Insights into Chitin-Degradation Potential of Shewanella khirikhana JW44 with Emphasis on Characterization and Function of a Chitinase Gene SkChi65
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain Source, Genome Sequencing, and Functional Annotation
2.2. Analysis of Genes Associated with Chitin Degradation
2.3. Gene Cloning
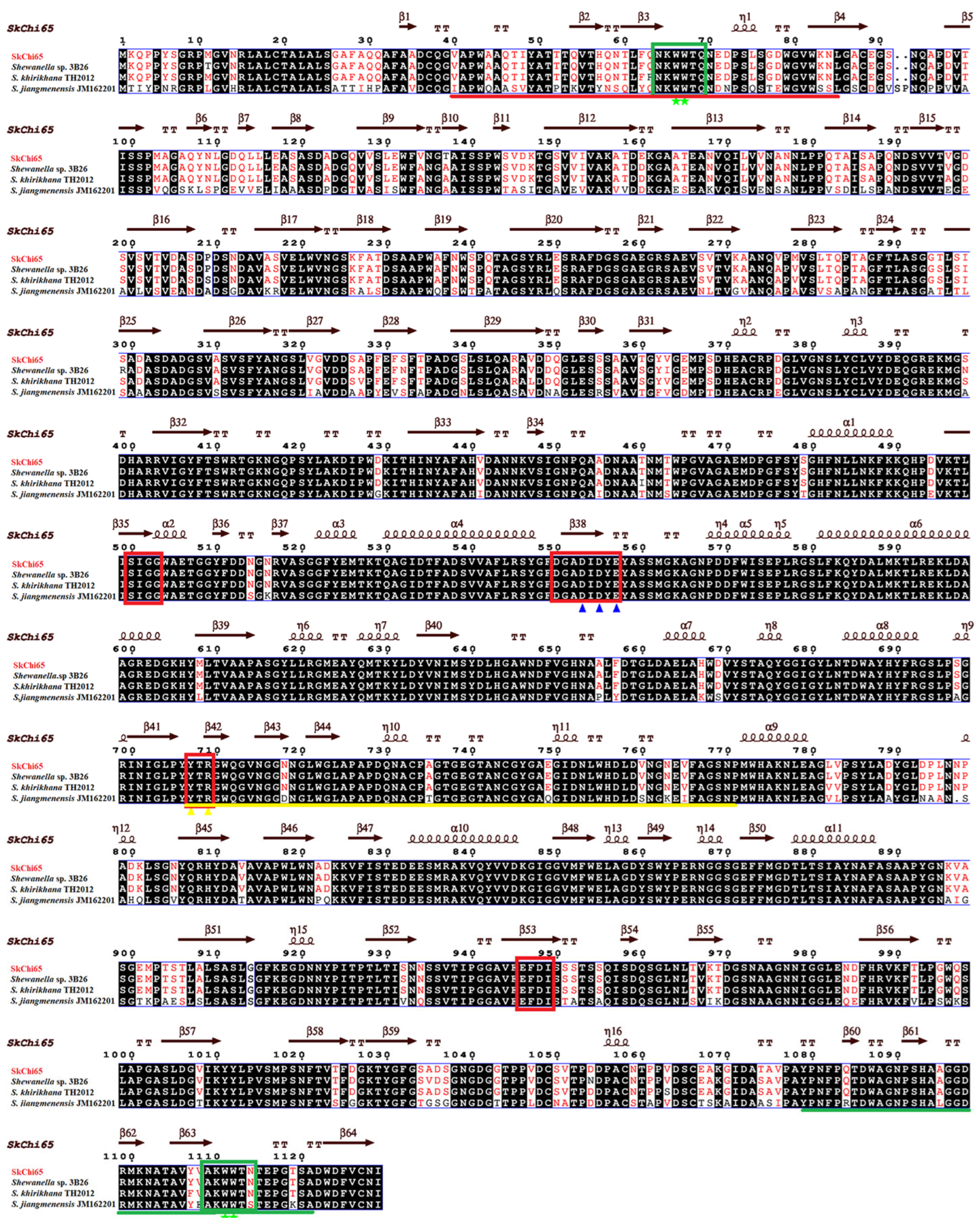
2.4. Bioinformatic Analysis
2.5. Optimization of Expression
2.6. Analysis of Solubility and Purification
2.7. Enzyme Activity Assay
2.8. Biochemical Characterization
2.9. Analysis of Hydrolysis Products from Chitooligomers
3. Results
3.1. Analysis of Chitin-Utilization Genes in the JW44 Genome
3.2. Phylogeny of SkChi65 with Other Chitinases
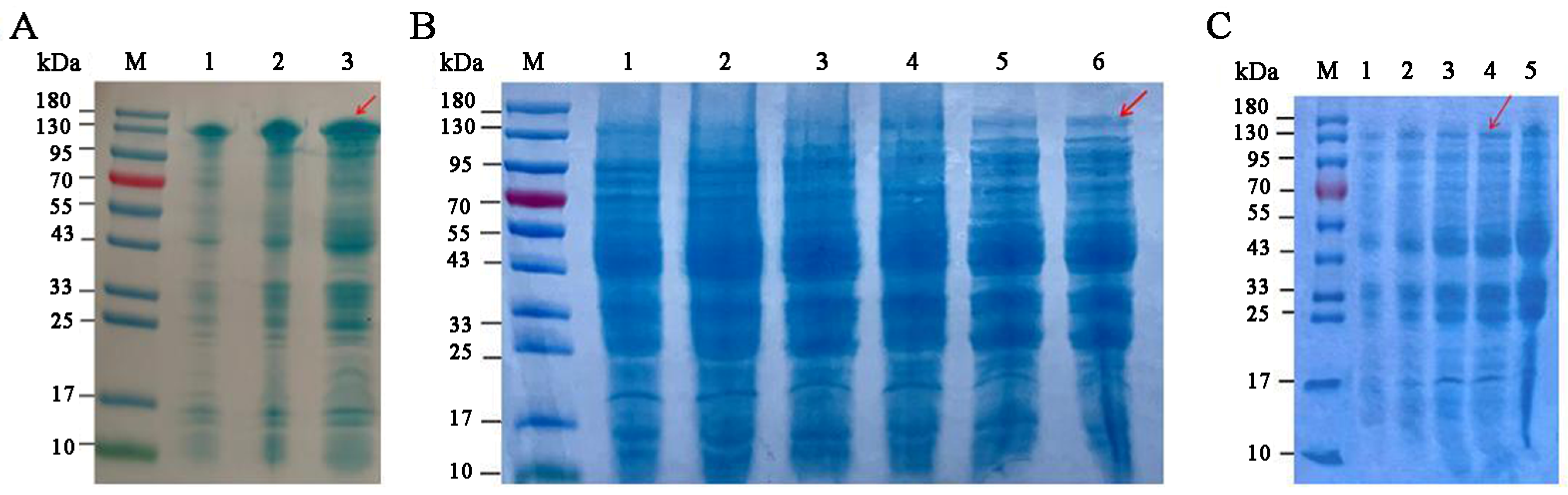
3.3. Optimization of SkChi65 Expression
3.4. Solubility and Purification of SkChi65
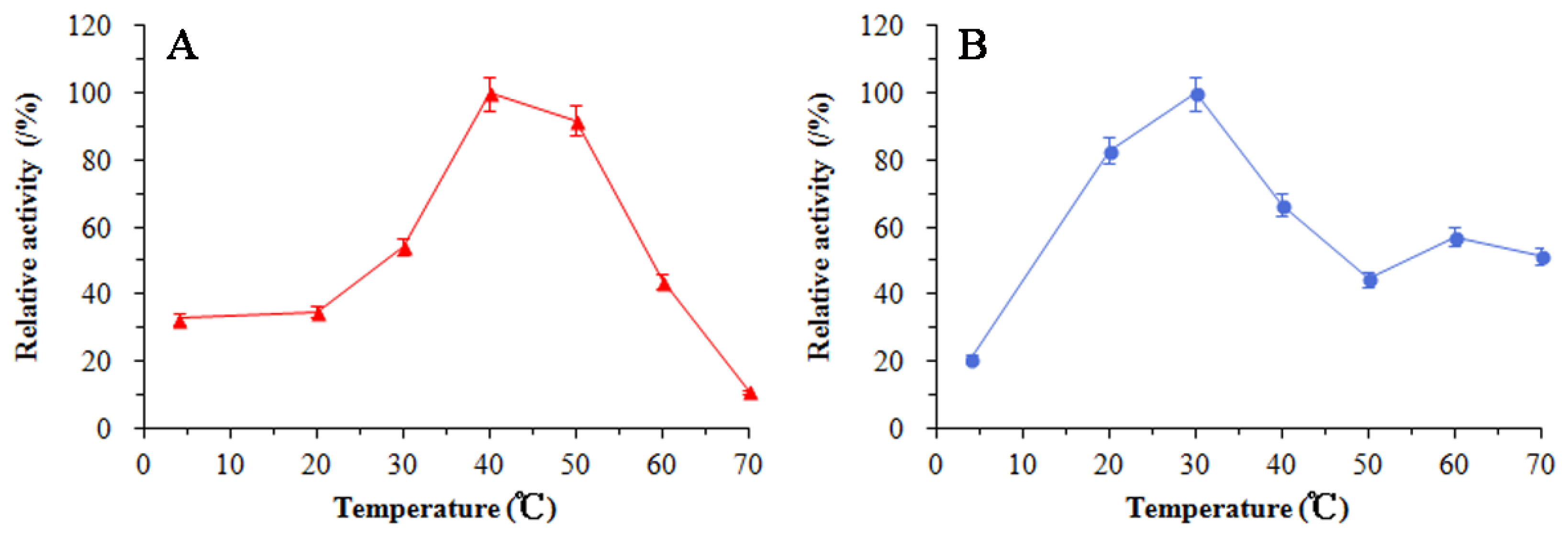
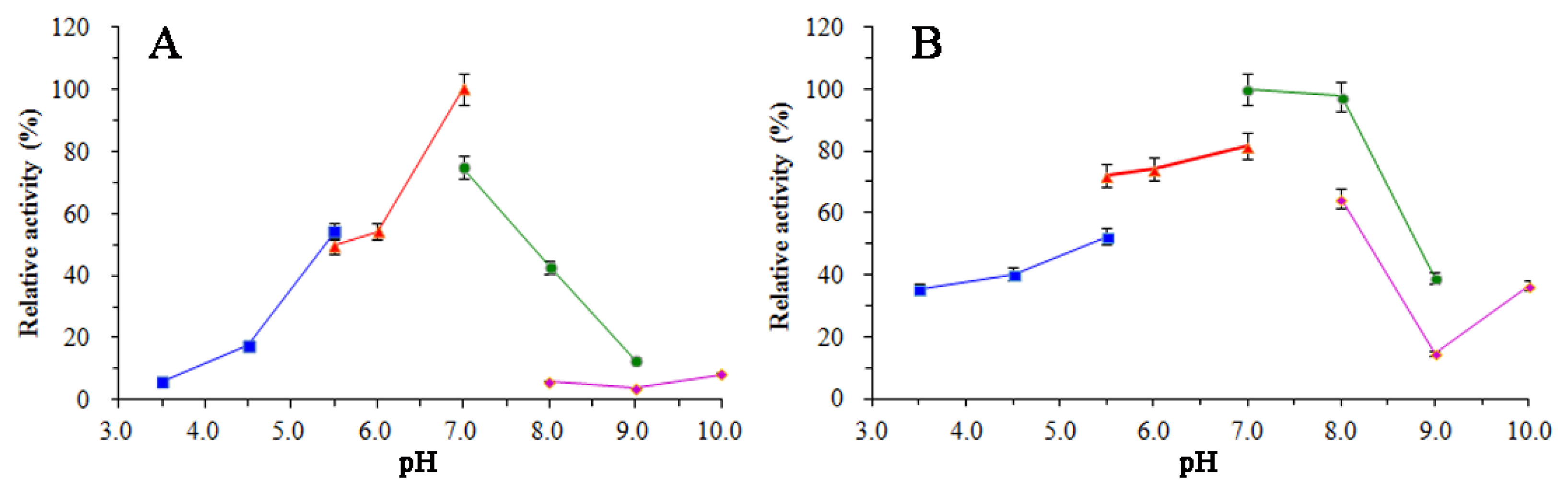
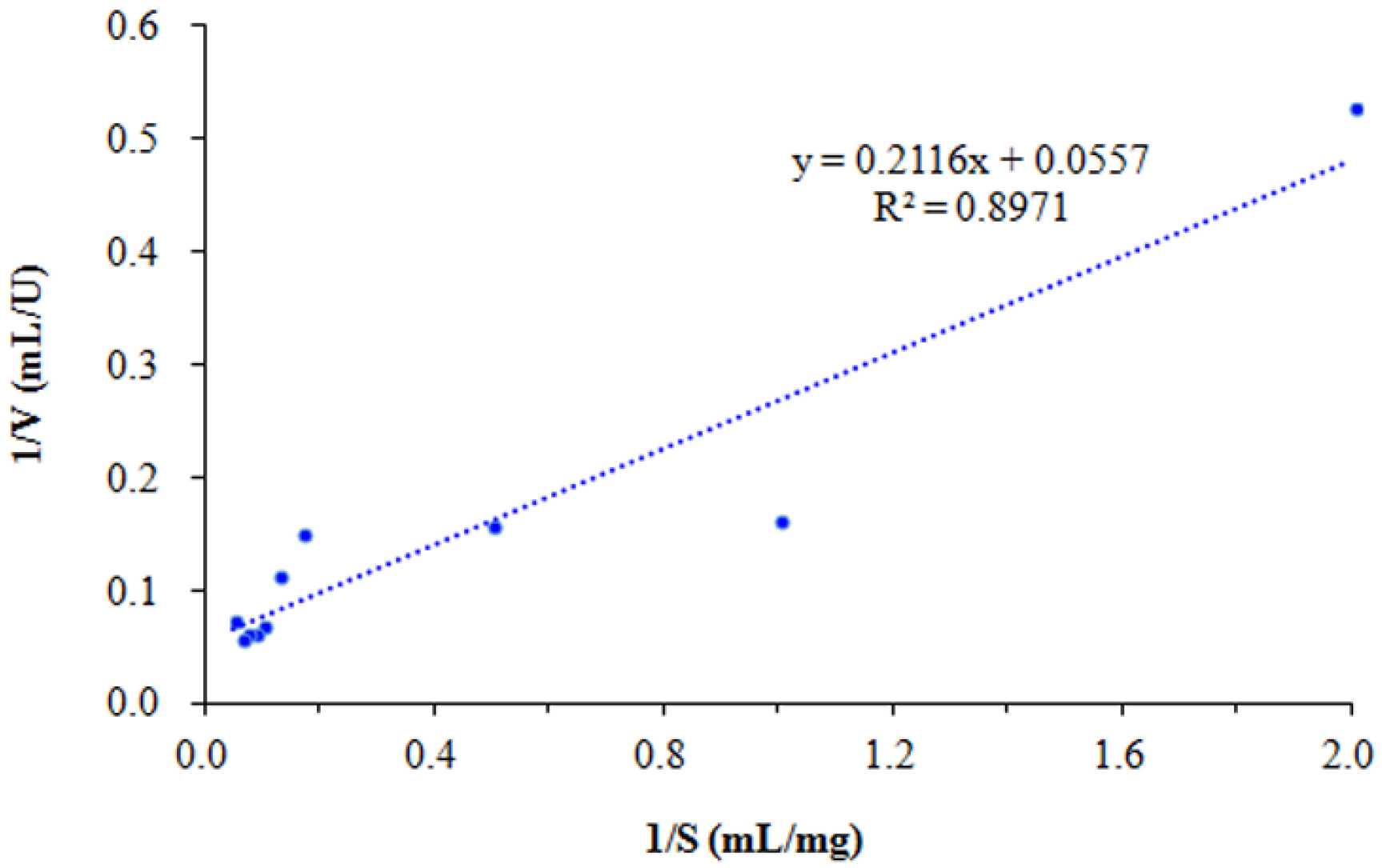
3.5. Enzymatic Properties of SkChi65
3.6. Analysis of Substrate Specificity and Hydrolysis Product
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.N.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and eco-friendly approaches for the extraction of chitin and chitosan: A review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef] [PubMed]
- Meibom, K.L.; Li, X.B.; Nielsen, A.T.; Wu, C.Y.; Roseman, S.; Schoolnik, G.K. The Vibrio cholerae chitin utilization program. Proc. Natl. Acad. Sci. USA 2004, 101, 2524–2529. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, S.T.; Barzkar, N. Marine bacterial chitinase as sources of energy, eco-friendly agent, and industrial biocatalyst. Int. J. Biol. Macromol. 2018, 120, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Schmitz, C.; González Auza, L.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of chitin to defined chitosan oligomers: Current status and future prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef] [PubMed]
