Abstract
The present work assessed the experimental susceptibility of Nyssomyia antunesi and Lutzomyia longipalpis to Leishmania (Viannia) lainsoni and L. (V.) lindenbergi. A L. (Leishmania) chagasi–Lu. longipalpis combination was used as a susceptible control. Wild-caught Ny. antunesi and laboratory-bred Lu. longipalpis were membrane-fed on blood with a 5 × 106/mL log-phase promastigote culture suspension and dissected on days 2 and 8 post-blood meal (pbm) for analysis focused on the assessment of parasitoses, as well as placement and promastigote morphotyping. Survival curves were constructed. In all combinations, promastigotes were observed on day 8 pbm. For both Leishmania species, in Lu. longipalpis, the presence of parasites was observed up to the stomodeal valve, while in Ny. antunesi, the presence of parasites was observed up to the cardia. There were no significant differences in parasitosis between L. (V.) lainsoni and L. (V.) lindenbergi in either Ny. antunesi or Lu. longipalpis. Six morphological promastigote forms were distinguished in Giemsa-stained gut smears. The survival curves of all combinations decreased and were affected differently by several Lu. longipalpis–parasite combinations, as well with Lu. longipalpis–uninfected blood. These findings stress Lu. longipalpis as experimentally susceptible to Leishmania spp. and suggest the putative susceptibility of Ny. antunesi to L. (V.) lainsoni and L. (V.) lindenbergi.
1. Introduction
Phlebotomines (Diptera: Psychodidae) are medically important insects implicated in the transmission of several pathogens, mainly Leishmania protozoa (Kinetoplastea: Trypanosomatidae) [1], which are the agents of leishmaniases, a group of neglected tropical diseases affecting millions of people worldwide [2].
Leishmania parasites have a digenetic life cycle and infect a wide range of vertebrate reservoir hosts and invertebrate vectors, mainly phlebotomines (Diptera: Psychodidae) [3,4,5]. The development of Leishmania within the phlebotomines is a complex process: after the ingestion of infected blood, amastigotes (nonflagellated forms) differ from promastigotes (flagellated forms) inside the insect’s gut [6], overcoming adverse conditions such as physico-chemical barriers and excretion flow [7,8,9,10]. Several subtypes of promastigote forms are recognized according to their morphology, including procyclic, haptomone, nectomonad, paramastigote and metacyclic [11], the latter of which are infective to vertebrate hosts [12,13].
A fundamental aspect in determining whether phlebotomines are incriminated by the transmission of Leishmania is the differentiation of infectious metacyclic forms [14]. In this same sense, the parasitosis and placement of late-stage infection constitute important parameters for evaluating the vector competence of a particular phlebotomine species for the developmental success of a given Leishmania sp. [15].
In the Brazilian Amazon, a particular tegumentary leishmaniasis (TL) transmission scenario occurs mainly because of the etiology of L. (Leishmania) amazonensis, L. (Viannia) lainsoni and L. (V.) lindenbergi in the forest fragments of Belém city [16]. In these TL foci, with respect to L. (L.) amazonensis, Bichromomyia flaviscutellata has well-established vector evidence [17]; for L. (V.) lainsoni, species of Trichophoromyia, particularly Th. Ubiquitalis and Th. brachipyga, have been shown to be involved in transmission [18]; ultimately, for L. (V.) lindenbergi, Nyssomyia antunesi has received attention due to its abundance, dominance, spatiotemporal convergence with human disease, blood feeding on human and potential reservoirs of Leishmania [19,20]. Despite the lack of evidence of true and species-specific identifiable Leishmania infection, the vector role of Ny. antunesi remains undefined, not advancing on suspect status.
However, studies on the interactions between Leishmania and its vectors are required to advance the understanding of the processes involved in parasite development and transmission [6]. Some parasite–vector combinations have been studied under laboratory conditions; however, the majority of binomials inferred by field evidence still require laboratory investigation. Therefore, the present study aimed to fill the gap in vector knowledge on the development of L. (V.) lainsoni and L. (V.) lindenbergi in Ny. antunesi and Lu. longipalpis. A L. (L.) chagasi–Lu. longipalpis combination was used as a ‘positive control’.
2. Materials and Methods
2.1. Parasites
The World Health Organization reference strains of three different Leishmania species maintained in the cryobank of the ‘Ralph Lainson’ Leishmaniases Laboratory, Instituto Evandro Chagas (IEC), Belém, Brazil, were used: L. (V.) lainsoni (MHOM/BR/1981/M6426), L. (V.) lindenbergi (MHOM/BR/1996/M15729) and L. (L.) chagasi (MHOM/BR/1981/M6445). Promastigotes were cultured in Schneider’s insect medium (SIM) supplemented with 100 U/mL penicillin, 100 g/mL streptomycin and 10% heat-inactivated fetal bovine serum. To carry out the subsequent experimental infection of phlebotomines, low-passage parasites were used. Before being mixed with the blood, the samples were washed by centrifugation (2400× g for 5 min) and resuspended in a sterile container with a saline solution [5].
2.2. Phlebotomines
Wild-caught Ny. antunesi were obtained from the Bosque Rodrigues Alves-Jardim Botânico da Amazônia (1°25′48″ S; 48°27′25″ W), an urban park of Belém city in which the phlebotomine fauna has already been surveyed [21]. Captures were performed with CDC light traps set 1.5 m above ground level (n = 4) and 20 m above ground level (n = 2), operating from 6:00 p.m. to 6:00 a.m., from May to August 2023. The phlebotomines were visually screened, aspirated from the primary cage in the field, and transported to the laboratory under 80 ± 10% relative humidity and 10% glucose solution offered ad libitum [22,23]. The phlebotomines were immediately transferred to a secondary nylon cage. Congested, gravid or semigravid females were excluded from the experiments [24].
Laboratory-bred Lu. longipalpis from an established Amazonian closed colony (Abaetetuba F236) were used. For the tests, adult female specimens 5–9 days old were used [5,25] and supplied with 10% glucose solution ad libitum [26] up to 24 h before the assays [27,28].
2.3. Parasite–Vector Systems
Experimental infections were carried out according to the artificial blood feeding protocol proposed by Sánchez Uzcátegui et al. [24]. Briefly, the groups of both wild-caught Ny. antunesi and laboratory-bred Lu. longipalpis were artificially fed in 30 cm3 nylon cages for 3 h through a sausage membrane installed in a circulator device containing previously heat-inactivated serum (56 °C for 1 h) human blood and 5 × 106/mL promastigotes [5] from log-phase cultures [29]. For Lu. longipalpis, females that had fed on uninfected blood were also assessed. The engorged females were confined to 200 mL flasks, and the recipients were lined with moistened filter paper and given a 10% sucrose diet until dissection [5]. Females from each species/experiment were divided into two groups for dissection: one group was dissected before the females defecated (early stage of infection) on day 2 post-blood meal (pbm), and the other group was dissected after defecation (late stage of infection) on day 8 pbm [5,25].
2.4. Parasite Detection and Development
Phlebotomines were monitored daily to account for dead females, and survival curves were constructed. Dead females were dissected, and only Leishmania-positive females were counted [30]. The proportion survived (lx) was calculated according to Rabinovich [31]. Survivorship curves were obtained for different parasite–vector combinations and were compared by the log-rank test using BioEstat 5.3 software [32]. On days 2 and 8 pbm, the females were removed from the oviposition glasses using a Castro aspirator, and placed at 4 °C for thermal immobilization by cooling. The females were washed once with a 0.9% NaCl solution plus 5% neutral detergent, and twice with 0.9% NaCl for subsequent dissection. Phlebotomines were placed in a drop of phosphate-buffered saline (PBS) on a microscope slide, and the head was separated from the thorax before the intestine was extracted through the apex of the abdomen. The intestines were individually observed under an optical microscope and examined to determine the development of flagellates in the guts [33] following the taxonomic statement of Lainson and Shaw [34], and classified with the semiquantitative parasitosis scale described by Myskova et al. [15], whereby parasite loads were graded as absent (0 parasites per gut), weak (less than 100 parasites per gut), moderate (100–1000 parasites per gut) or heavy (more than 1000 parasites per gut). Promastigotes from the gut were Giemsa-stained and microscopically assessed to infer distinguishable evolutive forms based on morphologic/morphometric criteria modified by Ticha et al. [5], focusing on identifying metacyclic-like forms on day 8 pbm. All experiments were repeated at least three times for each parasite–vector combination. Parasitoses were compared using the G test with BioEstat 5.3 software [32]. In all statistical analysis, p ≤ 0.05 was considered to indicate a 95% confidence interval.
2.5. Ethical Approval
The capture and processing of invertebrate fauna (phlebotomines) were authorized by the ‘Sistema de Autorização e Informação em Biodiversidade’ under protocol no. 70142-2. Animals used for the blood feeding of phlebotomine colonies were maintained and handled at the Instituto Evandro Chagas animal facility, in accordance with institutional guidelines and Brazilian legislation (Federal Law no. 11.794, 8 October 2008). In vivo blood feeding standard operational procedures were approved by the Ethics Committee on Animal Use (CEUA/IEC), under certificate no. 30/2021.
