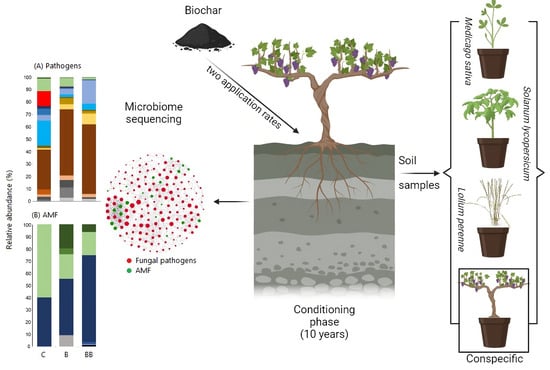
Long-Term Application of Biochar Mitigates Negative Plant–Soil Feedback by Shaping Arbuscular Mycorrhizal Fungi and Fungal Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Conditioning Phase and Biochar Application
2.2. Response Phase
2.3. Soil Chemical and Microbial Properties
2.4. Data Analysis
3. Results
3.1. Chemical Properties of the Conditioned Soils
3.2. Plants’ Performances in the Response Phase
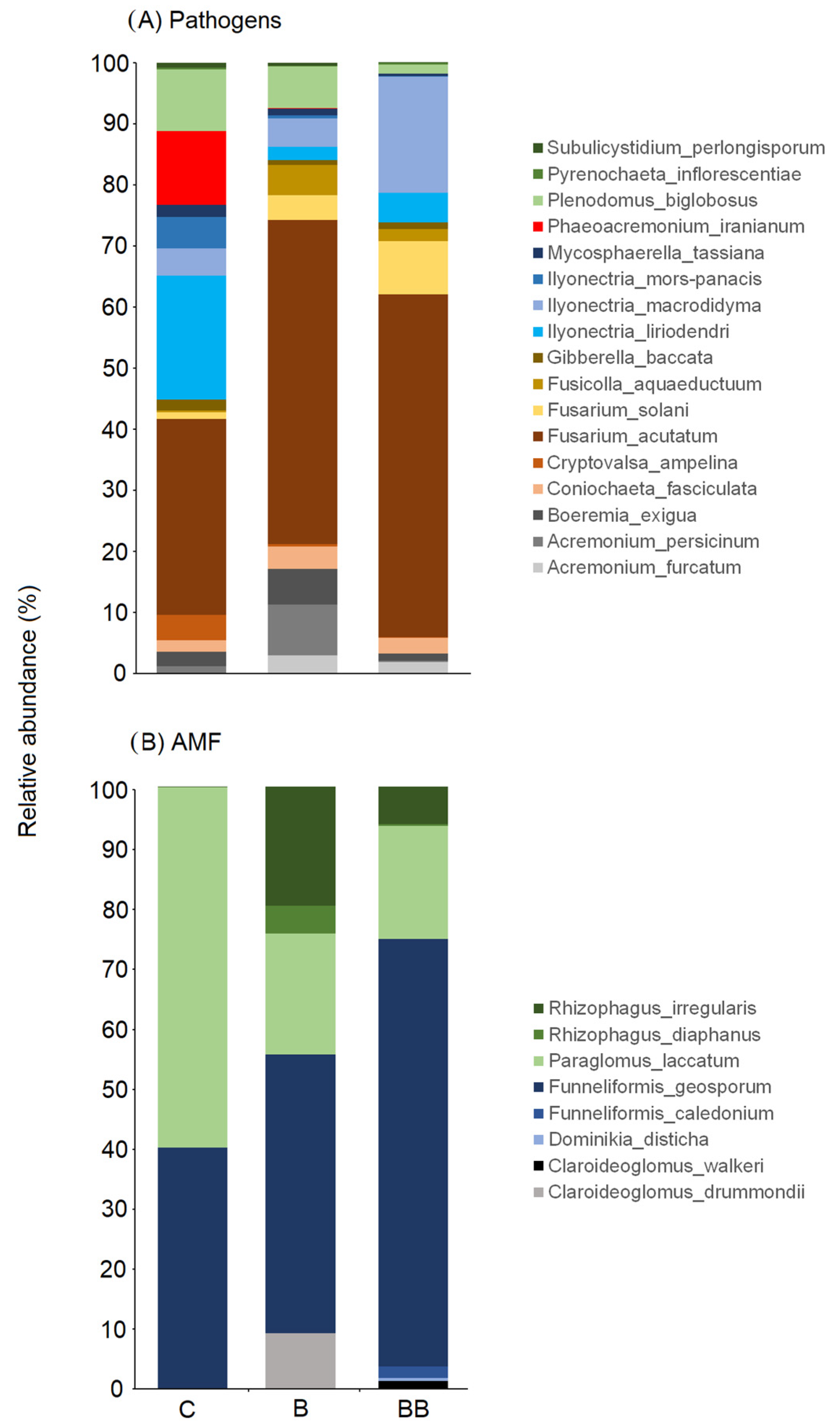
3.3. Fungal Pathogens and AMF Community Composition
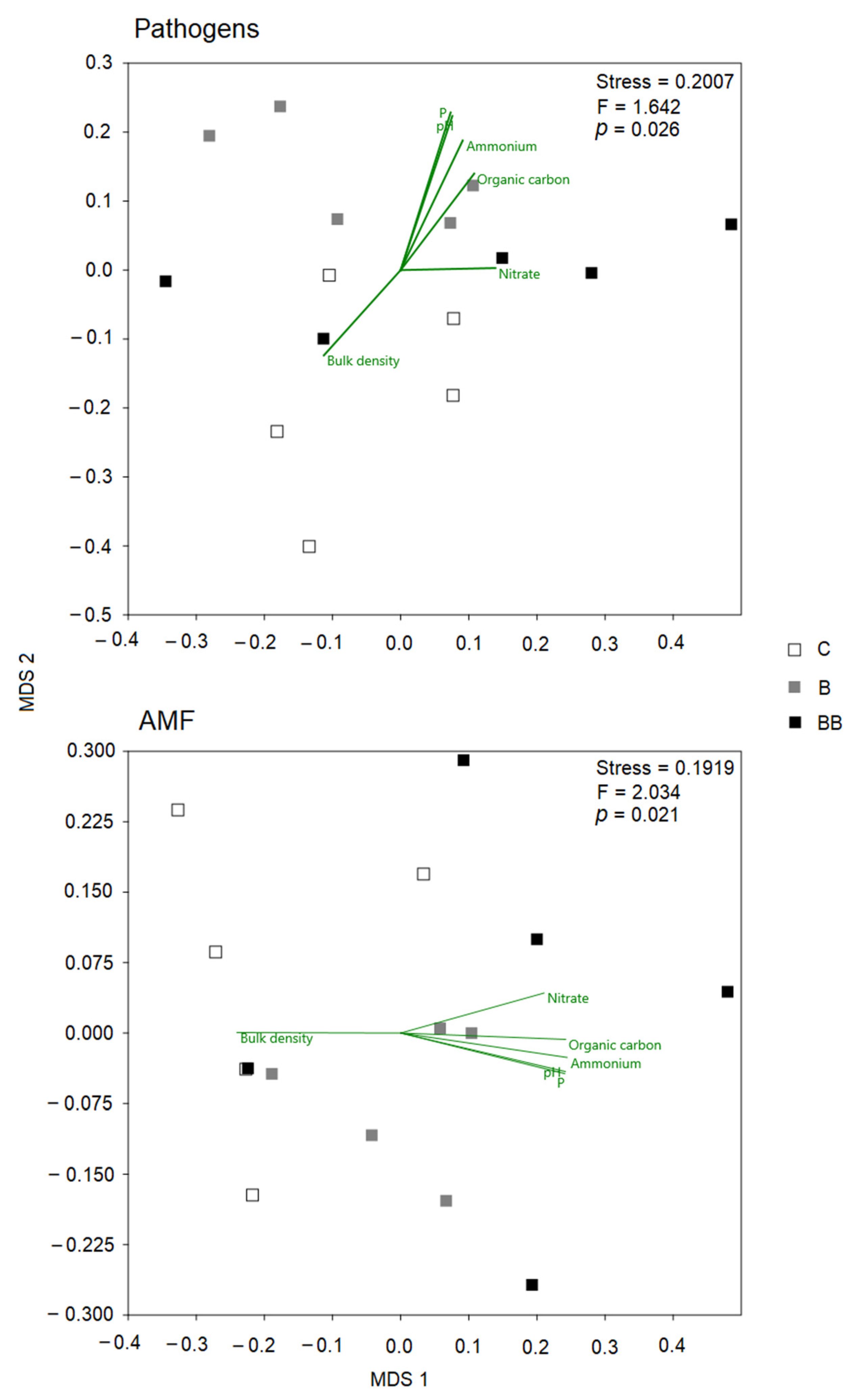
3.4. Linking Pathogens and AMF to Soil Chemical Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bever, J.D.; Westover, K.M.; Antonovics, J. Incorporating the soil community into plant population dynamics: The utility of the feedback approach. J. Ecol. 1997, 85, 561–573. [Google Scholar] [CrossRef]
- Van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant-soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Klironomos, J.N. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 2002, 417, 67–70. [Google Scholar] [CrossRef]
- Schroeder, J.W.; Dobson, A.; Mangan, S.A.; Petticord, D.F.; Herre, E.A. Mutualist and pathogen traits interact to affect plant community structure in a spatially explicit model. Nat. Commun. 2020, 11, 2204. [Google Scholar] [CrossRef]
- Wolfe, B.E.; Klironomos, J.N. Breaking new ground: Soil communities and exotic plant invasion. Bioscience 2005, 55, 477–487. [Google Scholar] [CrossRef]
- Dòstalek, T.; Münzbergová, Z.; Kladivová, A.; Macel, M. Plant–soil feedback in native vs. invasive populations of a range expanding plant. Plant Soil 2016, 399, 209–220. [Google Scholar] [CrossRef]
- Ghorbani, R.; Wilcockson, S.; Koocheki, A.; Leifert, C. Soil management for sustainable crop disease control: A review. Environ. Chem. Lett. 2008, 6, 149–162. [Google Scholar] [CrossRef]
- Dickie, I.A.; Koele, N.; Blum, J.D.; Gleason, J.D.; McGlone, M.S. Mycorrhizas in changing ecosystems. Bot.-Bot. 2014, 92, 149–160. [Google Scholar] [CrossRef]
- Idbella, M.; Bonanomi, G.; De Filippis, F.; Amor, G.; Chouyia, F.E.; Fechtali, T.; Mazzoleni, S. Contrasting effects of Rhizophagus irregularis versus bacterial and fungal seed endophytes on Trifolium repens plant-soil feedback. Mycorrhiza 2021, 31, 103–115. [Google Scholar] [CrossRef]
- Bennett, J.A.; Maherali, H.; Reinhart, K.O.; Lekberg, Y.; Hart, M.M.; Klironomos, J. Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 2017, 355, 181–184. [Google Scholar] [CrossRef]
- International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil; IBI Biochar Standards: Canandaigua, NY, USA, 2012. [Google Scholar]