- Taokaew, S.; Kriangkrai, W. Chitinase-assisted bioconversion of chitinous waste for development of value-added chito-oligosaccharides products. Biology 2023, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Oyeleye, A.; Normi, Y.M. Chitinase: Diversity, limitations, and trends in engineering for suitable applications. Biosci. Rep. 2018, 38, BSR2018032300. [Google Scholar] [CrossRef] [PubMed]
- Poria, V.; Rana, A.; Kumari, A.; Grewal, J.; Pranaw, K.; Singh, S. Current perspectives on chitinolytic enzymes and their agro-industrial applications. Biology 2021, 10, 1319. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Yang, S.H. Microbial chitinases: Properties, current state and biotechnological applications. World J. Microbiol. Biotechnol. 2019, 35, 144. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Microbial chitinases: Properties, enhancement and potential applications. Protoplasma 2021, 258, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Greene, L.H. Sequence and structural analysis of the chitinase insertion domain reveals two conserved motifs involved in chitin-binding. PLoS ONE 2010, 5, e8654. [Google Scholar] [CrossRef] [PubMed]
- Swiontek Brzezinska, M.; Jankiewicz, U.; Burkowska, A.; Walczak, M. Chitinolytic microorganisms and their possible application in environmental protection. Curr. Microbiol. 2014, 68, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.B.; Dang, Y.R.; Liu, S.S.; Huang, K.X.; Qin, Q.L.; Chen, X.L.; Zhang, Y.Z.; Wang, Y.J.; Li, P.Y. Identification and characterization of three chitinases with potential in direct conversion of crystalline chitin into N,N’-diacetylchitobiose. Mar. Drugs 2022, 20, 165. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Fong, S.S. Bacterial chitinase: Nature and perspectives for sustainable bioproduction. Bioresour. Bioprocess. 2015, 2, 31. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Wu, Y.J.; Chiang, T.Y.; Kuo, C.Y.; Shrestha, K.L.; Chao, C.F.; Huang, Y.C.; Chuankhayan, P.; Wu, W.G.; Li, Y.K.; et al. Crystal structures of Bacillus cereus NCTU2 chitinase complexes with chitooligomers reveal novel substrate binding for catalysis: A chitinase without chitin binding and insertion domains. J. Biol. Chem. 2010, 285, 31603–31615. [Google Scholar] [CrossRef]
