; Ameni, G.; Medhin, G.; Gumi, B.; Hagos, Y.; Wieland, B. Poultry disease occurrences and their impacts in Ethiopia. Trop. Anim. Health Prod. 2021, 53, 54. [Google Scholar] [CrossRef]
- Mezal, E.H.; Sabol, A.; Khan, M.A.; Ali, N.; Stefanova, R.; Khan, A.A. Isolation and molecular characterization of Salmonella enterica serovar Enteritidis from poultry house and clinical samples during 2010. Food Microbiol. 2014, 38, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on major food-borne zoonotic bacterial pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef]
- Wilson, R.L.; Elthon, J.; Clegg, S.; Jones, B.D. Salmonella enterica serovars Gallinarum and Pullorum expressing Salmonella enterica serovar Typhimurium type 1 fimbriae exhibit increased invasiveness for mammalian cells. Infect. Immun. 2000, 68, 4782–4785. [Google Scholar] [CrossRef] [PubMed]
- Beyene, G.; Nair, S.; Asrat, D.; Mengistu, Y.; Engers, H.; Wain, J. Multidrug resistant Salmonella Concord is a major cause of salmonellosis in children in Ethiopia. J. Infect. Dev. Ctries. 2011, 5, 023–033. [Google Scholar] [CrossRef] [PubMed]
- Feasey, N.A.; Dougan, G.; Kingsley, R.A.; Heyderman, R.S.; Gordon, M.A. Invasive non-typhoidal Salmonella disease: An emerging and neglected tropical disease in Africa. Lancet 2012, 379, 2489–2499. [Google Scholar] [CrossRef]
- Li, S.; He, Y.; Mann, D.A.; Deng, X. Global spread of Salmonella Enteritidis via centralized sourcing and international trade of poultry breeding stocks. Nat. Commun. 2021, 12, 5109. [Google Scholar] [CrossRef] [PubMed]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Gast, R.; Humphrey, T.J.; Van Immerseel, F. Mechanisms of egg contamination by Salmonella Enteritidis. FEMS Microbiol. Rev. 2009, 33, 718–738. [Google Scholar] [CrossRef] [PubMed]
- Al-Abadi, I.K.M.; Al-Mayah, A.A.S. Isolation and identification of Salmonella spp. from chicken and chicken environment in Basrah province. Afr. J. Biol. Sci. 2011, 7, 33–43. [Google Scholar]
- Moraes, D.M.C.; Duarte, S.C.; Bastos, T.S.A.; Rezende, C.L.G.; Leandro, N.S.M.; Café, M.B.; Andrade, M.A. Detection of Salmonella spp. by conventional bacteriology and by quantitative polymerase-chain reaction in commercial egg structures. Braz. J. Poult. Sci. 2016, 18, 117–124. [Google Scholar] [CrossRef]
- Liljebjelke, K.A.; Hofacre, C.L.; White, D.G.; Ayers, S.; Lee, M.D.; Maurer, J.J. Diversity of antimicrobial resistance phenotypes in Salmonella isolated from commercial poultry farms. Front. Vet. Sci. 2017, 4, 96. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J. International Collaboration on Enteric Disease “Burden of Illness” Studies. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef]
- Muvhali, M.; Smith, A.M.; Rakgantso, A.M.; Keddy, K.H. Investigation of Salmonella Enteritidis outbreaks in South Africa using multi-locus variable-number tandem-repeats analysis, 2013–2015. BMC Infect. Dis. 2017, 17, 661. [Google Scholar] [CrossRef] [PubMed]
- Glenn, L.M.; Lindsey, R.L.; Frank, J.F.; Meinersmann, R.J.; Englen, M.D.; Fedorka-Cray, P.J.; Frye, J.G. Analysis of antimicrobial resistance genes detected in multidrug-resistant Salmonella enterica serovar Typhimurium isolated from food animals. Microb. Drug Resist. 2011, 17, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, S. Typhoid fever in sub-Saharan Africa: Challenges of diagnosis and management of infections. J. Infect. Dev. Ctries. 2008, 2, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, G. Prevalence of human Salmonellosis in Ethiopia: A systematic review and meta-analysis. BMC Infect. Dis. 2014, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Waktole, H.; Muluneh, T.; Miressa, Y.; Ayane, S.; Berhane, G.; Kabeta, T.; Mideksa, B.; Amenu, K.; Ashenafi, H.; Antonissen, G. Quantitative assessment of major biosecurity challenges of poultry production in central Ethiopia. Animals 2023, 13, 3719. [Google Scholar] [CrossRef] [PubMed]
