High-Throughput Screening Method Using Escherichia coli Keio Mutants for Assessing Primary Damage Mechanism of Antimicrobials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Growth
2.2. Nanoparticle Preparation
2.3. Viability Assay
2.4. Cell Viability Controls
2.5. Protein Model Analysis
2.6. Reporter Strains
3. Results
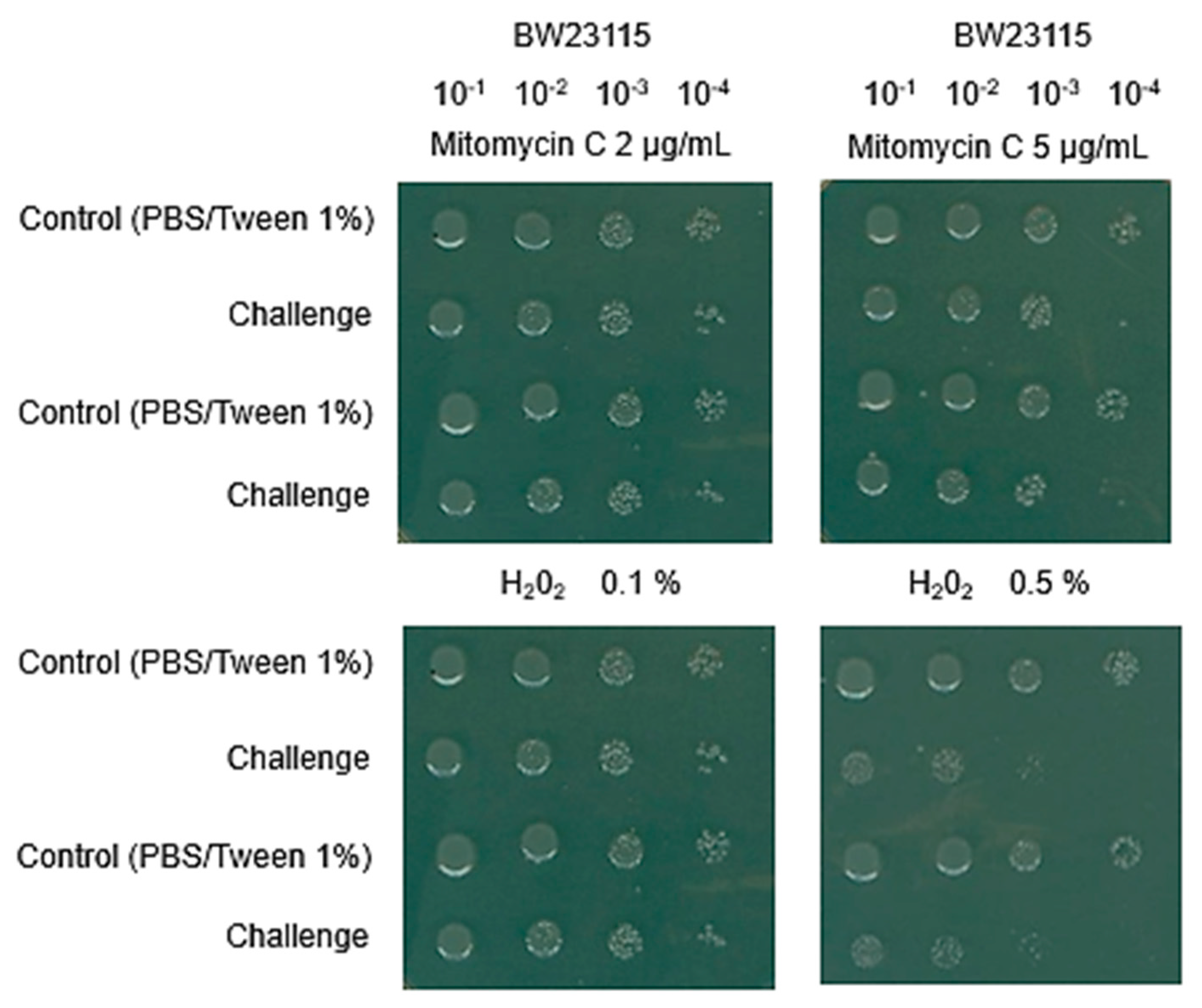
3.1. Keio Mutants Exhibit the Expected Phenotype to Damaging Agents
3.2. Silver NPs Generate Double-Strand Breaks in the DNA but No ROS
3.3. Keio Collection Mutants Serves as Chassis for Bioreporter Strains
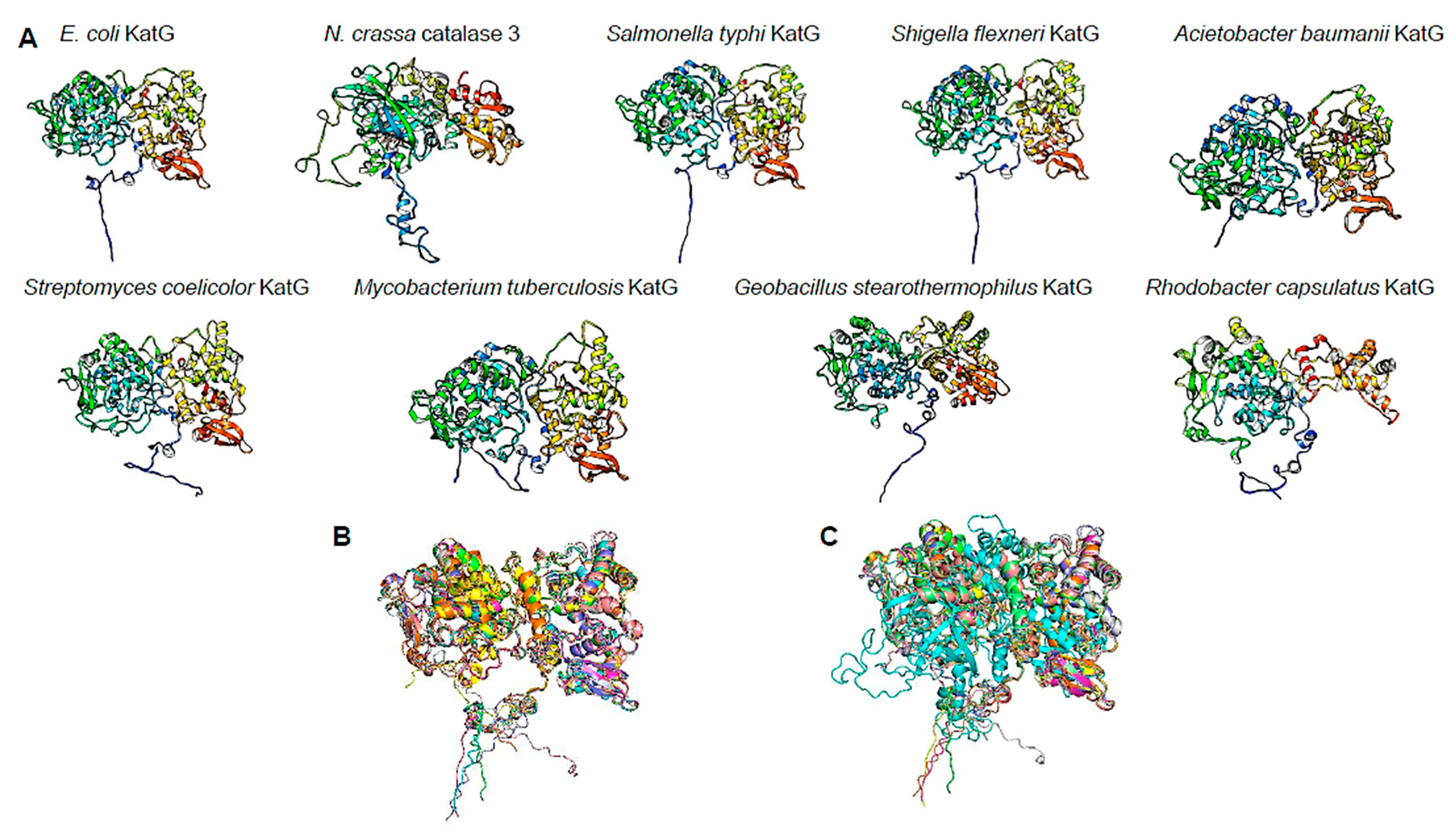
3.4. ΔkatG Phenotype May Be Related to Uncharacterized Function of This Catalase
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davis, M.; Whittaker, A.; Lindgren, M.; Djerf-Pierre, M.; Manderson, L.; Flowers, P. Understanding media publics and the antimicrobial resistance crisis. Glob. Public Health 2018, 13, 1158–1168. [Google Scholar] [CrossRef]
- Fariña, N. Bacterial resistance. A global public health problem with a difficult solution. Memorias del Inst. Investig. en Ciencias la Salud. 2016, 14, 06–07. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Peterson, E.; Kaur, P. Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef]
- Chellat, M.F.; Raguž, L.; Riedl, R. Targeting Antibiotic Resistance. Angew. Chem. Int. Ed. 2016, 55, 6600–6626. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.; Hou, J.; Sun, X.; Wang, Y.; Li, L.; Yang, F.; Yao, Y.; An, Y. Mobilome affect the dissemination of antibiotic resistance genes (ARGs) of clinical importance into the natural environment. Environ. Res. 2023, 243, 117801. [Google Scholar] [CrossRef]
