Alkaline-Tolerant Bacillus cereus 12GS: A Promising Polyhydroxybutyrate (PHB) Producer Isolated from the North of Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Substrates
2.2. Sample Collection and Isolation of Bacterial Strains
2.3. Qualitative Screening of PHB-Producer Bacterial Strains
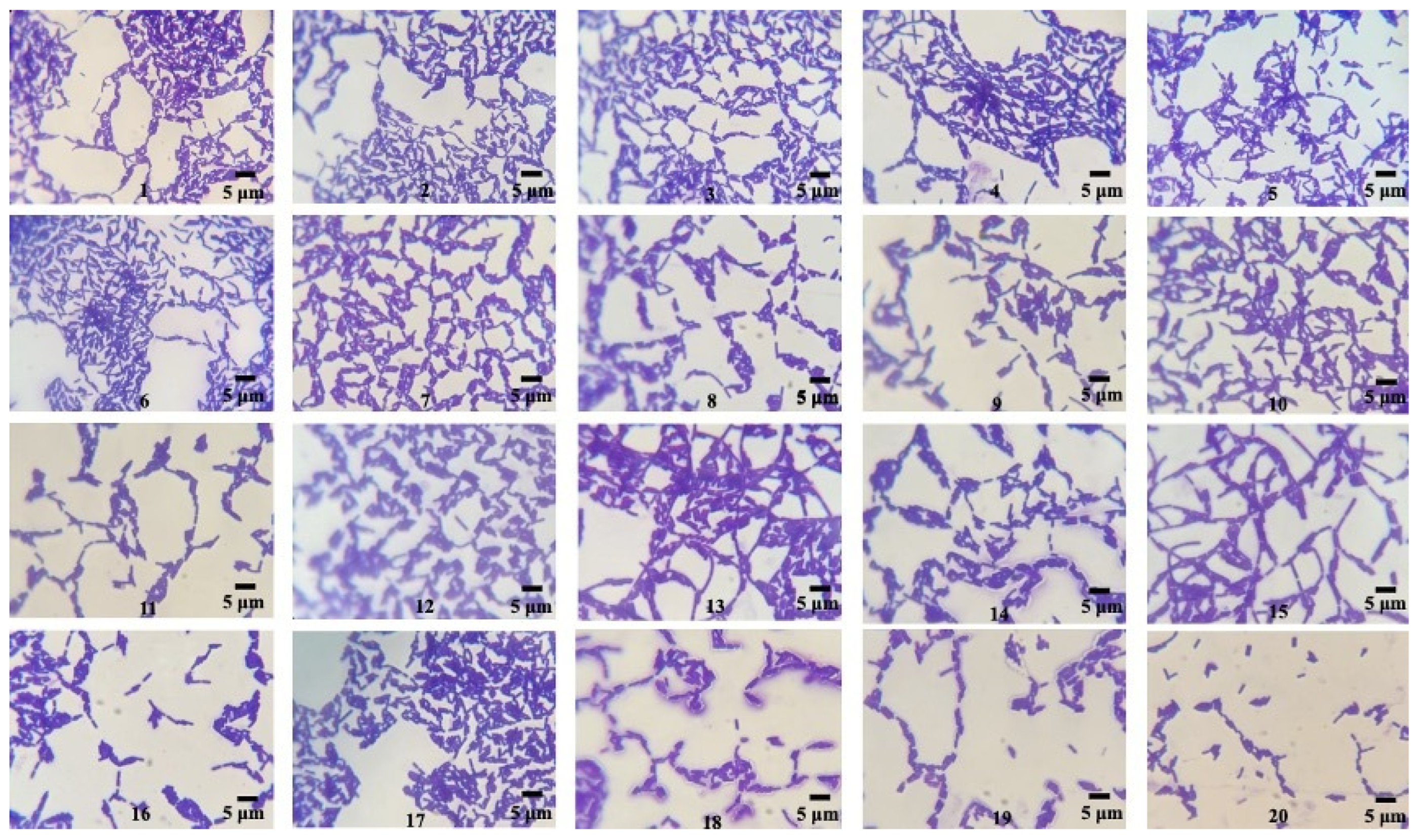
2.3.1. Sudan Black B Staining
2.3.2. Nile Blue A Staining
2.4. Quantitative Screening of PHB-Producer Bacterial Strains
2.4.1. Evaluation of Biomass Generation and PHB Production
2.4.2. PHB Extraction Protocol
2.4.3. Quantification of Biomass Generation
2.4.4. Quantification of PHB Accumulation Percentage
2.5. Statistical Analysis
2.6. Biochemical and Molecular Characterization
2.6.1. Biochemical Characterization
2.6.2. Molecular Characterization
DNA Extraction
Phylogenetic Analysis by 16S rRNA Sequencing
2.7. Optimization of PHB Production and Kinetic Studies
2.7.1. Optimization of Incubation Parameters
2.7.2. Analysis of Bacterial Growth Kinetics
2.8. PHB Characterization
2.8.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.8.2. Differential Scanning Calorimetry (DSC)
3. Results and Discussions
3.1. Isolation and Qualitative Screening
3.2. Quantitative Screening of the Bacterial Isolates
3.3. Biochemical and Molecular Characterization
3.4. Optimization of PHB Production and Kinetic Studies
3.5. PHB Characterization
3.5.1. Fourier-Transform Infrared Spectroscopy (FTIR)
3.5.2. Differential Scanning Calorimetry (DSC)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Horton, A.A. Plastic pollution: When do we know enough? J. Hazard. Mater. 2022, 422, 126885. [Google Scholar] [CrossRef] [PubMed]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A.; Kumar, S. Green product innovation: A means towards achieving global sustainable product within biodegradable plastic industry. J. Clean. Prod. 2022, 363, 132506. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Kalina, M.; Machovsky, M.; Enev, V.; Jakesova, M.; Sobkova, M.; Marova, I. Enzymatic Hydrolysis of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Scaffolds. Materials 2020, 13, 2992. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, J.; Loh, X. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Puppi, D.; Pecorini, G.; Chiellini, F. Biomedical Processing of Polyhydroxyalkanoates. Bioengineering 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Markl, E. PHB—Bio Based and Biodegradable Replacement for PP: A Review. Nov. Tech. Nutr. Food Sci. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Thirumala, M.; Reddy, S.V.; Mahmood, S.K. Production and characterization of PHB from two novel strains of Bacillus spp. isolated from soil and activated sludge. J. Ind. Microbiol. Biotechnol. 2010, 37, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. SpringerPlus 2016, 5, 762. [Google Scholar] [CrossRef] [PubMed]
- Boey, J.Y.; Mohamad, L.; Khok, Y.S.; Tay, G.S.; Baidurah, S. A Review of the Applications and Biodegradation of Polyhydroxyalkanoates and Poly(lactic acid) and Its Composites. Polymers 2021, 13, 1544. [Google Scholar] [CrossRef]
