A Point Mutation in Cassette Relieves the Repression Regulation of CcpA Resulting in an Increase in the Degradation of 2,3-Butanediol in Lactococcus lactis
Abstract
1. Introduction
2. Methods
2.1. Bacterial Strains, Plasmids, and Culture Conditions
2.2. Biolog Phenotype Microarray Metabolic Profiling
2.3. Real-Time Fluorescence Quantitative PCR
2.4. L. lactis N8-2 Genome Complete Map Sequencing
2.5. Detection of Promoter Activity Using Cat as a Reporter Gene
2.6. Detection of Promoter Activity Using Red Fluorescent Protein as a Reporter Gene
2.7. Analysis of the Functional Region Responsible for Initiating Transcription and Structural Characteristics of the butBA Gene Cluster
2.8. Preparation of a Fluorescent Probe DNA
2.9. Construction of Recombinant Plasmids and Subsequent Induction of Expression for Purification of the CcpA Protein
2.10. Micro-Scale Thermophoresis (MST)
2.11. Construction of a Bacterial Two-Plasmid Fluorescence Hybridization System
2.12. Determination of Growth Trends and Fluorescence Values in a Two-Plasmid Fluorescent Hybridization Assay
2.13. Determination of the Metabolism of 2,3-Butanediol at Different Concentrations
2.14. Determination of the Metabolism of 2,3-Butanediol at Optimal Concentrations
2.15. Statistical Analysis
3. Results and Discussion
3.1. Microarray Analysis of Mutants’ Phenotypes
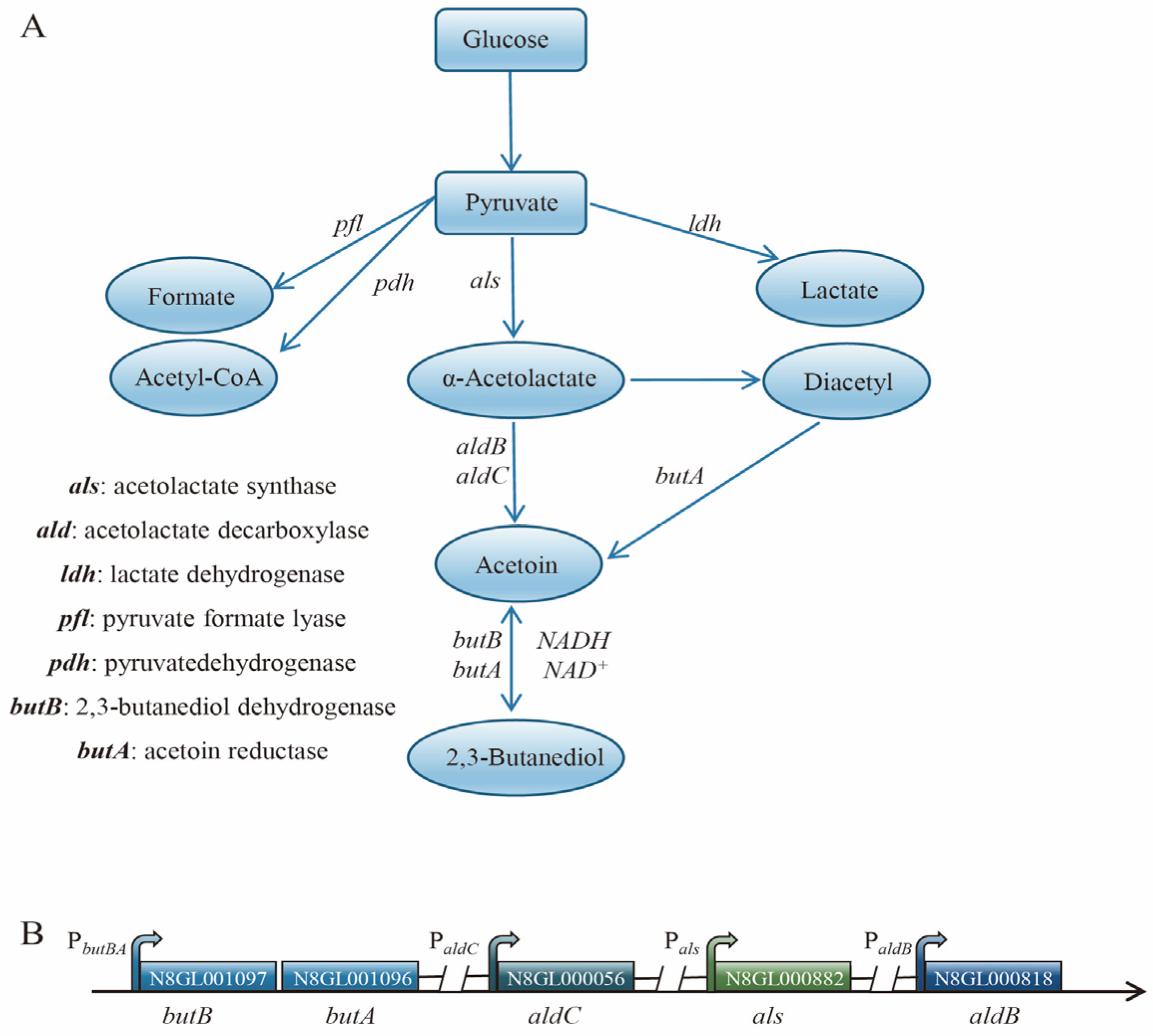
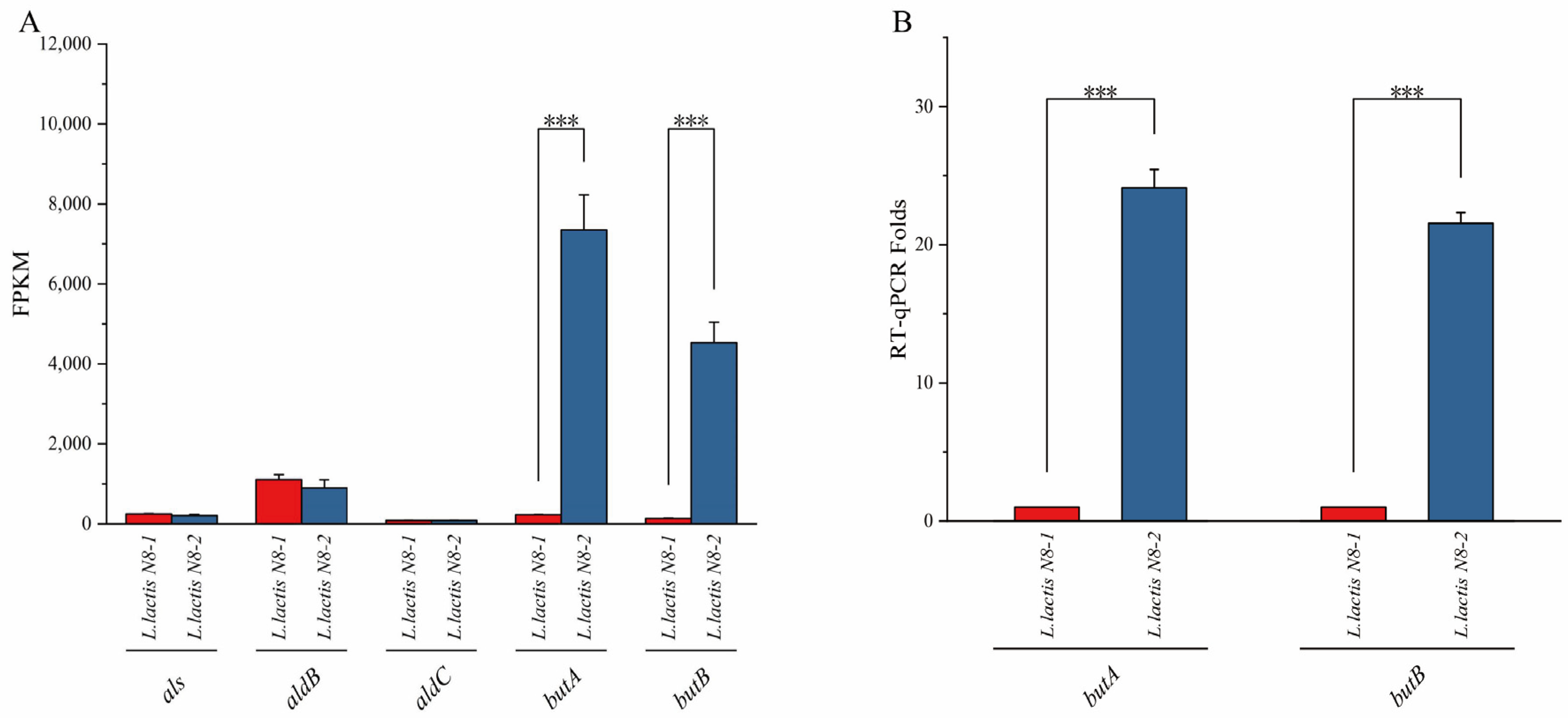
3.2. Analysis of the Metabolic Pathways of 2,3-Butanediol and the Identification of Differentially Expressed Genes in L. lactis N8
3.3. Whole-Genome Complete Map Sequencing of L. lactis N8-2
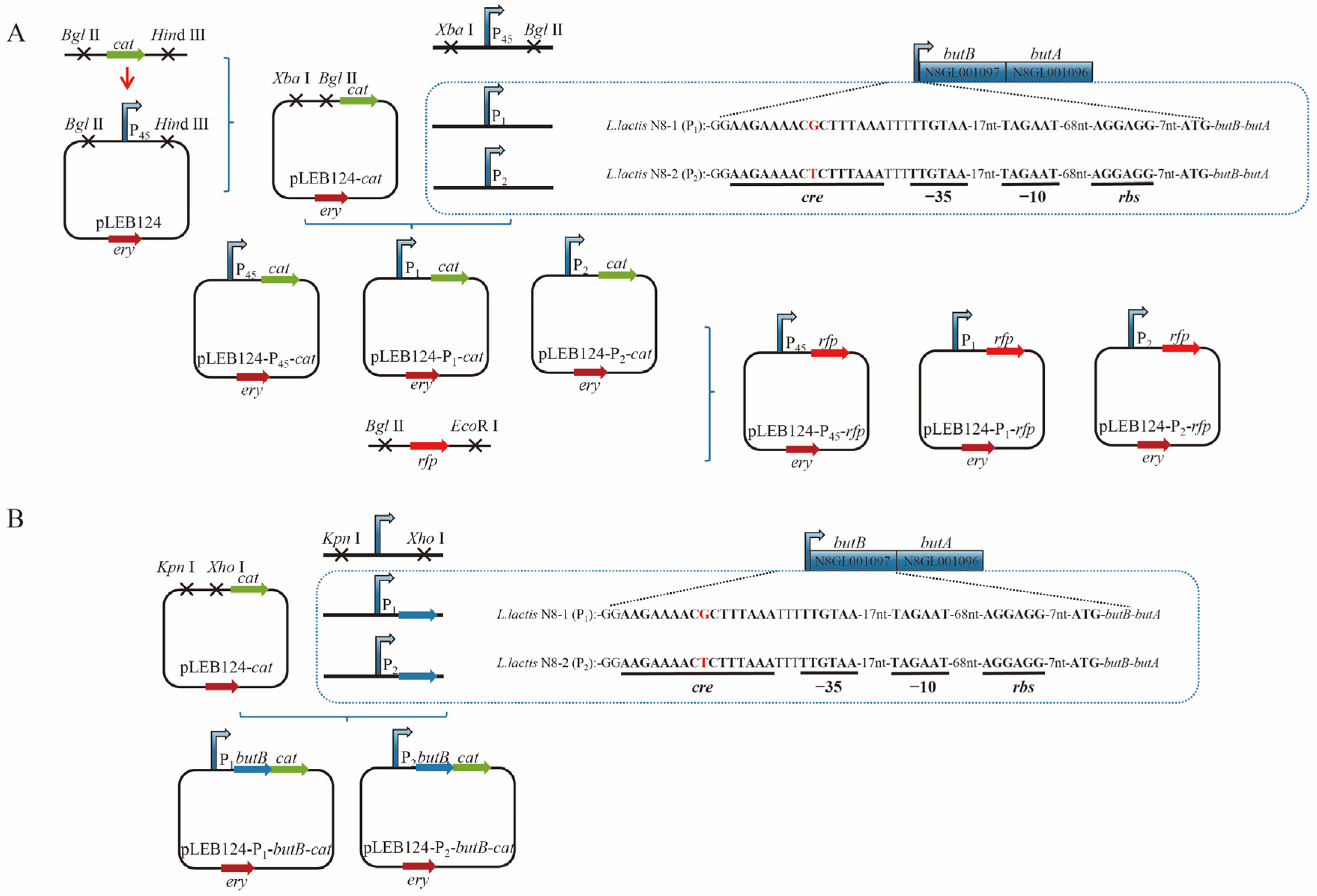
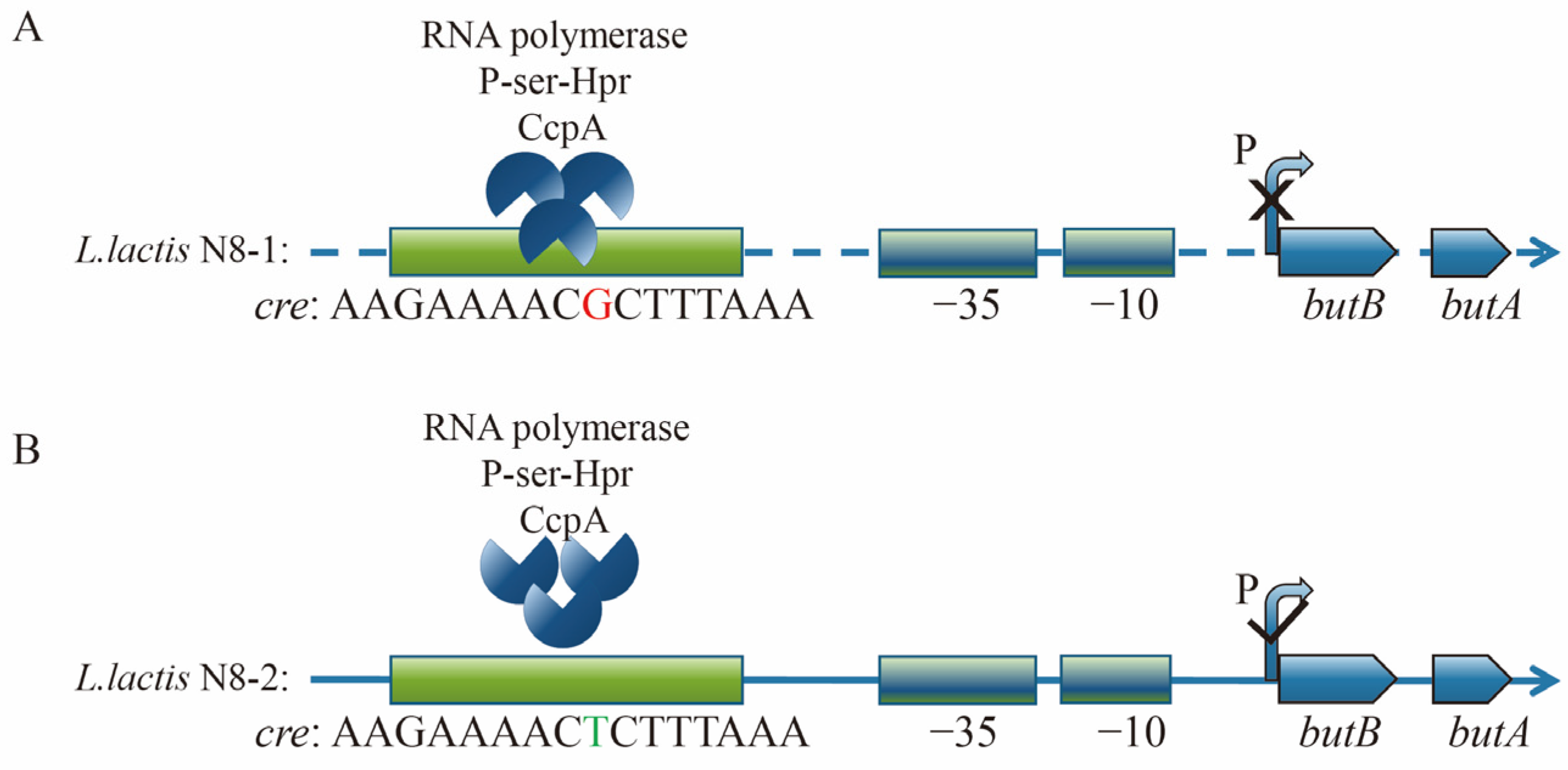
3.4. Analysis of the Promoter Region of the butBA Gene in L. lactis N8
3.5. Preliminary Testing of the Impact of Point Mutations on Promoter Activity
3.6. Structural Analysis of the Transcriptionally Active Functional Region of the butBA Gene Cluster
3.7. Mechanistic Analysis and Exploration of butBA Promoter Point Mutation Resulting in Enhanced Expression of Its Gene Cluster
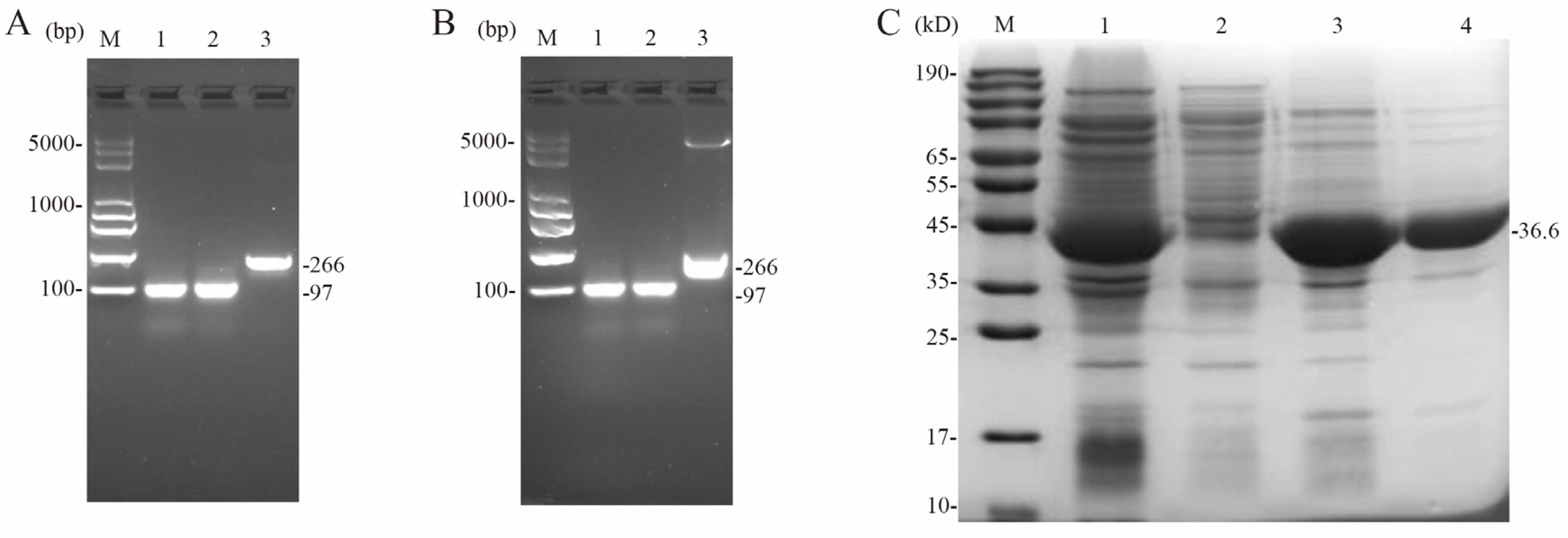
3.8. Preparation of Fluorescent Probes and Purification of CcpA Proteins
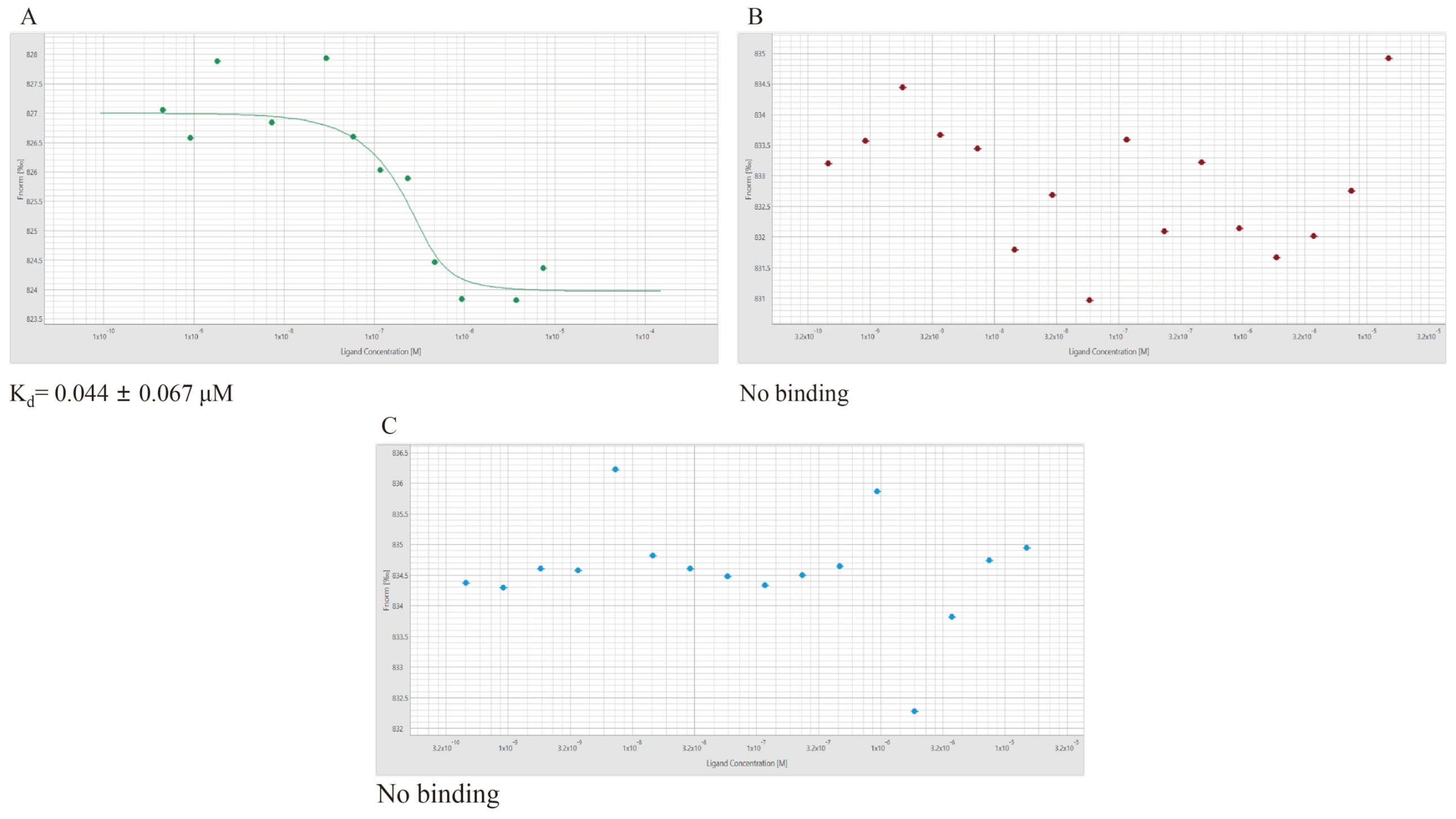
3.9. Micro-Scale Thermophoresis (MST)
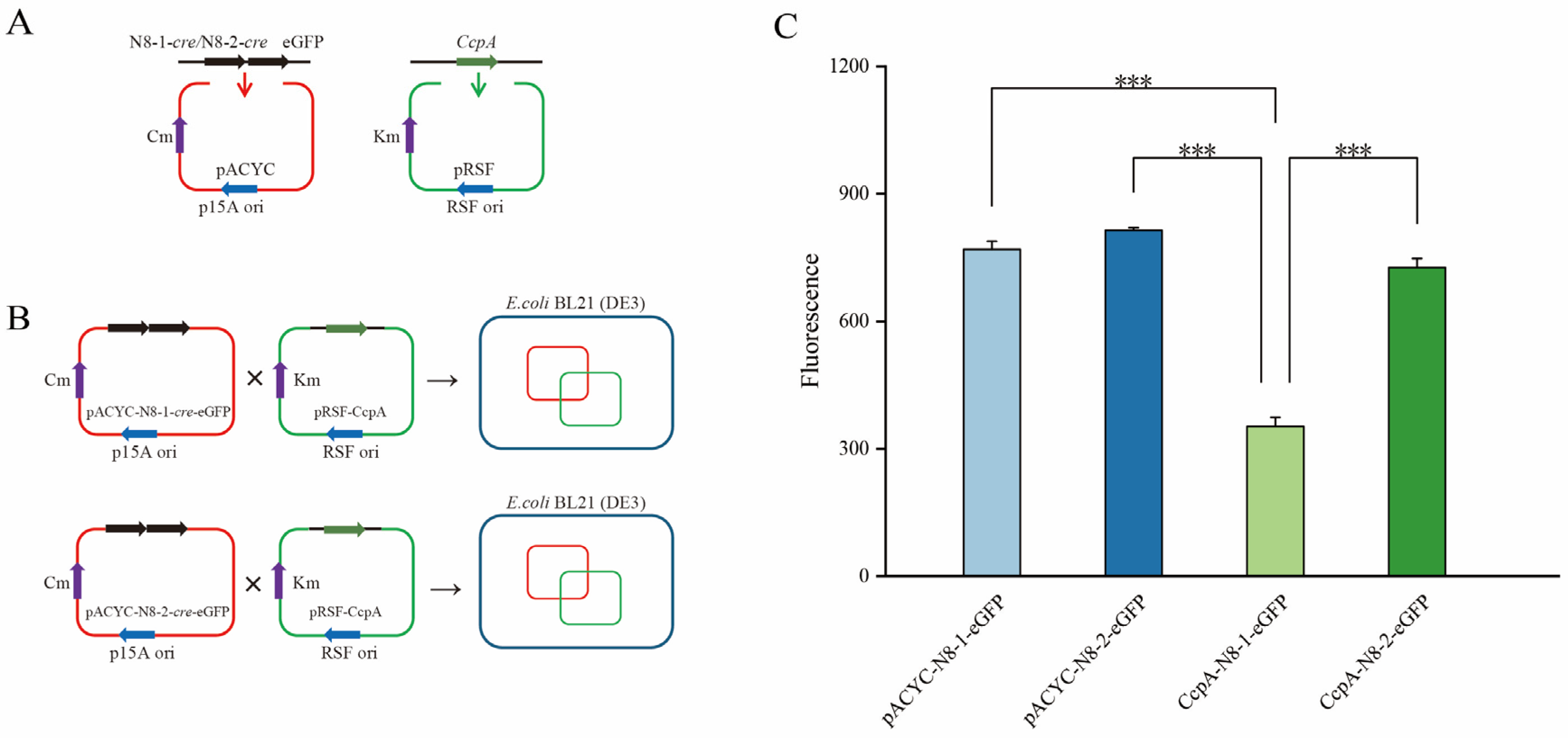
3.10. Construction of a Bacterial System for Fluorescent Hybridization Using Two Plasmids
3.11. Determination of Growth Trends and Fluorescence Values in Two-Hybrid Plasmid Strains
3.12. A Comparative Analysis of the Metabolic Responses of L. lactis N8-2 and L. lactis N8-1 to Varying Concentrations of 2,3-Butanediol
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| cre | catabolite responsive element |
| CcpA | Carbon catabolite protein A |
| cat | chloramphenicol |
| rfp | red fluorescent protein |
| MST | Micro-scale thermophoresis |