- Crump, J.A.; Luby, S.P.; Mintz, E.D. The global burden of typhoid fever. Bull. World Health Organ. 2004, 82, 346–353. [Google Scholar] [PubMed]
- Eguale, T.; Gebreyes, W.A.; Asrat, D.; Alemayehu, H.; Gunn, J.S.; Engidawork, E. Non-typhoidal Salmonella serotypes, antimicrobial resistance and co-infection with parasites among patients with diarrhea and other gastrointestinal complaints in Addis Ababa, Ethiopia. BMC Infect. Dis. 2015, 15, 497. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Rahman, H.; Qasim, M.; Khan, T.A.; Ullah, W.; Jie, Y. Molecular detection and antimicrobial resistance profile of zoonotic Salmonella Enteritidis isolated from broiler chickens in Kohat, Pakistan. J. Chin. Med. Assoc. 2017, 80, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Dagnew, B.; Alemayehu, H.; Medhin, G.; Eguale, T. Prevalence and antimicrobial susceptibility of Salmonella in poultry farms and in-contact humans in Adama and Modjo towns, Ethiopia. Microbiol. Open 2020, 9, e1067. [Google Scholar] [CrossRef] [PubMed]
- Ramtahal, M.A.; Amoako, D.G.; Akebe, A.L.; Somboro, A.M.; Bester, L.A.; Essack, S.Y. A public health insight into Salmonella in poultry in Africa: A review of the past decade: 2010–2020. Microb. Drug Resist. 2022, 28, 710–733. [Google Scholar] [CrossRef] [PubMed]
- De Mesquita Souza Saraiva, M.; Lim, K.; do Monte, D.F.M.; Givisiez, P.E.N.; Alves, L.B.R.; de Freitas Neto, O.C.; Kariuki, S.; Júnior, A.B.; de Oliveira, C.J.B.; Gebreyes, W.A. Antimicrobial resistance in the globalized food chain: A One Health perspective applied to the poultry industry. Braz. J. Microbiol. 2022, 53, 465–486. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Aarestrup, F.M. Veterinary drug usage and antimicrobial resistance in bacteria of animal origin. Basic Clin. Pharmacol. Toxicol. 2005, 96, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Munang’andu, H.M.; Kabilika, S.H.; Chibomba, O.; Munyeme, M.; Muuka, G.M. Bacteria isolations from broiler and layer chicks in Zambia. J. Pathog. 2012, 2012, 520564. [Google Scholar] [CrossRef] [PubMed]
- Hedman, H.D.; Vasco, K.A.; Zhang, L. A review of antimicrobial resistance in poultry farming within low-resource settings. Animals 2020, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; Bachmeier, J.; Bisgaard, M. New strategies to prevent and control avian pathogenic Escherichia coli (APEC). Avian Pathol. 2021, 50, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.; Kenney, P.B. Screening of Salmonella isolates from a turkey production facility for antibiotic resistance. Poult. Sci. 2002, 81, 1496–1500. [Google Scholar] [CrossRef]
- Jain, S.; Chen, J. Antibiotic resistance profiles and cell surface components of Salmonellae. J. Food Prot. 2006, 69, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, C.G.; Macklin, K.S.; Kumar, S.; Bailey, M.; Ebner, P.E.; Oliver, H.F.; Singh, M. Prevalence and antimicrobial resistance patterns of Salmonella isolated from poultry farms in southeastern United States. Poult. Sci. 2018, 97, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, R.; Zhao, C.; Tiseo, K.; Pires, J.; Van Boeckel, T.P. Predictive Mapping of Antimicrobial Resistance for Escherichia coli, Salmonella, and Campylobacter in Food-Producing Animals, Europe, 2000–2021. Emerg. Infect. Dis. 2024, 30, 96. [Google Scholar] [PubMed]
- NMA—National Meteorological Agency. Annual Meteorological Reports; NMA: Addis Ababa, Ethiopia, 2021.