- Jeffery, S.; Bezemer, M.; Cornelissen, G.; Kuyper, T.W.; Lehmann, J.; Mommer, L.; Sohi, S.; Van De Voorde, T.F.; Wardle, D.; Van Groenigen, J.W. The way forward in biochar research: Targeting trade-offs between the potential wins. GCB Bioenergy 2013, 7, 1–13. [Google Scholar] [CrossRef]
- Iacomino, G.; Sarker, T.C.; Ippolito, F.; Bonanomi, G.; Vinale, F.; Staropoli, A.; Idbella, M. Biochar and compost application either alone or in combination affects vegetable yield in a volcanic Mediterranean soil. Agronomy 2022, 12, 1996. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Abd-ElGawad, A.M.; Iacomino, G.; Nappi, A.; Grauso, L.; Idbella, M. Plant-growth promotion by biochar-organic amendments mixtures explained by selective chemicals adsorption of inhibitory compounds. J. Environ. Chem. Eng. 2023, 11, 109009. [Google Scholar] [CrossRef]
- Kończak, M.; Oleszczuk, P. Application of biochar to sewage sludge reduces toxicity and improve organisms growth in sewage sludge-amended soil in long term field experiment. Sci. Total Environ. 2018, 625, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yuan, Y.; Huang, H.; Ye, C.; Guo, C.; Xu, Y.; Wang, W.; He, X.; Liu, Y.; Zhu, S. Steaming combined with biochar application eliminates negative plant-soil feedback for sanqi cultivation. Soil Tillage Res. 2019, 189, 189–198. [Google Scholar] [CrossRef]
- Bruun, S.; Jensen, E.S.; Jensen, L.S. Microbial mineralization and assimilation of black carbon: Dependency on degree of thermal alteration. Org. Geochem. 2008, 39, 839–845. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Kolton, M.; Graber, E.R.; Tsehansky, L.; Elad, Y.; Cytryn, E. Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol. 2017, 213, 1393–1404. [Google Scholar] [CrossRef]
- Pandit, N.R.; Mulder, J.; Hale, S.E.; Martinsen, V.; Schmidt, H.P.; Cornelissen, G. Biochar improves maize growth by alleviation of nutrient stress in a moderately acidic low-input Nepalese soil. Sci. Total Environ. 2018, 625, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Cytryn, E.; Meller Harel, Y.; Lew, B.; Graber, E.R. The biochar effect: Plant resistance to biotic stresses. Phytopathol. Mediterr. 2011, 50, 335–349. [Google Scholar]
- Bonanomi, G.; Jesu, G.; Zotti, M.; Idbella, M.; d’Errico, G.; Laudonia, S.; Vinale, F.; Abd-ElGawad, A. Biochar-derived smoke-water exerts biological effects on nematodes, insects, and higher plants but not fungi. Sci. Total Environ. 2021, 750, 142307. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, G.; Idbella, M.; Laudonia, S.; Vinale, F.; Bonanomi, G. The suppressive effects of biochar on above- and belowground plant pathogens and pests: A review. Plants 2022, 11, 3144. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, O.N.; Haynes, R.J. Comparison of the effects of conventional organic amendments and biochar on the chemical, physical and microbial properties of coal fly ash as a plant growth medium. Environ. Earth Sci. 2011, 66, 1987–1997. [Google Scholar] [CrossRef]