| TTC | 2,3,5-triphenyl-2H-tetrazolium chloride |
References
- Song, A.A.-L.; In, L.L.A.; Lim, S.H.E.; Rahim, R.A. A review on Lactococcus lactis: From food to factory. Microbial Cell Factories 2017, 16, 55. [Google Scholar] [CrossRef]
- Campos, G.M.; Americo, M.F.; Freitas, A.d.S.; Barroso, F.A.L.; Dutra, J.d.C.F.; Quaresma, L.S.; Cordeiro, B.F.; Laguna, J.G.; de Jesus, L.C.L.; Fontes, A.M.; et al. Lactococcus lactis as an Interleukin Delivery System for Prophylaxis and Treatment of Inflammatory and Autoimmune Diseases. Probiotics Antimicrob. Proteins 2023, 16, 352–366. [Google Scholar] [CrossRef]
- Qiao, W.; Liu, F.; Wan, X.; Qiao, Y.; Li, R.; Wu, Z.; Saris, P.E.J.; Xu, H.; Qiao, M. Genomic Features and Construction of Streamlined Genome Chassis of Nisin Z Producer Lactococcus lactis N8. Microorganisms 2022, 10, 47. [Google Scholar] [CrossRef]
- Qiao, M.Q.; Saris, P.E.J. Evidence for a role of NisT in transport of the lantibiotic nisin produced by Lactococcus lactis N8. FEMS Microbiol. Lett. 1996, 144, 89–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simsek, O.; Saris, P.E.J. Cycle changing the medium results in increased nisin productivity per cell in Lactococcus lactis. Biotechnol. Lett. 2009, 31, 415–421. [Google Scholar] [CrossRef]
- Qiao, W.; Qiao, Y.; Liu, F.; Zhang, Y.; Li, R.; Wu, Z.; Xu, H.; Saris, P.E.J.; Qiao, M. Engineering Lactococcus lactis as a multi-stress tolerant biosynthetic chassis by deleting the prophage-related fragment. Microb. Cell Factories 2020, 19, 225. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, M.F.; Cacace, G.; Peluso, A.; Zotta, T.; Muscariello, L.; Vastano, V.; Parente, E.; Siciliano, R.A. Effect of inactivation of ccpA and aerobic growth in Lactobacillus plantarum: A proteomic perspective. J. Proteom. 2012, 75, 4050–4061. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, J.W.; Zhang, G.F.; Liu, L.B. Effect of the absence of the CcpA gene on growth, metabolic production, and stress tolerance in Lactobacillus delbrueckii ssp. bulgaricus. J. Dairy Sci. 2016, 99, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zotta, T.; Ricciardi, A.; Guidone, A.; Sacco, M.; Muscariello, L.; Mazzeo, M.F.; Cacace, G.; Parente, E. Inactivation of ccpA and aeration affect growth, metabolite production and stress tolerance in Lactobacillus plantarum WCFS1. Int. J. Food Microbiol. 2012, 155, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, L.; Li, C. Effects of ccpA gene deficiency in Lactobacillus delbrueckii subsp. bulgaricus under aerobic conditions as assessed by proteomic analysis. Microb. Cell Factories 2020, 19, 9. [Google Scholar] [CrossRef]
- Presecan-Siedel, E.; Galinier, A.; Longin, R.; Deutscher, J.; Danchin, A.; Glaser, P.; Martin-Verstraete, I. Catabolite regulation of the pta gene as part of carbon flow pathways in Bacillus subtilis. J. Bacteriol. 1999, 181, 6889–6897. [Google Scholar] [CrossRef] [PubMed]