3. Results
3.1. Susceptibility of Ny. antunesi to L. (V.) lainsoni and L. (V.) lindenbergi
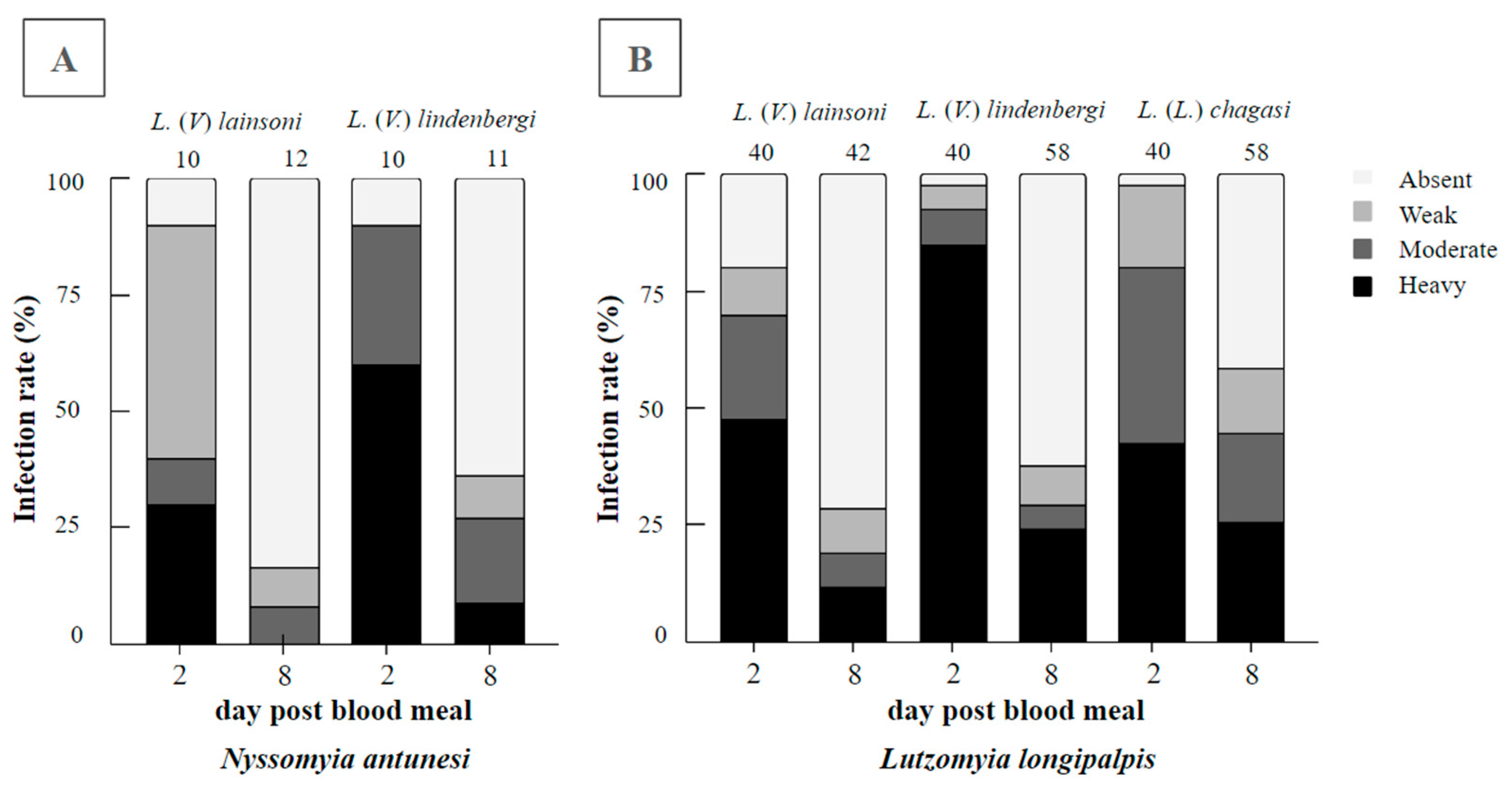
In total, 43 blood-fed Ny. antunesi females were dissected, 22 of which were exposed to L. (V.) lainsoni and 21 to L. (V.) lindenbergi. On day 2 pbm, the infection rates were 90% for both parasite–vector combinations, recording weak, moderate and heavy parasitoses, respectively; 50%, 10% and 30%, respectively, for L. (V.) lainsoni; and 0%, 30% and 60%, respectively, for L. (V.) lindenbergi. Promastigotes were limited to the endoperitrophic space within the ingested blood meal. On day 8 pbm, the infection rates were 16% and 36% for L. (V.) lainsoni and L. (V.) lindenbergi, respectively. For L. (V.) lainsoni, parasitoses were assessed as 8% weak and 8% moderate, whereas for L. (V.) lindenbergi, parasitoses were assessed as 9% weak, 18% moderate and 9% heavy (Figure 1A). The results from the assessment of parasitoses between these Leishmania species were not significant (G test = 2.2148, df = 3, p = 0.5290).
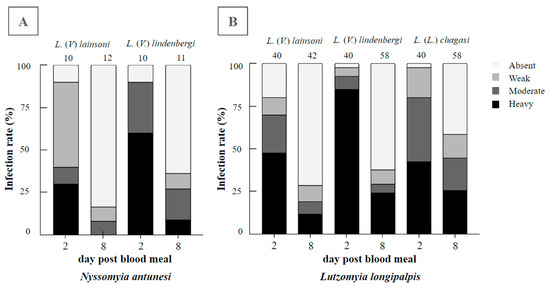
Figure 1.
Infection rates and parasitoses of Leishmania (Viannia) lindenbergi (MHOM/BR/1996/M15729) and L. (V.) lainsoni (MHOM/BR/1981/M6426) in (A) Nyssomyia antunesi and (B) Lutzomyia longipalpis. The well-known susceptible L. (L.) chagasi–Lu. longipalpis combination was also performed as the control. Intestines were dissected on days 2 and 8 post-blood meal (pbm). Parasitoses were classified into four categories: weak (less than 100 parasites per gut), moderate (100–1000 parasites per gut) and heavy (more than 1000 parasites per gut). The number of females evaluated can be found above the columns.
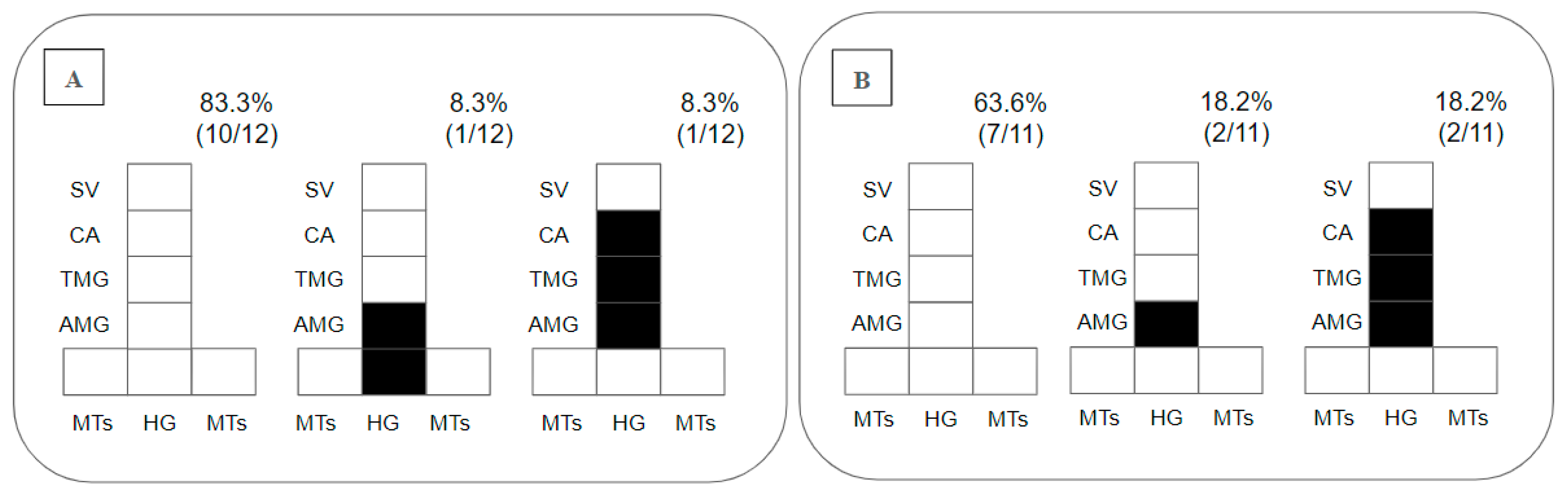
Regarding the placement of parasites in the gut, as promastigotes were limited to the endoperitrophic space on day 2 pbm, this parameter was only considered on day 8 pbm, when 83% of the Ny. antunesi–L. (V.) lainsoni combinations did not sustain infection; 8.3% presented peripylarian development with colonization in the hindgut (HG) and abdominal midgut (AMG); and 8.3% presented suprapylarian development, with colonization in the AMG, thoracic midgut (TMG) and cardia (CA) (Figure 2A). In Ny. antunesi–L. (V.) lindenbergi combinations, 63.6% of the strains did not sustain infection, and 36% presented suprapylarian development, 18.2% colonization in the AMG and 18.2% suprapylarian development with colonization in the AMG, TMG and CA (Figure 2B).
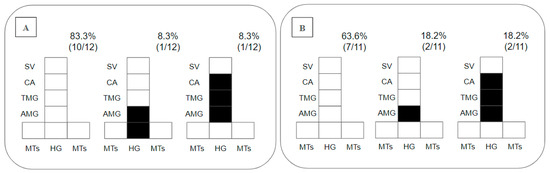
Figure 2.