- Aeschelmann, F.; Carus, M. Biobased Building Blocks and Polymers in the World: Capacities, Production, and Applications–Status Quo and Trends towards 2020. Ind. Biotechnol. 2015, 11, 154–159. [Google Scholar] [CrossRef]
- Gurieff, N.; Lant, P. Comparative life cycle assessment and financial analysis of mixed culture polyhydroxyalkanoate production. Bioresour. Technol. 2007, 98, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Maity, S.; Dash, H.R.; Das, S.; Pattnaik, S.; Rath, C.C.; Samantaray, D. Bacillus and biopolymer: Prospects and challenges. Biochem. Biophys. Rep. 2017, 12, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Nazina, T.N.; Tourova, T.P.; Poltaraus, A.B.; Novikova, E.V.; Grigoryan, A.A.; Ivanova, A.E.; Lysenko, A.M.; Petrunyaka, V.V.; Osipov, G.A.; Belyaev, S.S.; et al. Taxonomic study of aerobic thermophilic bacilli: Descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. th. Int. J. Syst. Evol. Microbiol. 2001, 51 Pt 2, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Adebajo, S.O.; Bankole, P.O.; Ojo, A.E.; Akintokun, P.O. Screening and optimization of polyhydroxybutyrate (PHB) by Lysinibacillus fusiformis from diverse environmental sources. Microbe 2024, 2, 100043. [Google Scholar] [CrossRef]
- Thapa, C.; Shakya, P.; Shrestha, R.; Pal, S.; Manandhar, P. Isolation of Polyhydroxybutyrate (PHB) Producing Bacteria, Optimization of Culture Conditions for PHB production, Extraction and Characterization of PHB. Nepal J. Biotechnol. 2019, 6, 62–68. [Google Scholar] [CrossRef]
- Mesquita, D.P.; Amaral, A.L.; Leal, C.; Oehmen, A.; Reis, M.A.; Ferreira, E.C. Polyhydroxyalkanoate granules quantification in mixed microbial cultures using image analysis: Sudan Black B versus Nile Blue A staining. Anal. Chim. Acta 2015, 865, 8–15. [Google Scholar] [CrossRef]
- Tekin, E.; Ates, M.; Kahraman, Ö. Poly-3-hydroxybutyrate-producing extreme halophilic archaeon: Haloferax sp. Ma10 isolated from çamaltı saltern, İzmir. Turk. J. Biol. 2012, 36, 303–312. [Google Scholar] [CrossRef]
- Meijering, E.; Jacob, M.; Sarria, J.C.; Steiner, P.; Hirling, H.; Unser, M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytom. Part A J. Int. Soc. Anal. Cytol. 2004, 58, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Masood, F.; Abdul-Salam, M.; Yasin, T.; Hameed, A. Effect of glucose and olive oil as potential carbon sources on production of PHAs copolymer and tercopolymer by Bacillus cereus FA11. 3 Biotech 2017, 7, 87. [Google Scholar] [CrossRef]
- Martínez-Herrera, R.E.; Alemán-Huerta, M.E.; Almaguer-Cantú, V.; Rosas-Flores, W.; Martínez-Gómez, V.J.; Quintero-Zapata, I.; Rivera, G.; Rutiaga-Quiñones, O.M. Efficient recovery of thermostable polyhydroxybutyrate (PHB) by a rapid and solvent-free extraction protocol assisted by ultrasound. Int. J. Biol. Macromol. 2020, 164, 771–782. [Google Scholar] [CrossRef]