- Dempster, N.; Gleeson, B.; Solaiman, M.; Jones, L.; Murphy, V. Decreased soil microbial biomass and nitrogen olonizationi with eucalyptus biochar addition to a coarse textured soil. Plant Soil 2011, 354, 311–324. [Google Scholar] [CrossRef]
- Galvez, A.; Sinicco, T.; Cayuela, M.L.; Mingorance, M.D.; Fornasier, F.; Mondini, C. Short term effects of bioenergy by-products on soil C and N dynamics, nutrient availability and biochemical properties. Agric. Ecosyst. Environ. 2012, 160, 3–14. [Google Scholar] [CrossRef]
- Ventura, M.; Zhang, C.; Baldi, E.; Fornasier, F.; Sorrenti, G.; Panzacchi, P.; Tonon, G. Effect of biochar addition on soil respiration partitioning and root dynamics in an apple orchard. Eur. J. Soil Sci. 2014, 65, 186–195. [Google Scholar] [CrossRef]
- Kolton, M.; Meller Harel, Y.; Pasternak, Z.; Graber, E.R.; Elad, Y.; Cytryn, E. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microbiol. 2011, 77, 4924–4930. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Cao, L.; Zhang, R. Bacterial and fungal taxon changes in soil microbial community composition induced by short-term biochar amendment in red oxidized loam soil. World J. Microbiol. Biotechnol. 2014, 30, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Warnock, D.D.; Mummey, D.L.; Mcbride, B.; Major, J.; Lehmann, J.; Rillig, M.C. Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: Results from growth-chamber and field experiments. Appl. Soil Ecol. 2010, 46, 450–456. [Google Scholar] [CrossRef]
- Elmer, W.H.; Pignatello, J.J. Effect of biochar amendments on mycorrhizal associations and Fusarium crown and root rot of asparagus in replant soils. Plant Dis. 2011, 95, 960–966. [Google Scholar] [CrossRef]
- Ameloot, N.; Sleutel, S.; Case, S.D.C.; Alberti, G.; McNamara, N.P.; Zavalloni, C.; Vervisch, B.; delle Vedove, G.; De Neve, S. C mineralization and microbial activity in four biochar field experiments several years after incorporation. Soil Biol. Biochem. 2014, 78, 195–203. [Google Scholar] [CrossRef]
- Elad, Y.; David, D.R.; Harel, Y.M.; Borenshtein, M.; Ben Kalifa, H.; Silber, A.; Graber, E.R. Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent. Phytopathology 2010, 100, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Graber, E.R.; Frenkel, O.; Jaiswal, A.K.; Elad, Y. How may biochar influence severity of diseases caused by soilborne pathogens. Carbon Manag. 2014, 5, 169–183. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Kardol, P. Getting plant—Soil feedbacks out of the greenhouse: Experimental and conceptual approaches. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 2008; pp. 449–472. [Google Scholar]