- Lluka, T.; Stokes, J.M. Antibiotic discovery in the artificial intelligence era. Ann. N. Y. Acad. Sci. 2023, 1519, 74–93. [Google Scholar] [CrossRef]
- Aguilar-Vega, L.; López-Jácome, L.E.; Franco, B.; Muñoz-Carranza, S.; Vargas-Maya, N.; Franco-Cendejas, R.; Hernández-Durán, M.; Otero-Zúñiga, M.; Campo-Beleño, C.; Jiménez-Cortés, J.G.; et al. Antibacterial properties of phenothiazine derivatives against multidrug-resistant Acinetobacter baumannii strains. J. Appl. Microbiol. 2021, 131, 2235–2243. [Google Scholar] [CrossRef]
- Wahab, S.; Salman, A.; Khan, Z.; Khan, S.; Krishnaraj, C.; Yun, S.I. Metallic Nanoparticles: A Promising Arsenal against Antimicrobial Resistance-Unraveling Mechanisms and Enhancing Medication Efficacy. Int. J. Mol. Sci. 2023, 24, 14897. [Google Scholar] [CrossRef] [PubMed]
- Skóra, B.; Krajewska, U.; Nowak, A.; Dziedzic, A.; Barylyak, A.; Kus-Liśkiewicz, M. Noncytotoxic silver nanoparticles as a new antimicrobial strategy. Sci. Rep. 2021, 11, 13451. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, H.; Li, X.; Liu, W. What happens when nanoparticles encounter bacterial antibiotic resistance? Sci Total Environ. 2023, 876, 162856. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Garrido, K.G.; Ramírez-Montiel, F.B.; Reyes-Vidal, Y.; Bacame-Valenzuela, F.J.; Padilla-Vaca, F.; Palma-Tirado, L.; Estecez, M.; España-Sánchez, B.L. Antibacterial behavior and bacterial resistance analysis of P. aeruginosa in contact with copper nanoparticles/Comportamiento antibacteriano y análisis de resistencia bacteriana de P. aeruginosa al contacto con nanopartículas de cobre. Mex. J. Biotechnol. 2023, 8, 1–20. [Google Scholar] [CrossRef]
