Abstract
The vaginal microbiota can be classified into five major community state types (CSTs) based on the bacterial content. However, the link between different CST subtypes and vaginal infection remains unclear. Here, we analyzed 2017 vaginal microbiota samples from women of a reproductive age with vaginal infections that were published in the last decade. We found that L. iners was the most dominant in 34.8% of the vaginal samples, followed by L. crispatus (21.2%). CST I was common in healthy individuals, whereas CST III and IV were associated with dysbiosis and infection. CST III-B, IV-A, IV-B, and IV-C0 were prevalent in patients with bacterial vaginosis (BV). Based on the relative abundance of bacteria at the (sub)genus level, a random forest classifier was developed to predict vaginal infections with an area under the curve of 0.83. We further identified four modules of co-occurring bacterial taxa: L. crispatus, Gardnerella, Prevotella, and Bacteroides. The functional prediction revealed that nucleotide biosynthesis pathways were upregulated in patients with human papilloma virus, and carbohydrate degradation pathways were downregulated in patients with BV. Overall, our study identified the bacterial signatures of healthy and infected vaginal microbiota, providing unique insights into the clinical diagnosis and health status prediction of women of a reproductive age.
1. Introduction
The vaginal microbiota of healthy women is dominated by a single Lactobacillus species []. In general, Lactobacillus spp. inhibits the proliferation of exotic bacterial species, such as opportunistic pathogens, in the vagina by producing lactic acid, hydrogen peroxide, and bacteriocins []. In dysbiosis, other facultative or strictly anaerobic microorganisms such as Gardnerella and Prevotella spp. can also inhabit the vagina [].
To better illustrate the structure of the vaginal microbiota, a clustering algorithm based on the composition and relative abundance of vaginal bacteria at the species level, called community state types (CSTs), was proposed [], in which the vaginal microbiota was classified into five CSTs based on four Lactobacillus species. CST I was dominated by L. crispatus, CST II by L. gasseri, CST III by L. iners, and CST V by L. jensenii. CST IV was exceptional, because it was not dominated by any Lactobacillus species; instead, it consisted of different anaerobic species, such as Gardnerella vaginalis and Prevotella spp. CST IV was classified into three independent CSTs based on the type of dominant anaerobic species: CST IV-A, CST IV-C, and CST IV-D []. Since then, CSTs have been widely used to describe the vaginal microbiome, which may be associated with the health status of the female reproductive system. Recently, the CST scheme has been extended to 13 subtypes named VALENCIA, which is short for the VAginaL community state typE Nearest CentroId clAssifier [], where CST I is classified into CST I-A and CST I-B, CST III is classified into CST III-A and CST III-B, and CST IV is classified into CST IV-A, IV-B, IV-C0, IV-C1, IV-C2, IV-C3, and IV-C4. CST IV-A is represented by a high to moderate abundance of BVAB1 and G. vaginalis; CST IV-B is represented by a high to moderate abundance of G. vaginalis and A. vaginae; and CST IV-C1, C2, C3, and C4 are dominated by Prevotella spp., Enterococcus spp., Bifidobacterium spp., and Straphylococcus spp., respectively. This new classification can be applied to any cohort study to allow for comparative analysis between studies.
The composition of the vaginal microbiota is dynamic and influenced by ethnicity, sexual activity, age, and lifestyle []. More specifically, the predominant CSTs among different ethnic groups differ significantly, with African and Hispanic women having a lower abundance of Lactobacillus and a higher proportion of CST IV than Caucasian and Asian women []. Estrogen levels, throughout the life of women, starting from baby, child, puberty, and reproductive age (menstruation and pregnancy) to menopause, promote changes in the vaginal microbiota, as they are correlated with the abundance of Lactobacillus spp. and the vaginal pH [,,,,]. CST Ⅰ, CST III, and CST IV are widely observed in women of a reproductive age, whereas CST V is uncommon [,]. Large cohort studies in Europe and North America have investigated the characteristics of vaginal microbiota composition [,]. The lack of systematic studies from Africa, Asia, and Oceania has resulted in a failure to provide a global baseline for a healthy vaginal microbiota.
In recent years, the association between a disturbed vaginal microbiota and gynecological and obstetric outcomes has been widely studied, especially bacterial vaginosis (BV) [,,], sexually transmitted infections (STIs) [,,], and preterm births [,]. CST IV consists of various strictly anaerobic bacteria, such as Gardnerella vaginalis, BVAB1/2/3, Prevotella spp., Atopobium spp., and Megasphaera spp., and is associated with different types of vaginal dysbiosis or infections, including aerobic vaginitis (AV), BV, STIs such as human papilloma virus (HPV), herpes simplex virus (HSV), and Chlamydia trachomatis (CT) infections, and vulvovaginal candidiasis (VVC) []. However, CST IV-associated infections do not always present with symptoms [], raising questions about whether CST IV or any subtype of CST IV increases the risk of adverse health outcomes.
The accumulated sequencing data of the vaginal microbiota enabled us to explore the features of the vaginal microbiota under different health conditions. Here, we present a combined analysis of 16S rRNA amplicon sequencing data of 2017 vaginal microbiomes from 14 studies to investigate dysbiosis or infection-associated microbial signatures, thus contributing to the overall understanding of the healthy vaginal microbiota and future diagnosis of vaginal infection with microbial signatures.
2. Materials and Methods
2.1. Data Sources and Study Population
The 16S rRNA gene sequencing data of vaginal microbiota from women with different types of vaginal infection and healthy women were downloaded by searching published research using the keywords “16S rRNA gene sequencing and vagina”. The inclusion criteria for the studies were as follows: (1) studies containing publicly available 16S rRNA gene sequencing data using Illumina sequencing platforms, (2) studies using vaginal swabs as sample sources, and (3) studies containing publicly available metadata, especially group information for each sample. A total of 14 studies with accessible raw data and metadata of vaginal samples were selected for this study [,,,,,,,,,,,,,]. For longitudinal studies in which women were treated with probiotics or medicines after diagnosis, only the baseline samples were selected. In total, 2017 samples from healthy individuals and six types of infections from different areas were used in our analyses. The details of the included studies and their samples are presented in Table 1.