- Suginta, W.; Sirimontree, P.; Sritho, N.; Ohnuma, T.; Fukamizo, T. The chitin-binding domain of a GH-18 chitinase from Vibrio harveyi is crucial for chitin-chitinase interactions. Int. J. Biol. Macromol. 2016, 93, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Zees, A.C.; Pyrpassopoulos, S.; Vorgias, C.E. Insights into the role of the (alpha + beta) insertion in the TIM-barrel catalytic domain, regarding the stability and the enzymatic activity of chitinase A from Serratia marcescens. Biochim. Biophys. Acta 2009, 1794, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Hernández, E.O.; Casados-Vázquez, L.E.; Brieba, L.G.; Torres-Larios, A.; Jimenez-Sandoval, P.; Barboza-Corona, J.E. The crystal structure of the chitinase ChiA74 of Bacillus thuringiensis has a multidomain assembly. Sci. Rep. 2019, 9, 2591. [Google Scholar] [CrossRef]
- Wang, Y.J.; Jiang, W.X.; Zhang, Y.S.; Cao, H.Y.; Zhang, Y.; Chen, X.L.; Li, C.Y.; Wang, P.; Zhang, Y.Z.; Song, X.Y.; et al. Structural insight into chitin degradation and thermostability of a novel endochitinase from the glycoside hydrolase family 18. Front. Microbiol. 2019, 10, 2457. [Google Scholar] [CrossRef]
- Makhdoumi, A.; Dehghani-Joybari, Z.; Mashreghi, M.; Jamialahmadi, K.; Asoodeh, A. A novel halo-alkali-tolerant and thermotolerant chitinase from Pseudoalteromonas sp. DC14 isolated from the Caspian Sea. Int. J. Environ. Sci. Technol. 2015, 12, 3895–3904. [Google Scholar] [CrossRef]
- Fu, X.; Yan, Q.; Wang, J.; Yang, S.; Jiang, Z. Purification and biochemical characterization of novel acidic chitinase from Paenicibacillus barengoltzii. Int. J. Biol. Macromol. 2016, 91, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Cheba, B.A.; Zaghloul, T.I.; EL-Mahdy, A.R.; EL-Massry, M.H. Effect of pH and temperature on Bacillus sp. R2 chitinase activity and stability. Proc. Technol. 2016, 22, 471–477. [Google Scholar] [CrossRef]