- Liu, B.; Liu, D.; Chen, T.; Wang, X.; Xiang, H.; Wang, G.; Cai, R. iTRAQ-based quantitative proteomic analysis of the antibacterial mechanism of silver nanoparticles against multidrug-resistant Streptococcus suis. Front. Microbiol. 2023, 14, 1293363. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.M.; Karam, K.; Khan, T.; Wahab, S.; Ullah, S.; Sadiq, M. Reactive oxygen species induced oxidative damage to DNA, lipids, and proteins of antibiotic-resistant bacteria by plant-based silver nanoparticles. 3 Biotech 2023, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Campo-Beleño, C.; Villamizar-Gallardo, R.A.; López-Jácome, L.E.; González, E.E.; Muñoz-Carranza, S.; Franco, B.; Morales-Espinosa, R.; Coria-Jimenez, R.; Franco-Cendejas, R.; Hernández-Durán, M.; et al. Biologically synthesized silver nanoparticles as potent antibacterial effective against multidrug-resistant Pseudomonas aeruginosa. Lett. Appl. Microbiol. 2022, 75, 680–688. [Google Scholar] [CrossRef]
- Mora-Garduño, J.D.; Tamayo-Nuñez, J.; Padilla-Vaca, F.; Ramírez-Montiel, F.B.; Rangel-Serrano, Á.; Santos-Escobar, F.; Gutiérrez-Corona, F.; Páramo-Pérez, I.; Anaya-Velázquez, F.; García-Contreras, R.; et al. Chromogenic Escherichia coli reporter strain for screening DNA damaging agents. AMB Express 2022, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Kamat, S.; Kumari, M. Emergence of microbial resistance against nanoparticles: Mechanisms and strategies. Front. Microbiol. 2023, 14, 1102615. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef]
- Yamamoto, N.; Nakahigashi, K.; Nakamichi, T.; Yoshino, M.; Takai, Y.; Touda, Y.; Furubayashi, A.; Kinjyo, S.; Dose, H.; Hasegawa, M.; et al. Update on the Keio collection of Escherichia coli single-gene deletion mutants. Mol. Syst. Biol. 2009, 5, 335. [Google Scholar] [CrossRef]