Diagramatic placement of Leishmania spp. in the gut of Nyssomyia antunesi on day 8 post-blood meal (pbm). (A): Leishmania (Viannia) lainsoni (MHOM/BR/1981/M6426); (B): L. (V.) lindenbergi (MHOM/BR/1996/M15729). HG, hindgut; MTs, Malpighian tubules; AMG, abdominal midgut; TMG, thoracic midgut; CA, cardia; SV, stomodeal valve. Percent distribution of localization patterns among the infected females is shown in the top right of each diagram.
3.2. Susceptibility of Lu. longipalpis to L. (V.) lainsoni and L. (V.) lindenbergi
In total, 180 blood-fed Lu. longipalpis females were dissected, 82 were exposed to L. (V.) lainsoni and 98 were exposed to L. (V.) lindenbergi. On day 2 pbm, the infection rates were 81% for L. (V.) lainsoni and 98% for L. (V.) lindenbergi, with promastigotes found in the endoperitrophic space only within the ingested blood meal. Parasitoses were assessed as 10% weak, 23% moderate and 48% heavy for L. (V.) lainsoni, whereas parasitosis were assessed as 5% weak, 8% moderate and 85% heavy for L. (V.) lindenbergi. On day 8 pbm, the infection rates were 29% for L. (V.) lainsoni and 38% for L. (V.) lindenbergi, with parasitoses assessed as 10% weak, 7% moderate and 12% heavy for L. (V.) lainsoni, whereas parasitosis were assessed as 9% weak, 5% moderate and 24% heavy for L. (V.) lindenbergi (Figure 1B). The results from the assessment of parasitoses between these Leishmania species were not significant (G test = 1.7129, df = 3, p = 0.6341).
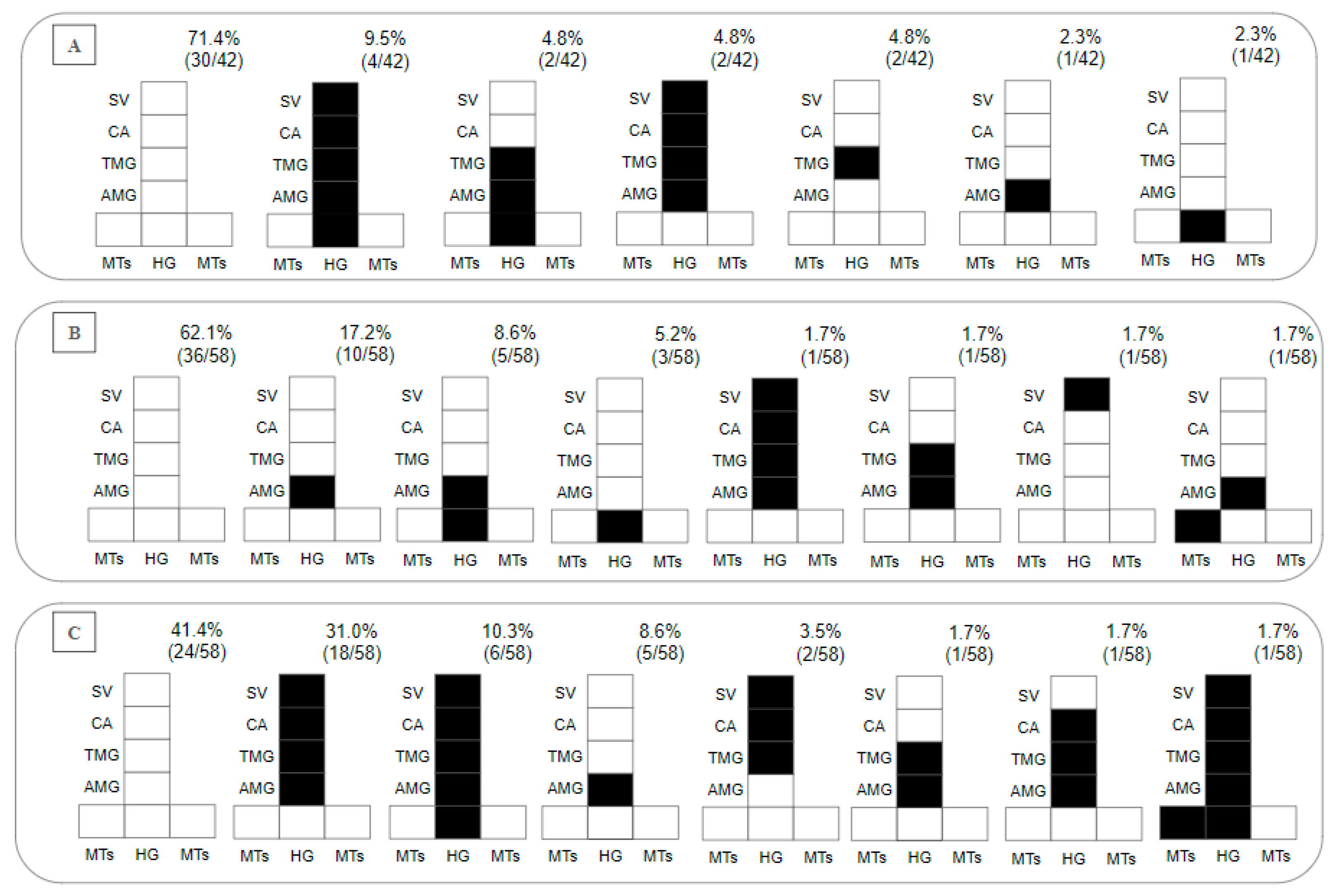
Regarding the presence of parasites in the gut on day 8 pbm, within 28.7% of the Lu. longipalpis–L. (V.) lainsoni positive combinations, 2.4% presented with hypopylarian, 14.3% with peripylarian and 12% with suprapylarian development. The peripylarian patterns included colonization in the HG, AMG, TMG, CA and stomodeal valve (SV) (9.5%), and in the HG, AMG and TMG (4.8%). The suprapylarian patterns included colonization in the AMG, TMG, CA and SV (4.8%), and in the TMG (4.8%) and AMG (2.3%) (Figure 3A). Within 37.8% of the Lu. longipalpis–L. (V.) lindenbergi positive combinations, hypopylarian (5.2%), peripylarian (10.3%) and suprapylarian (22.3%) development was observed. The hipopylarian pattern included colonization in the HG (5.2%); peripylarian comprised colonization in the HG and AMG (8.6%), and MTs and AMG (1.7%); and more frequent suprapylarian colonization was in the AMG (17.2%), while other infection patterns did not exceed 2% (Figure 3B).
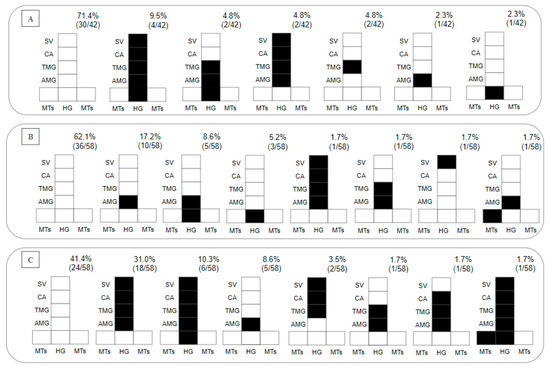
Figure 3.
Diagramatic placement of (A) Leishmania (Viannia) lainsoni (MHOM/BR/1981/M6426), (B) L. (V.) lindenbergi (MHOM/BR/1996/M15729) and (C) L. (L.) chagasi (MHOM/BR/1981/M6445) in the gut of Lu. longipalpis on the 8th day post-blood meal (pbm). HG, hindgut; MTs, Malpighian tubules; AMG, abdominal midgut; TMG, thoracic midgut; CA, cardia; SV, stomodeal valve. In the upper right part of each diagram is the percentage distribution obtained for each Leishmania species within the intestine of Lu. longipalpis.
3.3. Susceptibility of Lu. longipalpis to L. (L.) chagasi (Control Experiment)
In total, 98 Lu. longipalpis exposed to L. (L.) chagasi were dissected. On day 2 pbm, the infection rate was 99%, recording 18% weak, 38% moderate and 43% heavy parasitoses. On day 8 pbm, the infection rate was 59%, recording 14% weak, 19% moderate and 26% heavy parasitoses. Peripylarian (12.1%) and suprapylarian (44.8%) development was observed. Peripylarian pattern comprised colonization in the HG, AMG, TMG, CA and SV (10.3%); suprapylarian comprised colonization in the AMG, TMG, CA and SV (31%), exclusively in the AMG (8.6%), and TMG, CA and SV (3.5%); and other infection patterns did not exceed 2% (Figure 3C).
On the other hand, the results of the evaluation of parasites among the Leishmania species were significant on day 8 pbm (Table 1).

Table 1.
Summary statistics for the comparison of parasitosis on day 8 post-blood meal in different parasite–vector combinations. Significant differences are highlighted in bold.