Table 1.
Characteristics of the datasets in this study.
2.2. 16S rRNA Data Processing
Because the targeted regions of the 16S rRNA gene sequencing data downloaded from public databases were the V3–V4 or V4 regions, the V4 region of these sequences was extracted for integrated analysis. Briefly, the pair-end reads were merged into long sequences using QIIME2 (version 2020.8.0) []. The V4 regions were extracted using Cutadapt (version 2.3) by marking the 16S rRNA sequences of the 515F and 806R primers with single-end reads or merged pair-end reads []. Subsequently, the deblur plugin in QIIME2 was used to denoise the sequencing reads and generate amplicon sequence variants (ASVs). Next, the ASVs were taxonomically classified using the downloaded naïve Bayes classifier, trained using the 16S rRNA V4 sequences in the SILVA database (silva-138-99-515-806-nb-classifier-download) for each dataset. Samples with less than 1000 mapped reads were filtered for analysis.
2.3. Lactobacillus Subgenus Reclassification
ASVs generated by 16S rRNA gene sequencing can only be accurately classified to the genus level, whereas the analysis of vaginal Lactobacillus requires species-level classification. Therefore, we reclassified the ASVs of Lactobacillus into subgenera according to the method proposed by France et al. []. Briefly, species of the Lactobacillus genus were reclassified into nine subgenera based on the maximum-likelihood phylogenetic tree, constructed using amino acid sequences of 100 single-copy core genes of representative genomes of all Lactobacillus species. This method can differentiate the four major vaginal Lactobacillus species into different Lactobacillus subgenera.
2.4. VALENCIA Clustering Analysis
The VALENCIA is a clustering scheme based on the nearest centroid classification algorithm, which was used for reproducible and rigorous classification of vaginal microbiota []. The vaginal microbiota was classified into 5 CSTs: CST I (L. crispatus- dominated), II (L. gasseri-dominated), III (L. iners-dominated), IV (Non-Lactobacillus-dominated), and V (L. jensenii-dominated). The CSTs were further classified into 13 subtypes based on the percentage and type of dominant species. Specifically, CST I was classified into CST I-A and I-B, CST III was classified into CST III-A and III-B, and CST IV was classified as CST IV-A, IV-B, IV-C0, IV-C1, IV-C2, IV-C3, and IV-C4. To map our data to the CSTs defined by VALENCIA, we manually modified the subgenus of Lactobacillus into the VALENCIA taxon names.
2.5. t-SNE Analysis of the Vaginal Microbiota
To investigate the structure of the vaginal microbiota within our samples, t-SNE was performed on the relative abundances of the (sub)genus in each sample using the Bray–Curtis distance metric to calculate distances using the TSNE function in the sklearn.manifold module. Samples were labeled with CSTs, CST subtypes, the most dominant taxa, the second most dominant taxa, and the abundances of the most and second most dominant taxa.
2.6. Network Analysis
To explore the correlations between vaginal taxa, we performed a network correlation analysis using SparCC with 1000 bootstrap replicates to estimate the p-values []. Only species that were present in at least 10% of all samples were retained for network analysis. The network was visualized using the igraph package in R.
2.7. Functional Prediction of the Microbiota
The relative abundances of the (sub)genera were calculated based on the ASV tables. The metagenomic functional composition of the vaginal microbiota was predicted based on the relative abundance of the (sub)genus using PICRUSt2 []. The relative abundance of metabolic pathways was annotated using the MetaCyc database. The relative abundances of pathways between the healthy and disease groups were compared using the ggpicrust2 package in R. Statistical analysis was performed using the embedded DESeq2 method.
2.8. Random Forest Model
To assess the performance of vaginal microbiota in predicting vaginal infection, random forest models were trained based on the relative abundance of vaginal microbiota in each sample using the RandomForest Classifier function in the sklearn.ensemble module. First, all the samples were randomly split into training (70%) and test datasets (30%). To obtain the best (sub)genus for classification and the best combination of hyperparameters in each model, feature and hyperparameter selections were performed and embedded into a random search pipeline with 10-fold cross-validation. Finally, the performance of the model was evaluated using the area under the curve (AUC), accuracy, precision, and sensitivity, which were calculated using roc_auc_score, accuracy_score, precision_score, and recall_score functions in the sklearn.metrics module, respectively.
2.9. Statistical Analysis
Intergroup comparisons were performed using Wilcoxon rank sum tests in R. BH adjustment was used to control the FDR in multiple hypothesis tests. All statistical tests were two-sided. p-values < 0.05 were regarded as significant after adjusting for multiple testing.
3. Results
3.1. Lactobacillus Species Were Dominant in the Vagina
We collected 2017 sequencing data of the 16S rRNA gene of vaginal swabs from 14 studies to characterize the vaginal microbiota of women of a reproductive age (Table 1), including samples from healthy controls and aerobic vaginitis (AV), bacterial vaginosis (BV), vulvovaginal candidiasis (VVC)-, Chlamydia trachomatis (CT)-, human papilloma virus (HPV)-, and herpes simplex virus (HSV)-infected individuals from different continents. After sequencing quality assessment, a set of 1941 samples was selected for further analysis, including 315 samples from Africa, 566 from Asia, 396 from Europe, 197 from North America, and 467 from Oceania (Figure 1A,B).
