Lysin and Lytic Phages Reduce Vibrio Counts in Live Feed and Fish Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vibrio Strains
2.2. Lytic Phage and Endolysin
2.3. Lytic Activity of Endolysin on Vibrio Strains
2.4. Vibrio Reduction in Bioassay Using Rotifers
2.5. Vibrio Reduction in Bioassay Using Fish Larvae
3. Results
3.1. Lytic Activity of Lysin against Vibrio Strains
3.2. Phage CH20 against Vibrio Strain GV09
3.3. Reduction in Vibrio Load in Rotifers
3.4. Reduction in Vibrio Load in Fish Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cahill, M.M. Bacterial flora of fishes: A review. Microb. Ecol. 1990, 19, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.H.; Olafsen, J.A. Bacterial interactions in early life stages of marine cold water fish. Microb. Ecol. 1999, 38, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Vadstein, O.; Bergh, Ø.; Gatesoupe, F.J.; Galindo-Villegas, J.; Mulero, V.; Picchietti, S.; Defoirdt, T. Microbiology and immunology of fish larvae. Rev. Aquacult. 2013, 5, S1–S25. [Google Scholar] [CrossRef]
- Hurtado, L.; Miranda, C.D.; Rojas, R.; Godoy, F.A.; Añazco, M.A.; Romero, J. Live Feeds Used in the Larval Culture of Red Cusk Eel, Genypterus chilensis, Carry High Levels of Antimicrobial-Resistant Bacteria and Antibiotic-Resistance Genes (ARGs). Animals 2020, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Skjermo, J.; Vadstein, O. Characterization of the Bacterial Flora of Mass Cultivated Brachionus plicatilis. Hydrobiologia 1993, 255, 185–191. [Google Scholar] [CrossRef]
- Vadstein, O.; Attramadal, K.J.K.; Bakke, I.; Forberg, T.; Olsen, Y.; Verdegem, M.; Giatsis, C.; Skjermo, J.; Aasen, I.M.; Gatesoupe, F.-J.; et al. Managing the Microbial Community of Marine Fish Larvae: A Holistic Perspective for Larviculture. Front. Microbiol. 2018, 9, 1820. [Google Scholar] [CrossRef] [PubMed]
- Munro, P.D.; Birkbeck, T.H.; Barbour, A. Influence of Rate of Bacterial Colonisation of the Gut of Turbot Larvae on Larval Survival. In Fish Farming Technology; Reinertsen, H., Dahle, L.A., Jørgensen, L., Tvinnereim, K., Eds.; AA Balkema: Rotterdam, The Netherlands, 1993; pp. 85–92. [Google Scholar]
- Dhert, P.; Rombaut, G.; Suantika, G.; Sorgeloos, P. Advancement of rotifer culture and manipulation techniques in Europe. Aquaculture 2001, 200, 129–146. [Google Scholar] [CrossRef]
- Benavente, G.P.; Gatesoupe, F.J. Bacteria associated with cultured rotifers and Artemia are detrimental to larval turbot, Scophthalmus maximus L. Aquac. Eng. 1988, 7, 289–293. [Google Scholar] [CrossRef]
- Gatesoupe, F.J. Further Advances in the Nutritional and Antibacterial Treatments of Rotifers as Food for Turbot Larvae, Scophthalmus maximus L. In Aquaculture—A Biotechnology in Progress; De Pauw, N., Ackerfots, H., Wilkins, N., Eds.; European Aquaculture Society: Bredene, Belgium, 1989; Volume 2, pp. 721–730. [Google Scholar]
- Verdonck, L.; Grisez, L.; Sweetman, E.; Minkoff, G.; Sorgeloos, P.; Ollevier, F.; Swings, J. Vibrios associated with routine productions of Brachionus plicatilis. Aquaculture 1997, 149, 203–214. [Google Scholar] [CrossRef]
- Rønneseth, A.; Castillo, D.; D’Alvise, P.; Tønnesen, Ø.; Haugland, G.; Grotkjær, T.; Engell-Sørensen, K.; Nørremark, L.; Bergh, Ø.; Wergeland, H.I.; et al. Comparative assessment of Vibrio virulence in marine fish larvae. J. Fish Dis. 2017, 40, 1373–1385. [Google Scholar] [CrossRef]