- Sarba, E.J.; Kudama, K.; Dandecha, M.; Megersa, L.; Borena, B.M.; Gebremdhin, E.Z. Prevalence, organ distribution and antimicrobial susceptibility profile of Salmonella isolated from chickens purchased from markets in selected districts of West Shoa, Ethiopia. Ethiop. Vet. J. 2020, 24, 73–89. [Google Scholar] [CrossRef]
- Thrusfield, M. Veterinary Epidemiology, 3rd ed.; Blackwell Science Ltd.: Oxford, UK, 2007; pp. 233–250. [Google Scholar]
- Office International Épizooties (OIÉ). Terrestrial Manual. In Laboratory Methodologies for Bacterial Antimicrobial Susceptibility Testing; OIÉ: Paris, France, 2012; pp. 1–11. [Google Scholar]
- 6579:2002/A1:2007; Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Detection of Salmonella spp. Amendment 1: Annex D: Detection of Salmonella spp. in Animal Faeces and in Environmental Samples from the Primary Production Stage. International Organization for Standardization (ISO): Geneva, Switzerland, 2007.
- Daquigan, N.; Grim, C.J.; White, J.R.; Hanes, D.E.; Jarvis, K.G. Early recovery of Salmonella from food using a 6-hour non-selective pre-enrichment and reformulation of tetrathionate broth. Front. Microbiol. 2016, 7, 235212. [Google Scholar] [CrossRef] [PubMed]
- Djeffal, S.; Mamache, B.; Elgroud, R.; Hireche, S.; Bouaziz, O. Prevalence and Risk Factors for Salmonella spp. Contamination in Broiler Chicken Farms and Slaughterhouses in the Northeast of Algeria. Vet. World 2018, 11, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Macwilliams, M.P. Indole Test Protocol; American Society for Microbiology: Washington, DC, USA, 2016; pp. 1–9. [Google Scholar]
- Park, S.H.; Ryu, S.; Kang, D.H. Development of an improved selective and differential medium for isolation of Salmonella spp. J. Clin. Microbiol. 2012, 50, 3222–3226. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.E.; Sao, S. Isolation and Identification of Micro Organisms. World J. Pharm. Res. 2015, 4, 2043–2057. [Google Scholar]
- Mcdevitt, S. Methyl Red and Voges-Proskauer Test Protocols. Am. Soc. Microbiol. 2016, 8, 1–9. Available online: https://asm.org/getattachment/0c828061-9d6f-4ae7-aea3-66e1a8624aa0/Methyl-Red-and-Voges-Proskauer-Test-Protocols.pdf (accessed on 12 March 2021).