3.4. Morphology of L. (V.) lainsoni and L. (V.) lindenbergi Promastigotes in Ny. antunesi
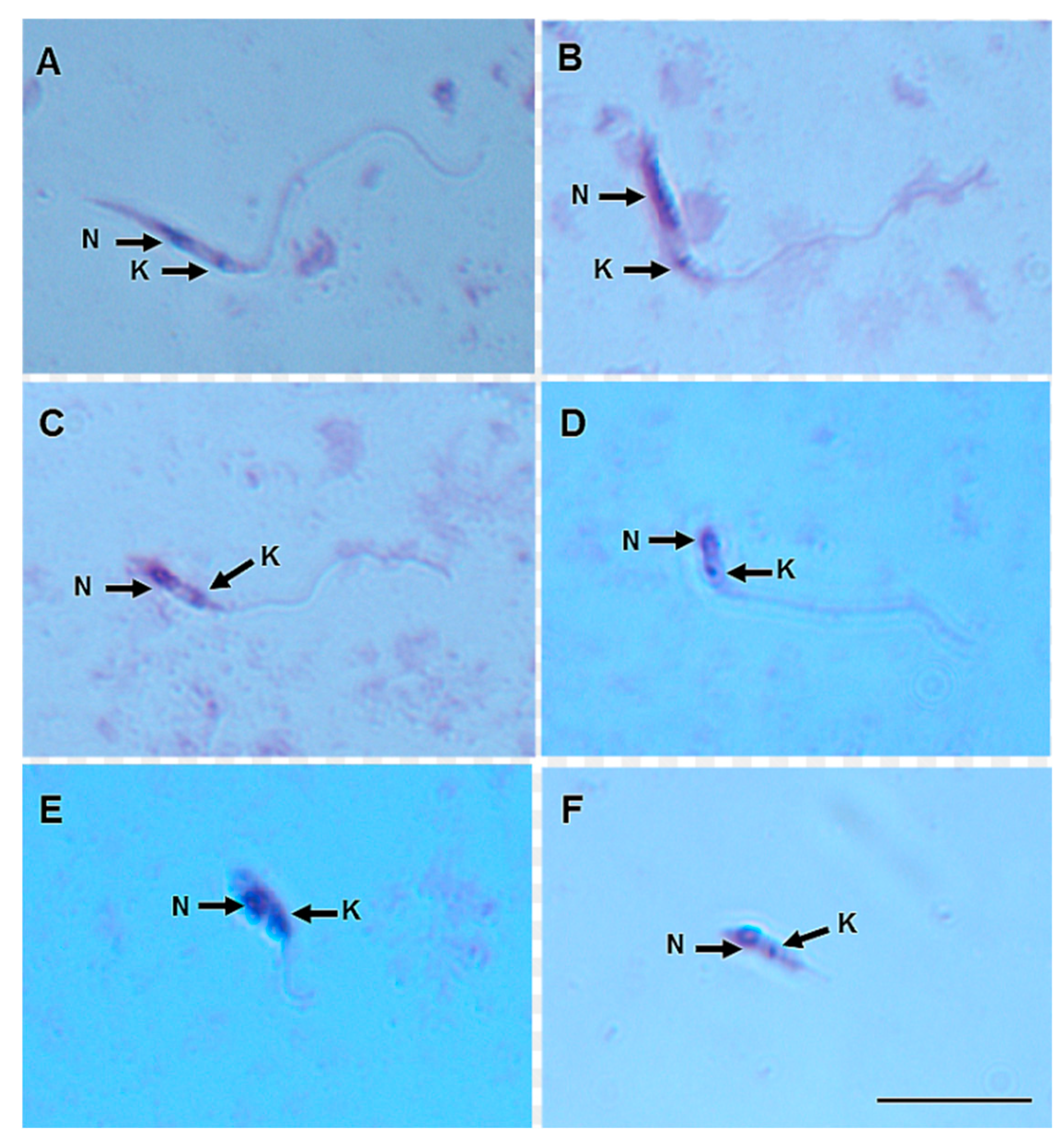
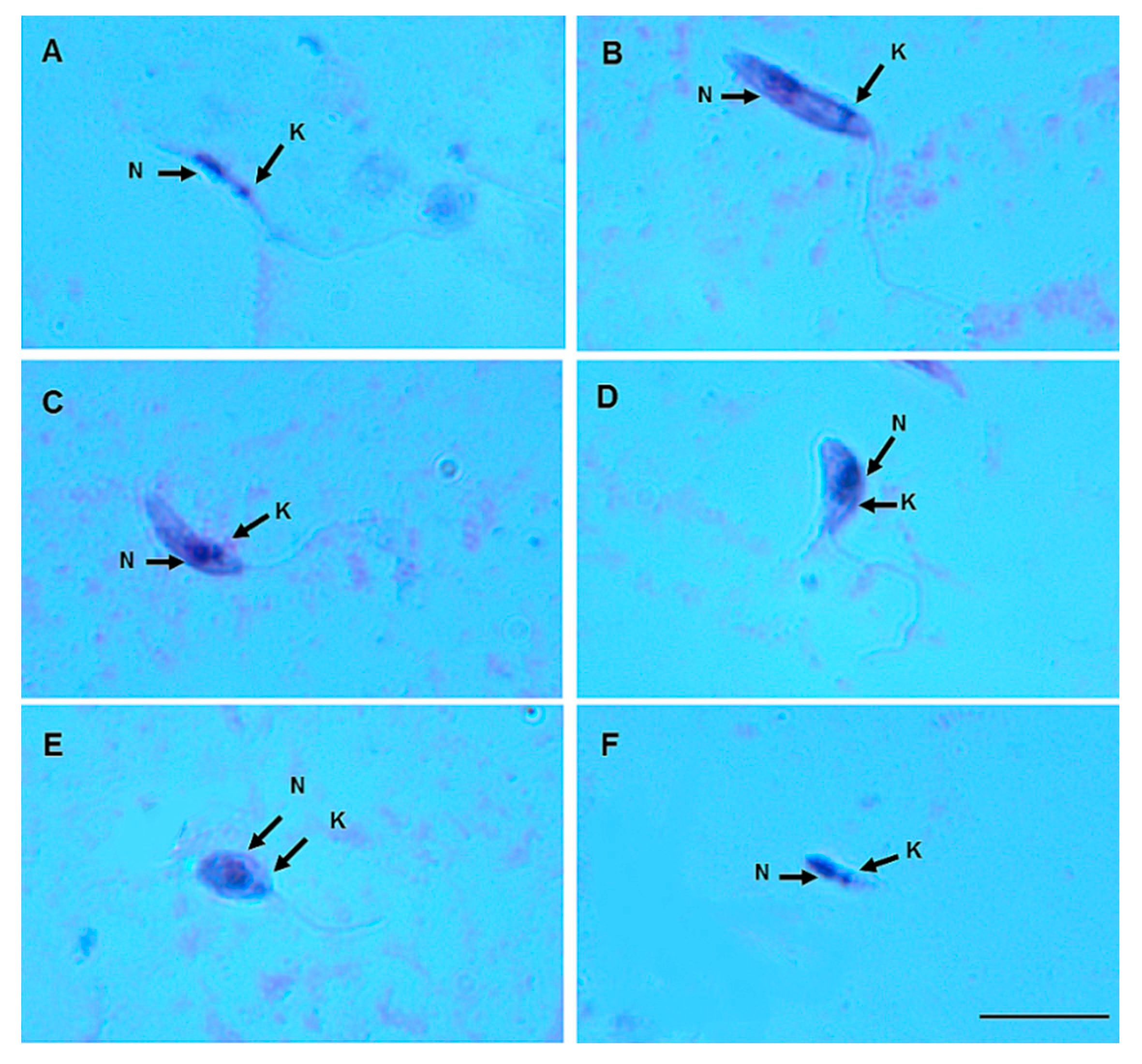
On day 8 pbm, six promastigote morphotypes of L. (V.) lindenbergi (Figure 4) and L. (V.) lainsoni (Figure 5) were observed in the gut of Ny. antunesi: elongated nectomonad, short nectomonad, metacyclic promastigote, rounded metacyclic promastigote, rounded paramastigote and haptomonad.
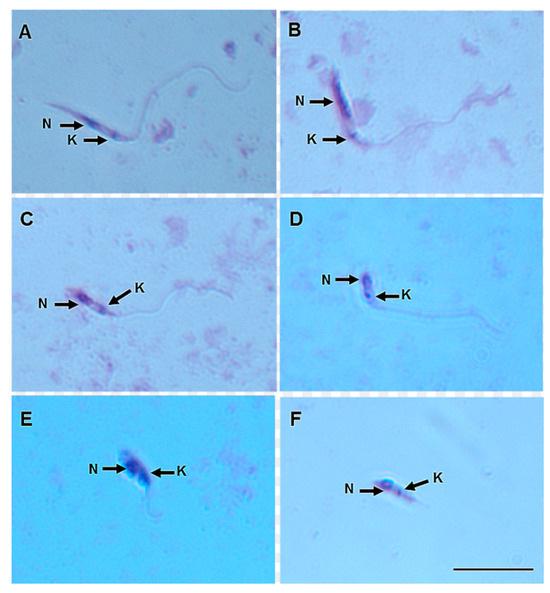
Figure 4.
Leishmania (Viannia) lindenbergi (MHOM/BR/96/M15729) morphological form development in the gut of Nyssomyia antunesi: (A) elongated nectomonad; (B) short nectomonad; (C) metacyclic promastigote; (D) rounded metacyclic promastigote; (E) rounded paramastigote; (F) haptomonad. N, nucleus; K, kinetoplast (stained by Giemsa). Bar = 10 μm.
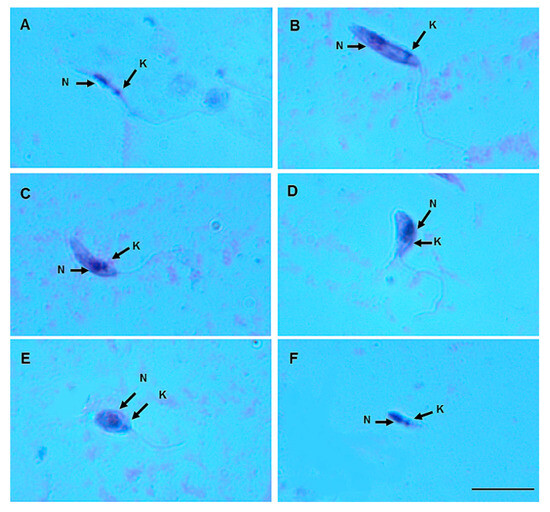
Figure 5.