- Aramvash, A.; Banadkuki, N.G.; Zavareh, F.M.; Turchi, S.H. An Environmentally Friendly and Efficient Method for Extraction of PHB Biopolymer with Non-Halogenated Solvents. J. Microbiol. Biotechnol. 2015, 25, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Pagliano, G.; Ventorino, V.; Panico, A.; Pepe, O. Integrated systems for biopolymers and bioenergy production from organic waste and by-products: A review of microbial processes. Biotechnol. Biofuels 2017, 10, 113. [Google Scholar] [CrossRef]
- Arocha-Garza, H.F.; Canales-Del Castillo, R.; Eguiarte, L.E.; Souza, V.; De la Torre-Zavala, S. High diversity and suggested endemicity of culturable Actinobacteria in an extremely oligotrophic desert oasis. PeerJ 2017, 5, e3247. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Garibay, J.A.; Aguilar, C.N.; Rodríguez-Herrera, R.; Guerrero-Olazarán, M.; Viader-Salvadó, J.M. Tannase sequence from a xerophilic Aspergillus niger Strain and production of the enzyme in Pichia pastoris. Mol. Biotechnol. 2015, 57, 439–447. [Google Scholar] [CrossRef]
- Mohammed, S.; Behera, H.T.; Dekebo, A.; Ray, L. Optimization of the culture conditions for production of Polyhydroxyalkanoate and its characterization from a new Bacillus cereus sp. BNPI-92 strain, isolated from plastic waste dumping yard. Int. J. Biol. Macromol. 2020, 156, 1064–1080. [Google Scholar] [CrossRef]
- Xiao, Z.; Liao, X.; Long, Z.; Li, M. Effect of cutting parameters on surface roughness using orthogonal array in hard turning of AISI 1045 steel with YT5 tool. Int. J. Adv. Manuf. Technol. 2017, 93, 273–282. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, H.; Aggarwal, A. Taguchi-fuzzy multi output optimization (MOO) in high speed CNC turning of AISI P-20 tool steel. Expert Syst. Appl. 2011, 38, 6822–6828. [Google Scholar] [CrossRef]
- Amit; Nayak, J.K.; Ghosh, U.K. Microalgal remediation of anaerobic pretreated pharmaceutical wastewater for sustainable biodiesel production and electricity generation. J. Water Process Eng. 2020, 35, 101192. [Google Scholar] [CrossRef]
- Quintero-Silva, M.J.; Suárez-Rodríguez, S.J.; Gamboa-Suárez, M.A.; Blanco-Tirado, C.; Combariza, M.Y. Polyhydroxyalkanoates Production from Cacao Fruit Liquid Residues Using a Native Bacillus megaterium Strain: Preliminary Study. J. Polym. Environ. 2023, 32, 1289–1303. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, R.N.; Varjani, S.; Nair, S.; Kim, D.S.; Shin, H.S.; et al. Developing Microbial Co-Culture System for Enhanced Polyhydroxyalkanoates (PHA) Production Using Acid Pretreated Lignocellulosic Biomass. Polymers 2022, 14, 726. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Das, S.; Mohapatra, S.; Tripathi, A.D.; Akthar, J.; Pati, S.; Pattnaik, S.; Samantaray, D.P. Growth associated polyhydroxybutyrate production by the novel Zobellellae tiwanensis strain DD5 from banana peels under submerged fermentation. Int. J. Biol. Macromol. 2020, 153, 461–469. [Google Scholar] [CrossRef]
- Lu, Z.; Guo, W.; Liu, C. Isolation, identification and characterization of novel Bacillus subtilis. J. Vet. Med. Sci. 2018, 80, 427–433. [Google Scholar] [CrossRef]
- Aljuraifani, A.A.; Berekaa, M.M.; Ghazwani, A.A. Bacterial biopolymer (polyhydroxyalkanoate) production from low-cost sustainable sources. MicrobiologyOpen 2019, 8, e00755. [Google Scholar] [CrossRef]