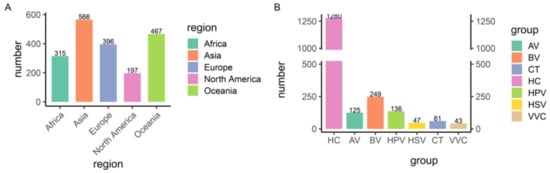
Figure 1.
Overview of the 1941 samples. (A) Geographic distribution of the samples. (B) Number of samples in each disease group. HC, healthy control; AV, aerobic vaginitis; BV, bacterial vaginosis; VVC, vulvovaginal candidiasis; CT, Chlamydia trachomatis; HPV, human papilloma virus; HSV, herpes simplex virus.
For comparative analysis, we used sequences of the V4 region of the 16S rRNA gene to profile the vaginal microbiota. Subsequently, the V4 region sequences of the Lactobacillus genus were reclassified to the subgenus level, resulting in nine subgenus groups that included the L. crispatus, L. iners, L. gasseri, and L. jensenii groups, and five other uncommon groups, the L. delbrueckii, L. pasteurii, L. apis, L. sp002417825, and L. sp002418055 groups []. The results of the comparative analysis showed that the Lactobacillus genus was dominant in the vaginal microbiota by 58.6%, with L. iners group being the most prevalent (34.8%), followed by the L. crispatus (21.2%), L. gasseri (1.6%), and L. jensenii groups (1.0%) (Figure 2A); the genus Gardnerella occupied 15.5%, followed by Prevotella (6.1%), Sneathia (3.4%), Streptococcus (1.5%), Atopobium (1.4%), and Megasphaera (0.15%), and the remaining genera occupied 13.3% (Figure 2A).
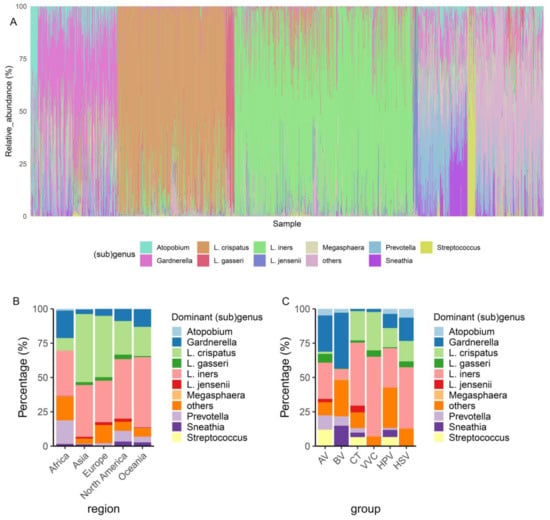
Figure 2.
Overview of the dominant taxa in the vaginal microbiota. (A) The relative abundance of the vaginal microbiota in each sample. (B) The distribution of the most dominant taxa in each region of healthy individuals. (C) The distribution of the most dominant taxa in each disease group. AV, aerobic vaginitis; BV, bacterial vaginosis; VVC, vulvovaginal candidiasis; CT, Chlamydia trachomatis; HPV, human papilloma virus; HSV, herpes simplex virus.
Among healthy individuals, L. crispatus was dominant in Asia and Europe, whereas L. iners was dominant in Africa, North America, and Oceania (Figure 2B). The percentages of Gardnerella- and Prevotella-dominated vaginal microbiota were higher in Africa than in other regions (Figure 2B). Among the vaginal microbiota with infections, the dominance of L. iners was widely identified in the CT, VVC, HPV, and HSV patient groups, indicating that L. iners was susceptible to vaginal infections. A L. crispatus-dominated microbiota was the second most common after L. iners in the CT, VVC, HPV, and HSV patient groups, whereas a Gardnerella-dominated microbiota was less common in these patient groups (Figure 2C). A Gardnerella-dominated microbiota was the most prevalent in patients with AV and BV, followed by L. iners and Prevotella (Figure 2C). An L. crispatus-dominated microbiota was absent in patients with BV. In summary, the dominant bacterial groups in healthy individuals from different continents differed, with L. cripatus and L. iners being the most common and dominant taxa. An L. iners-dominated microbiota was also common in patients with CT, VVC, HPV, and HSV infections, and Gardnerella was enriched in patients with BV.
3.2. CST III-A Was Enriched in Patients with Vaginal Infections
Next, we compared the subtypes of vaginal microbiota between different groups based on VALENCIA, which is a newly developed CST classifier. The 13 subtypes of CSTs defined by VALENCIA were identified in 1941 samples. The prevalence of CST I was much higher in healthy women than in the patient groups, except for VVC, whereas the CST III and IV were more prevalent in the patient groups (Figure 3A). The enrichment of CST III varied in different diseases: CST III-B was enriched in patients with AV and BV, whereas CST III-A was enriched in patients with CT, VVC, HPV, and HSV. In contrast, CST IV was more enriched in patients with BV and HPV. Notably, CST IV-A, IV-B, and IV-C0 were more prevalent in patients with BV (Figure 3B). Thus, we inferred that G. vaginalis-dominated CSTs, such as CST IV-A and IV-B, were representative of patients with BV, and that women with CST III-A were more likely to develop vaginal infections.
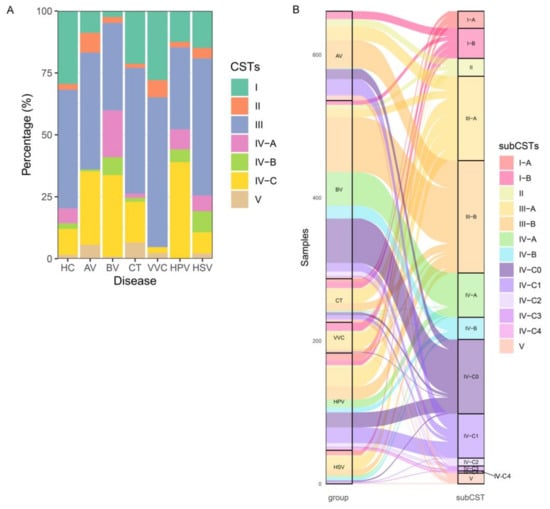
Figure 3.