- Islam, M.; Fakhruzzaman, M. Isolation and Identification of Escherichia coli and Salmonella from poultry litter and Feed. Int. J. Nat. Soc. Sci. 2018, 1, 1–7. [Google Scholar]
- Afendy, M.A.; Son, R. Pre-enrichment effect on PCR detection of Salmonella Enteritidis in artificially-contaminated raw chicken meat. Int. Food Res. J. 2015, 22, 2571. [Google Scholar]
- Alvarez, J.; Sota, M.; Vivanco, A.B.; Perales, I.; Cisterna, R.; Rementeria, A.; Garaizar, J. Development of a multiplex PCR technique for detection and epidemiological typing of Salmonella in human clinical samples. J. Clin. Microbiol. 2004, 42, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard, 8th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32, pp. 1–38. [Google Scholar]
- Odey, T.O.J.; Tanimowo, W.O.; Afolabi, K.O.; Jahid, I.K.; Reuben, R.C. Antimicrobial use and resistance in food animal production: Food safety and associated concerns in Sub-Saharan Africa. Int. Microbiol. 2023, 27, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Tufa, T.B.; Regassa, F.; Amenu, K.; Stegeman, J.A.; Hogeveen, H. Livestock producers’ knowledge, attitude, and behavior (KAB) regarding antimicrobial use in Ethiopia. Front. Vet. Sci. 2023, 10, 1167847. [Google Scholar] [CrossRef] [PubMed]
- STATA Corp. Stata Statistical Software: Release 17; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- Ansari-Lari, M.; Hosseinzadeh, S.; Manzari, M.; Khaledian, S. Survey of Salmonella in commercial broiler farms in Shiraz, southern Iran. Prev. Vet. Med. 2022, 198, 105550. [Google Scholar] [CrossRef] [PubMed]
- Odoch, T.; Wasteson, Y.; L’Abée-Lund, T.; Muwonge, A.; Kankya, C.; Nyakarahuka, L.; Skjerve, E. Prevalence, antimicrobial susceptibility and risk factors associated with non-typhoidal Salmonella on Ugandan layer hen farms. BMC Vet. Res. 2017, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- DTU Findit. Annual Report on Zoonoses in Denmark 2018; DTU Findit: Lyngby, Denmark, 2018; pp. 1–64. [Google Scholar]
- Witkowska, D.; Kuncewicz, M.; Żebrowska, J.P.; Sobczak, J.; Sowińska, J. Prevalence of Salmonella spp. in broiler chicken flocks in northern Poland in 2014–2016. Ann. Agric. Environ. Med. 2021, 25, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Le Bouquin, S.; Allain, V.; Rouxel, S.; Petetin, I.; Picherot, M.; Michel, V.; Chemaly, M. Prevalence and risk factors for Salmonella spp. contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med. 2010, 97, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Jibril, A.H.; Okeke, I.N.; Dalsgaard, A.; Kudirkiene, E.; Akinlabi, O.C.; Bello, M.B.; Olsen, J.E. Prevalence and risk factors of Salmonella in commercial poultry farms in Nigeria. PLoS ONE 2020, 15, e0238190. [Google Scholar] [CrossRef] [PubMed]
- Barua, H.; Biswas, P.K.; Olsen, K.E.; Christensen, J.P. Prevalence and characterization of motile Salmonella in commercial layer poultry farms in Bangladesh. PLoS ONE 2012, 7, e35914. [Google Scholar] [CrossRef] [PubMed]
- Authority, European Food Safety. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, 12. [Google Scholar]
- Eguale, T. Non-typhoidal Salmonella serovars in poultry farms in central Ethiopia: Prevalence and antimicrobial resistance. BMC Vet. Res. 2018, 14, 217. [Google Scholar] [CrossRef] [PubMed]
- Geresu, M.A.; Wayuo, B.A.; Kassa, G.M. Occurrence and antimicrobial susceptibility profile of Salmonella isolates from animal origin food items in selected areas of Arsi zone, southeastern Ethiopia, 2018/19. Int. J. Microbiol. 2021, 2021, 6633522. [Google Scholar]