Leishmania (Viannia) lainsoni (MHOM/BR/1981/M6426) morphological form development in the gut of Nyssomyia antunesi: (A) elongated nectomonad; (B) short nectomonad; (C) metacyclic promastigote; (D) rounded metacyclic promastigote; (E) rounded paramastigote; (F) haptomonad. N, nucleus; K, kinetoplast (stained by Giemsa). Bar = 10 μm.
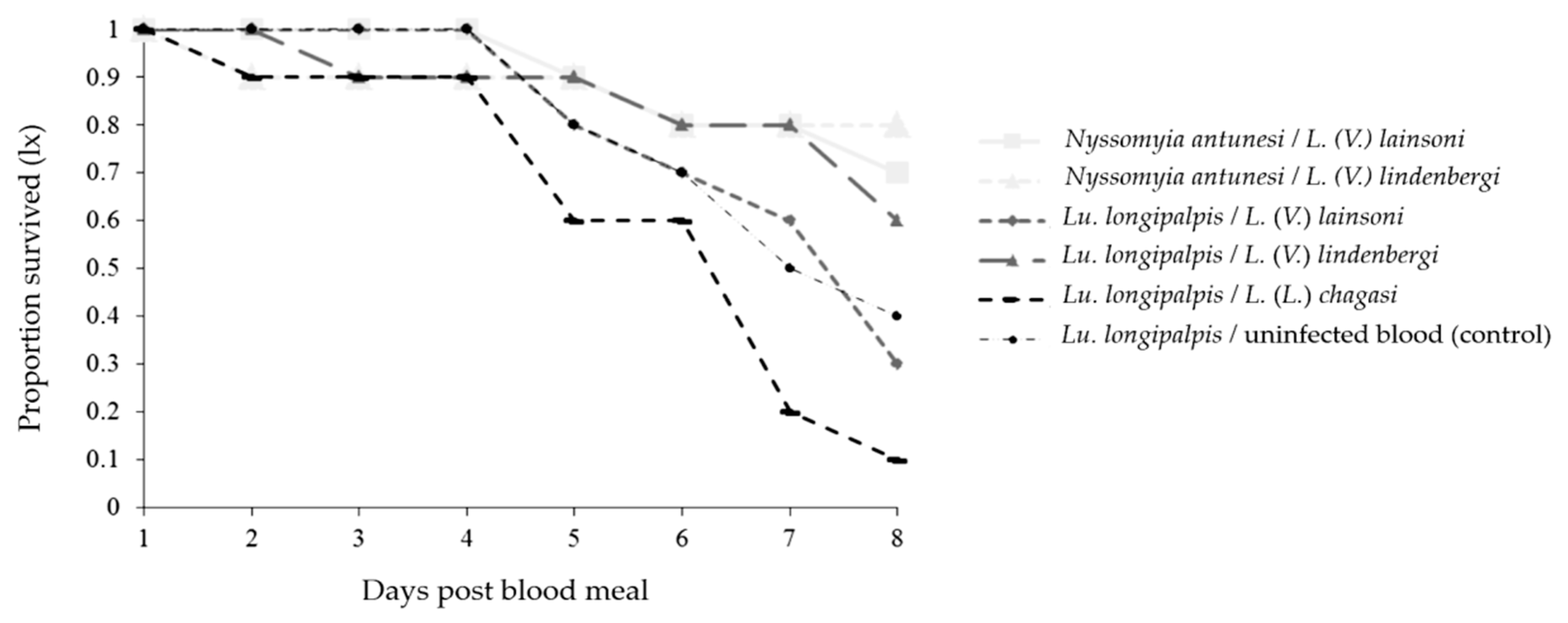
Survival Curves
The survival of both phlebotomine species decreased up to day 8 pbm for all combinations (Figure 6). The survival of Lu. longipalpis was affected differently in some Lu. longipalpis combinations, and the survival was higher when the phlebotomine species were infected with L. (V.) lindenbergi than when they were infected with L. (V.) lainsoni (p < 0.0001) or L. (L.) chagasi (p < 0.0001). Moreover, the survival was higher when the phlebotomine species were infected with L. (V.) lainsoni compared with L. (L.) chagasi (p < 0.0001); and when they were infected with uninfected blood compared with those infected with L. (V.) lindenbergi (p = 0.0246), or L. (L.) chagasi (p < 0.0001) (Table 2).
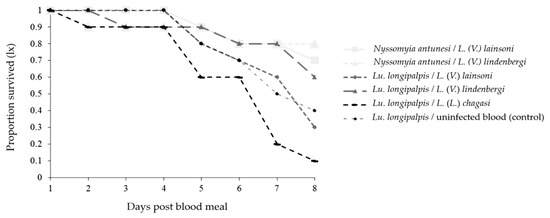
Figure 6.
Survival curves of Nyssomyia antunesi and Lutzomyia longipalpis blood-fed on a Leishmania spp. suspension (or not) up to day 8 post-blood meal.

Table 2.
Log-rank test significance on the comparison of survival curves obtained for the different parasite–vector combinations up to day 8 post-blood meal. Significant differences are highlighted in bold.
4. Discussion
Several parasite–vector combinations were studied under laboratory conditions to evaluate interaction patterns, including the ability of vectors to support the late-stage development of parasites, suggesting the well-recognized classification of restrictive and permissive vectors. In the former category, phlebotomines present a remarkable specificity for a single (or some closely related) Leishmania species; while in the latter, phlebotomines allow for the development of a broad range of apart-related Leishmania species [35,36]. In this sense, the present study assessed Ny. antunesi and Lu. longipalpis in the development of medically important parasites in the Amazon biome, L. (V.) lainsoni and L. (V.) lindenbergi, until day 8 pbm, when late-stage promastigote forms were supposed to colonize the foregut, thus providing advanced inferences on their susceptibility.
After exhaustive attempts, the unsuccessful colonization of Ny. antunesi led the researchers to challenge wild-caught specimens. Although the unknown life status of these specimens and an apparently low number of assessments may compromise experimental reproducibility, it is believed that the field background brought about by nature adds pivotal elements for genuine parasite–vector interactions. Nyssomyia antunesi has been recognized as a suspected vector of L. (V.) lindenbergi based on some eco-epidemiological evidence [19,20,21], although no natural infection has been ascribed to this parasite. The present findings demonstrate that Ny. antunesi can develop at least a small population of late-stage promastigotes of both L. (V.) lainsoni and L. (V.) lindenbergi (a taxonomically distinct species) with no difference in parasitosis on day 8 pbm, suggesting that this species could be further investigated as a possible permissive vector, although it has never been found to be naturally infected by L. (V.) lainsoni in wild-caught specimens that have been examined. Interestingly, the present findings support early microscopic and current molecular-based evidence, which suggests that Ny. antunesi can harbor Trypanosoma sp. [37,38], Porcisia sp. [39], and a wide range of Leishmania spp. [40,41,42,43,44,45,46,47,48,49]. Most of these detections do not provide evidence of late-stage promastigote forms, which are insufficient to characterize Ny. antunesi as a true vector. Other supporting information for the present results is related to O-glycosylated proteins with N-acetylgalactosamine (GalNAc) epitopes, which are likely reported exclusively for permissive species [35], as has been suggested to be present in the midgut epithelial cells of Ny. antunesi [50].
On day 8 pbm, L. (V.) lainsoni and L. (V.) lindenbergi were observed up to the cardia of Ny. antunesi, probably because these Leishmania species need more time to advance to the stomodeal valve under laboratory conditions, as has been suggested for the binomial Phlebotomus arabicus–L. (L.) infantum [15]. On several occasions, it was noted that Ny. antunesi only partially fed under experimental conditions (Sánchez-Uzcátegui, personal observation), which could possibly be important information from an epidemiological point of view. In this sense, multiple bloodmeals during a single gonadotrophic cycle have been reported for Lu. longipalpis [51], which has a potential impact on survival and Leishmania transmission, as suggested by Killick-Kendrick [52], for Ph. papatasi and L. major. Moreover, this characteristic would improve vector competence since the development of a successful infection in wild phlebotomines is a gradual process that depends on the parasite’s action, which is amplified and enhanced by the ingestion of multiple blood meals [53]. Thus, all these facts add weight to the hypothesis that Ny. antunesi is an important vector from a medical point of view, but other criteria still need to be evaluated.
When evaluating the experimental infection of Lu. longipalpis with L. (V.) lainsoni and L. (V.) lindenbergi, it has been demonstrated that both parasite species can develop up to day 8 pbm with no difference in parasitosis between these combinations. Preliminary experimental infections have already been performed and the descriptions of these experiments with the two Leishmania species have focused on determining the developmental pattern for taxonomic purpose [19,38], thus not extending to the observation of late-stage promastigote forms. Lutzomyia longipalpis is well known as the major natural vector of L. (L.) chagasi [54], which is laboratory-supported as a permissive vector and competent for experimentally transmitting L. (V.) braziliensis [55], L. (L.) chagasi [56], L. (L.) mexicana [57], L. (L.) major [58,59] and L. (L.) amazonensis [60].
On day 8 pbm, parasitosis in Lu. longipalpis was higher in L. (L.) chagasi than in L. (V.) lainsoni and L. (V.) lindenbergi, reinforcing the status of the ancient and well-established L. (L.) chagasi–Lu. longipalpis natural binomial [54,61], herein regarded as the control experiment. Naturally, this combination [6,56,62,63,64] is overexploited due to its medical importance, as well as the manageable laboratory adaptation of Lu. longipalpis and consequent successful establishment of colonies [65], with effective rates of artificial blood feeding [22,24,66]. In addition, the results from developmental studies of L. (L.) chagasi in other phlebotomine species were also verified [67,68,69].
