Molecular Mechanisms of Lacticaseibacillus rhamnosus, LGG® Probiotic Function
Abstract
1. Introduction
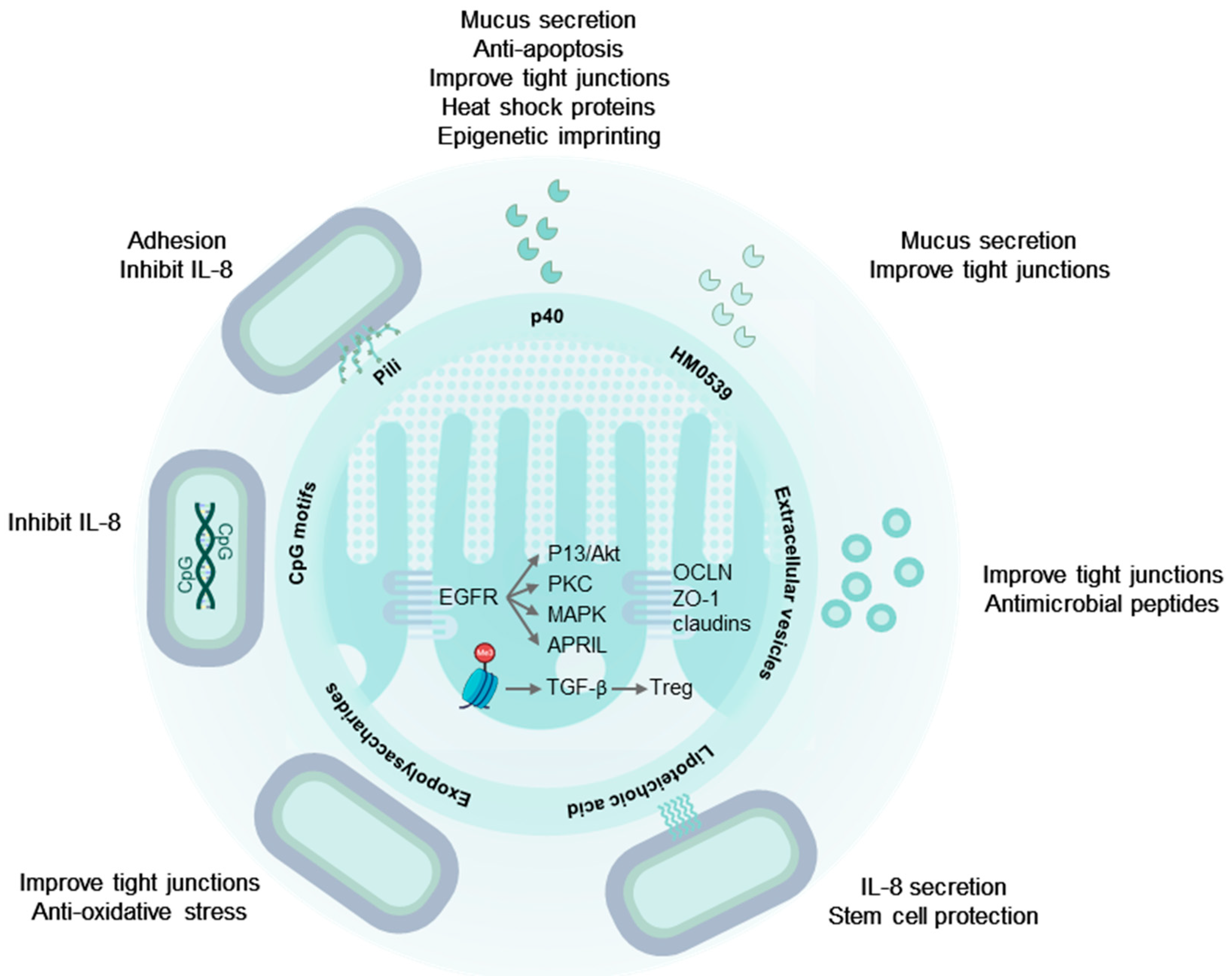
2. The Digestive Tract and the Intestinal Immune System Are Targets of LGG®
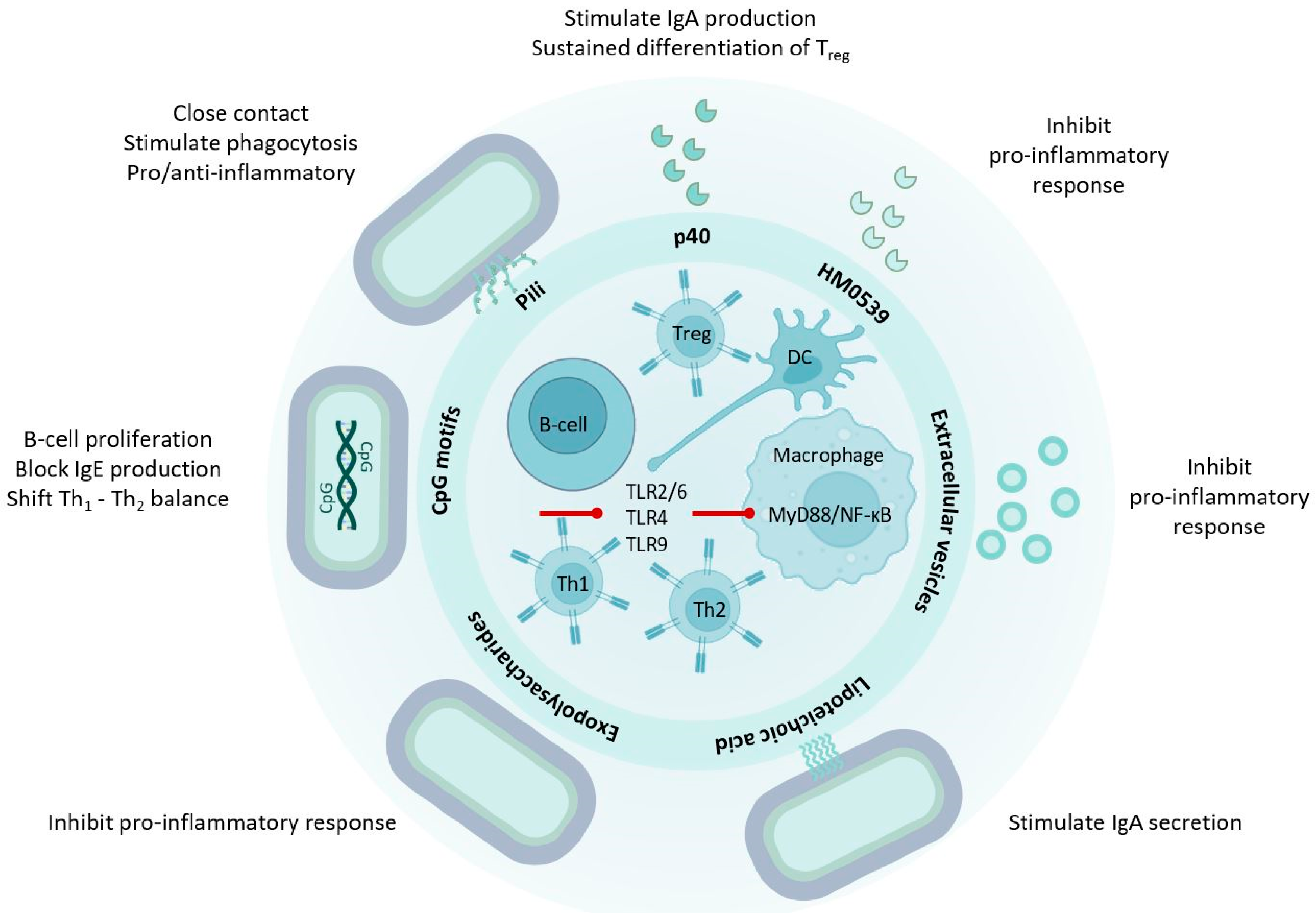
3. LGG® Communicates with the Human Host through Molecular Mechanisms
4. Secreted Proteins from LGG® Have Probiotic Effects
4.1. p40 and p75 Improve Intestinal Epithelial Integrity
4.2. p40 Is Immune Regulatory
4.3. HM0539: A Secreted Protein with Probiotic Effects
5. Extracellular Vesicles
6. LGG® Surface Molecules with Probiotic Effects
6.1. Pili and Other Proteinaceous Adhesins
6.2. Lectin-like Cell Wall Proteins
6.3. Exopolysaccharides
6.4. Lipoteichoic Acids
6.5. Peptidoglycan
7. LGG® Genomic DNA and Unmethylated CpG-Rich DNA Motifs
8. Comparison of LGG® Effector Molecules and Overall Effects
9. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dronkers, T.M.G.; Ouwehand, A.C.; Rijkers, G.T. Global Analysis of Clinical Trials with Probiotics. Heliyon 2020, 6, e04467. [Google Scholar] [CrossRef]
- Capurso, L. Thirty Years of Lactobacillus rhamnosus GG A Review. J. Clin. Gastroenterol. 2019, 53 (Suppl. S1), S1–S41. [Google Scholar] [CrossRef]
- Hojsak, I.; Abdović, S.; Szajewska, H.; Milošević, M.; Krznarić, Ž.; Kolaček, S. Lactobacillus GG in the Prevention of Nosocomial Gastrointestinal and Respiratory Tract Infections. Pediatrics 2010, 125, e1171–e1177. [Google Scholar] [CrossRef]
- Rautava, S.; Kalliomäki, M.; Isolauri, E. Probiotics during Pregnancy and Breast-Feeding Might Confer Immunomodulatory Protection against Atopic Disease in the Infant. J. Allergy Clin. Immunol. 2002, 109, 119–121. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Salminen, S.; Arvilommi, H.; Kero, P.; Koskinen, P.; Isolauri, E. Probiotics in Primary Prevention of Atopic Disease: A Randomised Placebo-Controlled Trial. Lancet 2001, 357, 1076–1079. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Salminen, S.; Poussa, T.; Arvilommi, H.; Isolauri, E. Probiotics and Prevention of Atopic Disease: 4-Year Follow-up of a Randomised Placebo-Controlled Trial. Lancet 2003, 361, 1869–1871. [Google Scholar] [CrossRef] [PubMed]
- De Vrese, M.; Rautenberg, P.; Laue, C.; Koopmans, M.; Herremans, T.; Schrezenmeir, J. Probiotic Bacteria Stimulate Virus-Specific Neutralizing Antibodies Following a Booster Polio Vaccination. Eur. J. Nutr. 2005, 44, 406–413. [Google Scholar] [CrossRef]
- Davidson, L.E.; Fiorino, A.M.; Snydman, D.R.; Hibberd, P.L. Lactobacillus GG as an Immune Adjuvant for Live-Attenuated Influenza Vaccine in Healthy Adults: A Randomized Double-Blind Placebo-Controlled Trial. Eur. J. Clin. Nutr. 2011, 65, 501–507. [Google Scholar] [CrossRef]
- van den Nieuwboer, M.; van de Burgwal, L.H.M.; Claassen, E. A Quantitative Key-Opinion-Leader Analysis of Innovation Barriers in Probiotic Research and Development: Valorisation and Improving the Tech Transfer Cycle. PharmaNutrition 2016, 4, 9–18. [Google Scholar] [CrossRef]
- Zeilstra, D.; Younes, J.A.; Brummer, R.J.; Kleerebezem, M. Perspective: Fundamental Limitations of the Randomized Controlled Trial Method in Nutritional Research: The Example of Probiotics. Adv. Nutr. 2018, 9, 561–571. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Cooney, J.C. Probiotic Bacteria Influence the Composition and Function of the Intestinal Microbiota. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 175285. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in Fecal Microbiota Composition by Probiotic Supplementation in Healthy Adults: A Systematic Review of Randomized Controlled Trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef]
- Bäckhed, F.; Fraser, C.M.; Ringel, Y.; Sanders, M.E.; Sartor, R.B.; Sherman, P.M.; Versalovic, J.; Young, V.; Finlay, B.B. Defining a Healthy Human Gut Microbiome: Current Concepts, Future Directions, and Clinical Applications. Cell Host Microbe 2012, 12, 611–622. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- McFarland, L. Use of Probiotics to Correct Dysbiosis of Normal Microbiota Following Disease or Disruptive Events: A Systematic Review. BMJ Open 2014, 4, e005047. [Google Scholar] [CrossRef]
- Booijink, C.C.G.M.; El-Aidy, S.; Rajilić-Stojanović, M.; Heilig, H.G.H.J.; Troost, F.J.; Smidt, H.; Kleerebezem, M.; De Vos, W.M.; Zoetendal, E.G. High Temporal and Inter-Individual Variation Detected in the Human Ileal Microbiota. Environ. Microbiol. 2010, 12, 3213–3227. [Google Scholar] [CrossRef]
- Matute, S.P.; Lyavoo, S. Exploring the Gut Microbiota: Lifestyle Choices, Disease Associations, and Personal Genomics. Front. Nutr. 2023, 10, 1225120. [Google Scholar] [CrossRef]
- Sartorius, N. The Meanings of Health and Its Promotion. Croat. Med. J. 2006, 47, 662–664. [Google Scholar]
- Binnendijk, K.H.; Rijkers, G.T. What Is a Health Benefit? An Evaluation of EFSA Opinions on Health Benefits with Reference to Probiotics. Benef. Microbes 2013, 4, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Segers, M.E.; Lebeer, S. Towards a Better Understanding of Lactobacillus rhamnosus GG—Host Interactions. Microb. Cell Fact. 2014, 13, S7. [Google Scholar] [CrossRef]