- Turinsky, A.J.; Moir-Blais, T.R.; Grundy, F.J.; Henkin, T.M. Bacillus subtilis ccpA gene mutants specifically defective in activation of acetoin biosynthesis. J. Bacteriol. 2000, 182, 5611–5614. [Google Scholar] [CrossRef]
- Peng, Q.; Zhao, X.; Wen, J.; Huang, M.; Zhang, J.; Song, F. Transcription in the acetoin catabolic pathway is regulated by AcoR and CcpA in Bacillus thuringiensis. Microbiol. Res. 2020, 235, 126438. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Lu, Y.; Yu, H.; Than, H. Comparative Transcriptional Analysis of Lactobacillus plantarum and Its ccpA-Knockout Mutant Under Galactooligosaccharides and Glucose Conditions. Front. Microbiol. 2019, 10, 1584. [Google Scholar] [CrossRef] [PubMed]
- Catone, M.V.; Palomino, M.M.; Legisa, D.M.; Fina Martin, J.; Monedero Garcia, V.; Ruzal, S.M.; Allievi, M.C. Lactic acid production using cheese whey based medium in a stirred tank reactor by a ccpA mutant of Lacticaseibacillus casei. World J. Microbiol. Biotechnol. 2021, 37, 61. [Google Scholar] [CrossRef] [PubMed]
- Monedero, V.; Maze, A.; Boel, G.; Zuniga, M.; Beaufils, S.; Hartke, A.; Deutscher, J. The phosphotransferase system of Lactobacillus casei: Regulation of carbon metabolism and connection to cold shock response. J. Mol. Microbiol. Biotechnol. 2007, 12, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Price, C.E.; dos Santos, F.B.; Hesseling, A.; Uusitalo, J.J.; Bachmann, H.; Benavente, V.; Goel, A.; Berkhout, J.; Bruggeman, F.J.; Marrink, S.-J.; et al. Adaption to glucose limitation is modulated by the pleotropic regulator CcpA, independent of selection pressure strength. BMC Evol. Biol. 2019, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrzak-Piekarczyk, T.; Polak, J.; Jezierska, B.; Renault, P.; Bardowski, J. Genetic characterization of the CcpA-dependent, cellobiose-specific PTS system comprising CelB, PtcB and PtcA that transports lactose in Lactococcus lactis IL1403. Int. J. Food Microbiol. 2011, 145, 186–194. [Google Scholar] [CrossRef]
- Maina, S.; Prabhu, A.A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on bio-based 2,3-butanediol and acetoin production: Recent progress and advances. Biotechnol. Adv. 2022, 54, 107783. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Bae, J.-M.; Kim, S.-J. Enantiopure meso-2,3-butanediol production by metabolically engineered Saccharomyces cerevisiae expressing 2,3-butanediol dehydrogenase from Klebsiella oxytoca. J. Biotechnol. 2022, 354, 1–9. [Google Scholar] [CrossRef]
- Kim, B.; Park, A.R.; Song, C.W.; Song, H.; Kim, J.-C. Biological Control Efficacy and Action Mechanism of Klebsiella pneumoniae JCK-2201 Producing Meso-2,3-Butanediol Against Tomato Bacterial Wilt. Front. Microbiol. 2022, 13, 914589. [Google Scholar] [CrossRef] [PubMed]