- Evangeline, S.; Sridharan, T.B. Biosynthesis and statistical optimization of polyhydroxyalkanoate (PHA) produced by Bacillus cereus VIT-SSR1 and fabrication of biopolymer films for sustained drug release. Int. J. Biol. Macromol. 2019, 135, 945–958. [Google Scholar] [CrossRef]
- Bhagowati, P.; Pradhan, S.; Dash, H.R.; Das, S. Production, optimization and characterization of polyhydroxybutyrate, a biodegradable plastic by Bacillus spp. Biosci. Biotechnol. Biochem. 2015, 79, 1454–1463. [Google Scholar] [CrossRef]
- Amiri Kojuri, S.; Issazadeh, K.; Heshmatipour, Z.; Mirpour, M.; Zarrabi, S. Production of Bioplastic (Polyhydroxybutyrate) with Local Bacillus megaterium Isolated from Petrochemical Wastewater. Iran. J. Biotechnol. 2021, 19, e2849. [Google Scholar] [CrossRef]
- Thammasittirong, A.; Saechow, S.; Thammasittirong, S.N. Efficient polyhydroxybutyrate production from Bacillus juice substrate thuringiensis using sugarcane. Turk. J. Biol. Turk Biyol. Derg. 2017, 41, 992–1002. [Google Scholar] [CrossRef]
- Yadav, J.; Balabantaray, S.; Patra, N. Statistical optimization of fermentation conditions for the improved production of poly-β-hydroxybutyrate from Bacillus subtilis. Chem. Eng. Commun. 2017, 204, 1122–1128. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, H.J.; Kim, S.H.; Suh, M.J.; Cho, J.Y.; Ham, S.; Jeon, J.M.; Yoon, J.J.; Bhatia, S.K.; Gurav, R.; et al. Screening of the strictly xylose-utilizing Bacillus sp. SM01 for polyhydroxybutyrate and its co-culture with Cupriavidus necator NCIMB 11599 for enhanced production of PHB. Int. J. Biol. Macromol. 2021, 181, 410–417. [Google Scholar] [CrossRef]
- Alshehrei, F. Production of Polyhydroxybutyrate (PHB) by Bacteria Isolated from Soil of Saudi Arabia. J. Pure Appl. Microbiol. 2019, 13, 897–904. [Google Scholar] [CrossRef]
- El-Kadi, S.M.; Elbagory, M.; El-Zawawy, H.A.H.; El-Shaer, H.F.A.; Shoukry, A.A.; El-Nahrawy, S.; Omara, A.E.; Ali, D.F.I. Biosynthesis of Poly-ß-Hydroxybutyrate (PHB) from Different Bacterial Strains Grown on Alternative Cheap Carbon Sources. Polymers 2021, 13, 3801. [Google Scholar] [CrossRef]
- Masood, F.; Hasan, F.; Ahmed, S.; Hameed, A. Biosynthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from Bacillus cereus FA11 isolated from TNT-contaminated soil. Ann. Microbiol. 2012, 62, 1377–1384. [Google Scholar] [CrossRef]
- Amiri, S.; Mohammadi Zeydi, M.; Amiri, N. Bacillus cereus saba.zh, a novel bacterial strain for the production of bioplastic (polyhydroxybutyrate). Braz. J. Microbiol. 2021, 52, 2117–2128. [Google Scholar] [CrossRef]
- Pirttijärvi, T.S.; Andersson, M.A.; Salkinoja-Salonen, M.S. Properties of Bacillus cereus and other bacilli contaminating biomaterial-based industrial processes. Int. J. Food Microbiol. 2000, 60, 231–239. [Google Scholar] [CrossRef]
- Javaid, H.; Nawaz, A.; Riaz, N.; Mukhtar, H.; -Ul-Haq, I.; Shah, K.A.; Khan, H.; Naqvi, S.M.; Shakoor, S.; Rasool, A.; et al. Biosynthesis of Polyhydroxyalkanoates (PHAs) by the Valorization of Biomass and Synthetic Waste. Molecules 2020, 25, 5539. [Google Scholar] [CrossRef]