- Ali, A.; Tan, H.Y.; Kaiko, G.E. Role of the Intestinal Epithelium and Its Interaction with the Microbiota in Food Allergy. Front. Immunol. 2020, 11, 604054. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E. V Fast Renewal of the Distal Colonic Mucus Layers by the Surface Goblet Cells as Measured by in Vivo Labeling of Mucin Glycoproteins. PLoS ONE 2012, 7, e41009. [Google Scholar] [CrossRef]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; De Vos, W.M.; et al. Homeostasis of the Gut Barrier and Potential Biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Van Itallie, C.M. Physiology and Function of the Tight Junction. Cold Spring Harb. Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight Junctions: From Simple Barriers to Multifunctional Molecular Gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, C.T.; Powell, D.N.; Kalman, D. Layered Defense: How Mucus and Tight Junctions Seal the Intestinal Barrier. J. Mol. Med. 2017, 95, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Rowart, P.; Wu, J.; Caplan, M.J.; Jouret, F. Implications of AMPK in the Formation of Epithelial Tight Junctions. Int. J. Mol. Sci. 2018, 19, 2040. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.A.; Li, J.S. Gut in Diseases: Physiological Elements and Their Clinical Significance. World J. Gastroenterol. 2003, 9, 2385–2389. [Google Scholar] [CrossRef]
- De Punder, K.; Pruimboom, L. Stress Induces Endotoxemia and Low-Grade Inflammation by Increasing Barrier Permeability. Front. Immunol. 2015, 6, 223. [Google Scholar] [CrossRef]
- Hollander, D. Intestinal Permeability, Leaky Gut, and Intestinal Disorders. Curr. Gastroenterol. Rep. 1999, 1, 410–416. [Google Scholar] [CrossRef]
- Fasano, A. All Disease Begins in the (Leaky) Gut: Role of Zonulin-Mediated Gut Permeability in the Pathogenesis of Some Chronic Inflammatory Diseases. F1000Research 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut Microbiota and Intestinal Trans-Epithelial Permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of the Intestinal Barrier by Nutrients: The Role of Tight Junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- Yarandi, S.S.; Peterson, D.A.; Treisman, G.J.; Moran, T.H.; Pasricha, P.J. Modulatory Effects of Gut Microbiota on the Central Nervous System: How Gut Could Play a Role in Neuropsychiatric Health and Diseases. J. Neurogastroenterol. Motil. 2016, 22, 201–212. [Google Scholar] [CrossRef]
- Slyepchenko, A.; Maes, M.; Jacka, F.N.; Köhler, C.A.; Barichello, T.; McIntyre, R.S.; Berk, M.; Grande, I.; Foster, J.A.; Vieta, E.; et al. Gut Microbiota, Bacterial Translocation, and Interactions with Diet: Pathophysiological Links between Major Depressive Disorder and Non-Communicable Medical Comorbidities. Psychother. Psychosom. 2016, 86, 31–46. [Google Scholar] [CrossRef]
- Simkin, D.R. Microbiome and Mental Health, Specifically as It Relates to Adolescents. Curr. Psychiatry Rep. 2019, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Van Ijzendoorn, S.C.D.; Derkinderen, P. The Intestinal Barrier in Parkinson’s Disease: Current State of Knowledge. J. Park. Dis. 2019, 9, S323–S329. [Google Scholar] [CrossRef]
- Han, S.K.; Kim, D.H. Lactobacillus Mucosae and Bifidobacterium Longum Synergistically Alleviate Immobilization Stress-Induced Anxiety/Depression in Mice by Suppressing Gut Dysbiosis. J. Microbiol. Biotechnol. 2019, 29, 1369–1374. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W.; et al. A Novel Postbiotic from Lactobacillus rhamnosus GG with a Beneficial Effect on Intestinal Barrier Function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, L.; Dou, X.; Wang, C.; Zhang, W.; Gao, K.; Liu, J.; Wang, H. Lactobacillus Reuteri ZJ617 Maintains Intestinal Integrity via Regulating Tight Junction, Autophagy and Apoptosis in Mice Challenged with Lipopolysaccharide. Oncotarget 2017, 8, 77489–77499. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Liu, L.; Cao, H.; Moore, D.J.; Washington, M.K.; Wang, B.; Peek, R.M.; Acra, S.A.; Polk, D.B.; Jr, R.M.P.; et al. Neonatal Colonization of Mice with LGG Promotes Intestinal Mucosal Development and Decreases Susceptibility to Colitis in Adulthood. Mucosal Immunol. 2017, 10, 117–127. [Google Scholar] [CrossRef]
- Ritze, Y.; Bardos, G.; Claus, A.; Ehrmann, V.; Bergheim, I.; Schwiertz, A.; Bischoff, S.C. Lactobacillus rhamnosus GG Protects against Non-Alcoholic Fatty Liver Disease in Mice. PLoS ONE 2014, 9, e80169. [Google Scholar] [CrossRef] [PubMed]
- Donato, K.A.; Gareau, M.G.; Wang, Y.J.J.; Sherman, P.M. Lactobacillus rhamnosus GG Attenuates Interferon-g and Tumour Necrosis Factor-a-Induced Barrier Dysfunction and pro-Inflammatory Signalling. Microbiology 2010, 156, 3288–3297. [Google Scholar] [CrossRef]
- Orlando, A.; Linsalata, M.; Notarnicola, M.; Tutino, V.; Russo, F. Lactobacillus GG Restoration of the Gliadin Induced Epithelial Barrier Disruption: The Role of Cellular Polyamines. BMC Microbiol. 2014, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Gotteland, M.; Cruchet, S.; Verbeke, S. Effect of Lactobacillus Ingestion on the Gastrointestinal Mucosal Barrier Alterations Induced by Indometacin in Humans. Aliment. Pharmacol. Ther. 2001, 15, 11–17. [Google Scholar] [CrossRef]