- Inoue, T.; Shingaki, R.; Hirose, S.; Waki, K.; Mori, H.; Fukui, K. Genome-wide screening of genes required for swarming motility in Escherichia coli K-12. J. Bacteriol. 2007, 189, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Tohsato, Y.; Baba, T.; Mazaki, Y.; Ito, M.; Wanner, B.L.; Mori, H. Environmental dependency of gene knockouts on phenotype microarray analysis in Escherichia coli. J. Bioinform. Comput. Biol. 2010, 8 (Suppl. S1), 83–99. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, R.; Tamura, T.; Nakayashiki, T.; Tanaka, Y.; Muto, A.; Wanner, B.L.; Mori, H. Colony-live--a high-throughput method for measuring microbial colony growth kinetics--reveals diverse growth effects of gene knockouts in Escherichia coli. BMC Microbiol. 2014, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.; Datsenko, K.A.; Ess, S.C.; Zhalnina, M.V.; Wanner, B.L.; Cramer, W.A. Genome-wide screens: Novel mechanisms in colicin import and cytotoxicity. Mol. Microbiol. 2009, 73, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Bertani, G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef] [PubMed]
- Tajima, F.; Nei, M. Estimation of evolutionary distance between nucleotide sequences. Mol. Biol. Evol. 1984, 1, 269–285. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, J.; Xu, J. Alignment of distantly related protein structures: Algorithm, bound and implications to homology modeling. Bioinformatics 2011, 27, 2537–2545. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Peng, J.; Xu, J. Protein structure alignment beyond spatial proximity. Sci. Rep. 2013, 3, 1448. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, L.; DeLano, W. PyMOL. 2020. Available online: http://www.pymol.org/pymol (accessed on 12 January 2021).