- Petrova, P.; Petlichka, S.; Petrov, K. New Bacillus spp. with potential for 2,3-butanediol production from biomass. J. Biosci. Bioeng. 2020, 130, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Gao, T.; Bai, R.; Yang, J.; Xu, Y.; Chu, W.; Jiang, K.; Zhang, J.; Xu, F.; Zhao, H. Regulation of carbon flux and NADH/NAD plus supply to enhance 2,3-buta-nediol production in Enterobacter aerogenes. J. Biotechnol. 2022, 358, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, X.; Tong, T.; Ge, J. Acetic acid acting as a signaling molecule in the quorum sensing system increases 2,3-butanediol production in Saccharomyces cerevisiae. Prep. Biochem. Biotechnol. 2022, 52, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Gao, S.; Yu, Y.; Yang, H. Microbial production of 2,3-butanediol by a newly-isolated strain of Serratia marcescens. Biotechnol. Lett. 2014, 36, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, V.; Liu, J.; Dantoft, S.H.; Solem, C.; Jensen, P.R. Synthesis of (3R)-acetoin and 2,3-butanediol isomers by metabolically engineered Lactococcus lactis. Sci. Rep. 2016, 6, 36769. [Google Scholar] [CrossRef]
- Garcia-Quintans, N.; Repizo, G.; Martin, M.; Magni, C.; Lopez, P. Activation of the diacetyl/acetoin pathway in Lactococcus lactis subsp lactis bv. diacetylactis CRL264 by acidic growth. Appl. Environ. Microbiol. 2008, 74, 1988–1996. [Google Scholar] [CrossRef]
- Qiao, M.Q.; Immonen, T.; Koponen, O.; Saris, P.E.J. The Cellular Location and Effect on Nisin Immunity of the Nisi Protein from Lactococcus-Lactis N8 Expressed in Escherichia-Coli and L-Lactis. FEMS Microbiol. Lett. 1995, 131, 75–80. [Google Scholar] [CrossRef]
- Tsigkrimani, M.; Bakogianni, M.; Paramithiotis, S.; Bosnea, L.; Pappa, E.; Drosinos, E.H.; Skandamis, P.N.; Mataragas, M. Microbial Ecology of Artisanal Feta and Kefalograviera Cheeses, Part I: Bacterial Community and Its Functional Characteristics with Focus on Lactic Acid Bacteria as Determined by Culture-Dependent Methods and Phenotype Microarrays. Microorganisms 2022, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Preston-Mafham, J.; Boddy, L.; Randerson, P.F. Analysis of microbial community functional diversity using sole-carbon-source utilisation profiles—A critique. FEMS Microbiol. Ecol. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Garland, J.L.; Mills, A.L. Classification and Characterization of Heterotrophic Microbial Communities on the Basis of Patterns of Community-Level Sole-Carbon-Source Utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Neves, A.R.; Fonseca, L.L.; Pool, W.A.; Kok, J.; Kuipers, O.P.; Santos, H. Characterization of the individual glucose uptake systems of Lactococcus lactis: Mannose-PTS, cellobiose-PTS and the novel GlcU permease. Mol. Microbiol. 2009, 71, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Sibakov, M.; Koivula, T.; Vonwright, A.; Palva, I. Secretion of Tem Beta-Lactamase with Signal Sequences Isolated from the Chromosome of Lactococcus-Lactis Subsp Lactis. Appl. Environ. Microbiol. 1991, 57, 341–348. [Google Scholar] [CrossRef]