- Martínez-Herrera, R.E.; Alemán-Huerta, M.E.; Rutiaga-Quiñones, O.M.; de Luna-Santillana ED, J.; Elufisan, T.O. A comprehensive view of Bacillus cereus as a polyhydroxyalkanoate (PHA) producer: A promising alternative to Petroplastics. Process Biochem. 2023, 129, 281–292. [Google Scholar] [CrossRef]
- Philip, S.; Sengupta, S.; Keshavarz, T.; Roy, I. Effect of impeller speed and pH on the production of poly(3-hydroxybutyrate) using Bacillus cereus SPV. Biomacromolecules 2009, 10, 691–699. [Google Scholar] [CrossRef]
- Mascarenhas, J.; K, A. Production and Optimization of Polyhydroxyalkaonoate Obtained from Bacillus megaterium JHA. J. Appl. Biotechnol. Rep. 2021, 8, 346–360. [Google Scholar] [CrossRef]
- Mokhtarani, N.; Ganjidoust, H.; Vasheghani Farahani, E. Effect of process variables on the production of Polyhydroxyalkanoates by activated sludge. Iran. J. Environ. Health Sci. Eng. 2012, 9, 6. [Google Scholar] [CrossRef]
- Ronďošová, S.; Legerská, B.; Chmelová, D.; Ondrejovič, M.; Miertuš, S. Optimization of Growth Conditions to Enhance PHA Production by Cupriavidus necator. Fermentation 2022, 8, 451. [Google Scholar] [CrossRef]
- García, G.; Sosa-Hernández, J.E.; Rodas-Zuluaga, L.I.; Castillo-Zacarías, C.; Iqbal, H.; Parra-Saldívar, R. Accumulation of PHA in the Microalgae Scenedesmus sp. under Nutrient-Deficient Conditions. Polymers 2020, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Ramarao, M.; King, F.L.; Sivakumar, A.; Manikandan, V.; Vijayakumar, M.; Subbiah, R. Optimizing GMAW parameters to achieve high impact strength of the dissimilar weld joints using Taguchi approach. Mater. Today Proc. 2021, 50, 861–866. [Google Scholar] [CrossRef]
- Hamid, H.; Rehman, Z.U.; Basheer, S.; Akbar, A.; Taj, M. Prodcution of Polyhydroxy Butyrate (PHB) from Soil Bacterium (Bacillus megaterium TISTR 1814) with Cantaloupe Waste Extract as Potential Carbon Source. J. Med. Life Sci. 2020, 3, 6–11. [Google Scholar]
- Manikandan, N.; Pakshirajan, K.; Pugazhenthi, G. A closed-loop biorefinery approach for polyhydroxybutyrate (PHB) production using sugars from carob pods as the sole raw material and downstream processing using the co-product lignin. Bioresour. Technol. 2020, 307, 123247. [Google Scholar] [CrossRef] [PubMed]
- Ahmady-Asbchin, S.; Rezaee, H.; Safari, M.; Zamanifar, P.; Siyamiyan, D. Production and optimization of polyhydroxybutyrate (PHB) from Bacillus megaterium as biodegradable plastic. Eur. J. Biol. Res. 2020, 10, 26–34. [Google Scholar] [CrossRef]
- Patel, S.K.; Singh, M.; Kalia, V.C. Hydrogen and Polyhydroxybutyrate Producing Abilities of Bacillus spp. From Glucose in Two Stage System. Indian J. Microbiol. 2011, 51, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Yustinah; Hidayat, N.; Alamsyah, R.; Roslan, A.M.; Hermansyah, H.; Gozan, M. Production of polyhydroxybutyrate from oil palm empty fruit bunch (OPEFB) hydrolysates by Bacillus cereus suaeda B-001. Biocatal. Agric. Biotechnol. 2019, 18, 101019. [Google Scholar] [CrossRef]