- Zaki, M.S.; Fahmy, H.A.; Khedr, M.H.; Goha, M.; Attia, A.S. The prevalence of Salmonella species as a biosecurity indicator in poultry farms in Sharkia governorate, Egypt. Zagazig Vet. J. 2023, 51, 295–309. [Google Scholar]
- Abdi, R.D.; Mengstie, F.; Beyi, A.F.; Beyene, T.; Waktole, H.; Mammo, B.; Abunna, F. Determination of the sources and antimicrobial resistance patterns of Salmonella isolated from the poultry industry in Southern Ethiopia. BMC Infect. Dis. 2017, 17, 352. [Google Scholar] [CrossRef] [PubMed]
- Abunna, F.; Bedasa, M.; Beyene, T.; Ayana, D.; Mamo, B.; Duguma, R. Salmonella: Isolation and antimicrobial susceptibility tests on isolates collected from poultry farms in and around Modjo, Central Oromia, and Ethiopia. J. Anim. Poult. Sci. 2016, 5, 21–35. [Google Scholar]
- Alali, W.Q.; Thakur, S.; Berghaus, R.D.; Martin, M.P.; Gebreyes, W.A. Prevalence and distribution of Salmonella in organic and conventional broiler poultry farms. Foodborne Pathog. Dis. 2010, 7, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Kumar, D.; Hussain, S.; Pathak, A.; Shukla, M.; Kumar, V.P.; Singh, S.P. Prevalence, antimicrobial resistance and virulence genes characterization of non-typhoidal Salmonella isolated from retail chicken meat shops in Northern India. Food Control 2019, 102, 104–111. [Google Scholar] [CrossRef]
- Mridha, D.; Uddin, M.N.; Alam, B.; Akhter, A.T.; Islam, S.S.; Islam, M.S.; Kabir, S.L. Identification and characterization of Salmonella spp. from samples of broiler farms in selected districts of Bangladesh. Vet. World 2020, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Bayu, Z.; Asrade, B.; Kebede, N.; Sisay, Z.; Bayu, Y. Identification and characterization of Salmonella species in whole egg purchased from local markets in Addis Ababa, Ethiopia. J. Vet. Med. Anim. Health 2013, 5, 133–137. [Google Scholar]
- Abda, S.; Haile, T.; Abera, M. Isolation, identification antimicrobial susceptibility and associated risk factors of Salmonella in semi-intensive poultry farms of Kafa zone, Southwest Ethiopia. Vet. Anim. Sci. 2021, 14, 100206. [Google Scholar] [CrossRef] [PubMed]
- Zahli, R.; Scheu, A.K.; Abrini, J.; Copa-Patiño, J.L.; Nadia, A.; Nadia, S.S.; Soliveri, J. Salmonella spp: Prevalence, antimicrobial resistance and molecular typing of strains isolated from poultry in Tetouan-Morocco. LWT 2022, 153, 112359. [Google Scholar] [CrossRef]
- Blog: Salmonella Prevalence in Europe. 2021. Available online: https://www.calier.com/en/blog/Salmonella-prevalence-europe (accessed on 19 February 2024).
- Authority, European Food Safety. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA J. 2022, 20, 3. [Google Scholar]
- Van Immerseel, F.; De Buck, J.; Pasmans, F.; Bohez, L.; Boyen, F.; Haesebrouck, F.; Ducatelle, R. Intermittent long-term shedding and induction of carrier birds after infection of chickens early posthatch with a low or high dose of Salmonella Enteritidis. Poult. Sci. 2004, 83, 1911–1916. [Google Scholar] [CrossRef]
- Rose, N.; Beaudeau, F.; Drouin, P.; Toux, J.Y.; Rose, V.; Colin, P. Risk factors for Salmonella persistence after cleansing and disinfection in French broiler-chicken houses. Prev. Vet. Med. 2000, 44, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Gast, R.K.; Porter, R.E., Jr. Salmonella infections. Dis. Poult. 2020, 16, 717–753. [Google Scholar]