- Francavilla, R.; Miniello, V.; Magista, A.M.; De Canio, A.; Bucci, N.; Gagliardi, F.; Lionetti, E.; Castellaneta, S.; Polimeno, L.; Peccarisi, L.; et al. A Randomized Controlled Trial of Lactobacillus GG in Children with Functional Abdominal Pain. Pediatrics 2010, 126, e1445–e1452. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, K.N.C.; Sowmyanarayanan, T.V.; Paul, A.; Babji, S.; Ajjampur, S.S.R.; Priyadarshini, S.; Sarkar, R.; Balasubramanian, K.A.; Wanke, C.A.; Ward, H.D.; et al. Immune Response and Intestinal Permeability in Children with Acute Gastroenteritis Treated with Lactobacillus rhamnosus GG: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2014, 58, 1107–1115. [Google Scholar] [CrossRef]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the Gut Microbiome and Mucosal Immune System. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef]
- Vighi, G.; Marcucci, F.; Sensi, L.; Di Cara, G.; Frati, F. Allergy and the Gastrointestinal System. Clin. Exp. Immunol. 2008, 153, 3–6. [Google Scholar] [CrossRef]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human Gut-Associated Lymphoid Tissues (GALT); Diversity, Structure, and Function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Rogovskii, V. Immune Tolerance as the Physiologic Counterpart of Chronic Inflammation. Front. Immunol. 2020, 11, 2061. [Google Scholar] [CrossRef] [PubMed]
- Maskrey, B.H.; Megson, I.L.; Whitfield, P.D.; Rossi, A.G. Mechanisms of Resolution of Inflammation: A Focus on Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2011, 31, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Brain, S.D.; Buckley, C.D.; Gilroy, D.W.; Haslett, C.; O’Neill, L.A.J.; Perretti, M.; Rossi, A.G.; Wallace, J.L. Resolution of Inflammation: State of the Art, Definitions and Terms. FASEB J. 2006, 21, 672271. [Google Scholar] [CrossRef]
- Mann, E.R.; Landy, J.D.; Bernardo, D.; Peake, S.T.C.; Hart, A.L.; Al-Hassi, H.O.; Knight, S.C. Intestinal Dendritic Cells: Their Role in Intestinal Inflammation, Manipulation by the Gut Microbiota and Differences between Mice and Men. Immunol. Lett. 2013, 150, 30–40. [Google Scholar] [CrossRef]
- Lorenzatti, A.J. Anti-Inflammatory Treatment and Cardiovascular Outcomes: Results of Clinical Trials Anti-Inflammatory Treatment and Cardiovascular Outcomes. Eur. Cardiol. 2021, 16, e15. [Google Scholar] [CrossRef] [PubMed]
- Coates, L.C.; FitzGerald, O.; Helliwell, P.S.; Paul, C. Psoriasis, Psoriatic Arthritis, and Rheumatoid Arthritis: Is All Inflammation the Same? Semin. Arthritis Rheum. 2016, 46, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer’s Disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12050. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Wan, Y.Y. Intricacies of TGF-β Signaling in Treg and Th17 Cell Biology. Cell Mol. Immunol. 2023, 20, 1002–1022. [Google Scholar] [CrossRef]
- Vignali, D.A.A.; Collison, L.W.; Workman, C.J. How Regulatory T Cells Work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef]
- Tanoue, T.; Atarashi, K.; Honda, K. Development and Maintenance of Intestinal Regulatory T Cells. Nat. Rev. Immunol. 2016, 16, 295–309. [Google Scholar] [CrossRef]
- Deng, Y.; McDonald, O.G.; Means, A.L.; Peek, R.M.; Washington, M.K.; Acra, S.A.; Polk, D.B.; Yan, F. Exposure to P40 in Early Life Prevents Intestinal Inflammation in Adulthood through Inducing a Long-Lasting Epigenetic Imprint on TGFβ. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 1327–1345. [Google Scholar] [CrossRef]
- Guo, M.; Liu, H.; Yu, Y.; Zhu, X.; Xie, H.; Wei, C.; Mei, C.; Shi, Y.; Zhou, N.; Qin, K.; et al. Lactobacillus rhamnosus GG Ameliorates Osteoporosis in Ovariectomized Rats by Regulating the Th17/Treg Balance and Gut Microbiota Structure. Gut Microbes 2023, 15, 2190304. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.Y.; Li, Q.-H.; Su, H.; Sun, X. Lactobacillus rhamnosus GG Induced Protective Effect on Allergic Airway Inflammation Is Associated with Gut Microbiota. Cell Immunol. 2018, 332, 77–84. [Google Scholar] [CrossRef]
- Chen, R.C.; Xu, L.M.; Du, S.J.; Huang, S.S.; Wu, H.; Dong, J.J.; Huang, J.R.; Wang, X.D.; Feng, W.K.; Chen, Y.P. Lactobacillus rhamnosus GG Supernatant Promotes Intestinal Barrier Function, Balances Treg and TH17 Cells and Ameliorates Hepatic Injury in a Mouse Model of Chronic-Binge Alcohol Feeding. Toxicol. Lett. 2016, 241, 103–110. [Google Scholar] [CrossRef]
- Khailova, L.; Baird, C.H.; Rush, A.A.; McNamee, E.N.; Wischmeyer, P.E. Lactobacillus rhamnosus GG Improves Outcome in Experimental Pseudomonas Aeruginosa Pneumonia: Potential Role of Regulatory T Cells. Shock 2013, 40, 496–503. [Google Scholar] [CrossRef]
- Feleszko, W.Ã.; Jaworska, J.Ã.; Rha, R.D.; Steinhausen, S.; Avagyan, A.; Jaudszus, A.; Ahrens, B.; Groneberg, D.A.; Wahn, U.; Hamelmann, E. Probiotic-Induced Suppression of Allergic Sensitization and Airway Inflammation Is Associated with an Increase of T Regulatory-Dependent Mechanisms in a Murine Model of Asthma. Clin. Exp. Allergy 2007, 37, 498–505. [Google Scholar] [CrossRef]
- Shen, X.; Liu, L.; Peek, R.M.; Acra, S.A.; Moore, D.J.; Wilson, K.T.; He, F.; Polk, D.B.; Yan, F. Supplementation of P40, a Lactobacillus rhamnosus GG-Derived Protein, in Early Life Promotes Epidermal Growth Factor Receptor-Dependent Intestinal Development and Long-Term Health Outcomes. Mucosal Immunol. 2018, 11, 1316–1328. [Google Scholar] [CrossRef]