- Baronti, S.; Vaccari, F.P.; Miglietta, F.; Calzolari, C.; Lugato, E.; Orlandini, S.; Pini, R.; Zulian, C.; Genesio, L. Impact of biochar application on plant water relations in Vitis vinifera (L.). Eur. J. Agron. 2014, 53, 38–44. [Google Scholar] [CrossRef]
- Genesio, L.; Miglietta, F.; Baronti, S.; Vaccari, F.P. Biochar increases vineyard productivity without affecting grape quality: Results from a four years field experiment in Tuscany. Agric. Ecosyst. Environ. 2015, 201, 20–25. [Google Scholar] [CrossRef]
- Idbella, M.; Baronti, S.; Giagnoni, L.; Renella, G.; Becagli, M.; Cardelli, R.; Maienza, A.; Vaccari, F.P.; Giuliano Bonanomi, G. Long-term effects of biochar on soil chemistry, biochemistry, and microbiota: Results from a 10-year field vineyard experiment. Appl. Soil Ecol. 2024, 195, 105217. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community data sets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Bastian, M.; Jacomy, M. Gephi: An Open-Source Software for Exploring and Manipulating Networks. In Proceedings of the International AAAI Conference on Weblogs and Social Media, San Jose, CA, USA, 17–20 May 2009. [Google Scholar]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Z.; Yang, K.; Wang, P.; Wang, H.; Guo, L.; Zhu, S.; Zhu, Y.; He, X. Biochar Application Alleviated Negative Plant-Soil Feedback by Modifying Soil Microbiome. Front. Microbiol. 2020, 11, 799. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.H. Benefits and limitations of biochar amendment in agricultural soils: A review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Mia, S.; van Groenigen, J.W.; van de Voorde, T.F.J.; Oram, N.J.; Bezemer, T.M.; Mommer, L.; Jeffery, S. Biochar application rate affects biological nitrogen fixation in red clover conditional on potassium availability. Agric. Ecosyst. Environ. 2014, 191, 83–91. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.W.; Inyang, M.D. Synthesis, characterization, and environmental implications of graphene-coated biochar. Sci. Total Environ. 2012, 435–436, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Peighami-Ashnaei, S. Grapevine, esca complex, and environment: The disease triangle. Phytopathol. Mediterr 2019, 58, 17–37. [Google Scholar]
- Whitelaw-Weckert, M.A.; Nair, N.G.; Lamont, R.; Alonso, M.; Priest, M.J.; Huang, R. Root infection of Vitis vinifera by Cylindrocarpon olonizatio in Australia. Australas. Plant Pathol. 2007, 36, 403–406. [Google Scholar] [CrossRef]
- Petit, E.; Gubler, W.D. First report of Cylindrocarpon liriodendra causing black foot disease of grapevines in California. Plant Disease. 2007, 91, 1060. [Google Scholar] [CrossRef] [PubMed]