Overview of the CSTs in the vaginal microbiota. (A) The CSTs of the vaginal microbiota in each sample. (B) The distribution of CST subtypes in each disease group. HC, healthy control; AV, aerobic vaginitis; BV, bacterial vaginosis; VVC, vulvovaginal candidiasis; CT, Chlamydia trachomatis; HPV, human papilloma virus; HSV, herpes simplex virus.
3.3. CST III-B Components Were Complex and Associated with Patients with BV
The enrichment of CST III in both healthy individuals and individuals with a disease prompted us to compare the vaginal microbiota structures of these samples. The t-SNE analysis showed that Lactobacillus-dominated CST samples formed one cluster and were separated from the cluster formed by non-Lactobacillus-dominated CST samples. However, a group of CST III-B samples was found between the two clusters (Figure 4A,B). CSV III-B was originally defined as the vaginal microbiota in which L. iners was less abundant but still dominant; however, three subgroups of CST III-B were observed under t-SNE analysis that we further assigned as CST III-B0, III-B1, and III-B2. In CST III-B0, L. iners and Gardnerella were co-dominant with comparable relative abundances (Figure 4C–F). The CST III-B1 samples, which consisted of Gardnerella, Sneathia, Prevotella, and other non-Lactobacillus genera, merged into the cluster formed by the non-Lactobacillus-dominant CST samples, indicating that these samples were not properly clustered or defined by VALENCIA (Figure 4B–F). Most CST III-B1 samples belonged to the BV group, explaining why CST III-B was enriched in patients with BV (Figure 4G,H). The intermediate group between CST I-B and III-A, which we assigned as CST III-B2, was co-dominated by L. iners and L. crispatus, with relative abundances ranging from 40% to 60% (Figure 4B–F). Therefore, patients with BV tend to process Gardnerella-related vaginal microbiota compared with healthy individuals and patients with other types of infections.
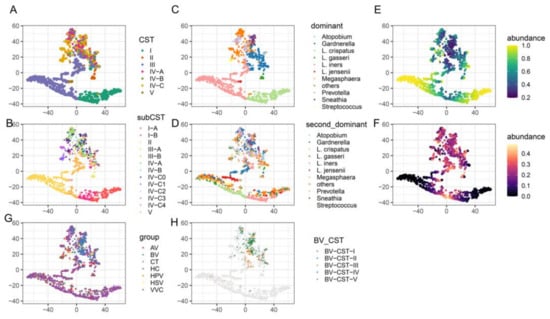
Figure 4.
t-SNE plot of microbiome samples. Samples are colored according to the CSTs (A), 13 subtypes of CSTs (B), dominant taxa (C), second most dominant taxa (D), abundance of the dominant taxa (E), abundance of the second most dominant taxa (F), disease group (G), and CSTs of the BV samples (H). AV, aerobic vaginitis; BV, bacterial vaginosis; VVC, vulvovaginal candidiasis; CT, Chlamydia trachomatis; HPV, human papilloma virus; HSV, herpes simplex virus.
3.4. The Potential Use of a Prediction Model Based on the Vaginal Microbiota in Clinical Diagnosis
To explore the potential of diagnosing vaginal infections based on the composition of the vaginal microbiota, a random forest model was employed to distinguish between vaginal dysbiosis and infections in healthy individuals. We constructed a random forest model with a relative abundance of vaginal microbiota and clinical metadata, which achieved good performance on the test dataset, as indicated by an AUC of 83% (Figure S1, Table S1). The accuracy and precision of the model were 79% and 76%, respectively, and the sensitivity of the model was 57%. The low sensitivity indicated that vaginal infections were difficult to diagnose when only the relative abundance of the vaginal microbiota was provided. However, the accuracy, precision, and sensitivity were improved when we used the model for BV prediction alone, with 92%, 83%, and 67%, respectively, indicating that the characteristics of the vaginal microbiota of patients with BV were more distinguishable than those of other vaginal infections (Figure S2, Table S2).
3.5. Functional Profiling of Metabolic Pathways in Vaginal Microbiota of Patient Groups
We further investigated the metabolic profiles of the vaginal microbiota in each disease group by performing metabolic pathway annotations using the MetaCyc database. Of the 1941 samples, 1372 were successfully annotated with at least one pathway. All HSV samples failed to be annotated to any pathway and were not used for subsequent comparative analysis, which was performed between the healthy group and each patient group. A total of 1071 differential pathways were identified, of which 218 were identified in the AV group, 282 in the BV group, 193 in the HPV group, 243 in the VVC group, and 135 in the CT group (p < 0.05). Specifically, the nucleotide biosynthesis and degradation pathways (de novo purine nucleotide biosynthesis and pyrimidine nucleotide salvage) were significantly elevated in the HPV group (Figure 5). The VVC and CT groups shared several downregulated pathways, including amino acid biosynthesis (L-lysine biosynthesis), carbohydrate degradation (galactose degradation), and fermentation (fermentation to lactate) (Figure 5). Moreover, the AV and BV groups showed similar patterns of metabolic profiles, where fatty acid and lipid biosynthesis pathways, such as phosphatidylglycerol biosynthesis and CDP-diacylglycerol biosynthesis, carrier biosynthesis pathways, such as coenzyme A biosynthesis, and pyruvate fermentation pathway were all downregulated. The lactose and sucrose degradation pathways were exclusively downregulated in the BV group (Figure 5). Overall, the differential pathways in HPV-infected individuals were characterized by enhanced nucleotide biosynthesis pathways, whereas carbohydrate metabolic pathways were downregulated in patients with VVC and CT and fatty acid, and lipid biosynthesis pathways were downregulated in patients with AV and BV.