- Del Egido, L.L.; Navarro-Miro, D.; Martinez-Heredia, V.; Toorop, P.E.; Iannetta, P.P.M. A Spectrophotometric Assay for Robust Viability Testing of Seed Batches Using 2,3,5-Triphenyl Tetrazolium Chloride: Using Hordeum vulgare L. as a Model. Front. Plant Sci. 2017, 8, 747. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Zapata, L.; Alvarez, J.C.; Romero-Tabarez, M.; Silby, M.W.; Traxler, M.F.; Behie, S.W.; Pessotti, R.d.C.; Villegas-Escobar, V. Inducible Antibacterial Activity in the Bacillales by Triphenyl Tetrazolium Chloride. Sci. Rep. 2020, 10, 5563. [Google Scholar] [CrossRef]
- Connor, C.H.; Zucoloto, A.Z.; Munnoch, J.T.; Yu, I.-L.; Corander, J.; Hoskisson, P.A.; McDonald, B.; McNally, A. Multidrug-resistant E. coli encoding high genetic diversity in carbohydrate metabolism genes displace commensal E. coli from the intestinal tract. PLoS Biol. 2023, 21, e3002329. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.H.; Rathnasingh, C.; Lee, H.J.; Seung, D. Identification of acetoin reductases involved in 2,3-butanediol pathway in Klebsiella oxytoca. J. Biotechnol. 2014, 172, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Feklistov, A.; Barinova, N.; Sevostyanova, A.; Heyduk, E.; Bass, I.; Vvedenskaya, I.; Kuznedelov, K.; Merkiene, E.; Stavrovskaya, E.; Klimasauskas, S.; et al. A basal promoter element recognized by free RNA polymerase σ subunit determines promoter recognition by RNA polymerase holoenzyme. Mol. Cell 2006, 23, 97–107. [Google Scholar] [CrossRef]
- Novichkov, P.S.; Kazakov, A.E.; Ravcheev, D.A.; Leyn, S.A.; Kovaleva, G.Y.; Sutormin, R.A.; Kazanov, M.D.; Riehl, W.; Arkin, A.P.; Dubchak, I.; et al. RegPrecise 3.0-A resource for genome-scale exploration of transcriptional regulation in bacteria. BMC Genom. 2013, 14, 745. [Google Scholar] [CrossRef]
- Zomer, A.L.; Buist, G.; Larsen, R.; Kok, J.; Kuipers, O.P. Time-resolved determination of the CcpA regulon of Lactococcus lactis subsp cremoris MG1363. J. Bacteriol. 2007, 189, 1366–1381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, T.; Qin, C.; Xu, H.; Qiao, M. A Novel Non-Coding RNA CsiR Regulates the Ciprofloxacin Resistance in Proteus vulgaris by Interacting with emrB mRNA. Int. J. Mol. Sci. 2021, 22, 10627. [Google Scholar] [CrossRef]
- Tachon, S.; Michelon, D.; Chambellon, E.; Cantonnet, M.; Mezange, C.; Henno, L.; Cachon, R.; Yvon, M. Experimental conditions affect the site of tetrazolium violet reduction in the electron transport chain of Lactococcus lactis. Microbiology 2009, 155, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Browning, D.F.; Butala, M.; Busby, S.J.W. Bacterial Transcription Factors: Regulation by Pick “N” Mix. J. Mol. Biol. 2019, 431, 4067–4077. [Google Scholar] [CrossRef] [PubMed]