- Auger, J.; Esterio, M.; Pérez, I. First report of black foot disease of grapevine caused by Cylindrocarpon macrodidymum in Chile. Plant Dis. 2007, 91, 470. [Google Scholar] [CrossRef] [PubMed]
- Halleen, F.; Schroers, H.J.; Groenewald, J.Z.; Crous, P.W. Novel species of Cylindrocarpon (Neonectria) and Campylocarpon gen. nov. Associated with black foot disease of grapevines (Vitis spp.). Stud. Mycol. 2004, 50, 431–455. [Google Scholar]
- Cabral, A.; Rego, C.; Nascimento, T.; Oliveira, H.; Groenewald, Z.; Crous, P.W. Multi-gene analysis and morphology reveal novel Ilyonectria species associated with black foot disease of grapevines. Fungal Biol. 2012, 116, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Petit, E.; Barriault, E.; Baumgartner, K.; Wilcox, W.F.; Rolshausen, P.E. Cylindrocarpon species associated with black-foot of grapevines in northeastern United States and southeastern Canada. Am. J. Enol. Vitic. 2011, 62, 177–183. [Google Scholar] [CrossRef]
- Alániz, S.; León, M.; Vicent, A.; García-Jiménez, J.; Abad-Campos, P.; Armengol, J. Characterization of Cylindrocarpon species associated with black foot disease of grapevines in Spain. Plant Dis. 2007, 91, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Abreo, E.; Martinez, S.; Bettucci, L.; Lupo, S. Morphological and molecular olonizationion of Campylocarpon and Cylindrocarpon spp. Associated with black foot disease of grapevines in Uruguay. Australas. Plant Pathol. 2010, 39, 446–452. [Google Scholar] [CrossRef]
- Savaş, N.G.; Akgül, D.S.; Albaz, E.A. First Report of Ilyonectria olonizatio Associated with Black Foot Disease of Grapevine in Turkey. Plant Dis. 2015, 99, 1855. [Google Scholar] [CrossRef]
- Mostert, L.; Halleen, F.; Creaser, M.L.; Crous, P.W. Cryptovalsa ampelina, a forgotten shoot and cane pathogen of grapevines. Australas. Plant Pathol. 2004, 33, 295–299. [Google Scholar] [CrossRef]
- Luque, J.; Sierra, D.; Torres, E.; Garcia, F. Cryptovalsa ampelina on grapevines in N.E. Spain: Identification and pathogenicity. Phytopathol. Mediterr. 2006, 45, S101–S109. [Google Scholar]
- Gramaje, D.; Armengol, J.; Colino, M.I.; Santiago, R.; Moralejo, E.; Olmo, D.; Luque, J.; Mostert, L. First Report of Phaeoacremonium inflatipes, P. iranianum, and P. sicilianum Causing Petri Disease of Grapevine in Spain. Plant Dis. 2009, 93, 964. [Google Scholar] [CrossRef]
- Liu, Z.; Latunde-Dada, A.O.; Hall, A.M.; Fitt, B.D.L. Phoma stem canker disease on oilseed rape (Brassica napus) in China is caused by Leptosphaeria biglobosa ‘brassicae’. Eur. J. Plant Pathol. 2014, 140, 841–857. [Google Scholar] [CrossRef]
- King, K.M.; West, J.S. Detection of the Phoma pathogens Plenodomus biglobosus subclades ‘brassicae’ and ‘canadensis’ on wasabi, and ‘canadensis’ in Europe. Eur. J. Plant Pathol. 2022, 162, 751–756. [Google Scholar] [CrossRef]