- Mahmud, M.S.; Bari, M.L.; Hossain, M.A. Prevalence of Salmonella serovars and antimicrobial resistance profiles in poultry of Savar area, Bangladesh. Foodborne Pathog. Dis. 2011, 8, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Soria, M.C.; Soria, M.A.; Bueno, D.J.; Godano, E.I.; Gómez, S.C.; ViaButron, I.A.; Rogé, A.D. Salmonella spp. contamination in commercial layer hen farms using different types of samples and detection methods. Poult. Sci. 2017, 96, 2820–2830. [Google Scholar] [CrossRef] [PubMed]
- Fagbamila, I.O.; Barco, L.; Mancin, M.; Kwaga, J.; Ngulukun, S.S.; Zavagnin, P.; Muhammad, M. Salmonella serovars and their distribution in Nigerian commercial chicken layer farms. PLoS ONE 2017, 12, e0173097. [Google Scholar] [CrossRef] [PubMed]
- Yhiler, N.Y.; Bassey, B.E. Antimicrobial susceptibility patterns of Salmonella species from sources in poultry production settings in Calabar, Cross River state. Niger. Am. J. Health Res. 2015, 3, 76–81. [Google Scholar] [CrossRef][Green Version]
- García, C.; Soriano, J.M.; Benítez, V.; Catalá-Gregori, P. Assessment of Salmonella spp. in feces, cloacal swabs, and eggs (eggshell and content separately) from a laying hen farm. Poult. Sci. 2011, 90, 1581–1585. [Google Scholar] [CrossRef] [PubMed]
- El-Tras, W.F.; Tayel, A.A.; Samir, A. Potential zoonotic pathways of Salmonella Enteritidis in laying farms. Vector-Borne Zoonotic Dis. 2010, 10, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Borsoi, A.; Santos, L.R.D.; Diniz, G.S.; C Salle, C.T.P.; Moraes, H.L.S.; Nascimento, V.P.D. Salmonella fecal excretion control in broiler chickens by organic acids and essential oils blend feed added. Braz. J. Poult. Sci. 2011, 13, 65–69. [Google Scholar] [CrossRef]
- Jajere, S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World 2019, 12, 504. [Google Scholar] [CrossRef]
- Folorunso, O.R.; Kayode, S.; Onibon, V.O. Poultry farm hygiene: Microbiological quality assessment of drinking water used in layer chickens managed under the battery cage and deep litter systems at three poultry farms in southwestern Nigeria. Pak. J. Biol. Sci. 2014, 17, 74–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lapuz, R.; Tani, H.; Sasai, K.; Shirota, K.; Katoh, H.; Baba, E. The role of roof rats (Rattus rattus) in the spread of Salmonella Enteritidis and S. Infantis contamination in layer farms in eastern Japan. Epidemiol. Infect. 2008, 136, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Elkenany, R.; Elsayed, M.M.; Zakaria, A.I.; El-Sayed, S.A.E.S.; Rizk, M.A. Antimicrobial resistance profiles and virulence genotyping of Salmonella enterica serovars recovered from broiler chickens and chicken carcasses in Egypt. BMC Vet. Res. 2019, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Olobatoke, R.Y.; Mulugeta, S.D. Incidence of non-typhoidal Salmonella in poultry products in the North West Province, South Africa. South Afr. J. Sci. 2015, 111, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huong, L.Q.; Reinhard, F.; Padungtod, P.; Hanh, T.T.; Kyule, M.N.; Baumann, M.P.; Zessin, K.H. Prevalence of Salmonella in retail chicken meat in Hanoi, Vietnam. Ann. NY Acad. Sci. 2006, 1081, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Abdellah, C.; Fouzia, R.F.; Abdelkader, C.; Rachida, S.B.; Mouloud, Z. Prevalence and anti-microbial susceptibility of Salmonella isolates from chicken carcasses and giblets in Meknès, Morocco. Afr. J. Microbiol. Res. 2009, 3, 215–219. [Google Scholar]