- Xue, J.; Wen, C.; Wang, L.; Ning, W.; Dai, S.; Xue, M. Cloning expression and enzymatic properties of chitinase gene PpchiC from Pseudoalteromonas piscicida C923. Microbiol. China 2023, 50, 91–106. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhao, Y.; Tan, H.D.; Chi, N.Y.; Zhang, Q.F.; Du, Y.G.; Yin, H. Characterisation of a chitinase from Pseudoalteromonas sp. DL-6, a marine psychrophilic bacterium. Int. J. Biol. Macromol. 2014, 70, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Behera, P.K.; Madhuprakash, J. Efficient conversion of crystalline chitin to N-acetylglucosamine and N,N’-diacetylchitobiose by the enzyme cocktail produced by Paenibacillus sp. LS1. Carbohydr. Polym. 2020, 250, 116889. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.Y.; Gu, T.Y.; Ma, R.; Yao, W.; Huang, Y.Y.; Gu, J.G.; Zhao, G.G. Biochemical characterization of a GH19 chitinase from Streptomyces alfalfae and its applications in crystalline chitin conversion and biocontrol. Int. J. Biol. Macromol. 2021, 167, 193–201. [Google Scholar] [CrossRef]
- Zou, Y.; Robbens, J.; Heyndrickx, M.; Debode, J.; Raes, K. Quantification of extracellular proteases and chitinases from marine bacteria. Curr. Microbiol. 2020, 77, 3927–3936. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, I.; Silva, R.; Meunier, L.; Valente, S.M.; Lago-Lestón, A.; Keller-Costa, T.; Costa, R. Functional metagenomics reveals differential chitin degradation and utilization features across free-living and host-associated marine microbiomes. Microbiome 2021, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Han, D.S.; Choi, S.J.; Choi, S.W.; Kim, D.S.; Bai, D.H.; Yu, J.H. Purification, characterization, and primary structure of a chitinase from Pseudomonas sp. YHS-A2. Appl. Microbiol. Biotechnol. 2000, 54, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Gao, Q.; Yan, R.; Liu, L.; Wang, L.; Wen, B.; Wen, C. Comparative genomic analysis of shrimp-pthogenic Vibrio parahaemolyticus LC and intraspecific strains with emphasis on virulent factors of mobile genetic elements. Microorganisms 2023, 11, 2752. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Imanaka, T.; Fukui, T.; Fujiwara, S. Chitinase from Thermococcus kodakaraensis KOD1. Methods Enzymol. 2001, 330, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.E.; Gevers, D.; Vahora, N.M.; Polz, M.F. Conservation of the chitin utilization pathway in the Vibrionaceae. Appl. Environ. Microbiol. 2008, 74, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yu, M.; Wang, X.; Zhang, X.H. Comparative genomic analysis reveals the evolution and environmental adaptation strategies of vibrios. BMC Genom. 2018, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Huang, X.; Xue, J.; He, R.; Liang, G.; Liang, H.; Liu, J.; Wen, C. Comparative genomic analysis of seven Vibrio alginolyticus strains isolated from shrimp larviculture water with emphasis on chitin utilization. Front. Microbiol. 2022, 13, 925747. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, Y.S.; Choi, Y.L. Cloning, purification, and characterization of an organic solvent-tolerant chitinase, MtCh509, from Microbulbifer thermotolerans DAU221. Biotechnol. Biofuels 2018, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Synstad, B.; Gåseidnes, S.; Van Aalten, D.M.; Vriend, G.; Nielsen, J.E.; Eijsink, V.G. Mutational and computational analysis of the role of conserved residues in the active site of a family 18 chitinase. Eur. J. Biochem. 2004, 271, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Timmis, K.N.; Golyshin, P.N. Characterization of a chitinolytic enzyme from Serratia sp. KCK isolated from kimchi juice. Appl. Microbiol. Biotechnol. 2007, 75, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.S.; Yan, J.H.; Tang, J.Y.; Tao, Y.M.; Xie, X.L.; Wang, Y.; Wei, X.Q.; Yan, Q.H.; Chen, Q.X. Cloning and tissue expressions of seven chitinase family genes in Litopenaeus vannamei. Fish Shellfish Immunol. 2010, 29, 75–81. [Google Scholar] [CrossRef] [PubMed]
- García-Fraga, B.; da Silva, A.F.; López-Seijas, J.; Sieiro, C. Functional expression and characterization of a chitinase from the marine archaeon Halobacterium salinarum CECT 395 in Escherichia coli. Appl. Microbiol. Biotechnol. 2014, 98, 2133–2143. [Google Scholar] [CrossRef] [PubMed]