- Masood, F.; Chen, P.; Yasin, T.; Hasan, F.; Ahmad, B.; Hameed, A. Synthesis of poly-(3-hydroxybutyrate-co-12 mol % 3-hydroxyvalerate) by Bacillus cereus FB11: Its characterization and application as a drug carrier. J. Mater. Sci. Mater. Med. 2013, 24, 1927–1937. [Google Scholar] [CrossRef]
- Kynadi, A.S.; Suchithra, T.V. Rubber Seed Oil as a Novel Substrate for Polyhydroxyalkanoates Accumulation in Bacillus cereus. CLEAN–Soil Air Water 2017, 45, 1600572. [Google Scholar] [CrossRef]
- Lathwal, P.; Nehra, K.; Singh, M.; Jamdagni, P.; Rana, J.S. Optimization of Culture Parameters for Maximum Polyhydroxybutyrate Production by Selected Bacterial Strains Isolated from Rhizospheric Soils. Pol. J. Microbiol. 2015, 64, 227–239. [Google Scholar] [CrossRef]
- Sharma, P.; Bajaj, B.K. Cost-effective-substrates for production of poly-β-hydroxybutyrate by a newly isolated Bacillus cereus PS-10. J. Environ. Biol. 2015, 36, 1297–1304. [Google Scholar]
- Hagagy, N.; Saddiq, A.A.; Tag, H.M.; Selim, S.; AbdElgawad, H.; Martínez-Espinosa, R.M. Characterization of Polyhydroxybutyrate, PHB, Synthesized by Newly Isolated Haloarchaea Halolamina spp. Molecules 2022, 27, 7366. [Google Scholar] [CrossRef]
- Chang, Y.C.; Reddy, M.V.; Imura, K.; Onodera, R.; Kamada, N.; Sano, Y. Two-Stage Polyhydroxyalkanoates (PHA) Production from Cheese Whey Using Acetobacter pasteurianus C1 and Bacillus sp. CYR1. Bioengineering 2021, 8, 157. [Google Scholar] [CrossRef]
- Rehman, A.; Aslam, A.; Masood, R.; Aftab, M.N. Production and characterization of a thermostable bioplastic (Poly-s-hydroxybutyrate) from Bacillus cereus NRRL-b-3711. Pak. J. Bot. 2016, 48, 349–356. [Google Scholar]
- Pati, S.; Maity, S.; Dash, A.; Jema, S.; Mohapatra, S.; Das, S.; Samantaray, D.P. Biocompatible PHB Production from Bacillus Species under Submerged and Solid-State Fermentation and Extraction through Different Downstream Processing. Curr. Microbiol. 2020, 77, 1203–1209. [Google Scholar] [CrossRef]
- Oliveira, F.C.; Dias, M.L.; Castilho, L.R.; Freire, D.M.G. Characterization of poly(3-hydroxybutyrate) produced by Cupriavidus necator in solid-state fermentation. Bioresour Technol. 2007, 98, 633–638. [Google Scholar] [CrossRef]
- Penkhrue, W.; Jendrossek, D.; Khanongnuch, C.; Pathom-Aree, W.; Aizawa, T.; Behrens, R.L.; Lumyong, S. Response surface method for polyhydroxybutyrate (PHB) bioplastic accumulation in Bacillus drentensis BP17 using pineapple peel. PLoS ONE 2020, 15, e0230443. [Google Scholar] [CrossRef]
- Hong, S.G.; Hsu, H.W.; Ye, M.T. Thermal properties and applications of low molecular weight polyhydroxybutyrate. J. Therm. Anal. Calorim 2013, 111, 1243–1250. [Google Scholar] [CrossRef]
- Sathya, A.B.; Sivasubramanian, V.; Santhiagu, A.; Sebastian, C.; Sivashankar, R. Production of Polyhydroxyalkanoates from Renewable Sources Using Bacteria. J. Polym. Environ. 2018, 26, 3995–4012. [Google Scholar] [CrossRef]
- Martínez-Herrera, R.E.; Alemán-Huerta, M.E.; Flores-Rodríguez, P.; Almaguer-Cantú, V.; Valencia-Vázquez, R.; Rosas-Flores, W.; Medrano-Roldán, H.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, O.M. Utilization of Agave durangensis leaves by Bacillus cereus 4N for polyhydroxybutyrate (PHB) biosynthesis. Int. J. Biol. Macromol. 2021, 175, 199–208. [Google Scholar] [CrossRef] [PubMed]