- Hansberg, W. Monofunctional Heme-Catalases. Antioxidants 2022, 11, 2173. [Google Scholar] [CrossRef]
- Hansberg, W.; Nava-Ramírez, T.; Rangel-Silva, P.; Díaz-Vilchis, A.; Mendoza-Oliva, A. Large-Size Subunit Catalases Are Chimeric Proteins: A H2O2 Selecting Domain with Catalase Activity Fused to a Hsp31-Derived Domain Conferring Protein Stability and Chaperone Activity. Antioxidants 2022, 11, 979. [Google Scholar] [CrossRef]
- Nava-Ramírez, T.; Gutiérrez-Terrazas, S.; Hansberg, W. The Molecular Chaperone Mechanism of the C-Terminal Domain of Large-Size Subunit Catalases. Antioxidants 2023, 12, 839. [Google Scholar] [CrossRef]
- Belkin, S. Microbial whole-cell sensing systems of environmental pollutants. Curr. Opin. Microbiol. 2003, 6, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Elad, T.; Belkin, S. Reporter Gene Assays in Ecotoxicology. Adv. Biochem. Eng. Biotechnol. 2017, 157, 135–157. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeong, H.; Lee, S.J. Synthetic biology for microbial heavy metal biosensors. Anal. Bioanal. Chem. 2018, 410, 1191–1203. [Google Scholar] [CrossRef]
- Liu, C.; Yu, H.; Zhang, B.; Liu, S.; Liu, C.G.; Li, F.; Song, H. Engineering whole-cell microbial biosensors: Design principles and applications in monitoring and treatment of heavy metals and organic pollutants. Biotechnol. Adv. 2022, 60, 108019. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Schillereff, D.N.; Chiverrell, R.C.; Tefsen, B.; Wells, M. Whole-cell biosensors for determination of bioavailable pollutants in soils and sediments: Theory and practice. Sci. Total Environ. 2022, 811, 152178. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.M.; Zhang, X.; Holmgren, A. A new mechanism of action for the anticancer drug mitomycin C: Mechanism-based inhibition of thioredoxin reductase. Chem. Res. Toxicol. 2012, 25, 1502–1511. [Google Scholar] [CrossRef]
- Konstantinov, S.M.; Berger, M.R. Alkylating Agents. In Encyclopedia of Molecular Pharmacology; Offermanns, S., Rosenthal, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Gad, S.E.; Mitomycin, C. (Eds.) Philip Wexler, Encyclopedia of Toxicology, 3rd ed.; Academic Press: Vienna, Austria, 2014; pp. 354–356. ISBN 9780123864550. [Google Scholar] [CrossRef]
- Giacomoni, P.U. Survival and induction of recA protein in mitomycin C-treated Escherichia coli rec, lex, or uvr strains. J. Biol. Chem. 1983, 258, 13653–13657. [Google Scholar] [CrossRef]
- Giacomoni, P.U. Induction by mitomycin C of recA protein synthesis in bacteria and spheroplasts. J. Biol. Chem. 1982, 257, 14932–14936. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.K.; Alvarado, C.L.; Sutton, M.D. Role of the SOS Response in the Generation of Antibiotic Resistance In Vivo. Antimicrob. Agents Chemother. 2021, 65, e0001321. [Google Scholar] [CrossRef]
- Crane, J.K.; Catanzaro, M.N. Role of Extracellular DNA in Bacterial Response to SOS-Inducing Drugs. Antibiotics 2023, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- Bryant, F.R. Construction of a recombinase-deficient mutant recA protein that retains single-stranded DNA-dependent ATPase activity. J. Biol. Chem. 1988, 263, 8716–8723. [Google Scholar] [CrossRef]
- Al Mamun, A.A.; Lombardo, M.J.; Shee, C.; Lisewski, A.M.; Gonzalez, C.; Lin, D.; Nehring, R.B.; Saint-Ruf, C.; Gibson, J.L.; Frisch, R.L.; et al. Identity and function of a large gene network underlying mutagenic repair of DNA breaks. Science 2012, 338, 1344–1348. [Google Scholar] [CrossRef]
- Kouzminova, E.A.; Rotman, E.; Macomber, L.; Zhang, J.; Kuzminov, A. RecA-dependent mutants in Escherichia coli reveal strategies to avoid chromosomal fragmentation. Proc. Natl. Acad. Sci. USA 2004, 101, 16262–16267. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Takemoto, T.; Mito, S.; Yonei, S. Induction of repair capacity for oxidatively damaged DNA as a component of peroxide stress response in Escherichia coli. J. Radiat. Res. 1996, 37, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M.A.; Nair, S.; Abratt, V.R.; Zappe, H.; Steyn, L.M. Involvement of the N- and C-terminal domains of Mycobacterium tuberculosis KatG in the protection of mutant Escherichia coli against DNA-damaging agents. Microbiology 1999, 145 Pt 8, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.J.; Gu, M.B. An Escherichia coli biosensor capable of detecting both genotoxic and oxidative damage. Appl. Microbiol. Biotechnol. 2004, 64, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.D.; Cook, C.O.; Goodwin, D.C. Properties of catalase-peroxidase lacking its C-terminal domain. Biochem. Biophys. Res. Commun. 2004, 320, 833–839. [Google Scholar] [CrossRef]