- Bulock, L.L.; Ahn, J.; Shinde, D.; Pandey, S.; Sarmiento, C.; Thomas, V.C.; Guda, C.; Bayles, K.W.; Sadykov, M.R. Interplay of CodY and CcpA in Regulating Central Metabolism and Biofilm Formation in Staphylococcus aureus. J. Bacteriol. 2022, 204, e0061721. [Google Scholar] [CrossRef] [PubMed]
- Seidel, G.; Diel, M.; Fuchsbauer, N.; Hillen, W. Quantitative interdependence of coeffectors, CcpA and cre in carbon catabolite regulation of Bacillus subtilis. FEBS J. 2005, 272, 2566–2577. [Google Scholar] [CrossRef] [PubMed]
- Lanza, A.M.; Alper, H.S. Global strain engineering by mutant transcription factors. Methods Mol. Biol. 2011, 765, 253–274. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, Y.; Wang, J. Engineering of transcriptional regulators enhances microbial stress tolerance. Biotechnol. Adv. 2013, 31, 986–991. [Google Scholar] [CrossRef]
- Cai, D.; Zhu, J.; Zhu, S.; Lu, Y.; Zhang, B.; Lu, K.; Ma, J.; Ma, X.; Chen, S. Metabolic Engineering of Main Transcription Factors in Carbon, Nitrogen, and Phosphorus Metabolisms for Enhanced Production of Bacitracin in Bacillus licheniformis. ACS Synth. Biol. 2019, 8, 866–875. [Google Scholar] [CrossRef] [PubMed]



| Strains or Plasmids | Relevant Descriptions | Reference |
|---|---|---|
| Strains | ||
| L. lactis N8 | Wild-type (WT) Nisin Z producer | [28] |
| L. lactis N8-1 | The first DNA region L1 deletion in L. lactis N8 | [6] |
| L. lactis N8-2 | The L2 deletion in L. lactis N8-1 | Lab stork |
| E. coli DH5α | Cloning host; F-φ80 lacZΔM15 endA1 recA1 endA1 hsdR17 (rK-mK+) supE44 thi-1 gyrA 96 relA1 Δ(lacZYA-argF)U169 deoR λ- | Lab stork |
| BL21(DE3) | F-ompT hsdSB(rB-m-) gal dcm (DE3) | Lab stork |
| N8-1-P45-cat | Emr, L. lactis N8-1 derivative containing pLEB124-P45-cat | This study |
| N8-1-P1-cat | Emr, L. lactis N8-1 derivative containing pLEB124-P1-cat | This study |
| N8-1-P2-cat | Emr, L. lactis N8-1 derivative containing pLEB124-P2-cat | This study |
| N8-2-P45-cat | Emr, L. lactis N8-2 derivative containing pLEB124-P45-cat | This study |
| N8-2-P1-cat | Emr, L. lactis N8-2 derivative containing pLEB124-P1-cat | This study |
| N8-2-P2-cat | Emr, L. lactis N8-2 derivative containing pLEB124-P2-cat | This study |
| N8-1-P45-rfp | Emr, L. lactis N8-1 derivative containing pLEB124-P45-rfp | This study |
| N8-1-P1-rfp | Emr, L. lactis N8-1 derivative containing pLEB124-P1-rfp | This study |
| N8-1-P2-rfp | Emr, L. lactis N8-1 derivative containing pLEB124-P2-rfp | This study |
| N8-2-P45-rfp | Emr, L. lactis N8-2 derivative containing pLEB124-P45-rfp | This study |
| N8-2-P1-rfp | Emr, L. lactis N8-2 derivative containing pLEB124-P1-rfp | This study |
| N8-2-P2-rfp | Emr, L. lactis N8-2 derivative containing pLEB124-P2-rfp | This study |