| Parameter | Factor | Level | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Agitation speed (rpm) | A | 110 | 120 | 130 | 140 | 150 |
| pH | B | 6 | 6.5 | 7 | 7.5 | 8 |
| Temperature (°C) | C | 28 | 30 | 32 | 34 | 36 |
| Inoculum (%) | D | 1 | 2 | 3 | 4 | 5 |
| Stainings of PHB (Qualitative Screening) | ||
|---|---|---|
| Strain | Sudan Black B (Production) | Nile Blue A (Integrated Density) |
| B1 | (++) | 44,013.228 |
| B2 | (+++) | 166,705.709 |
| B3 | (+++) | 728,499.860 |
| B4 | (++) | 31,617.513 |
| B5 | (++) | 87,029.191 |
| B6 | (++) | 8140.101 |
| B7 | (+) | 2472.227 |
| B8 | (+) | 2551.696 |
| B9 | (++) | 7311.102 |
| B10 | (+) | 4247.510 |
| B11 | (++) | 173,663.663 |
| B12 | (+++) | 1,695,242.386 |
| B13 | (++) | 123,182.626 |
| B14 | (++) | 72,675.122 |
| B15 | (+) | 5060.914 |
| B16 | (+) | 4856.413 |
| B17 | (+++) | 175,356.035 |
| B18 | (+++) | 347,629.345 |
| B19 | (+) | 5420.557 |
| B20 | (++) | 29,617.563 |
| Strain | Biomass g/L | PHB g/L | % PHB |
|---|---|---|---|
| B1 | 0.96 ± 0.18 e | 0.39 ± 0.08 a | 40.7 a |
| B2 | 1.67 ± 0.3 b,c,d,e | 0.43 ± 0.15 a | 25.8 a |
| B3 | 1.49 ± 0.07 d,e | 0.59 ± 0.18 a | 39.8 a |
| B4 | 1.4 ± 0.1 d,e | 0.39 ± 0.14 a | 28.1 a |
| B5 | 1.84 ± 0.22 b,c,d,e | 0.52 ± 0.1 a | 29.8 a |
| B6 | 1.73 ± 0.11 b,c,d,e | 0.31 ± 0.05 a | 18 a |
| B9 | 1.88 ± 0.07 b,c,d,e | 0.48 ± 0.14 a | 25.7 a |
| B11 | 1.95 ± 0.26 b,c,d,e | 0.41 ± 0.01 a | 21.1 a |
| B12 | 4.6 ± 0.7 a | 0.65 ± 0.15 a | 14.2 a |
| B13 | 2.81 ± 0.5 b | 0.49 ± 0.16 a | 17.3 a |
| B14 | 2.48 ± 0.27 b,c,d | 0.44 ± 0.09 a | 17.6 a |
| B17 | 2.69 ± 0.9 b,c | 0.63 ± 0.15 a | 18 a |
| B18 | 2.42 ± 0.17 b,c,d | 0.44 ± 0.14 a | 18.3 a |
| B20 | 1.63 ± 0.08 c,d,e | 0.64 ± 0.15 a | 39.1 a |
| Characterization | Bacillus sp. 12GS | Bacillus cereus saba.zh [43] |
|---|---|---|
| Shape | Rod | Rod |
| Gram staining | Gram-positive (+) | Gram-positive (+) |
| Sporulation | (+) | (+) |
| Oxidase | (+) | (+) |
| Catalase | (+) | (+) |
| Sulfur | (−) | — |
| Indol | (−) | (−) |
| Motility | (−) | — |
| Citrate | (−) | (+) |
| Triple Sugar Iron | (K/A) | (K/A) |
| Lysine Iron Agar | (−) | — |
| Motility Indole Ornithine | (−) | — |
| Urea | (+) | (−) |
| Methyl Red | (−) | (−) |
| Voges–Proskauer | (−) | (+) |
| Growth on NaCl 6.5% | (−) | — |
| Starch hydrolysis | (+) | (+) |
| Lecthinase | (+) | — |
| Experiment No. | Agitation Speed (rpm) | pH | Temperature (°C) | Inocule (%) | Biomass (g/L) | PHB (g/L) | % PHB |
|---|---|---|---|---|---|---|---|
| 1 | 110 | 6 | 28 | 1 | 1.89 ± 0.57 | 0.65 ± 0.12 | 52.24 |
| 2 | 110 | 6.5 | 30 | 2 | 1.86 ± 0.14 | 0.58 ± 0.21 | 31.35 |
| 3 | 110 | 7 | 32 | 3 | 1.93 ± 0.52 | 0.62 ± 0.13 | 31.92 |
| 4 | 110 | 7.5 | 34 | 4 | 1.7 ± 0.01 | 0.53 ± 0.15 | 30.9 |
| 5 | 110 | 8 | 36 | 5 | 1.33 ± 0.07 | 0.25 ± 0.12 | 19.19 |
| 6 | 120 | 6 | 30 | 3 | 1.82 ± 0.11 | 1.01 ± 0.05 | 55.45 |
| 7 | 120 | 6.5 | 32 | 4 | 2.29 ± 0.18 | 0.93 ± 0.2 | 40.37 |
| 8 | 120 | 7 | 34 | 5 | 1.96 ± 0.17 | 0.58 ± 0.04 | 29.61 |