- Lebeer, S.; Bron, P.A.; Marco, M.L.; Van Pijkeren, J.P.; O’Connell Motherway, M.; Hill, C.; Pot, B.; Roos, S.; Klaenhammer, T. Identification of Probiotic Effector Molecules: Present State and Future Perspectives. Curr. Opin. Biotechnol. 2018, 49, 217–223. [Google Scholar] [CrossRef]
- García, C.E.V.; Petrova, M.; Claes, I.J.J.; De Boeck, I.; Verhoeven, T.L.A.; Dilissen, E.; von Ossowski, I.; Palva, A.; Bullens, D.M.; Vanderleyden, J.; et al. Piliation of Lactobacillus rhamnosus GG Promotes Adhesion, Phagocytosis, and Cytokine Modulation in Macrophages. Appl. Environ. Microbiol. 2015, 81, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, S.; Liang, S.; Xiang, F.; Wang, X.; Lian, H.; Li, B.; Liu, F. Exopolysaccharides of Lactic Acid Bacteria: Structure, Biological Activity, Structure-Activity Relationship, and Application in the Food Industry: A Review. Int. J. Biol. Macromol. 2024, 257, 128733. [Google Scholar] [CrossRef]
- Duboux, S.; Golliard, M.; Muller, J.A.; Bergonzelli, G.; Bolten, C.J.; Mercenier, A.; Kleerebezem, M. Carbohydrate-Controlled Serine Protease Inhibitor (Serpin) Production in Bifidobacterium Longum Subsp. Longum. Sci. Rep. 2021, 11, 7236. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Xu, J.; Zhao, Z.; Guo, Y.; Zhang, H.; Jurutka, P.W.; Huang, D.; Cao, C.; Cheng, S. Dietary Lactobacillus rhamnosus GG Extracellular Vesicles Enhance Antiprogrammed Cell Death 1 (Anti-PD-1) Immunotherapy Efficacy against Colorectal Cancer. Food Funct. 2023, 14, 10314–10328. [Google Scholar] [CrossRef] [PubMed]
- Claes, I.J.J.; Schoofs, G.; Regulski, K.; Courtin, P.; Chapot-Chartier, M.-P.; Rolain, T.; Hols, P.; von Ossowski, I.; Reunanen, J.; de Vos, W.M.; et al. Genetic and Biochemical Characterization of the Cell Wall Hydrolase Activity of the Major Secreted Protein of Lactobacillus rhamnosus GG. PLoS ONE 2012, 7, e31588. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Li, F.; Liu, Y.; Jiang, M.; Zhang, L.; He, L.; Wilkey, D.W.; Merchant, M.; Zhang, X.; Deng, Z.-B.; et al. Exosome-Like Nanoparticles from Lactobacillus rhamnosus GG Protect Against Alcohol-Associated Liver Disease through Intestinal Aryl Hydrocarbon Receptor in Mice. Hepatol. Commun. 2021, 5, 846–864. [Google Scholar] [CrossRef] [PubMed]
- Yoda, K.; Miyazawa, K.; Hosoda, M.; Hiramatsu, M.; Yan, F.; He, F. Lactobacillus GG-Fermented Milk Prevents DSS-Induced Colitis and Regulates Intestinal Epithelial Homeostasis through Activation of Epidermal Growth Factor Receptor. Eur. J. Nutr. 2014, 53, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Claes, I.J.J.; Balog, C.I.A.; Schoofs, G.; Verhoeven, T.L.A.; Nys, K.; von Ossowski, I.; de Vos, W.M.; Tytgat, H.L.P.; Agostinis, P.; et al. The Major Secreted Protein Msp1/P75 Is O-Glycosylated in Lactobacillus rhamnosus GG. Microb. Cell Fact. 2012, 11, 15. [Google Scholar] [CrossRef]
- Bäuerl, C.; Abitayeva, G.; Sosa-Carrillo, S.; Mencher-Beltrán, A.; Navarro-Lleó, N.; Coll-Marqués, J.M.; Zúñiga-Cabrera, M.; Shaikhin, S.; Pérez-Martinez, G. P40 and P75 Are Singular Functional Muramidases Present in the Lactobacillus Casei/Paracasei/Rhamnosus Taxon. Front. Microbiol. 2019, 10, 1420. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Characterization of a Probiotic-Derived Soluble Protein Which Reveals a Mechanism of Preventive and Treatment Effects of Probiotics on Intestinal Inflammatory Diseases. Gur Microbes 2012, 3, 25–28. [Google Scholar] [CrossRef]
- Yan, F.; Cao, H.; Cover, T.L.; Washington, M.K.; Shi, Y.; Liu, L.; Chaturvedi, R.; Peek Jr, R.M.; Wilson, K.T.; Polk, D.B. Colon-Specific Delivery of a Probiotic-Derived Soluble Protein Ameliorates Intestinal Inflammation in Mice through an EGFR-Dependent Mechanism. J. Clin. Investig. 2011, 121, 2242–2253. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Liu, L.; Dempsey, P.J.; Tsai, Y.H.; Raines, E.W.; Wilson, C.L.; Cao, H.; Cao, Z.; Liu, L.; Polk, D.B. A Lactobacillus rhamnosus GG-Derived Soluble Protein, P40, Stimulates Ligand Release from Intestinal Epithelial Cells to Transactivate Epidermal Growth Factor Receptor. J. Biol. Chem. 2013, 288, 30742–30751. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, H.; Liu, L.; Wang, B.; Walker, W.A.; Acra, S.A.; Yan, F. Activation of Epidermal Growth Factor Receptor Mediates Mucin Production Stimulated by P40, a Lactobacillus rhamnosus GG-Derived Protein. J. Biol. Chem. 2014, 289, 20234–20244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Moore, D.J.; Shen, X.; Peek, R.M.; Acra, S.A.; Li, H.; Ren, X.; Polk, D.B.; Yan, F. An LGG-Derived Protein Promotes IgA Production through Upregulation of APRIL Expression in Intestinal Epithelial Cells. Mucosal Immunol. 2017, 10, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Yan, F.; Polk, D.B.; Rao, R.K. Probiotics Ameliorate the Hydrogen Peroxide-Induced Epithelial Barrier Disruption by a PKC- and MAP Kinase-Dependent Mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Higginbotham, J.N.; Liu, L.; Zhao, G.; Acra, S.A.; Peek, R.M.; Polk, D.B. Production of a Functional Factor, P40, by Lactobacillus rhamnosus GG Is Promoted by Intestinal Epithelial Cell- Secreted Extracellular Vesicles. Infect. Immun. 2019, 87, 1–17. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotic Bacterium Prevents Cytokine-Induced Apoptosis in Intestinal Epithelial Cells. J. Biol. Chem. 2002, 277, 50959–50965. [Google Scholar] [CrossRef]