- Kidibule, P.E.; Santos-Moriano, P.; Jiménez-Ortega, E.; Ramírez-Escudero, M.; Limón, M.C.; Remacha, M.; Plou, F.J.; Sanz-Aparicio, J.; Fernández-Lobato, M. Use of chitin and chitosan to produce new chitooligosaccharides by chitinase Chit42: Enzymatic activity and structural basis of protein specificity. Microb. Cell Fact. 2018, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhu, W.; Wang, J.; Zhou, Y.; Duan, Y.; Qu, M.; Yang, Q. The deduced role of a chitinase containing two nonsynergistic catalytic domains. Acta Crystallogr. Sect. D Struct. Biol. 2018, 74, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Hibi, T.; Suzuki, F.; Sugimoto, I.; Fujiwara, A.; Inaka, K.; Tanaka, H.; Ohta, K.; Fujii, Y.; Taketo, A.; et al. Crystal structure of chitinase ChiW from Paenibacillus sp. str. FPU-7 reveals a novel type of bacterial cell-surface-expressed multi-modular enzyme machinery. PLoS ONE 2016, 11, e0167310. [Google Scholar] [CrossRef] [PubMed]
- Nimlos, M.R.; Beckham, G.T.; Matthews, J.F.; Bu, L.T.; Himmel, M.E.; Crowley, M.F. Binding preferences, surface attachment, diffusivity, and orientation of a family 1 carbohydrate-binding module on cellulose. J. Biol. Chem. 2012, 287, 20603–20612. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Fan, L.; Deng, C.; Ma, C.; Zhang, C.; Zhao, L. Bioconversion of chitin into chitin oligosaccharides using a novel chitinase with high chitin-binding capacity. Int. J. Biol. Macromol. 2023, 244, 125241. [Google Scholar] [CrossRef] [PubMed]
- Uni, F.; Lee, S.; Yatsunami, R.; Fukui, T.; Nakamura, S. Mutational analysis of a CBM family 5 chitin-binding domain of an alkaline chitinase from Bacillus sp. J813. Biosci. Biotechnol. Biochem. 2012, 76, 530–535. [Google Scholar] [CrossRef]