| N8-1-P1-butB-cat | Emr, L. lactis N8-1 derivative containing pLEB124-P1-butB-cat | This study |
| N8-1-P2-butB-cat | Emr, L. lactis N8-1 derivative containing pLEB124-P2-butB-cat | This study |
| N8-2-P1-butB-cat | Emr, L. lactis N8-2 derivative containing pLEB124-P1-butB-cat | This study |
| N8-2-P2-butB-cat | Emr, L. lactis N8-2 derivative containing pLEB124-P1-butB-cat | This study |
| pACYC-N8-1-cre-rfp | Cmr, E. coli BL21 containing pACYC-N8-1-cre-rfp | This study |
| pACYC-N8-2-cre-rfp | Cmr, E. coli BL21 containing pACYC-N8-2-cre-rfp | This study |
| CcpA-N8-1-cre-eGFP | Kanr, Cmr, E. coli BL21containing pRSF-CcpA and pACYC-N8-1-cre-rfp | This study |
| CcpA-N8-2-cre-eGFP | Kanr, Cmr, E. coli BL21containing pRSF-CcpA and pACYC-N8-2-cre-rfp | This study |
| Plasmids | ||
| pEASY-T1 | Ampr, Blue-white screening vector | TransGen, Beijing, China |
| pLEB124 | Emr, expression vector | [28] |
| pETm3c | Ampr, expression vector | Lab stork |
| pNZ8048-rfp | Cmr, pNZ8048 derivative containing the rfp gene | Lab stork |
| pRSF-Dute | Kanr, pRSF-Duet is designed for the coexpression of two target ORFs. | Lab stork |
| pACYC-eGFP | Cmr, pACYC-eGFP is designed for the coexpression of two target genes. | Lab stork |
| pETm3c-CcpA | Ampr, pETm3c derivative containing the CcpA gene | This study |
| pEASY-N8-1-cre | Ampr, pEASY-T1 derivative containing the N8-1-cre gene | This study |
| pEASY-N8-2-cre | Ampr, pEASY-T1 derivative containing the N8-2-cre gene | This study |
| pEASY-N8-16s | Ampr, pEASY-T1 derivative containing the N8-16s gene | This study |
| pACYC-N8-1-cre-eGFP | Cmr, pACYC-eGFP derivative containing the N8-1-cre gene | This study |
| pACYC-N8-2-cre-eGFP | Cmr, pACYC-eGFP derivative containing the N8-2-cre gene | This study |
| pRSF-CcpA | Kanr, pRSF-Dute derivative containing the CcpA gene | This study |
| pLEB124-P45-cat | Emr, pLEB124 derivative containing the P45 and cat cassette | This study |
| pLEB124-P1-cat | Emr, pLEB124 derivative containing the P1 and cat cassette | This study |
| pLEB124-P2-cat | Emr, pLEB124 derivative containing the P2 and cat cassette | This study |
| pLEB124-P45-rfp | Emr, pLEB124 derivative containing the P45 and rfp cassette | This study |
| pLEB124-P1-rfp | Emr, pLEB124 derivative containing the P1 and rfp cassette | This study |
| pLEB124-P2-rfp | Emr, pLEB124 derivative containing the P2 and rfp cassette | This study |
| pLEB124-P1-butB-cat | Emr, pLEB124 derivative containing the P1 and butB and cat cassette | This study |
| pLEB124-P2-butB-cat | Emr, pLEB124 derivative containing the P2 and butB and cat cassette | This study |
| Primer | Primer Sequence (5′-3′) |
|---|---|
| cat-clone-F | GCTAGATCTAGGCATATCAAATGAA |
| cat-clone-R | GCTAAGCTTCCAATCATCAATTGCG |
| P45-F | GCTTCTAGACGTTAGGGGCTTGAAC |