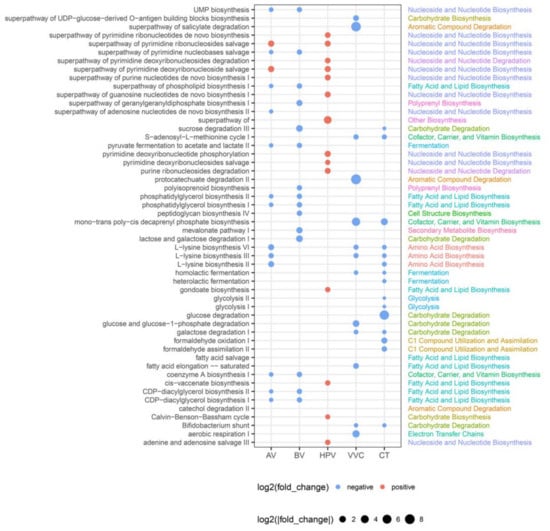
Figure 5.
Differential functional pathways between the disease group and healthy controls. The size of the dot represents |log2(fold change)|. If the dots are red, the pathway is upregulated in the disease group. If the dots are blue, the pathway is downregulated in the disease group. Only pathways with p < 0.05 are shown. AV, aerobic vaginitis; BV, bacterial vaginosis; VVC, vulvovaginal candidiasis; CT, Chlamydia trachomatis; HPV, human papilloma virus.
3.6. Colonization Resistance Can Be Explained by Bacterial Co-Occurrence Modules
Since we observed that L. iners co-occurred with different vaginal bacteria and was enriched in both healthy and disease groups, we investigated how different bacterial taxa in the vagina were correlated. Four bacterial co-occurrence modules of the correlated bacterial taxa were identified: L. crispatus, Gardnerella, Prevotella, and Bacteroides (Figure 6). Within the L. crispatus module, L. crispatus was strongly correlated with L. jensenii and L. gasseri (r = 0.4 and 0.5, p < 0.05). The bacterial taxa of the L. crispatus module were negatively correlated with the bacterial taxa in the Gardnerella and Prevotella modules (r between −0.25 and −0.04 and between −0.31 and −0.07, respectively) and positively correlated with the bacterial taxa of the Bacteroides module (r = 0.04–0.2). In addition, the bacterial taxa in the Prevotella module were positively correlated with the taxa in the Gardnerella module, except that Anaerococcus in the Prevotella module showed a negative correlation with Shuttleworthia in the Gardnerella module (r = −0.12–0.64). Interestingly, the L. iners group did not correlate with any bacterial taxa, indicating that the L. iners group coexisted with any bacterial taxa, which explains the three subgroups observed in CST III-B. In summary, the L. crispatus module showed evidence of colonization resistance to Gardnerella and Prevotella. However, L. iners did not show colonization resistance to any bacterial taxa in the vagina.
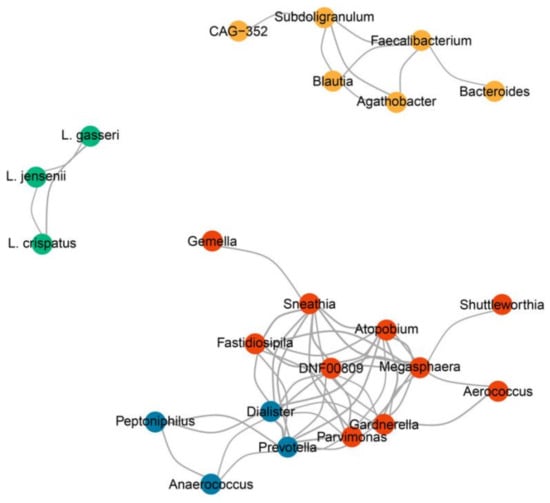
Figure 6.
Co-occurrence network of the vaginal microbiota. Four modules of the correlated vaginal microbiota were identified: L. crispatus (green), Gardnerella (red), Prevotella (blue), and Bacteroides (yellow) modules. The line thickness represents the degree of correlation. Only correlations between subgenera with |r > 0.4| and p < 0.05 are shown.
4. Discussion
The vaginal microbiota plays an important role in the health of women of a reproductive age [,]; however, the association between the vaginal microbiota and different types of vaginal infections is not clear. In this study, we systematically analyzed the characteristics of the vaginal microbiota of 1280 healthy and 661 infected women of a reproductive age. Unlike previous studies, we aimed to decipher the bacterial co-occurrence modules in the vaginal microbiota associated with infections to define the colonization resistance. The results were based on an assessment of previous studies of patients with six types of dysbiosis or infections in comparison with healthy individuals.
The L. iners-dominated vaginal microbiota was prevalent (34.8%) in both the healthy individuals and the disease groups and was observed in women of a reproductive age in Africa, North America, and Oceania, with the highest prevalence. The CST analysis further verified the enrichment of CST-III in the patient groups, where CST III-A was enriched in the VVC, CT, and HSV patient groups, and CST III-B was enriched in the BV patient group. Notably, in our study, CST III-B could be reclassified into three subgroups by t-SNE analysis, including CST III-B0, III-B1, and III-B2, owing to the differences in co-inhabitant bacterial taxa. CST III-B0 had L. iners cohabitating with G. vaginalis with comparable relative abundances; CST III-B1 was not dominated by L. iners but was composed of different types of non-Lactobacillus species; and CST III-B2 had L. iners cohabitating with L. crispatus with comparable relative abundances. CST III-B0 was previously described as a transition from L. crispatus to L. iners []. However, we inferred that CST III-B1 is a new subtype of CST IV that cannot be properly classified using VALENCIA. VALENCIA was constructed based on the vaginal microbiota of North American cohorts [], while our data consisted of individuals from different continents and with different health statuses, thus covering more diverse vaginal microbiota or non-Lactobacillus-dominated vaginal microbiota.