- Purushotham, P.; Sarma, P.V.; Podile, A.R. Multiple chitinases of an endophytic Serratia proteamaculans 568 generate chitin oligomers. Bioresour. Technol. 2012, 112, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Vaikuntapu, P.R.; Rambabu, S.; Madhuprakash, J.; Podile, A.R. A new chitinase-D from a plant growth promoting Serratia marcescens GPS5 for enzymatic conversion of chitin. Bioresour. Technol. 2016, 220, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Laribi-Habchi, H.; Bouacem, K.; Allala, F.; Jabeur, F.; Selama, O.; Mechri, S.; Yahiaoui, M.; Bouanane-Darenfed, A.; Jaouadi, B. Characterization of chitinase from Shewanella inventionis HE3 with bio-insecticidal effect against granary weevil, Sitophilus granarius Linnaeus (Coleoptera: Curculionidae). Process Biochem. 2020, 97, 222–233. [Google Scholar] [CrossRef]
- Laribi-Habchi, H.; Bouanane-Darenfed, A.; Drouiche, N.; Pauss, A.; Mameri, N. Purification, characterization, and molecular cloning of an extracellular chitinase from Bacillus licheniformis stain LHH100 isolated from wastewater samples in Algeria. Int. J. Biol. Macromol. 2015, 72, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Brurberg, M.B.; Nes, I.F.; Eijsink, V.G. Comparative studies of chitinases A and B from Serratia marcescens. Microbiology 1996, 142, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Vaaje-Kolstad, G.; Houston, D.R.; Rao, F.V.; Peter, M.G.; Synstad, B.; van Aalten, D.M.; Eijsink, V.G. Structure of the D142N mutant of the family 18 chitinase ChiB from Serratia marcescens and its complex with allosamidin. Biochim. Biophys. Acta 2004, 1696, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, J.; Yan, Q.; Jiang, Z.; Yang, S. Biochemical characterization of a novel acidic chitinase with antifungal activity from Paenibacillus xylanexedens Z2-4. Int. J. Biol. Macromol. 2021, 182, 1528–1536. [Google Scholar] [CrossRef]
- Jamek, S.B.; Nyffenegger, C.; Muschiol, J.; Holck, J.; Meyer, A.S.; Mikkelsen, J.D. Characterization of two novel bacterial type A exo-chitobiose hydrolases having C-terminal 5/12-type carbohydrate-binding modules. Appl. Microbiol. Biotechnol. 2017, 101, 4533–4546. [Google Scholar] [CrossRef] [PubMed]
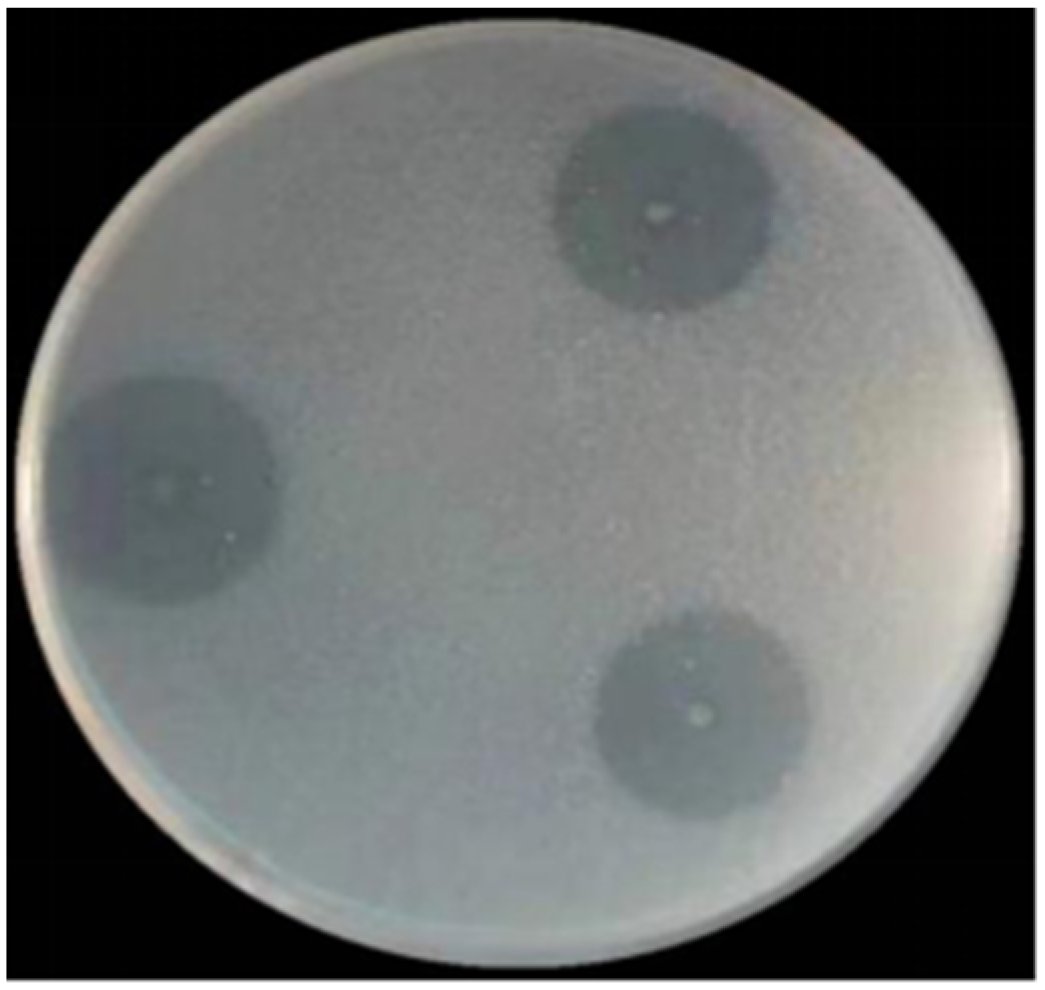