- Walsh, J.P.; McMullin, D.R.; Yeung, K.K.C.; Sumarah, M.W. Resorcylic acid lactones from the ginseng pathogen Ilyonectria mors-panacis. Phytochem. Lett. 2022, 48, 94–99. [Google Scholar] [CrossRef]
- Harel, Y.M.; Elad, Y.; Rav-David, D.; Borenstein, M.; Shulchani, R.; Lew, B.; Graber, E.R. Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil 2012, 357, 245–257. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Pane, C.; Scala, E. Suppression of soilborne fungal diseases with organic amendments. J. Plant Pathol. 2007, 89, 311–324. [Google Scholar]
- Akhter, A.; Hage-Ahmed, K.; Soja, G.; Steinkellner, S. Compost and biochar alter mycorrhization, tomato root exudation, and development of Fusarium oxysporum f. sp. Lycopersici. Front. Plant Sci. 2015, 6, 529. [Google Scholar] [CrossRef] [PubMed]
- Solaiman, Z.M.; Blackwell, P.; Abbott, L.K.; Storer, P. Direct and residual effect of biochar application on mycorrhizal root olonization, growth and nutrition of wheat. J. Soil Res. 2010, 48, 546–554. [Google Scholar] [CrossRef]
- Mickan, B.S.; Abbott, L.K.; Stefanova, K.; Solaiman, Z.M. Interactions between biochar and mycorrhizal fungi in a water-stressed agricultural soil. Mycorrhiza 2016, 26, 565–574. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil-Concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Zhao, J.; Luo, Y.; Novak, J.; Herbert, S.; Xing, B. Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour. Technol. 2013, 130, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 35–52. [Google Scholar] [CrossRef]
- Hammer, E.C.; Balogh-Brunstad, Z.; Jakobsen, I.; Olsson, P.A.; Stipp, S.L.S.; Rillig, M.C. A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol. Biochem. 2014, 77, 252–260. [Google Scholar] [CrossRef]
- Bowles, T.M.; Barrios-Masias, F.H.; Carlisle, E.A.; Cavagnaro, T.R.; Jackson, L.E. Effects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under deficit irrigation in field conditions. Sci. Total Environ. 2016, 566, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M. Arbuscular mycorrhizal fungi act as bio-stimulants in horticultural crops. Sci. Hort 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Thirkell, T.J.; Charters, M.D.; Elliott, A.J.; Sait, S.M.; Field, K.J. Are mycorrhizal fungi our sustainable saviours considerations for achieving food security. J. Ecol. 2017, 105, 921–929. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ahmed, N.; Ashraf, M.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Whipps, J.M. Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Can. J. Bot. 2004, 82, 1198–1227. [Google Scholar] [CrossRef]
- Matsubara, Y.; Hasegawa, N.; Fukui, H. Incidence of Fusarium root rot in asparagus seedlings infected with arbuscular mycorrhizal fungus as affected by several soil amendments. J. Jpn. Soc. Hortic. Sci. 2002, 71, 370–374. [Google Scholar] [CrossRef]