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble Proteins Produced by Probiotic Bacteria Regulate Intestinal Epithelial Cell Survival and Growth. Gasteroenterology 2007, 132, 562–575. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.; Peng, C.; Gui, Q.; Li, J.; Xu, Z.; Yang, Y. Lactobacillus rhamnosus GG Promotes Recovery of the Colon Barrier in Septic Mice through Accelerating ISCs Regeneration. Nutrients 2023, 15, 672. [Google Scholar] [CrossRef]
- Tipici, E.B.; Coskunpinar, E.; Altunkanat, D.; Cagatay, P.; Omer, B.; Palanduz, S.; Satman, I.; Aral, F. Lactobacillus GG Is Associated with Mucin Genes Expressions in Type 2 Diabetes Mellitus: A Randomized, Placebo-Controlled Trial. Eur. J. Nutr. 2023, 62, 2155–2164. [Google Scholar] [CrossRef]
- Han, X.; Lee, A.; Huang, S.; Gao, J.; Spence, J.R.; Owyang, C. Lactobacillus rhamnosus GG Prevents Epithelial Barrier Dysfunction Induced by Interferon-Gamma and Fecal Supernatants from Irritable Bowel Syndrome Patients in Human Intestinal Enteroids and Colonoids. Gut Microbes 2019, 10, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Drabik, K.A.; Waypa, T.S.; Musch, M.W.; Alverdy, J.C.; Schneewind, O.; Chang, E.B.; Petrof, E.O. Soluble Factors from Lactobacillus GG Activate MAPKs and Induce Cytoprotective Heat Shock Proteins in Intestinal Epithelial Cells. Am. J. Physiol. Cell Physiol. 2006, 290, C1018–C1030. [Google Scholar] [CrossRef] [PubMed]
- Donkor, O.N.; Ravikumar, M.; Proudfoot, O.; Day, S.L.; Apostolopoulos, V.; Paukovics, G.; Vasiljevic, T.; Nutt, S.L.; Gill, H. Cytokine Profile and Induction of T Helper Type 17 and Regulatory T Cells by Human Peripheral Mononuclear Cells after Microbial Exposure. Clin. Exp. Immunol. 2012, 167, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Pessi, T.; Sutas, Y.; Hurme, S.M.; Isolauri, E.; Allergy, E. Interleukin-10 Generation in Atopic Children Following Oral Lactobacillus rhamnosus GG. Clin. Exp. Allergy 2000, 30, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Ali, S.A.; Short, S.P.; Williams, C.S.; Goettel, J.A.; Washington, M.K.; Peek, R.M.; Acra, S.A.; Yan, F. Identification of a Functional Peptide of a Probiotic Bacterium-Derived Protein for the Sustained Effect on Preventing Colitis. Gut Microbes 2023, 15, 2264456. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H.; McMahon, R.J. Milk-Derived or Recombinant Transforming Growth Factor-Beta Has Effects on Immunological Outcomes: A Review of Evidence from Animal Experimental Studies. Clin. Exp. Allergy 2011, 41, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, S.; Lun, J.; Gao, J.; Gao, X.; Gong, Z.; Wan, Y.; He, X.; Cao, H. Inhibitory Effects of the Lactobacillus rhamnosus GG Effector Protein HM0539 on Inflammatory Response through the TLR4/MyD88/NF-κB Axis. Front. Immunol. 2020, 11, 551449. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Garrido, N.; Badia, J.; Baldomà, L. Microbiota-Derived Extracellular Vesicles in Interkingdom Communication in the Gut. J. Extracell. Vesicles 2021, 10, e12161. [Google Scholar] [CrossRef]
- Stentz, R.; Carvalho, A.L.; Jones, E.J.; Carding, S.R. Fantastic Voyage: The Journey of Intestinal Microbiota-Derived Microvesicles through the Body. Biochem. Soc. Trans. 2018, 46, 1021–1027. [Google Scholar] [CrossRef]
- Krzyżek, P.; Marinacci, B.; Vitale, I.; Grande, R. Extracellular Vesicles of Probiotics: Shedding Light on the Biological Activity and Future Applications. Pharmaceutics 2023, 15, 552. [Google Scholar] [CrossRef]
- Bäuerl, C.; Coll-Marqués, J.M.; Tarazona-González, C.; Pérez-Martínez, G. Lactobacillus Casei Extracellular Vesicles Stimulate EGFR Pathway Likely Due to the Presence of Proteins P40 and P75 Bound to Their Surface. Sci. Rep. 2020, 10, 19237. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhang, X.; Hao, H.; Liu, Q.; Zhou, Z.; Liang, X.; Liu, T.; Gong, P.; Zhang, L.; Zhai, Z.; et al. Lactobacillus rhamnosus GG Derived Extracellular Vesicles Modulate Gut Microbiota and Attenuate Inflammatory in DSS-Induced Colitis Mice. Nutrients 2021, 13, 3319. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, M.M.; Björkander, S.; Pang, Y.; Lundqvist, L.; Ndi, M.; Ott, M.; Escribá, I.B.; Jaeger, M.C.; Roos, S.; Sverremark-Ekström, E. Extracellular Membrane Vesicles from Lactobacilli Dampen IFN-γ Responses in a Monocyte-Dependent Manner. Sci. Rep. 2019, 9, 17109. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, E.; Mahmoodzadeh Hosseini, H.; Imani Fooladi, A.A. The Inhibitory Impacts of Lactobacillus rhamnosus GG-Derived Extracellular Vesicles on the Growth of Hepatic Cancer Cells. Microb. Pathog. 2017, 110, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Keyhani, G.; Hosseini, H.M.; Salimi, A. Effect of Extracellular Vesicles of Lactobacillus rhamnosus GG on the Expression of CEA Gene and Protein Released by Colorectal Cancer Cells. Iran. J. Microbiol. 