- Adhikari, P.; Cosby, D.E.; Cox, N.A.; Franca, M.S.; Williams, S.M.; Gogal Jr, R.M.; Kim, W.K. Effect of dietary fructooligosaccharide supplementation on internal organs Salmonella colonization, immune response, ileal morphology, and ileal immunohistochemistry in laying hens challenged with Salmonella Enteritidis. Poult. Sci. 2018, 97, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, A.E.; Basami, M.; Afshari, N.S. Identification of Salmonella spp. and Salmonella typhimurium by a multiplex PCR-based assay from poultry carcasses in Mashhad-Iran. Int. J. Vet. Res. 2009, 3, 43–48. [Google Scholar]
- Arkali, A.; Çetinkaya, B. Molecular identification and antibiotic resistance profiling of Salmonella species isolated from chickens in eastern Turkey. BMC Vet. Res. 2020, 16, 205. [Google Scholar] [CrossRef] [PubMed]
- Abatcha, M.G.; Effarizah, M.E.; Rusul, G. Prevalence, antimicrobial resistance, resistance genes and class 1 integrons of Salmonella serovars in leafy vegetables, chicken carcasses and related processing environments in Malaysian fresh food markets. Food Control 2018, 91, 170–180. [Google Scholar] [CrossRef]
- El-Aziz, D.M. Detection of Salmonella typhimurium in retail chicken meat and chicken giblets. Asian Pac. J. Trop. Biomed. 2013, 3, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Mollenhorst, H.; Van Woudenbergh, C.J.; Bokkers, E.G.M.; De Boer, I.J.M. Risk factors for Salmonella Enteritidis infections in laying hens. Poult. Sci. 2005, 84, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Namata, H.; Welby, S.; Aerts, M.; Faes, C.; Abrahantes, J.C.; Imberechts, H.; Mintiens, K. Identification of risk factors for the prevalence and persistence of Salmonella in Belgian broiler chicken flocks. Prev. Vet. Med. 2009, 90, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Rabsch, W.; Andrews, H.L.; Kingsley, R.A.; Prager, R.; Tschäpe, H.; Adams, L.G.; Bäumler, A.J. Salmonella enterica serotype Typhimurium and its host-adapted variants. Infect. Immun. 2002, 70, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Kidanemariam, A.; Engelbrecht, M.; Picard, J. Retrospective study on the incidence of Salmonella isolations in animals in South Africa, 1996 to 2006. J. South Afr. Vet. Assoc. 2010, 81, 37–44. [Google Scholar] [CrossRef]
- Agron, P.G.; Walker, R.L.; Kinde, H.; Sawyer, S.J.; Hayes, D.C.; Wollard, J.; Andersen, G.L. Identification by subtractive hybridization of sequences specific for Salmonella enterica serovar Enteritidis. Appl. Environ. Microbiol. 2001, 67, 4984–4991. [Google Scholar] [CrossRef] [PubMed]
- Adamu, K.; Sori, H.; Gelaye, E.; Belay, A.; Ayelet, G.; Yami, M.; Abayneh, T. Evaluation of the protective efficacy of Salmonella Gallinarum 9R strain vaccine against Salmonella strains isolated from cases suspected of salmonellosis outbreaks in poultry farms in central Ethiopia. Ethiop. Vet. J. 2017, 21, 102–116. [Google Scholar] [CrossRef][Green Version]
- Ramatla, T.A.; Mphuthi, N.; Ramaili, T.; Taioe, M.O.; Thekisoe, O.M.; Syakalima, M. Molecular detection of virulence genes in Salmonella spp. isolated from chicken faeces in Mafikeng, South Africa. J. South Afr. Vet. Assoc. 2020, 91, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Garedew, L.; Hagos, Z.; Addis, Z.; Tesfaye, R.; Zegeye, B. Prevalence and antimicrobial susceptibility patterns of Salmonella isolates in association with hygienic status from butcher shops in Gondar town, Ethiopia. Antimicrob. Resist. Infect. Control 2015, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Geresu, M.A.; Desta, W.Z. Carriage, risk factors, and antimicrobial resistance patterns of Salmonella isolates from raw beef in Jimma, Southwestern Ethiopia. Infect. Drug Resist. 