| P45-R | GCTAGATCTTTTTCACTATTCTAGGTTCC |
| rfp-clone-F | CTAGATCTCTCGAGATGGTTTCAAAAGGTGAAG |
| rfp-clone-R | GCCGAATTCCAATTGAACGTTTCAAGCC |
| PbutBA-F | GCTTCTAGACAATTAATAAGAATACAAAAAGTAA |
| PbutBA-R | GCTGCTAGATCT AAAAACGCCTCCTCTAT |
| PbutBA-butB-F | GCTGGTACCCAATTAATAAGAATACAAAAAGTAA |
| PbutBA-butB-R | GCTCTCGAGTTTATAGACCTTTACCAGTTG |
| CcpA-F | CGGGATCCATGGTAGAATCAACAACAACAAT |
| CcpA-R | CGAGCTCTTTGGTAGAACGACGAGAAAAGA |
| Cy5′ | AGCCAGTGGCGATAAG |
| N8-1/N8-2-cre-F | AGCCAGTGGCGATAAGTACGATAAAATATTTAAAAGTCT |
| N8-1/N8-2-cre-R | CTATCATTATTTCTAATAATTTAC |
| 16s-F | AGCCAGTGGCGATAAGGCGTTAGCTGCGATACAG |
| 16s-R | CATGTGTAGCGGTGAAATG |
| N8-1-cre-rfp-F | CGGGATCCCCAAGGAAGAAAACGCTTTAAATTTTTATGGTGAGCAAGGGCGAGGAGG |
| N8-2-cre-rfp-F | CGGGATCCCCAAGGAAGAAAACTCTTTAAATTTTTATGGTGAGCAAGGGCGAGGAGG |
| For the RT-qPCR | |
| Q-tufA-F | GACCTCTTGAGCGAATACGACT |
| Q-tufA-R | TTCTTCAACTTTAGCAACCCATT |
| Q-butA-F | ATTATCAACGCAACCTCACAAGC |
| Q-butA-R | TCCCCATTCATCATCTTTACCAG |
| Q-butB-F | CCGTCAGCAGAACATCCTAATC |
| Q-butB-R | TCAGCCAATCCTCCACCAT |
| T7-F | TAATACGACTCACTATAGGG |
| T7-R | TGCTAGTTATTGCTCAGCGG |
| Recombinant Plasmid | Host Bacterium MIC (μg/mL) | |
|---|---|---|
| L. lactis N8-1 | L. lactis N8-2 | |
| pLEB124-P45-cat | 10 | 10 |
| pLEB124-P1-cat | 2.5 | 2.5 |
| pLEB124-P2-cat | 2.5 | 2.5 |
| Recombinant Plasmid | Host Bacterium MIC (μg/mL) | |
|---|---|---|
| L. lactis N8-1 | L. lactis N8-2 | |
| pLEB124-P45-cat | 10 | 10 |
| pLEB124-P1-butB-cat | 7.5 | 7.5 |
| pLEB124-P2-butB-cat | 80 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Liu, F.; Qiao, W.; Dong, Y.; Yang, H.; Liu, F.; Xu, H.; Qiao, M. A Point Mutation in Cassette Relieves the Repression Regulation of CcpA Resulting in an Increase in the Degradation of 2,3-Butanediol in Lactococcus lactis. Microorganisms 2024, 12, 773. https://doi.org/10.3390/microorganisms12040773
Xu X, Liu F, Qiao W, Dong Y, Yang H, Liu F, Xu H, Qiao M. A Point Mutation in Cassette Relieves the Repression Regulation of CcpA Resulting in an Increase in the Degradation of 2,3-Butanediol in Lactococcus lactis. Microorganisms. 2024; 12(4):773. https://doi.org/10.3390/microorganisms12040773
Chicago/Turabian StyleXu, Xian, Fulu Liu, Wanjin Qiao, Yujie Dong, Huan Yang, Fengming Liu, Haijin Xu, and Mingqiang Qiao. 2024. "A Point Mutation in Cassette Relieves the Repression Regulation of CcpA Resulting in an Increase in the Degradation of 2,3-Butanediol in Lactococcus lactis" Microorganisms 12, no. 4: 773. https://doi.org/10.3390/microorganisms12040773
APA StyleXu, X., Liu, F., Qiao, W., Dong, Y., Yang, H., Liu, F., Xu, H., & Qiao, M. (2024). A Point Mutation in Cassette Relieves the Repression Regulation of CcpA Resulting in an Increase in the Degradation of 2,3-Butanediol in Lactococcus lactis. Microorganisms, 12(4), 773. https://doi.org/10.3390/microorganisms12040773