2022, 14, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Kankainen, M.; Paulin, L.; Tynkkynen, S.; von Ossowski, I.; Reunanen, J.; Partanen, P.; Satokari, R.; Vesterlund, S.; Hendrickx, A.P.A.; Lebeer, S.; et al. Comparative Genomic Analysis of Lactobacillus rhamnosus GG Reveals Pili Containing a Human- Mucus Binding Protein. Proc. Natl. Acad. Sci. USA 2009, 106, 17193–17198. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Claes, I.; Tytgat, H.L.P.; Verhoeven, T.L.A.; Marien, E.; von Ossowski, I.; Reunanen, J.; Palva, A.; de Vos, W.M.; De Keersmaecker, S.C.J.; et al. Functional Analysis of Lactobacillus rhamnosus GG Pili in Relation to Adhesion and Immunomodulatory Interactions with Intestinal Epithelial Cells. Appl. Environ. Microbiol. 2012, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Beaussart, A.; Alsteens, D.; Dupres, V.; Claes, I.; Von Ossowski, I.; De Vos, W.M.; Palva, A.; Lebeer, S.; Vanderleyden, J.; et al. Adhesion and Nanomechanics of Pili from the Probiotic Lactobacillus rhamnosus GG. ACS Nano 2013, 7, 3685–3697. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Ueno, S.; Sugiyama, M.; Yamamoto, Y.; Mukai, T. Lactobacillus rhamnosus GG SpaC Pilin Subunit Binds to the Carbohydrate Moieties of Intestinal Glycoconjugates. Anim. Sci. J. 2016, 87, 809–815. [Google Scholar] [CrossRef]
- Vélez, M.P.; Petrova, M.I.; Lebeer, S.; Verhoeven, T.L.A.; Claes, I.; Lambrichts, I.; Tynkkynen, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Characterization of MabA, a Modulator of Lactobacillus rhamnosus GG Adhesion and Biofilm Formation. Immunol. Med. Microbiol. 2010, 59, 386–398. [Google Scholar] [CrossRef]
- von Ossowski, I.; Satokari, R.; Reunanen, J.; Lebeer, S.; De Keersmaecker, S.C.J.; Vanderleyden, J.; de Vos, W.M.; Palva, A. Functional Characterization of a Mucus-Specific LPXTG Surface Adhesin from Probiotic Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 2011, 77, 4465–4472. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, H.L.P.; van Teijlingen, N.H.; Sullan, R.M.A.; Douillard, F.P.; Rasinkangas, P.; Messing, M.; Reunanen, J.; Satokari, R.; Vanderleyden, J.; Dufrêne, Y.F.; et al. Probiotic Gut Microbiota Isolate Interacts with Dendritic Cells via Glycosylated Heterotrimeric Pili. PLoS ONE 2016, 11, e0151824. [Google Scholar] [CrossRef] [PubMed]
- Mohammedsaeed, W.; Cruickshank, S.; McBain, A.J.; O’Neill, C.A. Lactobacillus rhamnosus GG Lysate Increases Re-Epithelialization of Keratinocyte Scratch Assays by Promoting Migration. Sci. Rep. 2015, 5, 16147. [Google Scholar] [CrossRef]
- El-Chami, C.; Choudhury, R.; Mohammedsaeed, W.; McBain, A.J.; Kainulainen, V.; Lebeer, S.; Satokari, R.; O’Neill, C.A. Multiple Proteins of Lacticaseibacillus Rhamnosus GG Are Involved in the Protection of Keratinocytes from the Toxic Effects of Staphylococcus Aureus. Front. Microbiol. 2022, 13, 875542. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, H.L.P.; Douillard, F.P.; Reunanen, J.; Rasinkangas, P.; Hendrickx, A.P.A.; Laine, P.K.; Paulin, L.; Satokari, R.; de Vos, W.M. Lactobacillus rhamnosus GG Outcompetes Enterococcus faecium via Mucus-Binding Pili: Evidence for a Novel and Heterospecific Probiotic Mechanism. Appl. Environ. Microbiol. 2016, 82, 5756–5762. [Google Scholar] [CrossRef]
- Petrova, M.I.; Imholz, N.C.E.E.; Verhoeven, T.L.A.; Balzarini, J.; Van Damme, E.J.M.M.; Schols, D.; Vanderleyden, J.; Lebeer, S. Lectin-Like Molecules of Lactobacillus rhamnosus GG Inhibit Pathogenic Escherichia coli and Salmonella Biofilm Formation. PLoS ONE 2016, 11, e0161337. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Hols, P.; Bernard, E.; Rolain, T.; Zhou, M.; Siezen, R.J.; Bron, P.A. The Extracellular Biology of the Lactobacilli. FEMS Microbiol. Rev. 2010, 34, 199–230. [Google Scholar] [CrossRef]
- Lebeer, S.; Verhoeven, T.L.A.; Francius, G.; Schoofs, G.; Lambrichts, I.; Dufrêne, Y.; Vanderleyden, J.; De Keersmaecker, S.C.J. Identification of a Gene Cluster for the Biosynthesis of a Long, Galactose-Rich Exopolysaccharide in Lactobacillus rhamnosus GG and Functional Analysis of the Priming Glycosyltransferase. Appl. Environ. Microbiol. 2009, 75, 3554–3563. [Google Scholar] [CrossRef]
- Burgain, J.; Scher, J.; Lebeer, S.; Vanderleyden, J.; Corgneau, M.; Guerin, J.; Caillet, C.; Duval, J.F.L.; Francius, G.; Gaiani, C. Impacts of PH-Mediated EPS Structure on Probiotic Bacterial Pili-Whey Proteins Interactions. Colloids Surf. B Biointerfaces 2015, 134, 332–338. [Google Scholar] [CrossRef]
- Lu, Y.; Han, S.; Zhang, S.; Wang, K.; Lv, L.; McClements, D.J.; Xiao, H.; Berglund, B.; Yao, M.; Li, L. The Role of Probiotic Exopolysaccharides in Adhesion to Mucin in Different Gastrointestinal Conditions. Curr. Res. Food Sci. 2022, 5, 581–589. [Google Scholar] [CrossRef]
- Koskenniemi, K.; Laakso, K.; Koponen, J.; Kankainen, M.; Greco, D.; Auvinen, P.; Savijoki, K.; Nyman, T.A.; Surakka, A.; Salusjärvi, T.; et al. Proteomics and Transcriptomics Characterization of Bile Stress Response in Probiotic Lactobacillus rhamnosus GG. Mol. Cell. Proteom. 2011, 10, M110.002741. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Claes, I.J.J.; Verhoeven, T.L.A.; Vanderleyden, J.; De Keersmaecker, S.C.J. Exopolysaccharides of Lactobacillus rhamnosus GG Form a Protective Shield against Innate Immune Factors in the Intestine. Microb. Biotechnol. 2011, 4, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Allonsius, C.N.; van den Broek, M.F.L.; De Boeck, I.; Kiekens, S.; Oerlemans, E.F.M.; Kiekens, F.; Foubert, K.; Vandenheuvel, D.; Cos, P.; Delputte, P.; et al. Interplay between Lactobacillus rhamnosus GG and Candida and the Involvement of Exopolysaccharides. Microb. Biotechnol. 2017, 10, 1753–1763. [Google Scholar] [CrossRef]