- Kumar, J.K.; Tabor, S.; Richardson, C.C. Proteomic analysis of thioredoxin-targeted proteins in Escherichia coli. Proc. Natl. Acad. Sci. USA 2004, 101, 3759–3764. [Google Scholar] [CrossRef]




| Strain | Genotype * | Antibiotic Resistance |
|---|---|---|
| BW25113 | Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, rph-1, Δ(rhaD-rhaB)568, hsdR514 | None |
| JW4019-2 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, rph-1, Δ(rhaD-rhaB)568, ΔuvrA753::kan, hsdR514 | Kanr |
| JW0762-2 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, ΔuvrB751::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Kanr |
| JW1898-1 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, ΔuvrC759::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Kanr |
| JW2146-1 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, Δnfo-786::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Kanr |
| JW2703-2 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, ΔmutS738::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Kanr |
| JW4128-1 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, rph-1, Δ(rhaD-rhaB)568, ΔmutL720::kan, hsdR514 | Kanr |
| JW2928-1 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, ΔmutY736::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Kanr |
| JW2564-2 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, Δung-748::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Kanr |
| JW2669-1 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, ΔrecA774::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Kanr |
| JW1100-1 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, Δmfd-739::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Kanr |
| JW5581-1 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, rph-1, ΔubiE778::kan, Δ(rhaD-rhaB)568, hsdR514 | Kanr |
| JW0669-2 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), Δfur-731::kan, λ-, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Kanr |
| JW3914-1 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, rph-1, Δ(rhaD-rhaB)568, ΔkatG729::kan, hsdR514 | Kanr |
| JW3879-1 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, rph-1, Δ(rhaD-rhaB)568, ΔsodA768::kan, hsdR514 | Kanr |
| JW0871-1 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, ΔtrxB786::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Kanr |
| JW3467-1 | F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, Δgor-756::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Kanr |
| DH5α (ThermoFisher) | F– φ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK–, mK+) phoA supE44 λ–thi-1 gyrA96 relA1 | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Álvarez, J.A.; Vicente-Gómez, M.; García-Contreras, R.; Wood, T.K.; Ramírez Montiel, F.B.; Vargas-Maya, N.I.; España-Sánchez, B.L.; Rangel-Serrano, Á.; Padilla-Vaca, F.; Franco, B. High-Throughput Screening Method Using Escherichia coli Keio Mutants for Assessing Primary Damage Mechanism of Antimicrobials. Microorganisms 2024, 12, 793. https://doi.org/10.3390/microorganisms12040793
Martínez-Álvarez JA, Vicente-Gómez M, García-Contreras R, Wood TK, Ramírez Montiel FB, Vargas-Maya NI, España-Sánchez BL, Rangel-Serrano Á, Padilla-Vaca F, Franco B. High-Throughput Screening Method Using Escherichia coli Keio Mutants for Assessing Primary Damage Mechanism of Antimicrobials. Microorganisms. 2024; 12(4):793. https://doi.org/10.3390/microorganisms12040793
Chicago/Turabian StyleMartínez-Álvarez, José A., Marcos Vicente-Gómez, Rodolfo García-Contreras, Thomas K. Wood, Fátima Berenice Ramírez Montiel, Naurú Idalia Vargas-Maya, Beatriz Liliana España-Sánchez, Ángeles Rangel-Serrano, Felipe Padilla-Vaca, and Bernardo Franco. 2024. "High-Throughput Screening Method Using Escherichia coli Keio Mutants for Assessing Primary Damage Mechanism of Antimicrobials" Microorganisms 12, no. 4: 793. https://doi.org/10.3390/microorganisms12040793