- Ina-Salwany, M.Y.; Al-Saari, N.; Mohamad, A.; Mursidi, F.A.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A review on disease development and prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef]
- Kong, W.; Huang, L.; Su, Y.; Qin, Y.; Ma, Y.; Xu, X.; Lin, M.; Zheng, J.; Yan, Q. Investigation of possible molecular mechanisms underlying the regulation of adhesion in Vibrio alginolyticus with comparative transcriptome analysis. Antonie Leeuwenhoek 2015, 107, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Austin, B.; Austin, D.A. Vibrionaceae representatives. Bact. Fish Pathog. 2012, 357–411. [Google Scholar] [CrossRef]
- Colorni, A.; Paperna, I.; Gordin, H. Bacterial infections in gilt-head sea bream Sparus aurata cultured at Elat. Aquaculture 1981, 23, 257–267. [Google Scholar] [CrossRef]
- Akayli, T.; Timur, G.; Aydemir, B.; Kiziler, A.R.; Coskun, O.; Albayrak, G.; Arican, E. Characterization of Vibrio alginolyticus isolates from diseased cultured gilthead sea bream, Sparus aurata. Isr. J. Aquac. 2008, 60, 89–94. [Google Scholar] [CrossRef]
- Prol-García, M.J.; Planas, M.; Pintado, J. Different colonization and residence time of Listonella anguillarum and Vibrio splendidus in the rotifer Brachionus plicatilis determined by real-time PCR and DGGE. Aquaculture 2010, 302, 26–35. [Google Scholar] [CrossRef]
- Snoussi, M.; Chaieb, K.; Mahmoud, R.; Bakhrouf, A. Quantitative study, identification and antibiotics sensitivity of some Vibrionaceae associated to a marine fish hatchery. Ann. Microbiol. 2006, 56, 289–293. [Google Scholar] [CrossRef]
- Davis, D.A.; Arnold, C.R. Tolerance of the rotifer Brachionus plicatilis to ozone and total oxidative residuals. Ozone Sci. Eng. 1997, 19, 457–469. [Google Scholar] [CrossRef]
- Theisen, D.D.; Stansell, D.D.; Woods, L.C., III. Disinfection of nauplii of Artemia franciscana by ozonation. Prog. Fish-Cultur. 1998, 60, 149–151. [Google Scholar] [CrossRef]
- Gomez-Gil-RS, B.; Abreu-Grobois, F.A.; Romero-Jarero, J.; De Los Herrera-Vega, M. Chemical disinfection of Artemia nauplii. J. World Aquacult. Soc. 1994, 25, 579–583. [Google Scholar] [CrossRef]
- Hameed, A.S.; Balasubramanian, G. Antibiotic resistance in bacteria isolated from Artemia nauplii and efficacy of formaldehyde to control bacterial load. Aquaculture 2000, 183, 195–205. [Google Scholar] [CrossRef]
- Munro, P. Partial decontamination of rotifers with ultraviolet radiation: The effect of changes in the bacterial load and flora of rotifers on mortalities in start-feeding larval turbot. Aquaculture 1999, 170, 229–244. [Google Scholar] [CrossRef]
- Lu, H.; Li, Z.; Elbaz, A.; Ni, S.Q. Synergistic Action of Phages and Lytic Proteins with Antibiotics: A Combination Strategy to Target Bacteria and Biofilms. BMC Microbiol. 2023, 23, 149. [Google Scholar] [CrossRef] [PubMed]
- Dy, R.; Rigano, L.A.; Fineran, P. Phage-based biocontrol strategies and their application in agriculture and aquaculture. Biochem. Soc. Trans. 2018, 46, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Higuera, G.; Bastías, R.; Tsertsvadze, G.; Romero, J.; Espejo, R.T. Recently discovered Vibrio anguillarum phages can protect against experimentally induced vibriosis in Atlantic salmon, Salmo salar. Aquaculture 2013, 392–395, 128–133. [Google Scholar] [CrossRef]