2021, 14, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Gemeda, B.A.; Amenu, K.; Magnusson, U.; Dohoo, I.; Hallenberg, G.S.; Alemayehu, G.; Wieland, B. Antimicrobial use in extensive smallholder livestock farming systems in Ethiopia: Knowledge, attitudes, and practices of livestock keepers. Front. Vet. Sci. 2020, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Siddiky, N.A.; Sarker, M.S.; Khan, M.S.R.; Begum, R.; Kabir, M.E.; Karim, M.R.; Samad, M.A. Virulence and antimicrobial resistance profiles of Salmonella enterica serovars isolated from chicken at wet markets in Dhaka, Bangladesh. Microorganisms 2021, 9, 952. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, S.; Shrestha, P.; Adhikari, B. Antimicrobial use in food animals and human health: Time to implement ‘One Health’ approach. Antimicrob. Resist. Infect. Control 2020, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, U.; Moodley, A.; Osbjer, K. Antimicrobial resistance at the livestock-human interface: Implications for Veterinary Services. Rev. Sci. Et Tech. (Int. Off. Epizoot.) 2021, 40, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Ejo, M.; Garedew, L.; Alebachew, Z.; Worku, W. Prevalence and antimicrobial resistance of Salmonella isolated from animal-origin food items in Gondar, Ethiopia. BioMed Res. Int. 2016, 2016, 4290506. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Maksoud, M.; Abdel-Khalek, R.; El-Gendy, A.; House, B.L.; Gamal, R.F.; Abdelhady, H.M. Genetic characterisation of multidrug-resistant Salmonella enterica serotypes isolated from poultry in Cairo, Egypt. Afr. J. Lab. Med. 2015, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Andoh, L.A.; Dalsgaard, A.; Obiri-Danso, K.; Newman, M.J.; Barco, L.; Olsen, J.E. Prevalence and antimicrobial resistance of Salmonella serovars isolated from poultry in Ghana. Epidemiol. Infect. 2016, 144, 3288–3299. [Google Scholar] [CrossRef] [PubMed]
- Kayode, F.; Folasade, O.; Frank, M.A.; Rene, S.H. Antimicrobial susceptibility and serovars of Salmonella from chickens and humans in Ibadan, Nigeria. J. Infect Dev. Ctries 2010, 4, 484–494. [Google Scholar]
- Dione, M.M.; Ieven, M.; Garin, B.; Marcotty, T.; Geerts, S. Prevalence and antimicrobial resistance of Salmonella isolated from broiler farms, chicken carcasses, and street-vended restaurants in Casamance, Senegal. J. Food Prot. 2009, 72, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Achi, C.; Holmes, M. Multidrug-resistance in Salmonella species isolated from poultry in Nigeria. Int. J. Infect. Dis. 2020, 101, 37–38. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Li, T.; Liu, F.; Cheng, Y.; Guo, X.; Zhang, T. Characterization of Salmonella spp. isolated from chickens in Central China. BMC Vet. Res. 2020, 16, 299. [Google Scholar] [CrossRef]
- Van, T.T.H.; Yidana, Z.; Smooker, P.M.; Coloe, P.J. Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. J. Glob. Antimicrob. Resist. 2020, 20, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Destaw, T.; Ayehu, M. A review on antibiotics residue in foods of animal origin. Austin J. Vet. Sci. Anim. Husb. 2022, 9, 1104. [Google Scholar] [CrossRef]
- Ohemu, G.P. Starved of ACTION: A Critical Look at the Antimicrobial Resistance Action Plans of African Countries. ACS Infect. Dis. 2022, 8, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- DACA: Drug Administration and Control Authority of Ethiopia. Antimicrobial Use, Resistance and Containment Baseline Survey Synthesis of Findings; DACA: Addis Ababa, Ethiopia, 2009.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).