As expected, the predominant gut development reported for the studied Leishmania species (i.e., peripylarian for L. (V.) lainsoni and L. (V.) lindenbergi, and suprapylarian for L. (L.) chagasi) is in agreement with the taxonomic positions of these species originally described by Lainson and Shaw [34]. Few specimens with hindgut development were recorded for all combinations, which was exclusively attributed to heavy parasite loads throughout the phlebotomine gut. On that gut site, only adhered promastigotes were considered, avoiding artifactual observation due to back-wash [70]. In contrast, in the midgut, free-living promastigotes were considered. Killick-Kendrick [71] has shown striking features separating metacyclic forms from others in the phlebotomine gut, including a lack of attachment to the epithelium, high motility, a small body size and the presence of a long free flagellum.
The survival curves significantly decreased for all combinations, but were differentially affected in Lu. longipalpis–parasite combinations and uninfected blood. The reduced longevity of experimentally Leishmania-infected phlebotomines was documented [30,72,73], without evidence of a strain-specific impact [30]. Moreover, the results reported herein support the classical hypothesis that successful transmission in nature also depends on equilibrate parasitosis, which is sufficient for the inoculum but within the limits of vector tolerance, preserving its longevity [74].
5. Conclusions
In summary, wild-caught Ny. antunesi and laboratory-bred Lu. longipalpis have been suggested to be experimentally susceptible to L. (V.) lainsoni and L. (V.) lindenbergi, which results in the development of at least a small population of late-stage parasites up to day 8 pbm in the cardia or stomodeal valve. The putative permissiveness of Ny. antunesi has not been discarded, however, still requiring further assessment. The unsuccessful establishment of life cycles with these parasite–vector combinations in nature may result from non-negligible ecological field-driven elements. Indeed, the putative susceptibility of phlebotomines suggested herein is worthy of important epidemiological consequences because it enables a successful adaptation of Leishmania. A successful colonization of Ny. antunesi could provide a considerable number of specimens that allow for an in vivo and in vitro assessment of Leishmania–phlebotomine interactions, and thus definitively determine its permissiveness and vector competence status.
Author Contributions
Study design: Y.d.V.S.U., F.T.S., T.V.d.S. and M.M.P.; data acquisition: Y.d.V.S.U., T.G.d.M. and R.R.F.; resources: F.T.S., T.V.d.S. and M.M.P.; data analysis: Y.d.V.S.U., F.T.S., T.G.d.M., R.R.F., T.V.d.S. and M.M.P.; manuscript—original draft: Y.d.V.S.U. and T.V.d.S.; manuscript—final version: Y.d.V.S.U., F.T.S., T.G.d.M., R.R.F., T.V.d.S. and M.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Instituto Evandro Chagas from the Brazilian Ministry of Health and the ‘Programa de Apoio à Publicação Qualificada’ (no. 01/2024), the Universidade Federal do Pará. T.G.d.M. and Y.d.V.S.U. received Master and PhD scholarships from the ‘Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Brasil’-CAPES (Financial code 001), respectively. R.R.F. received an international mobility scholarship supported by the ‘Edital do Programa de Doutorado-Sanduíche no Exterior’ from the CAPES (PDSE/PROPESP 01/2023; PDSE/CAPES 44/2022). M.M.P. received a research productivity grant from the ‘Conselho Nacional de Desenvolvimento Científico e Tecnológico’-CNPq (grant no. 302292/2017-9).
Data Availability Statement
All data supporting the conclusions are included within the article. The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank Edna de Freitas Leão, Fábio Márcio Medeiros da Silva Freire, Iorlando da Rocha Barata, Luciene Aranha da Silva Santos and Maria Sueli Barros Pinheiro (Instituto Evandro Chagas, Ministry of Health, Brazil) for their technical support with the field and laboratory work; Alexandre Mesquita, Valéria Batista Libonati, Sarah Santos Carneiro, Carlos Alberto Silva Sousa (Department of Management of Special Areas, Municipal Secretariat of Environment, Belém, Brazil) and the Bosque Rodrigues Alves staff for access to their logistical facilities; Lucivaldo João Conceição Ferreira and Raimundo Nonato Barbosa Pires for their technical support with the parasite cultures; and Eduardo José Melo dos Santos for his support with the statistical analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ready, P.D. Biology of phlebotomine sand flies as vectors of disease agents. Annu. Rev. Entomol. 2013, 58, 227–250. [Google Scholar] [CrossRef]
- Organização Pan-Americana da Saúde (OPAS)/Organização Mundial da Saúde (OMS). Leishmanioses. Available online: https://www.who.int/es/news-room/fact-sheets/detail/leishmaniasis (accessed on 24 April 2023).
- Becvar, T.; Vojtkova, B.; Siriyasatien, P.; Votypka, J.; Modry, D.; Jahn, P.; Modri, D.; Jahn, P.P.; Bates, P.; Carpenter, S.; et al. Experimental transmission of Leishmania (Mundinia) parasites by biting midges (Diptera: Ceratopogonidae). PLoS Pathogens. 2021, 17, e1009654. [Google Scholar] [CrossRef]
- Sunantaraporn, S.; Thepparat, A.; Phumee, A.; Sor-Suwan, S.; Boonserm, R.; Bellis, G.; Siriyasatien, P. Culicoides Latreille (Diptera: Ceratopogonidae) as potential vectors for Leishmania martiniquensis and Trypanosoma sp. in northern Thailand. PLoS Negl. Trop. Dis. 2021, 15, e0010014. [Google Scholar] [CrossRef]
- Ticha, L.; Kykalova, B.; Sadlova, J.; Gramiccia, M.; Gradoni, L.; Volf, P. Development of various Leishmania (Sauroleishmania) tarentolae strains in three Phlebotomus species. Microorganisms 2021, 9, 2256. [Google Scholar] [CrossRef]
- Freitas, V.C.; Parreiras, K.P.; Duarte, A.P.M.; Secundino, N.F.; Pimenta, P.F. Development of Leishmania (Leishmania) infantum chagasi in its natural sandfly vector Lutzomyia longipalpis. Am. J. Trop. Med. Hyg. 2012, 86, 606. [Google Scholar] [CrossRef]
- Borovsky, D.; Schlein, Y. Trypsin and chymotrypsin-like enzymes of the sandfly Phlebotomus papatasi infected with Leishmania and their possible role in vector competence. Med. Vet. Entomol. 1987, 1, 235–242. [Google Scholar] [CrossRef]
- Pimenta, P.F.P.; Modi, G.B.; Pereira, S.T.; Shahabuddin, M.; Sacks, D.L. A novel role for the peritrophic matrix in protecting Leishmania from the hydrolytic activities of the sand fly midgut. Parasitology 1997, 115, 359–369. [Google Scholar] [CrossRef]
- Pimenta, P.F.; Turco, S.J.; McConville, M.J.; Lawyer, P.G.; Perkins, P.V.; Sacks, D.L. Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science 1992, 256, 1812–1815. [Google Scholar] [CrossRef]
- Kamhawi, S.; Ramalho-Ortigao, M.; Pham, V.M.; Kumar, S.; Lawyer, P.G.; Turco, S.J.; Barillas-Mury, C.; Sacks, D.L.; Valenzuela, J.G. A role for insect galectins in parasite survival. Cell 2004, 119, 329–341. [Google Scholar] [CrossRef]
- Lawyer, P.G.; Ngumbi, P.M.; Anjili, C.O.; Odongo, S.O.; Mebrahtu, Y.B.; Githure, J.I.; Koech, D.K.; Roberts, C.R. Development of Leishmania major in Phlebotomus duboscqi and Sergentomyia schwetzi (Diptera: Psychodidae). Am. J. Trop. Med. Hyg. 1990, 43, 31–43. [Google Scholar] [CrossRef]
- Sacks, D.L. Metacyclogenesis in Leishmania promastigotes. Exp. Parasitol. 1989, 69, 100–103. [Google Scholar] [CrossRef]
- Rogers, M.E.; Chance, M.L.; Bates, P.A. The role of promastigote secretory gel in the origin and transmission of the infective stage of Leishmania mexicana by the sandfly Lutzomyia longipalpis. Parasitology 2002, 124, 495–507. [Google Scholar] [CrossRef]
- Nieves, E.; Pimenta, P.F. Development of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis in the sand fly Lutzomyia migonei (Diptera: Psychodidae). J. Med. Entomol. 2000, 37, 134–140. [Google Scholar] [CrossRef]
- Myskova, J.; Votypka, J.; Volf, P. Leishmania in sand flies: Comparison of quantitative polymerase chain reaction with other techniques to determine the intensity of infection. J. Med. Entomol. 2008, 45, 133–138. [Google Scholar] [CrossRef][Green Version]