- Kalatzis, P.G.; Bastías, R.; Kokkari, C.; Katharios, P. Isolation and Characterization of Two Lytic Bacteriophages, St2 and Grn1; Phage Therapy Application for Biological Control of Vibrio alginolyticus in Aquaculture Live Feeds. PLoS ONE 2016, 11, e0151101. [Google Scholar] [CrossRef] [PubMed]
- Katharios, P.; Kalatzis, P.G.; Kokkari, C.; Sarropoulou, E.; Middelboe, M. Isolation and characterization of a N4-like lytic bacteriophage infecting Vibrio splendidus, a pathogen of fish and bivalves. PLoS ONE 2017, 12, e0190083. [Google Scholar] [CrossRef]
- Rohde, C.; Resch, G.; Pirnay, J.P.; Blasdel, B.G.; Debarbieux, L.; Gelman, D.; Górski, A.; Hazan, R.; Huys, I.; Kakabadze, E.; et al. Expert opinion on three phage therapy related topics: Bacterial phage resistance, phage training and prophages in bacterial production strains. Viruses 2018, 10, E178. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Hao, H.; Xie, S.; Yuan, Z. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef]
- Fischetti, V.A. Bacteriophage endolysins: A novel anti-infective to control Gram-positive pathogens. Int. J. Med. Microbiol. 2010, 300, 357–362. [Google Scholar] [CrossRef]
- Fuenzalida, L.; Hernández, C.; Toro, J.; Rioseco, M.L.; Romero, J.; Espejo, R.T. Vibrio parahaemolyticus in Shellfish and Clinical Samples During Two Large Epidemics of Diarrhoea in Southern Chile. Environ. Microbiol. 2006, 8, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Rojas, R.; Miranda, C.D.; Opazo, R.; Romero, J. Characterization and pathogenicity of Vibrio splendidus strains associated with massive mortalities of commercial hatchery-reared larvae of scallop Argopecten purpuratus (Lamarck, 1819). J. Invertebr. Pathol. 2014, 124C, 61–69. [Google Scholar] [CrossRef]
- Li, M.; Jin, Y.; Lin, H.; Wang, J.; Jiang, X. Complete Genome of a Novel Lytic Vibrio parahaemolyticus Phage VPp1 and Characterization of Its Endolysin for Antibacterial Activities. J. Food Prot. 2018, 81, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Son, B.; Kong, M.; Lee, Y.; Ryu, S. Development of a Novel Chimeric Endolysin, Lys109 With Enhanced Lytic Activity Against Staphylococcus aureus. Front. Microbiol. 2021, 11, 615887. [Google Scholar] [CrossRef] [PubMed]
- Bastías, R.; Higuera, G.; Sierralta, W.; Espejo, R.T. A new group of cosmopolitan bacteriophages induce a carrier state in the pandemic strain of Vibrio parahaemolyticus. Environ. Microbiol. 2010, 12, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Opazo, R.; Plaza-Parrochia, F.; Cardoso dos Santos, G.R.; Carneiro, G.R.A.; Sardela, V.F.; Romero, J.; Valladares, L. Fasting Upregulates npy, agrp, and ghsr Without Increasing Ghrelin Levels in Zebrafish (Danio rerio) Larvae. Front. Physiol. 2019, 9, 1901. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Tian, Y.; Pang, M.; Yang, Z.; Bao, H.; Zhou, Y.; Sun, L.; Wang, R.; Zhang, H. A novel vibriophage vB_VhaS_PcB-1G capable of inhibiting virulent Vibrio harveyi pathogen. Aquaculture 2021, 542, 736854. [Google Scholar] [CrossRef]
- Cevallos-Urena, A.; Kim, J.Y.; Kim, B.S. Vibrio-infecting bacteriophages and their potential to control biofilm. Food Sci. Biotechnol. 2023, 32, 1719–1727. [Google Scholar] [CrossRef]
- Hendrix, R.W.; Casjens, S.R.; Lavigne, R. Family—Siphoviridae. In Virus Taxonomy; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 86–98. ISBN 9780123846846. [Google Scholar] [CrossRef]