| 9 | 120 | 7.5 | 36 | 1 | 2.06 ± 0.05 | 0.61 ± 0.09 | 29.52 |
| 10 | 120 | 8 | 28 | 2 | 2.6 ± 0.06 | 1.14 ± 0.07 | 43.76 |
| 11 | 130 | 6 | 32 | 5 | 2.16 ± 0.12 | 0.63 ± 0.22 | 32.33 |
| 12 | 130 | 6.5 | 34 | 1 | 2.03 ± 0.27 | 0.42 ± 0.11 | 20.5 |
| 13 | 130 | 7 | 36 | 2 | 1.45 ± 0.08 | 0.3 ± 0.14 | 20.48 |
| 14 | 130 | 7.5 | 28 | 3 | 2.27 ± 0.11 | 0.7 ± 0.14 | 30.77 |
| 15 | 130 | 8 | 30 | 4 | 2.52 ± 0.34 | 1.26 ± 0.15 | 50.03 |
| 16 | 140 | 6 | 34 | 2 | 2.02 ± 0.41 | 0.53 ± 0.1 | 26.18 |
| 17 | 140 | 6.5 | 36 | 3 | 2.28 ± 0.25 | 0.55 ± 0.4 | 42.07 |
| 18 | 140 | 7 | 28 | 4 | 2.61 ± 0.25 | 1.52 ± 0.13 | 58.35 |
| 19 | 140 | 7.5 | 30 | 5 | 2.96 ± 0.43 | 0.97 ± 0.22 | 32.75 |
| 20 | 140 | 8 | 32 | 1 | 3.26 ± 0.32 | 1.11 ± 0.04 | 34.09 |
| 21 | 150 | 6 | 34 | 4 | 3.42 ± 0.09 | 0.79 ± 0.19 | 23.10 |
| 22 | 150 | 6.5 | 28 | 5 | 4.33 ± 0.62 | 1.06 ± 0.33 | 24.46 |
| 23 | 150 | 7 | 30 | 1 | 2.6 ± 0.17 | 1.28 ± 0.14 | 48.78 |
| 24 | 150 | 7.5 | 32 | 2 | 2.95 ± 0.12 | 1.67 ± 0.23 | 56.60 |
| 25 | 150 | 8 | 34 | 3 | 2.2 ± 0.21 | 1.01 ± 0.16 | 45.98 |
| Conditions | Biomass (g/L) | PHB (g/L) | % PHB |
|---|---|---|---|
| Original (pH 6, 30 °C, 2% inocule, 150 rpm) | 4.6 ± 0.7 | 0.65 ± 0.15 | 14.2 |
| Optimized (pH 8, 30 °C, 4% inocule, 150 rpm) | 2.19 ± 0.05 | 1.91 ± 0.1 | 87.2 |
| Kinetic Parameter | Value |
|---|---|
| µ (h−1) | 0.07 |
| µmax (h−1) | 0.09 |
| Td (h) | 9.90 |
| Ks (g/L) | 5 |
| Y p/x (g/L*h) | 0.52 |
| Y x/s (g/L*h) | 0.22 |
| Y p/s (g/L*h) | 0.15 |
| Y x + p/s (g/L*h) | 0.37 |
| qs (g/L*h) | 0.09 |
| qp (g/L*h) | 0.014 |
| Qp (g/L*h) | 0.04 |
| Sample | Tm (°C) | ΔHm (J/g) | Tc (°C) | Td (°C) | Xc (%) |
|---|---|---|---|---|---|
| PHB control (Sigma-Aldrich) | 177.38 | 76.78 | 166.33 | 277.83 | 52.59 |
| PHB by B. cereus 12GS | 172.49 | 61.55 | 163.17 | 237.82 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San Miguel-González, G.d.J.; Alemán-Huerta, M.E.; Martínez-Herrera, R.E.; Quintero-Zapata, I.; de la Torre-Zavala, S.; Avilés-Arnaut, H.; Gandarilla-Pacheco, F.L.; de Luna-Santillana, E.d.J. Alkaline-Tolerant Bacillus cereus 12GS: A Promising Polyhydroxybutyrate (PHB) Producer Isolated from the North of Mexico. Microorganisms 2024, 12, 863. https://doi.org/10.3390/microorganisms12050863
San Miguel-González GdJ, Alemán-Huerta ME, Martínez-Herrera RE, Quintero-Zapata I, de la Torre-Zavala S, Avilés-Arnaut H, Gandarilla-Pacheco FL, de Luna-Santillana EdJ. Alkaline-Tolerant Bacillus cereus 12GS: A Promising Polyhydroxybutyrate (PHB) Producer Isolated from the North of Mexico. Microorganisms. 2024; 12(5):863. https://doi.org/10.3390/microorganisms12050863
Chicago/Turabian StyleSan Miguel-González, Gustavo de J., María E. Alemán-Huerta, Raul E. Martínez-Herrera, Isela Quintero-Zapata, Susana de la Torre-Zavala, Hamlet Avilés-Arnaut, Fátima L. Gandarilla-Pacheco, and Erick de J. de Luna-Santillana. 2024. "Alkaline-Tolerant Bacillus cereus 12GS: A Promising Polyhydroxybutyrate (PHB) Producer Isolated from the North of Mexico" Microorganisms 12, no. 5: 863. https://doi.org/10.3390/microorganisms12050863