- Gonçalves, L.P.; Santos, T.V.D.; Campos, M.B.; Lima, L.V.D.R.; Ishikawa, E.A.Y.; Silveira, F.T.; Ramos, P.K.S. Further insights into the eco-epidemiology of American cutaneous leishmaniasis in the Belem metropolitan region, Pará State, Brazil. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200255. [Google Scholar] [CrossRef]
- Ward, R.D.; Lainson, R.; Shaw, J.J. Experimental transmissions of Leishmania mexicana amazonesnis Lainson & Shaw, between hamsters by the bite of Lutzomyia flaviscutellata (Mangabeira). Trans. R. Soc. Trop. Med. Hyg. 1977, 71, 265–266. [Google Scholar]
- Vasconcelos dos Santos, T.; Silveira, F.T. Increasing putative vector importance of Trichophoromyia phlebotomines (Diptera: Psychodidae). Mem. Inst. Oswaldo Cruz. 2020, 115, e190284. [Google Scholar] [CrossRef] [PubMed]
- Silveira, F.T.; Ishikawa, E.A.Y.; De Souza, A.A.A.; Lainson, R. An outbreak of cutaneous leishmaniasis among soldiers in Belém, Pará State, Brazil, caused by Leishmania (Viannia) lindenbergi n. sp.—A new leishmanial parasite of man in the Amazon region. Parasite 2002, 9, 43–50. [Google Scholar] [CrossRef]
- Pimentel, A.C.; Sánchez Uzcátegui, Y.D.V.; De Lima, A.C.S.; Silveira, F.T.; Vasconcelos dos Santos, T.; Ishikawa, E.A.Y. Blood Feeding Sources of Nyssomyia antunesi (Diptera: Psychodidae): A Suspected Vector of Leishmania (Kinetoplastida: Trypanosomatidae) in the Brazilian Amazon. J. Med. Entomol. 2022, 59, 1847–1852. [Google Scholar] [CrossRef]
- Sánchez Uzcátegui, Y.D.; Vasconcelos Dos Santos, T.; Silveira, F.T.; Ramos, P.K.; Dos Santos, E.J.M.; Póvoa, M.M. Phlebotomines (Diptera: Psychodidae) from a urban park of Belém, Pará State, northern Brazil and potential implications in the transmission of American cutaneous leishmaniasis. J. Med. Entomol. 2020, 57, 281–288. [Google Scholar] [CrossRef]
- Ready, P.D. The feeding habits of laboratory-bred Lutzomyia longipalpis (Diptera: Psychodidae). J. Med. Entomol. 1978, 14, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, O.L.; Munstermann, L.E.; Cárdenas, R.; Gutiérrez, R.; Ferro, C. Definición de las condiciones de temperatura y almacenamiento adecuadas en la detección de ADN de Leishmania por PCR en flebotominos. Biomedica 2002, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Uzcátegui, Y.D.V.; Dos Santos, E.J.M.; Matos, E.R.; Silveira, F.T.; Vasconcelos dos Santos, T.; Póvoa, M.M. Artificial blood-feeding of phlebotomines (Diptera: Psychodidae: Phlebotominae): Is it time to repurpose biological membranes in light of ethical concerns? Parasites Vectors 2022, 15, 399. [Google Scholar] [CrossRef] [PubMed]
- Vaselek, S.; Volf, P. Experimental infection of Phlebotomus perniciosus and Phlebotomus tobbi with different Leishmania tropica strains. Int. J. Parasitol. 2019, 49, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Rowton, E.D.; Dorsey, K.M.; Armstrong, K.L. Comparison of in vitro (chicken-skin membrane) versus in vivo (live hamster) blood-feeding methods for maintenance of colonized Phlebotomus papatasi (Diptera: Psychodidae). J. Med. Entomol. 2008, 45, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Munstermann, L.E. Care, Maintenance, and Experimental Infection of Phlebotomine Sand Flies in Biology of Disease Vectors; Marquardt, W.H., Ed.; BEI Resources: Amsterdam, The Netherlands, 2005; pp. 757–762. Available online: https://www.beiresources.org/Portals/2/VectorResources/Methods%20in%20Sand%20fly%20Research.pdf (accessed on 13 December 2023).
- Lawyer, P.G.; Meneses, C.; Rowland, T.; Rowton, E.D. Sand fly rearing. In Care and Maintenance of Phlebotomine Sand Flies; WRAIR: Silver Spring, MD, USA, 2016; Chapter 3. [Google Scholar]
- Volf, P.; Volfova, V. Establishment and maintenance of sand fly colonies. J. Vector Ecol. 2011, 36, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Agrela, I.F.; Feliciangeli, M.D. Effect of Leishmania spp. infection on the survival, life expectancy, fecundity and fertility of Lutzomyia longipalpis sl. and Lutzomyia pseudolongipalpis. Mem. Inst. Oswaldo Cruz. 2015, 110, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, J.E. Mortalidad y tablas de vida. In Ecología de Poblaciones Animales; Eva, V., Ed.; Chesneau: Washington, DC, USA, 1978; pp. 39–60. [Google Scholar]
- Ayres, M.; Junior, A.M. BioEstat 20: Aplicações Estatísticas nas Áreas das Ciências Biológicas e Médicas p. xii–259; Civil Society Mamirauá: Belém, Brazil, 2000. [Google Scholar]
- De Souza, A.A.A.; Da Rocha Barata, I.; Silva, M.D.G.S.; Lima, J.A.N.; Jennings, Y.L.L.; Ishikawa, E.A.Y.; Prévot, G.; Ginouves, M.; Silveira, F.T.; Shaw, J.; et al. Natural Leishmania (Viannia) infections of phlebotomines (Diptera: Psychodidae) indicate classical and alternative transmission cycles of American cutaneous leishmaniasis in the Guiana Shield, Brazil. Parasite 2017, 24, 13. [Google Scholar] [CrossRef]
- Lainson, R.; Shaw, J.J. Evolution, classification and geographical distribution. In The Leishmaniases in Biology and Medicine; Peters, W., Killick-Kendrick, R., Eds.; Academic Press: London, UK, 1987; Volume 1, pp. 1–120. [Google Scholar]
- Volf, P.; Peckova, J. Sand flies and Leishmania: Specific versus permissive vectors. Trends Parasitol. 2007, 23, 91–92. [Google Scholar] [CrossRef]
- Cecílio, P.; Cordeiro-da-Silva, A.; Oliveira, F. Sand flies: Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun. Biol. 2022, 5, 305. [Google Scholar] [CrossRef]
- Lainson, R.; Shaw, J.J. The role of animals in the epidemiology of South American Leishmaniasis. In Biology of the Kinetoplastida; Lumsden, W.H.R., Evans, D.A., Eds.; Academic Press: London, UK; New York, NY, USA; San Francisco, CA, USA, 1979; pp. 1–116. [Google Scholar]
- Silveira, F.T.; Souza, A.A.; Lainson, R.; Shaw, J.J.; Braga, R.R.; Ishikawa, E.A. Cutaneous leishmaniasis in the Amazon region: Natural infection of the sandfly Lutzomyia ubiquitalis (Psychodidae: Phlebotominae) by Leishmania (Viannia) lainsoni in Pará State, Brazil. Mem. Inst. Oswaldo Cruz. 1991, 86, 127–130. [Google Scholar] [CrossRef]
- Thies, S.F.; De Morais Bronzoni, R.V.; Michalsky, É.M.; Dos Santos, E.S.; Da Silva, D.J.F.; Dias, E.S.; Damazo, A.S. Aspects on the ecology of phlebotomine sand flies and natural infection by Leishmania hertigi in the Southeastern Amazon Basin of Brazil. Acta Trop. 2018, 177, 37–43. [Google Scholar] [CrossRef]
- Ryan, L.; Silveira, F.T.; Lainson, R.; Shaw, J.J. Leishmanial infections in Lutzomyia longipalpis and Lu. antunesi (Diptera: Psychodidae) on the island of Marajó, Pará State, Brazil. Trans R Soc. Trop. Med. Hyg. 1984, 78, 547–548. [Google Scholar] [CrossRef]
- Vásquez-Trujillo, A.; Santamaría-Herreño, E.; González-Reina, A.E.; Buitrago-Álvarez, L.S.; Góngora-Orjuela, A.; Cabrera-Quintero, O.L. Lutzomyia antunesi as suspected vector of cutaneous leishmaniasis in the Orinoquian region of Colombia. Rev. Salud Publica 2008, 10, 625–632. [Google Scholar] [CrossRef]
- Vásquez-Trujillo, A.; Reina, A.E.G.; Orjuela, A.G.; Suárez, E.P.; Palomares, J.E.; Alvarez, L.S.B. Seasonal variation and natural infection of Lutzomyia antunesi (Diptera: Psychodidae: Phlebotominae), an endemic species in the Orinoquia region of Colombia. Mem. Inst. Oswaldo Cruz. 2013, 108, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Thies, S.F.; Ribeiro, A.L.M.; Michalsky, E.M.; Miyazaki, R.D.; Fortes-Dias, C.L.; Fontes, C.J.F.; Dias, E.S. Phlebotomine sandfly fauna and natural Leishmania infection rates in a rural area of Cerrado (tropical savannah) in Nova Mutum, State of Mato Grosso in Brazil. Rev. Soc. Bras. Med. Trop. 2013, 46, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Chagas, A.P.; Soares, D.C.; De Sousa, G.C.R.; Viana, R.B.; Rebelo, J.M.M.; Garcez, L.M. Aspectos ecológicos da fauna de flebotomíneos em focos de leishmaniose na Amazônia Oriental, Estado do Pará, Brasil. Rev. Pan-Amaz. Saude 2016, 7, 10. [Google Scholar] [CrossRef]