- Moineau, S. Bacteriophage. In Brenner’s Encyclopedia of Genetics: Second Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 280–283. [Google Scholar] [CrossRef]
- Ackermman, H.-W. Phage Classification and Characterization. In Bacteriophages; Clokie, M.R.J., Kropinski, A.M., Eds.; Humana Press: Totowa, NJ, USA, 2009; Volume 501. [Google Scholar] [CrossRef]
- Li, F.; Xing, S.; Fu, K.; Zhao, S.; Liu, J.; Tong, Y.; Zhou, L. Genomic and biological characterization of the Vibrio alginolyticus-infecting “Podoviridae” bacteriophage, vB_ValP_IME271. Virus Genes 2019, 55, 218–226. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, L.; Wang, M.; Wang, Q.; Zhang, X.; Han, Y.; Wang, M.; Jiang, T.; Shao, H.; McMinn, A. Complete genomic sequence of bacteriophage P23: A novel Vibrio phage isolated from the Yellow Sea, China. Virus Genes 2019, 55, 834–842. [Google Scholar] [CrossRef]
- Li, J.; Tian, F.; Hu, Y.; Lin, W.; Liu, Y.; Zhao, F.; Ren, H.; Pan, Q.; Shi, T.; Tong, Y. Characterization and genomic analysis of BUCT549, a novel bacteriophage infecting Vibrio alginolyticus with flagella as receptor. Front. Microbiol. 2021, 12, 668319. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Zhang, L.; Liao, J.; Zhang, D.; Wu, S.; Zhang, X.; Qin, Q.; Wei, J. Isolation and characterization of a newly discovered phage, V-YDF132, for lysing Vibrio harveyi. Viruses 2022, 14, 1802. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.; Zhao, J.; Wang, L.; Xie, G.; Huang, J.; Zhang, Y. A novel Vibriophage vB_VcaS_HC containing lysogeny-related gene has strong lytic ability against pathogenic bacteria. Virol. Sin. 2021, 36, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Gibb, B.; Hyman, P.; Schneider, C.L. The Many Applications of Engineered Bacteriophages—An Overview. Pharmaceuticals 2021, 14, 634. [Google Scholar] [CrossRef] [PubMed]
- Nachimuthu, R.; Madurantakam Royam, M.; Manohar, P.; Leptihn, S. Application of bacteriophages and endolysins in aquaculture as a biocontrol measure. In Biological Control; Academic Press Inc.: Cambridge, MA, USA, 2021; Volume 160. [Google Scholar] [CrossRef]
- Castillo, D.; Kauffman, K.; Hussain, F.; Kalatzis, P.; Rørbo, N.; Polz, M.F.; Middelboe, M. Widespread distribution of prophage-encoded virulence factors in marine Vibrio communities. Sci. Rep. 2018, 8, 9973. [Google Scholar] [CrossRef]
- Lal, T.M.; Sano, M.; Ransangan, J. Genome characterization of a novel vibriophage VpKK5 (Siphoviridae) specific to fish pathogenic strain of Vibrio parahaemolyticus. J. Basic Microbiol. 2016, 56, 872–888. [Google Scholar] [CrossRef] [PubMed]
- Matamp, N.; Bhat, S.G. Phage Endolysins as Potential Antimicrobials against Multidrug Resistant Vibrio alginolyticus and Vibrio parahaemolyticus: Current Status of Research and Challenges Ahead. Microorganisms 2019, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Cong, Y.; Lin, H.; Wang, J. Development of cationic peptide chimeric lysins based on phage lysin Lysqdvp001 and their antibacterial effects against Vibrio parahaemolyticus: A preliminary study. Int. J. Food Microbiol. 2021, 358, 109396. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ju, X.; Cong, Y.; Lin, H.; Wang, J. A Single Catalytic Endolysin Domain Plychap001: Characterization and Application to Control Vibrio Parahaemolyticus and Its Biofilm Directly. Foods 2022, 11, 1578. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Lin, H.; Wang, J.; Mao, X. The Vibrio parahaemolyticus-infecting bacteriophage qdvp001: Genome sequence and endolysin with a modular structure. Arch. Virol. 2016, 161, 2645–2652. [Google Scholar] [CrossRef]