- Calcagno, C.; Novero, M.; Genre, A.; Bonfante, P.; Lanfranco, L. The exudate from an arbuscular mycorrhizal fungus induces nitric oxide accumulation in Medicago truncatularoots. Mycorrhiza 2012, 22, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.G.; Kong, C.C.; Xie, Z.H. Role of abscisic acid in strigolactoneinduced salt stress tolerance in arbuscular mycorrhizal Sesbania cannabina seedlings. BMC Plant Biol. 2018, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Hause, B.; Maier, W.; Miersch, O.; Kramell, R.; Strack, D. Induction of jasmonate biosynthesis in arbuscular mycorrhizal barley roots. Plant Physiol. 2002, 130, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, C.; Paszkowski, U. Weights in the balance: Jasmonic acid and salicylic acid signaling in root-biotroph interactions. Mol. Plant-Microbe Interact. 2009, 22, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Shaul-Keinan, O.; Gadkar, V.; Ginzberg, I.; Grünzweig, J.M.; Chet, I.; Elad, Y.; Wininger, S.; Belausov, E.; Eshed, Y.; Atzmon, N.; et al. Hormone concentrations in tobacco roots change during arbuscular mycorrhizal colonization with Glomus intraradices. New Phytol. 2002, 154, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.J.; Wang, J.; He, X.Y.; Tian, C.J. Different respiration metabolism between mycorrhizal and non-mycorrhizal rice under low-temperature stress: A cry for help from the host. J. Agric. Sci. 2015, 153, 602–614. [Google Scholar] [CrossRef]
- Prayogo, C.; Jones, J.E.; Baeyens, J.; Bending, G.D. Impact of biochar on mineralization of C and N from soil and willow litter and its relationship with microbial community biomass and structure. Biol. Fertil. Soils 2014, 50, 695–702. [Google Scholar] [CrossRef]
- Wang, G.F.; Ma, Y.; Chenia, H.Y.; Govinden, R.; Luo, J.; Ren, G.D. Biochar mediated control of Phytophthora blight of pepper is closely related to the improvement of the rhizosphere fungal community. Front. Microbiol. 2020, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Helgason, T.; Fitter, A.H. Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum Glomeromycota). J. Exp. Bot. 2009, 60, 2465–2480. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2010, 102, 3488–3497. [Google Scholar] [CrossRef]
- Neumann, G.; Ludewig, U. Chapter 14—Rhizosphere chemistry influencing plant nutrition. In Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 545–585. [Google Scholar]
- Guo, Y.; George, E.; Marschner, H. Contribution of an arbuscular mycorrhizal fungus to the uptake of cadmium and nickel in bean and maize plants. Plant Soil 1996, 184, 195–205. [Google Scholar] [CrossRef]