- Ogawa, G.M.; Pereira Júnior, A.M.; Resadore, F.; Ferreira, R.D.G.M.; Medeiros, J.F.; Camargo, L.M.A. Sandfly fauna (Diptera: Psychodidae) from caves in the state of Rondônia, Brazil. Rev. Bras. Parasitol. Vet. 2016, 25, 61–68. [Google Scholar] [CrossRef]
- De Oliveira Leão, P.; Júnior, A.M.P.; De Paulo, P.F.M.; Carvalho, L.P.C.; Souza, A.B.N.; Da Silva, M.S.; Castro, T.S.; De Souza, M.T.F.; De Souza, M.M.R.; Melim, G.E.F.; et al. Vertical stratification of sand fly diversity in relation to natural infections of Leishmania sp. and blood-meal sources in Jamari National Forest, Rondônia State, Brazil. Parasit. Vectors 2020, 13, 422. [Google Scholar] [CrossRef]
- Araujo-Pereira, T.D.; Pita-Pereira, D.D.; Baia-Gomes, S.M.; Boité, M.; Silva, F.; Pinto, I.D.S.; De Sousa, R.L.T.; Fuzari, A.; De Souza, C.; Brazil, R.; et al. An overview of the sandfly fauna (Diptera: Psychodidae) followed by the detection of Leishmania DNA and blood meal identification in the state of Acre, Amazonian Brazil. Mem. Inst. Oswaldo Cruz. 2020, 115, e200157. [Google Scholar] [CrossRef]
- Costa, G.S.G.; Júnior, A.M.P.; Castro, T.S.; De Paulo, P.F.M.; Ferreira, G.E.M.; Medeiros, J.F. Sand fly fauna and molecular detection of Leishmania species and blood meal sources in different rural environments in western Amazon. Acta Trop. 2021, 224, 106150. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.C.G.; De Souza, E.A.; Barroso, E.P.; De Ávila, M.M.; Melchior, L.A.K.; Rocha, R.D.C.; Shimabukuro, P.H.F.; Galati, E.A.B.; Brilhante, A.F. Phlebotomine Fauna (Diptera: Psychodidae) and Infection by Leishmania spp. in Forest Fragments of a University Campus, Western Amazon. J. Med. Entomol. 2022, 60, 218–223. [Google Scholar]
- De Oliveira, D.M.S.; Da Silva, B.J.M.; De Sena, C.B.C.; Lima, J.A.N.; Dos Santos, T.V.; Silveira, F.T.; Silva, E.O. Comparative analysis of carbohydrate residues in the midgut of phlebotomines (Diptera: Psychodidae) from colony and field populations from Amazon, Brazil. Exp. Parasitol. 2016, 168, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Elnaiem, D.E.A.; Morton, I.; Brazil, R.; Ward, R.D. Field and laboratory evidence for multiple blood feeding by Lutzomyia longipalpis (Diptera: Psychodidae). Med. Vet. Entomol. 1992, 6, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Killick-Kendrick, R. The biology of Leishmania in phlebotomine sandflies. In Biology of the Kinetoplastida; Lumsden, W.H.R., Evans, A., Eds.; Academic Press: London, UK, 1979; Volume 2, p. 395460. [Google Scholar]
- Serafim, T.D.; Coutinho-Abreu, I.V.; Oliveira, F.; Meneses, C.; Kamhawi, S.; Valenzuela, J.G. Sequential blood meals promote Leishmania replication and reverse metacyclogenesis augmenting vector infectivity. Nat. Microbiol. 2018, 3, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Lainson, R.; Rangel, E.F. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil: A review. Mem. Inst. Oswaldo Cruz. 2005, 100, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.D.V.; Falcão, A.R. Experimental transmission of L. brasiliensis. I. Transmission through inoculation of P. longipalpis triturates. Rev. Inst. Med. Trop. 1962, 4, 159–162. [Google Scholar]
- Lainson, R.; Ward, R.; Shaw, J. Experimental transmission of Leishmania chagasi, causative agent of neotropical visceral leishmaniasis, by the sandfly Lutzomyia longipalpis. Nature 1977, 266, 628–630. [Google Scholar] [CrossRef]
- Da Costa, S.G.; Moraes, C.D.S.; Bates, P.; Dillon, R.; Genta, F.A. Development of Leishmania mexicana in Lutzomyia longipalpis in the absence of sugar feeding. Mem. Inst. Oswaldo Cruz. 2019, 114, e180482. [Google Scholar] [CrossRef] [PubMed]
- Myskova, J.; Svobodova, M.; Beverley, S.M.; Volf, P. A lipophosphoglycan-independent development of Leishmania in permissive sand flies. Microbes Infect. 2007, 9, 317–324. [Google Scholar] [CrossRef]
- Cecílio, P.; Pires, A.C.A.; Valenzuela, J.G.; Pimenta, P.F.; Cordeiro-da-Silva, A.; Secundino, N.F.; Oliveira, F. Exploring Lutzomyia longipalpis sand fly vector competence for Leishmania major parasites. J. Infect. Dis. 2020, 222, 1199–1203. [Google Scholar] [CrossRef]
- Silva, R.C.R.D.; Cruz, L.N.P.D.; Coutinho, J.M.D.S.; Fonseca-Alves, C.E.; Rebêlo, J.M.M.; Pereira, S.R.F. Experimental transmission of Leishmania (Leishmania) amazonensis to immunosuppressed mice through the bite of Lutzomyia longipalpis (Diptera: Psychodidae) results in cutaneous leishmaniasis. Rev. Inst. Med. Trop. 2021, 63, e81. [Google Scholar] [CrossRef] [PubMed]
- Silveira, F.T.; Corbett, C.E.P. Leishmania chagasi Cunha & Chagas, 1937: Indigenous or introduced? A brief review. Rev. Pan-Amaz. Saúde 2010, 1, 143–147. [Google Scholar]
- Soares, R.P.; Macedo, M.E.; Ropert, C.; Gontijo, N.F.; Almeida, I.C.; Gazzinelli, R.T.; Pimenta, P.F.P.; Turco, S.J. Leishmania chagasi: Lipophosphoglycan characterization and binding to the midgut of the sand fly vector Lutzomyia longipalpis. Mol. Biochem. Parasitol. 2002, 121, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, S.; Rogers, M.E.; Hamilton, J.G.; Bates, P.A.; Maingon, R.D. A real-time PCR assay to estimate Leishmania chagasi load in its natural sand fly vector Lutzomyia longipalpis. Trans R Soc. Trop. Med. Hyg. 2008, 102, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Secundino, N.F.; De Freitas, V.C.; Monteiro, C.C.; Pires, A.C.A.; David, B.A.; Pimenta, P.F. The transmission of Leishmania infantum chagasi by the bite of the Lutzomyia longipalpis to two different vertebrates. Parasit. Vectors 2012, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Lawyer, P.; Killick-Kendrick, M.; Rowland, T.; Rowton, E.; Volf, P. Laboratory colonization and mass rearing of phlebotomine sand flies (Diptera, Psychodidae). Parasite 2017, 24, 42. [Google Scholar] [CrossRef] [PubMed]
- Killick-Kendrick, R.; Leaney, A.J.; Ready, P.D. The establishment, maintenance and productivity of a laboratory colony of Lutzomyia longipalpis (Diptera: Psychodidae). J. Med. Entomol. 1977, 13, 429–440. [Google Scholar] [CrossRef]
- Saraiva, L.; Carvalho, G.M.; Gontijo, C.M.; Quaresma, P.F.; Lima, A.C.; Falcão, A.L.; Filho, J.D.A. Natural infection of Lutzomyia neivai and Lutzomyia sallesi (Diptera: Psychodidae) by Leishmania infantum chagasi in Brazil. J. Med. Entomol. 2009, 46, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, V.C.F.V.; Pruzinova, K.; Sadlova, J.; Volfova, V.; Myskova, J.; Filho, S.P.B.; Volf, P. Lutzomyia migonei is a permissive vector competent for Leishmania infantum. Parasit. Vectors 2016, 9, 159. [Google Scholar] [CrossRef]
- Galvis-Ovallos, F.; Ueta, A.E.; Marques, G.D.O.; Sarmento, A.M.C.; Araujo, G.; Sandoval, C.; Tomokane, T.Y.; Da Matta, V.L.R.; Laurenti, M.D.; Galati, E.A.B. Detection of Pintomyia fischeri (Diptera: Psychodidae) with Leishmania infantum (Trypanosomatida: Trypanosomatidae) promastigotes in a focus of visceral leishmaniasis in Brazil. J. Med. Entomol. 2021, 58, 830–836. [Google Scholar] [CrossRef]
- Lainson, R.; Ward, R.D.; Shaw, J.J. Leishmania in phlebotomine sandflies: VI. Importance of hindgut development in distinguishing between parasites of the Leishmania mexicana and L. braziliensis complexes. Proc. R Soc. Lond B Biol. Sci. 1977, 199, 309–320. [Google Scholar] [PubMed]
- Killick-Kendrick, R. The life-cycle of Leishmania in the sandfly with special reference to the form infective to the vertebrate host. Ann. Parasitol. Hum. Comparée 1990, 65, 37–42. [Google Scholar] [CrossRef] [PubMed]
- El Sawaf, B.M.; El Sattar, S.A.; Shehata, M.G.; Lane, R.P.; Morsy, T.A. Reduced longevity and fecundity in Leishmania-infected sand flies. Am. J. Trop. Med. Hyg. 1994, 51, 767–770. [Google Scholar] [CrossRef]
- Rogers, M.E.; Bates, P.A. Leishmania manipulation of sand fly feeding behavior results in enhanced transmission. PLoS Pathog. 2007, 3, e91. [Google Scholar] [CrossRef]
- Hamilton, J.G.C.; Hurd, H. Parasite manipulation of vector behaviour. In The Behavioural Ecology of Parasites; Lewis, E.E., Campbell, J.F., Sukhdeo, M.V., Eds.; CAB International: New York, NY, USA, 2002; pp. 259–281. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).