In terms of colonization resistance, we showed the ability of L. iners to cohabit with either L. crispatus, regarded as “good” vaginal bacteria [,], or G. vaginalis, regarded as “bad” vaginal bacteria [,]. L. iners did not show any positive or negative correlation with other vaginal genera in our co-occurrence network analysis or in a previous study []. We assumed that L. iners did not show colonization resistance to other vaginal bacterial taxa. In contrast, L. crispatus showed clear co-occurrence patterns with L. jensenii and L. gasseri and showed colonization resistance to Gardnerella and Prevotella, reinforcing the concept that L. crispatus contributes to a healthy and stable vaginal microbiota [,]. The features that the L. iners can co-inhabit with “good” L. crispatus or “bad” G. vaginalis is probably the reason why CST III-B was enriched in both healthy and infected individuals. We hypothesized that L. iners is a neutral bacterium, and its contribution to health depends on the cohabiting bacteria. The initial colonization of Gardnerella spp. in L. iners-dominated microbiota was more likely to develop into BV than in L. crispatus-dominated microbiota. Thus, the presence of L. iners is an alarming sign that the vaginal microbiota may have an unhealthy outcome. One possible explanation is that L. iners shares certain metabolic and physical interactions with L. crispatus and G. vaginalis. Another interpretation is that the strains of L. iners show divergent metabolic potential that can be shared with L. crispatus or G. vaginalis.
In previous studies, the association between G. vaginalis and BV has not been determined, because G. vaginalis has been detected in asymptomatic patients with BV, non-sex-experienced adolescent girls [], and healthy women []. In our cohort, 41% of the vaginal microbiota in patients with BV was dominated by Gardnerella, followed by Sneathia (15%), L. iners (8%), Prevotella (7%), and Atopobium (3%). Moreover, CSTs dominated by G. vaginalis (CST-IV A and IV B) were more abundant in patients with BV than in those with other types of vaginal infection. The abundance and prevalence of G. vaginalis were lower in healthy individuals than in patients with BV, implying that the abundance, rather than the presence of G. vaginalis, is more important for BV diagnosis. Combining Nugent scores [], Amsel criteria [], and our diagnostic model may further improve the accuracy of clinical diagnosis of BV. Furthermore, our co-occurrence analysis revealed a positive correlation within members of the Gardnerella module and between the Gardnerella and Prevotella modules, which indicated that the colonization of non-Lactobacillus species would promote the colonization of other non-Lactobacillus species.
Compared to BV, vaginal infections such as VVC, CT, and HSV did not show a specific pattern of vaginal dysbiosis but showed a higher percentage of Lactobacillus iners-dominated microbiota (CST III-A), indicating that the recovery of vaginal microbiota from non-Lactobacillus-dominated or L. iners-dominated to L. crispatus-dominated is essentially important to decrease recurrence and for the successful treatment of vaginal infections. The diagnostic model for predicting vaginal dysbiosis and infections did not show as good a performance as that predicting BV. We inferred that the (sub)genus-level profiling of vaginal microbiota using 16S rRNA gene sequencing is not enough for differentiating healthy vaginal microbiota and infected microbiota. In future studies, strain-level or species-level analysis of vaginal microbiota should be performed by using metagenomic sequencing to decipher the presence of different Lactobacillus strains in vagina microbiota and their contribution to women’s reproductive health.
5. Conclusions
In conclusion, the vaginal microbiota of healthy individuals and patients were characterized by different bacterial co-occurrence modules. The vaginal microbiota in CST-I, dominated by L. crispatus, was more stable and enriched in healthy individuals, showing a higher colonization resistance to Gardnerella and Prevotella. In contrast, CST-III, which was dominated by L. iners, showed no co-occurrence modules with no colonization resistance and was susceptible to infections; for example, CST III-A was enriched in patients with HPV, HSV, CT, and VVC, whereas CST III-B was enriched in patients with BV. BV is a typical dysbiosis with higher Gardnerella, dominated by CST subtypes, such as CST IV-A, IV-B, IV-C0, CST III-B0, and CST III-B1.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms12051030/s1: Figure S1: The ROC curve of the random forest model predicting vaginal infection; Figure S2: The ROC curve of the random forest model predicting bacterial vaginosis; Table S1: The raw data for constructing the random forest model predicting vaginal infection; Table S2: The raw data for constructing the random forest model predicting bacterial vaginosis.
Author Contributions
Conceptualization, W.D. and B.Z.; methodology, W.D. and Q.X.; software, W.D. and Q.L.; validation, Y.P.; formal analysis, W.D. and S.W.; investigation, Z.L. and X.W.; resources, N.L.; data curation, S.W.; writing—original draft preparation, W.D.; writing—review and editing, D.L. and B.Z.; visualization, W.D. and G.X.; supervision, D.L.; project administration, B.Z.; funding acquisition, B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the National Key Research and Development Program of China (2021YFC2301000), the General Program of National Natural Science Foundation of China (32170068), and the Research Project of Jinan Microecological Biomedicine Shandong Laboratory (JNL-2022013B).