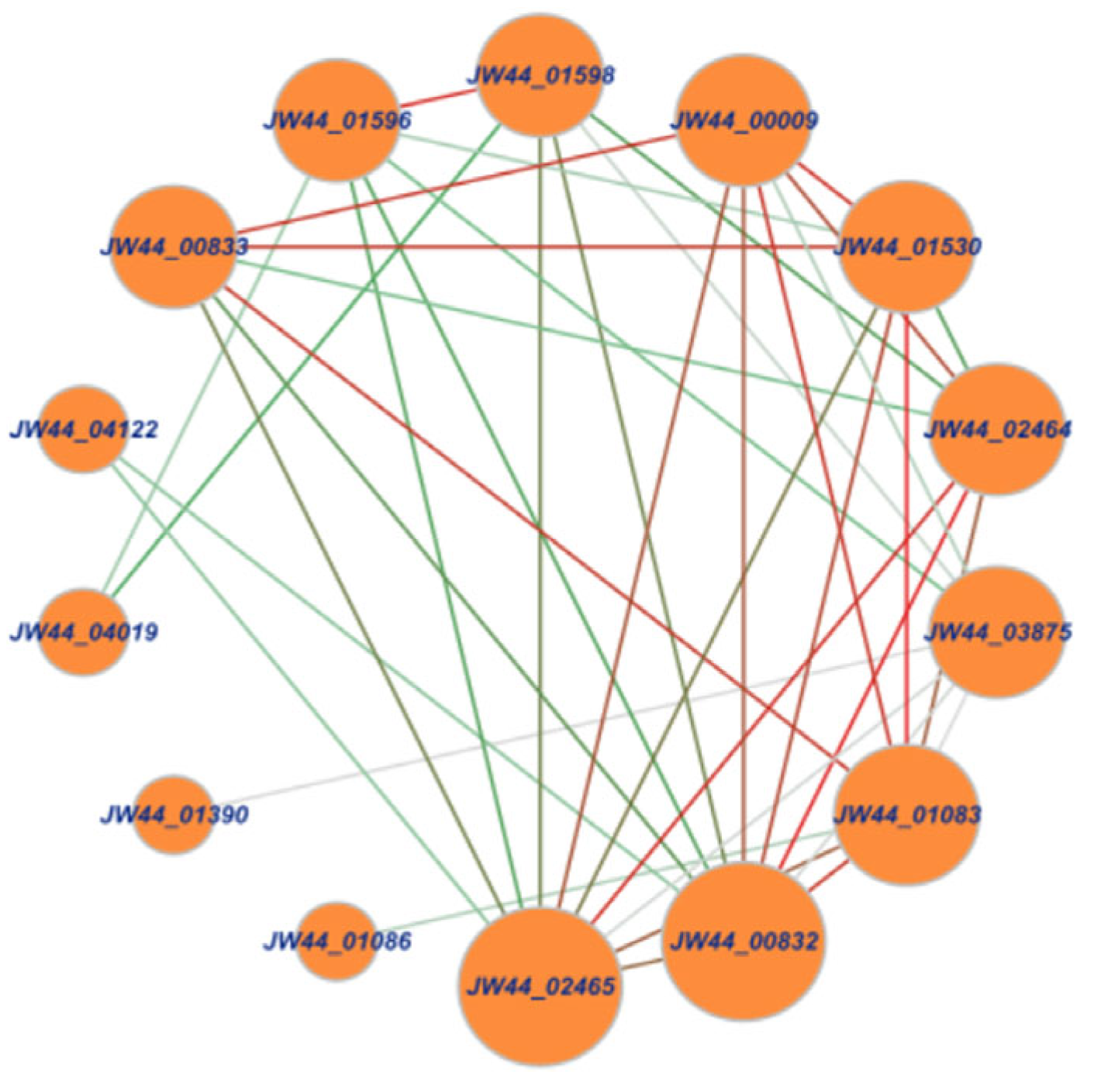
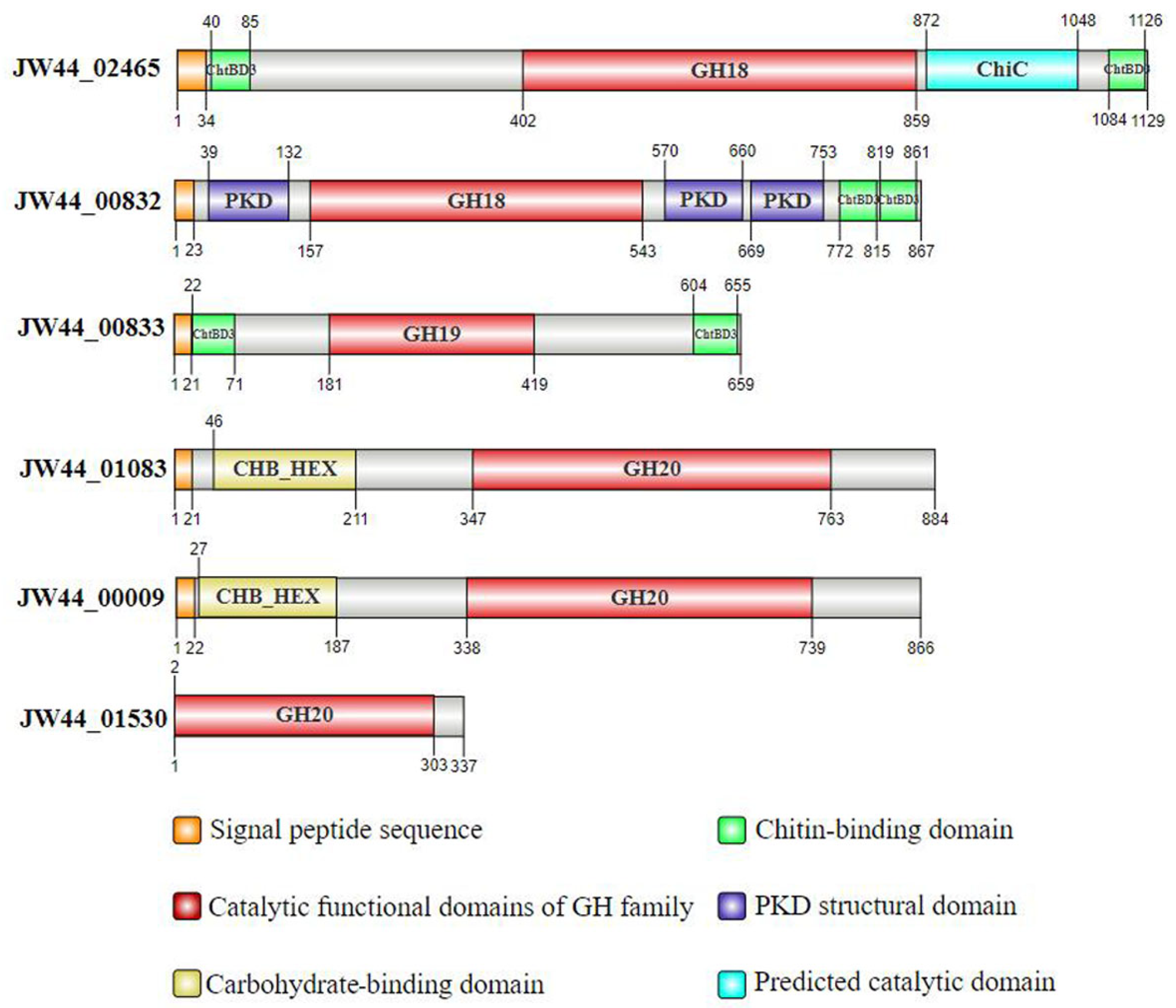
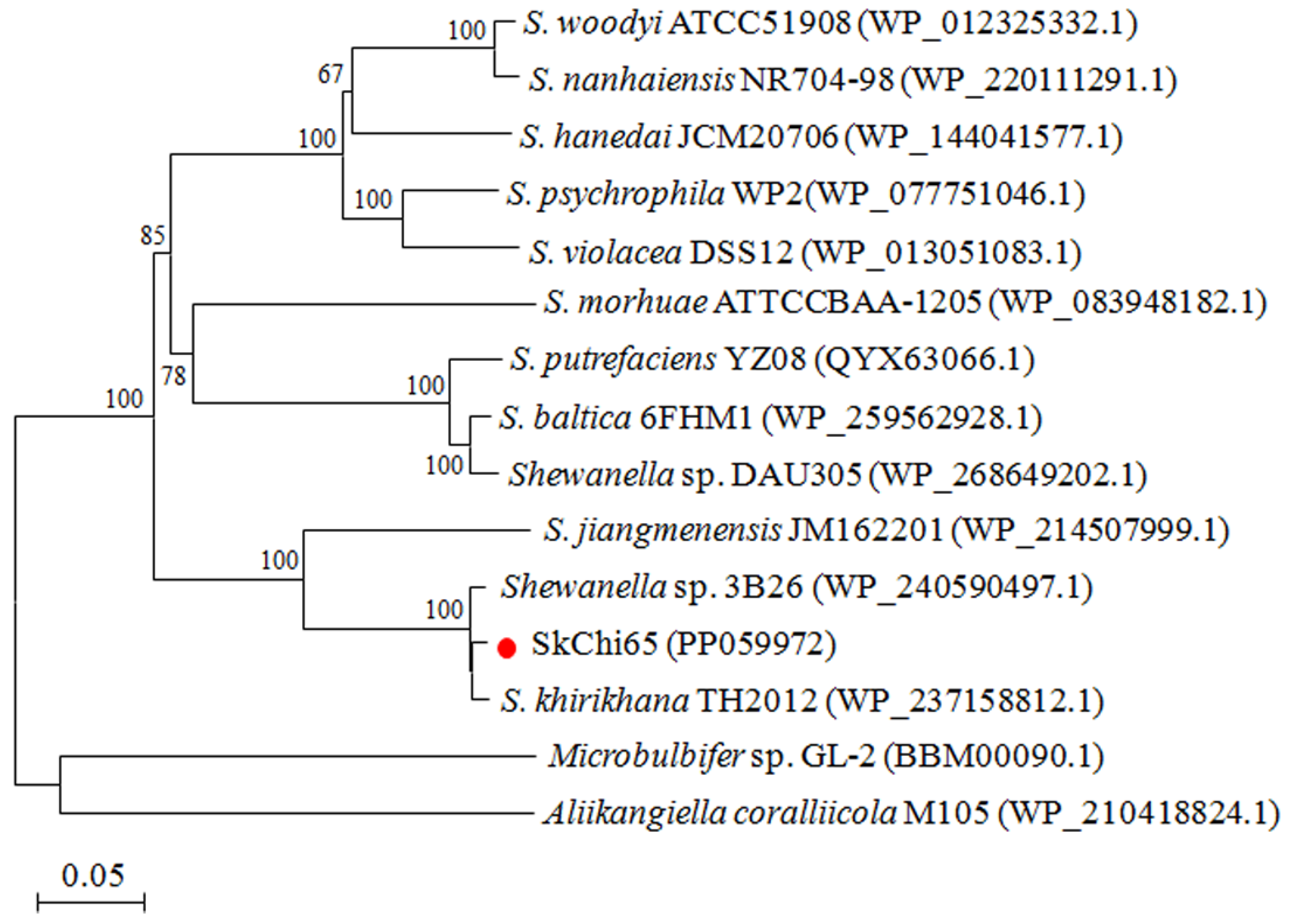



| Locus_Tag | Start | End | Size/aa | Mass/kDa | GH Family | EC Number | Product |
|---|---|---|---|---|---|---|---|
| JW44_02465 | * c2793077 | c2796466 | 1129 | 139.95 | GH18 | 3.2.1.202 | chitinase A |
| JW44_00832 | 950609 | 953212 | 867 | 91.32 | GH18 | 3.2.1.14 | chitinase A |
| JW44_00833 | 953285 | 955264 | 659 | 69.49 | GH19 | - | chitodextrinase |
| JW44_01083 | 1228958 | 1231612 | 884 | 97.20 | GH20 | 3.2.1.52 | β-N-acetylhexosaminidase |
| JW44_00009 | 13742 | 16342 | 866 | 96.14 | GH20 | 3.2.1.52 | chitobiase |
| JW44_01530 | 1751247 | 1752260 | 337 | 36.36 | GH20 | 3.2.1.52 | β-N-acetylhexosaminidase |
| Substrates (1%, w/v) | Specific Activity (U/mg) ** | Relative Activity (%) |
|---|---|---|
| Colloidal chitin | 3.89 ± 2.56 | 100.0 |
| Glycol chitosan (60% DD *) | 2.36 ± 0.57 | 60.7 |
| Chitin powder | 1.24 ± 0.47 | 31.9 |
| Chitosan (80% DD) | 0.63 ± 0.20 | 16.2 |
| Carboxylated chitosan (90% DD) | 0.30 ± 0.20 | 7.7 |
| Carboxymethyl cellulose | 0.00 | 0.0 |
| Microcrystalline cellulose | 0.00 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Xue, M.; Yan, R.; Xue, J.; Lu, Z.; Wen, C. Insights into Chitin-Degradation Potential of Shewanella khirikhana JW44 with Emphasis on Characterization and Function of a Chitinase Gene SkChi65. Microorganisms 2024, 12, 774. https://doi.org/10.3390/microorganisms12040774
Wang L, Xue M, Yan R, Xue J, Lu Z, Wen C. Insights into Chitin-Degradation Potential of Shewanella khirikhana JW44 with Emphasis on Characterization and Function of a Chitinase Gene SkChi65. Microorganisms. 2024; 12(4):774. https://doi.org/10.3390/microorganisms12040774
Chicago/Turabian StyleWang, Ling, Ming Xue, Rui Yan, Jiawei Xue, Zhipeng Lu, and Chongqing Wen. 2024. "Insights into Chitin-Degradation Potential of Shewanella khirikhana JW44 with Emphasis on Characterization and Function of a Chitinase Gene SkChi65" Microorganisms 12, no. 4: 774. https://doi.org/10.3390/microorganisms12040774