- Allonsius, C.N.; Vandenheuvel, D.; Oerlemans, E.F.M.; Petrova, M.I.; Donders, G.G.G.; Cos, P.; Delputte, P.; Lebeer, S. Inhibition of Candida Albicans Morphogenesis by Chitinase from Lactobacillus rhamnosus GG. Sci. Rep. 2019, 9, 2900. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Wang, C.; Liu, L.; Dou, X.; Liu, J.; Yuan, L.; Zhang, W.; Wang, H. Immunomodulation and Signaling Mechanism of Lactobacillus rhamnosus GG and Its Components on Porcine Intestinal Epithelial Cells Stimulated by Lipopolysaccharide. J. Microbiol. Immunol. Infect. 2017, 50, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; Wu, Q.; Gao, N.; Wang, Z.; Yang, Y.; Shan, A. Exopolysaccharides of Lactobacillus rhamnosus GG Ameliorate Salmonella Typhimurium-Induced Intestinal Inflammation via the TLR4/NF-ΚB/MAPK Pathway. J. Anim. Sci. Biotechnol. 2023, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Hekimi, S.; Lapointe, J.; Wen, Y. Taking a “Good” Look at Free Radicals in the Aging Process. Trends Cell Biol. 2011, 21, 569–576. [Google Scholar] [CrossRef]
- Blagov, A.V.; Orekhova, V.A.; Sukhorukov, V.N.; Melnichenko, A.A.; Orekhov, A.N. Potential Use of Antioxidant Compounds for the Treatment of Inflammatory Bowel Disease. Pharmaceuticals 2023, 16, 1150. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Gao, N.; Wang, Z.; Li, F.; Li, J.; Shan, A. Exopolysaccharides Produced by: Lactobacillus rhamnosus GG Alleviate Hydrogen Peroxide-Induced Intestinal Oxidative Damage and Apoptosis through the Keap1/Nrf2 and Bax/Bcl-2 Pathways in Vitro. Food Funct. 2021, 12, 9632–9641. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Z.; Li, Y.; Zhou, L.; Ding, Q.; Xu, L. Isolated Exopolysaccharides from Lactobacillus rhamnosus GG Alleviated Adipogenesis Mediated by TLR2 in Mice. Sci. Rep. 2016, 6, 36083. [Google Scholar] [CrossRef]
- Vélez, M.P.; Verhoeven, T.L.A.; Draing, C.; Von Aulock, S.; Pfitzenmaier, M.; Geyer, A.; Lambrichts, I.; Grangette, C.; Pot, B.; Vanderleyden, J.; et al. Functional Analysis of D-Alanylation of Lipoteichoic Acid in the Probiotic Strain Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 2007, 73, 3595–3604. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.H.; Baik, J.E.; Yang, J.S.; Kang, S.S.; Im, J.; Yun, C.H.; Kim, D.W.; Lee, K.; Chung, D.K.; Ju, H.R.; et al. Differential Immunostimulatory Effects of Gram-Positive Bacteria Due to Their Lipoteichoic Acids. Int. Immunopharmacol. 2009, 9, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, A.D.; Leoni, J.; Paz, M.L.; González Maglio, D.H. Lipoteichoic Acid from Lacticaseibacillus Rhamnosus GG Modulates Dendritic Cells and T Cells in the Gut. Nutrients 2022, 14, 723. [Google Scholar] [CrossRef] [PubMed]
- Claes, I.J.J.; Segers, M.E.; Verhoeven, T.L.A.; Dusselier, M.; Sels, B.F.; De Keersmaecker, S.C.J.; Vanderleyden, J.; Lebeer, S. Lipoteichoic Acid Is an Important Microbe-Associated Molecular Pattern of Lactobacillus rhamnosus GG. Microb. Cell Fact. 2012, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Claes, I.J.J.; Lebeer, S.; Shen, C.; Verhoeven, T.L.A.; Dilissen, E.; De Hertogh, G.; Bullens, D.M.A.; Ceuppens, J.L.; Van Assche, G.; Vermeire, S.; et al. Impact of Lipoteichoic Acid Modification on the Performance of the Probiotic Lactobacillus rhamnosus GG in Experimental Colitis. Clin. Exp. Immunol. 2010, 162, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Yun, C.H.; Han, S.H.; Song, K.D.; Kang, S.S. Inhibitory Effect of Lipoteichoic Acid Derived from Three Lactobacilli on Flagellin-Induced IL-8 Production in Porcine Peripheral Blood Mononuclear Cells. Probiotics Antimicrob. Proteins 2021, 13, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, M.A.; Riehl, T.E.; Rao, M.S.; Moon, C.; Ee, X.; Nava, G.M.; Walker, M.R.; Marinshaw, J.M.; Stappenbeck, T.S.; Stenson, W.F. Lactobacillus Probiotic Protects Intestinal Epithelium from Radiation Injury in a TLR-2/Cyclo-Oxygenase-2-Dependent Manner. Gut 2012, 61, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Riehl, T.E.; Alvarado, D.; Ee, X.; Zuckerman, A.; Foster, L.; Kapoor, V.; Thotala, D.; Ciorba, M.A.; Stenson, W.F. Lactobacillus rhamnosus GG Protects the Intestinal Epithelium from Radiation Injury through Release of Lipoteichoic Acid, Macrophage Activation and the Migration of Mesenchymal Stem Cells. Gut 2019, 68, 1003–1013. [Google Scholar] [CrossRef]
- Lam, E.K.Y.; Tai, E.K.K.; Koo, M.W.L.; Wong, H.P.S.; Wu, W.K.K.; Yu, L.; So, W.H.L.; Woo, P.C.Y.; Cho, C.H. Enhancement of Gastric Mucosal Integrity by Lactobacillus rhamnosus GG. Life Sci. 2007, 80, 2128–2136. [Google Scholar] [CrossRef]
- Weill, F.S.; Cela, E.M.; Paz, M.L.; Ferrari, A.; Leoni, J.; Maglio, D.H.G. Lipoteichoic Acid from Lactobacillus rhamnosus GG as an Oral Photoprotective Agent against UV-Induced Carcinogenesis. Br. J. Nutr. 2013, 109, 457–466. [Google Scholar] [CrossRef]