Data Availability Statement
The public data used in this study are available in the NCBI SRA database via the NCBI BioProject ID PRJNA511717, PRJEB29686, PRJNA398590, PRJNA592384, PRJNA735440, PRJNA310998, PRJNA523312, PRJEB63251, PRJNA391337, PRJNA730929, PRJNA518153, PRJNA826816, PRJNA831622, PRJNA566293, and PRJEB33108; and National Microbiology Data Center at https://nmdc.cn/resource/attachment/detail/NMDCX0000148 (accessed on 10 January 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Weinstein, L.; Bogin, M.; Howard, J.H.; Finkelstone, B.B. A survey of the vaginal flora at various ages, with special reference to the Doderlein bacillus. Am. J. Obstet. Gynecol. 1936, 32, 211–218. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Fredricks, D.N.; Fiedler, T.L.; Marrazzo, J.M. Molecular identification of bacteria associated with bacterial vaginosis. New Engl. J. Med. 2005, 353, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Freitas, A.C.; Bocking, A.; Hill, J.E.; Money, D.M.; the VOGUE Research Group. Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome 2018, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- France, M.T.; Ma, B.; Gajer, P.; Brown, S.; Humphrys, M.S.; Holm, J.B.; Waetjen, L.E.; Brotman, R.M.; Ravel, J. VALENCIA: A nearest centroid classification method for vaginal microbial communities based on composition. Microbiome 2020, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.M.; Bernstein, K.T.; Aral, S.O. Vaginal Microbiome and Its Relationship to Behavior, Sexual Health, and Sexually Transmitted Diseases. Obstet. Gynecol. 2017, 129, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Gosmann, C.; Anahtar, M.N.; Handley, S.A.; Farcasanu, M.; Abu-Ali, G.; Bowman, B.A.; Padavattan, N.; Desai, C.; Droit, L.; Moodley, A.; et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity 2017, 46, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.; Maibach, H. Lifetime changes in the vulva and vagina. Arch. Gynecol. Obstet. 2006, 273, 195–202. [Google Scholar] [CrossRef]
- Hammerschlag, M.R.; Alpert, S.; Rosner, I.; Thurston, P.; Semine, D.; McComb, D.; McCormack, W.M. Microbiology of the vagina in children: Normal and potentially pathogenic organisms. Pediatrics 1978, 62, 57–62. [Google Scholar] [CrossRef]
- Hickey, R.J.; Zhou, X.; Settles, M.L.; Erb, J.; Malone, K.; Hansmann, M.A.; Shew, M.L.; Van Der Pol, B.; Fortenberry, J.D.; Forney, L.J. Vaginal microbiota of adolescent girls prior to the onset of menarche resemble those of reproductive-age women. mBio 2015, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schutte, U.M.; Zhong, X.; Koenig, S.S.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132ra152. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; You, H.J.; Yu, J.; Sung, J.; Ko, G. Prevotella as a Hub for Vaginal Microbiota under the Influence of Host Genetics and Their Association with Obesity. Cell Host Microbe 2017, 21, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.G.; Parikh, H.I.; Brooks, J.P.; Edwards, D.J.; Arodz, T.J.; Edupuganti, L.; Huang, B.; Girerd, P.H.; Bokhari, Y.A.; Bradley, S.P.; et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 2019, 25, 1001–1011. [Google Scholar] [CrossRef]
- Lebeer, S.; Ahannach, S.; Gehrmann, T.; Wittouck, S.; Eilers, T.; Oerlemans, E.; Condori, S.; Dillen, J.; Spacova, I.; Vander Donck, L.; et al. A citizen-science-enabled catalogue of the vaginal microbiome and associated factors. Nat. Microbiol. 2023, 8, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Muzny, C.A.; Blanchard, E.; Taylor, C.M.; Aaron, K.J.; Talluri, R.; Griswold, M.E.; Redden, D.T.; Luo, M.; Welsh, D.A.; Van Der Pol, W.J.; et al. Identification of Key Bacteria Involved in the Induction of Incident Bacterial Vaginosis: A Prospective Study. J. Infect. Dis. 2018, 218, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.; Watt, A.P.; McKenna, J.P.; Coyle, P.V. Mycoplasma hominis and Gardnerella vaginalis display a significant synergistic relationship in bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Hoffman, N.G.; Morgan, M.T.; Matsen, F.A.; Fiedler, T.L.; Hall, R.W.; Ross, F.J.; McCoy, C.O.; Bumgarner, R.; Marrazzo, J.M.; et al. Bacterial communities in women with bacterial vaginosis: High resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE 2012, 7, e37818. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Tracy, J.K.; Zenilman, J.M.; Ravel, J.; Gravitt, P.E. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J. Infect. Dis. 2014, 210, 1723–1733. [Google Scholar] [CrossRef]
- Dareng, E.O.; Ma, B.; Famooto, A.O.; Adebamowo, S.N.; Offiong, R.A.; Olaniyan, O.; Dakum, P.S.; Wheeler, C.M.; Fadrosh, D.; Yang, H.; et al. Prevalent high-risk HPV infection and vaginal microbiota in Nigerian women. Epidemiol. Infect. 2016, 144, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Shannon, B.; Yi, T.J.; Perusini, S.; Gajer, P.; Ma, B.; Humphrys, M.S.; Thomas-Pavanel, J.; Chieza, L.; Janakiram, P.; Saunders, M.; et al. Association of HPV infection and clearance with cervicovaginal immunology and the vaginal microbiota. Mucosal Immunol. 2017, 10, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012. [Google Scholar] [CrossRef] [PubMed]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 4. [Google Scholar] [CrossRef]