- Seguel, A.; Cumming, J.R.; Klugh-Stewart, K.; Cornejo, P.; Borie, F. The role of arbuscular mycorrhizas in decreasing aluminium phytotoxicity in acidic soils: A review. Mycorrhiza 2013, 23, 167–183. [Google Scholar] [CrossRef]


| Unit | Value | |
|---|---|---|
| C | % | 77.81 |
| N | % | 0.91 |
| Al | mg·kg−1 | 268 |
| C/N | - | 63.53 |
| Ca | mg·kg−1 | 25,000 |
| Cu | mg·kg−1 | 97 |
| Fe | mg·kg−1 | 333 |
| K | mg·kg−1 | 13,900 |
| Mg | mg·kg−1 | 28,700 |
| Mn | mg·kg−1 | 84 |
| Na | mg·kg−1 | 11,900 |
| P | mg·kg−1 | 23,300 |
| S | mg·kg−1 | 481 |
| Zn | mg·kg−1 | 104 |
| pH | - | 9.8 |
| CEC | Cmolc·kg−1 | 101 |
| Max water absorption | gg−1 of d.m. | 4.53 |
| BET | m2·g−1 | 410 ± 6 |
| Total porosity | mm3·g−1 | 2722 |
| Transmission pores | mm3·g−1 | 318 |
| Storage pores | mm3·g−1 | 1997 |
| Residual pores | mm3·g−1 | 406 |
| Particle size distribution (mm): | ||
| 50–20 | % | 4.45 |
| 20–10 | % | 12.1 |
| 10–8 | % | 13.1 |
| 8–4 | % | 10.36 |
| 4–2 | % | 19.85 |
| 2–1 | % | 24.2 |
| <1 | % | 15.94 |
| Soil Parameters | Treatments | ||
|---|---|---|---|
| C Mean ± s.d. | B Mean ± s.d. | BB Mean ± s.d. | |
| pH | 6.33 ± 0.06 c | 6.83 ± 0.11 b | 7.07 ± 0.10 a |
| Bulk density (g·cm−3) | 1.63 ± 0.03 a | 1.59 ± 0.02 b | 1.53 ± 0.02 c |
| Organic carbon (g·kg−1) | 12.7 ± 0.67 c | 17.3 ± 1.06 b | 23.1 ± 1.15 a |
| Nitrate (NO3−-N (mg·kg−1)) | 1.51 ± 0.16 b | 1.78 ± 0.12 b | 5.88 ± 0.66 a |
| Ammonium (NH4+-N (mg·kg−1)) | 12.2 ± 0.42 b | 13.4 ± 0.30 a | 14.3 ± 1,29 a |
| P (mg·kg−1) | 147.4 ± 12.1 c | 262 ± 32.2 b | 313 ± 31.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idbella, M.; Baronti, S.; Vaccari, F.P.; Abd-ElGawad, A.M.; Bonanomi, G. Long-Term Application of Biochar Mitigates Negative Plant–Soil Feedback by Shaping Arbuscular Mycorrhizal Fungi and Fungal Pathogens. Microorganisms 2024, 12, 810. https://doi.org/10.3390/microorganisms12040810
Idbella M, Baronti S, Vaccari FP, Abd-ElGawad AM, Bonanomi G. Long-Term Application of Biochar Mitigates Negative Plant–Soil Feedback by Shaping Arbuscular Mycorrhizal Fungi and Fungal Pathogens. Microorganisms. 2024; 12(4):810. https://doi.org/10.3390/microorganisms12040810
Chicago/Turabian StyleIdbella, Mohamed, Silvia Baronti, Francesco Primo Vaccari, Ahmed M. Abd-ElGawad, and Giuliano Bonanomi. 2024. "Long-Term Application of Biochar Mitigates Negative Plant–Soil Feedback by Shaping Arbuscular Mycorrhizal Fungi and Fungal Pathogens" Microorganisms 12, no. 4: 810. https://doi.org/10.3390/microorganisms12040810
APA StyleIdbella, M., Baronti, S., Vaccari, F. P., Abd-ElGawad, A. M., & Bonanomi, G. (2024). Long-Term Application of Biochar Mitigates Negative Plant–Soil Feedback by Shaping Arbuscular Mycorrhizal Fungi and Fungal Pathogens. Microorganisms, 12(4), 810. https://doi.org/10.3390/microorganisms12040810