- Friedrich, A.D.; Campo, V.E.; Cela, E.M.; Morelli, A.E.; Shufesky, W.J.; Tckacheva, O.A.; Leoni, J.; Paz, M.L.; Larregina, A.T.; González Maglio, D.H. Oral Administration of Lipoteichoic Acid from Lactobacillus rhamnosus GG Overcomes UVB-Induced Immunosuppression and Impairs Skin Tumor Growth in Mice. Eur. J. Immunol. 2019, 49, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, C.; Shiraishi, T.; Chiou, T.Y.; Nakashima, Y.; Higashimura, Y.; Yokota, S.I.; Yamamoto, K.; Takahashi, T. Role of Lipoteichoic Acid from the Genus Apilactobacillus in Inducing a Strong IgA Response. Appl. Environ. Microbiol. 2022, 88, e0019022. [Google Scholar] [CrossRef] [PubMed]
- Schalich, K.; Rajagopala, S.; Das, S.; O’Connell, R.; Yan, F. Intestinal Epithelial Cell-Derived Components Regulate Transcriptome of Lactobacillus rhamnosus GG. Front. Microbiol. 2023, 13, 1051310. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-C.; Tomita, S.; Kleerebezem, M.; Bron, P.A. The Quest for Probiotic Effector Molecules—Unraveling Strain Specificity at the Molecular Level. Pharmacol. Res. 2013, 69, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.M.; Pot, B.; Grangette, C. Beneficial Effect of Probiotics in IBD: Are Peptidogycan and NOD2 the Molecular Key Effectors? Gut Microbes 2011, 2, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Zenhom, M.; Hyder, A.; De Vrese, M.; Heller, K.J.; Roeder, T.; Schrezenmeir, J. Peptidoglycan Recognition Protein 3 (PglyRP3) Has an Anti-Inflammatory Role in Intestinal Epithelial Cells. Immunobiology 2012, 217, 412–419. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like Receptor Recognizes Bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.D.; Kitazawa, H.; Shimosato, T.; Katoh, S.; Morita, H.; He, F.; Hosoda, M.; Saito, T. Strong Immunostimulation in Murine Immune Cells by Lactobacillus rhamnosus GG DNA Containing Novel Oligodeoxynucleotide Pattern. Cell Microbiol. 2005, 7, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Bornholdt, J.; Broholm, C.; Chen, Y.; Rago, A.; Sloth, S.; Hendel, J.; Melsæther, C.; Müller, C.V.; Nielsen, M.J.; Strickertsson, J.; et al. Personalized B Cell Response to the Lactobacillus rhamnosus GG Probiotic in Healthy Human Subjects: A Randomized Trial. Gut Microbes 2020, 12, 1854639. [Google Scholar] [CrossRef]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R.; Recto, M.S.T.; Castor, M.A.R.; Casis-Hao, R.J.; Nano, A.L.M. Comparative Effectiveness of Probiotic Strains on the Prevention of Pediatric Atopic Dermatitis: A Systematic Review and Network Meta-Analysis. Pediatr. Allergy Immunol. 2021, 32, 1255–1270. [Google Scholar] [CrossRef]
- Viljanen, M.; Savilahti, E.; Haahtela, T.; Korpela, R.; Poussa, T.; Tuure, T.; Kuitunen, M.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; et al. Probiotics in the Treatment of Atopic Eczema/Dermatitis Syndrome in Infants: A Double-Blind Placebo-Controlled Trial. Allergy Eur. J. Allergy Clin. Immunol. 2005, 60, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.D.; Tohno, M.; Kurosaki, D.; Shimosato, T.; He, F.; Hosoda, M.; Saito, T.; Kitazawa, H. Immunostimulatory Oligodeoxynucleotide Containing TTTCGTTT Motif from Lactobacillus rhamnosus GG DNA Potentially Suppresses OVA-Specific IgE Production in Mice. Scand. J. Immunol. 2008, 67, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mo, J.H.; Katakura, K.; Alkalay, I.; Rucker, A.N.; Liu, Y.T.; Lee, H.K.; Shen, C.; Cojocaru, G.; Shenouda, S.; et al. Maintenance of Colonic Homeostasis by Distinctive Apical TLR9 Signalling in Intestinal Epithelial Cells. Nat. Cell Biol. 2006, 8, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, D.; de Vrese, M.; Heller, K.J.; Schrezenmeir, J. Effect of Natural Commensal-Origin DNA on Toll-like Receptor 9 (TLR9) Signaling Cascade, Chemokine IL-8 Expression, and Barrier Integritiy of Polarized Intestinal Epithelial Cells. Inflamm. Bowel Dis. 2010, 16, 410–427. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, S.; Namai, F.; Ogita, T.; Sato, T.; Shimosato, T. Oral Priming with Oligodeoxynucleotide Particles from Lactobacillus rhamnosus GG Attenuates Symptoms of Dextran Sodium Sulfate-Induced Acute Colitis in Mice. Anim. Sci. J. 2020, 91, e13468. [Google Scholar] [CrossRef]
- Qi, S.R.; Cui, Y.J.; Liu, J.X.; Luo, X.; Wang, H.F. Lactobacillus rhamnosus GG Components, SLP, GDNA and CpG, Exert Protective Effects on Mouse Macrophages upon Lipopolysaccharide Challenge. Lett. Appl. Microbiol. 2020, 70, 118–127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leser, T.; Baker, A. Molecular Mechanisms of Lacticaseibacillus rhamnosus, LGG® Probiotic Function. Microorganisms 2024, 12, 794. https://doi.org/10.3390/microorganisms12040794
Leser T, Baker A. Molecular Mechanisms of Lacticaseibacillus rhamnosus, LGG® Probiotic Function. Microorganisms. 2024; 12(4):794. https://doi.org/10.3390/microorganisms12040794
Chicago/Turabian StyleLeser, Thomas, and Adam Baker. 2024. "Molecular Mechanisms of Lacticaseibacillus rhamnosus, LGG® Probiotic Function" Microorganisms 12, no. 4: 794. https://doi.org/10.3390/microorganisms12040794
APA StyleLeser, T., & Baker, A. (2024). Molecular Mechanisms of Lacticaseibacillus rhamnosus, LGG® Probiotic Function. Microorganisms, 12(4), 794. https://doi.org/10.3390/microorganisms12040794