- Wang, C.; Fan, A.; Li, H.; Yan, Y.; Qi, W.; Wang, Y.; Han, C.; Xue, F. Vaginal bacterial profiles of aerobic vaginitis: A case-control study. Diagn. Microbiol. Infect. Dis. 2020, 96, 114981. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, E.F.M.; Wuyts, S.; Bellen, G.; Wittouck, S.; De Boeck, I.; Ruban, K.; Allonsius, C.N.; van den Broek, M.F.L.; Donders, G.G.G.; Lebeer, S. The Dwindling Microbiota of Aerobic Vaginitis, an Inflammatory State Enriched in Pathobionts with Limited TLR Stimulation. Diagnostics 2020, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Plummer, E.L.; Sfameni, A.M.; Vodstrcil, L.A.; Danielewski, J.A.; Murray, G.L.; Fehler, G.; Fairley, C.K.; Garland, S.M.; Chow, E.P.F.; Hocking, J.S.; et al. Prevotella and Gardnerella Are Associated With Treatment Failure Following First-line Antibiotics for Bacterial Vaginosis. J. Infect. Dis. 2023, 228, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Lu, J.; Wang, J.; Xiao, B. Vaginal Lactobacillus iners abundance is associated with outcome in antibiotic treatment of bacterial vaginosis and capable of inhibiting Gardnerella. Front. Cell Infect. Microbiol. 2022, 12, 1033431. [Google Scholar] [CrossRef]
- Ceccarani, C.; Foschi, C.; Parolin, C.; D’Antuono, A.; Gaspari, V.; Consolandi, C.; Laghi, L.; Camboni, T.; Vitali, B.; Severgnini, M.; et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019, 9, 14095. [Google Scholar] [CrossRef]
- Oerlemans, E.; Ahannach, S.; Wittouck, S.; Dehay, E.; De Boeck, I.; Ballet, N.; Rodriguez, B.; Tuyaerts, I.; Lebeer, S. Impacts of Menstruation, Community Type, and an Oral Yeast Probiotic on the Vaginal Microbiome. mSphere 2022, 7, e0023922. [Google Scholar] [CrossRef] [PubMed]
- Bassis, C.M.; Allsworth, J.E.; Wahl, H.N.; Sack, D.E.; Young, V.B.; Bell, J.D. Effects of intrauterine contraception on the vaginal microbiota. Contraception 2017, 96, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.; Hayward, M.R.; Bloom, S.M.; Rocafort, M.; Ngcapu, S.; Mafunda, N.A.; Xu, J.; Xulu, N.; Dong, M.; Dong, K.L.; et al. Modeling the temporal dynamics of cervicovaginal microbiota identifies targets that may promote reproductive health. Microbiome 2021, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, Z.E.; Łaniewski, P.; Thomas, N.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. EBioMedicine 2019, 44, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Yang, Y.; Ren, J.; Zhou, H. Changes in vaginal microbiome after focused ultrasound treatment of high-risk human papillomavirus infection-related low-grade cervical lesions. BMC Infect. Dis. 2023, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chang, T.; Yuan, Q.; Wei, W.; Wang, P.; Song, X.; Yuan, H. Changes in the composition of gut and vaginal microbiota in patients with postmenopausal osteoporosis. Front. Immunol. 2022, 13, 930244. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.C.; Yap, P.S.X.; Chong, C.W.; Cheok, Y.Y.; Lee, C.Y.Q.; Tan, G.M.Y.; Sulaiman, S.; Hassan, J.; Sabet, N.S.; Looi, C.Y.; et al. Diversity of endocervical microbiota associated with genital Chlamydia trachomatis infection and infertility among women visiting obstetrics and gynecology clinics in Malaysia. PLoS ONE 2019, 14, e0224658. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, E.F.M.; Bellen, G.; Claes, I.; Henkens, T.; Allonsius, C.N.; Wittouck, S.; van den Broek, M.F.L.; Wuyts, S.; Kiekens, F.; Donders, G.G.G.; et al. Impact of a lactobacilli-containing gel on vulvovaginal candidosis and the vaginal microbiome. Sci. Rep. 2020, 10, 7976. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Watts, S.C.; Ritchie, S.C.; Inouye, M.; Holt, K.E. FastSpar: Rapid and scalable correlation estimation for compositional data. Bioinformatics 2019, 35, 1064–1066. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Delgado-Diaz, D.J.; Tyssen, D.; Hayward, J.A.; Gugasyan, R.; Hearps, A.C.; Tachedjian, G. Distinct Immune Responses Elicited From Cervicovaginal Epithelial Cells by Lactic Acid and Short Chain Fatty Acids Associated With Optimal and Non-optimal Vaginal Microbiota. Front. Cell Infect. Microbiol. 2020, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.G.; Al-Memar, M.; Marchesi, J.R.; Lee, Y.S.; Smith, A.; Chan, D.; Lewis, H.; Kindinger, L.; Terzidou, V.; Bourne, T.; et al. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl. Res. 2019, 207, 30–43. [Google Scholar] [CrossRef]
- France, M.T.; Mendes-Soares, H.; Forney, L.J. Genomic Comparisons of Lactobacillus crispatus and Lactobacillus iners Reveal Potential Ecological Drivers of Community Composition in the Vagina. Appl. Environ. Microbiol. 2016, 82, 7063–7073. [Google Scholar] [CrossRef]
- Witkin, S.S.; Mendes-Soares, H.; Linhares, I.M.; Jayaram, A.; Ledger, W.J.; Forney, L.J. Influence of Vaginal Bacteria and d- and l-Lactic Acid Isomers on Vaginal Extracellular Matrix Metalloproteinase Inducer: Implications for Protection against Upper Genital Tract Infections. mBio 2014, 5, 10–1128. [Google Scholar] [CrossRef]
- Cauci, S.; Culhane, J.F.; Di Santolo, M.; McCollum, K. Among pregnant women with bacterial vaginosis, the hydrolytic enzymes sialidase and prolidase are positively associated with interleukin-1beta. Am. J. Obstet. Gynecol. 2008, 198, 132.e1–132.e7. [Google Scholar] [CrossRef]
- Gelber, S.E.; Aguilar, J.L.; Lewis, K.L.; Ratner, A.J. Functional and phylogenetic characterization of Vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J. Bacteriol. 2008, 190, 3896–3903. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Soares, H.; Suzuki, H.; Hickey, R.J.; Forney, L.J. Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their environment. J. Bacteriol. 2014, 196, 1458–1470. [Google Scholar] [CrossRef]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef]
- Amsel, R.; Totten, P.A.; Spiegel, C.A.; Chen, K.C.; Eschenbach, D.; Holmes, K.K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 1983, 74, 14–22. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).