- Zermeño-Cervantes, L.A.; Makarov, R.; Lomelí-Ortega, C.O.; Martínez-Díaz, S.F.; Cardona-Félix, C.S. Recombinant LysVPMS1 as an endolysin with broad lytic activity against Vibrio parahaemolyticus strains associated to acute hepatopancreatic necrosis disease. Aquac. Res. 2018, 49, 1723–1726. [Google Scholar] [CrossRef]
- Sisson, H.M.; Jackson, S.A.; Fagerlund, R.D.; Warring, S.L.; Fineran, P.C. Gram-negative endolysins: Overcoming the outer membrane obstacle. Curr. Opin. Microbiol. 2024, 78, 102433. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Blanot, D.; De Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Alakomi, H.L.; Paananen, A.; Suihko, M.L.; Helander, I.M.; Saarela, M. Weakening effect of cell permeabilizers on Gram-negative bacteria causing biodeterioration. Appl. Environ. Microbiol. 2006, 72, 4695–4703. [Google Scholar] [CrossRef]
- Zermeño-Cervantes, L.A.; Martínez-Díaz, S.F.; Venancio-Landeros, A.A.; Cardona-Félix, C.S. Evaluating the efficacy of endolysins and membrane permeabilizers against Vibrio parahaemolyticus in marine conditions. Res. Microbiol. 2023, 174, 104104. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.M.; Chen, J.-H.; Zhang, R.; Liu, B. A comprehensive review of the applications of bacteriophage-derived endolysins for foodborne bacterial pathogens and food safety: Recent advances, challenges, and future perspective. Front. Microbiol. 2023, 14, 1259210. [Google Scholar] [CrossRef] [PubMed]
- Soto, F.; Lopez-Ramirez, M.A.; Jeerapan, I.; Esteban-Fernandez de Avila, B.; Mishra, R.K.; Lu, X.; Chai, I.; Chen, C.; Kupor, D.; Nourhani, A.; et al. Rotibot: Use of Rotifers as Self-Propelling Biohybrid Microcleaners. Adv. Funct. Mater. 2019, 29, 1900658. [Google Scholar] [CrossRef]
- Li, J.; Shen, H.; Zhou, H.; Shi, R.; Wu, C.; Chu, P.K. Antimicrobial micro/nanorobotic materials design: From passive combat to active therapy. Mater. Sci. Eng. R Rep. 2023, 152, 100712. [Google Scholar] [CrossRef]
- Vázquez-Salgado, L.; Olveira, J.G.; Dopazo, C.P.; Bandín, I. Role of rotifer (Brachionus plicatilis) and Artemia (Artemia salina) nauplii in the horizontal transmission of a natural nervous necrosis virus (NNV) reassortant strain to Senegalese sole (Solea senegalensis) larvae. Vet. Q. 2020, 40, 205–214. [Google Scholar] [CrossRef]
- Giménez, G.; Padrós, F.; Roque, A.; Estévez, A.; Furones, D. Bacterial load reduction of live prey for fish larval feeding using Ox-Aquaculture®. Aquac. Res. 2006, 37, 1130–1139. [Google Scholar] [CrossRef]
- Lomelí-Ortega, C.O.; Martínez-Díaz, S.F. Phage therapy against Vibrio parahaemolyticus infection in the whiteleg shrimp (Litopenaeus vannamei) larvae. Aquaculture 2014, 434, 208–211. [Google Scholar] [CrossRef]
- Silva, Y.J.; Costa, L.; Pereira, C.; Mateus, C.; Cunha, C.; Calado, R.; Gomes, N.C.M.; Pardo, M.A.; Hernandez, I.; Almeida, A. Phage therapy as an approach to prevent Vibrio anguillarum infections in fish larvae production. PLoS ONE 2014, 9, e114197. [Google Scholar] [CrossRef]
- Vallés, R.; Roque, A.; Caballero, A.; Estévez, A. Use of Ox-Aquaculture® for disinfection of live prey and meagre larvae, Argyrosomus regius (Asso, 1801). Aquac. Res. 2015, 46, 413–419. [Google Scholar] [CrossRef]
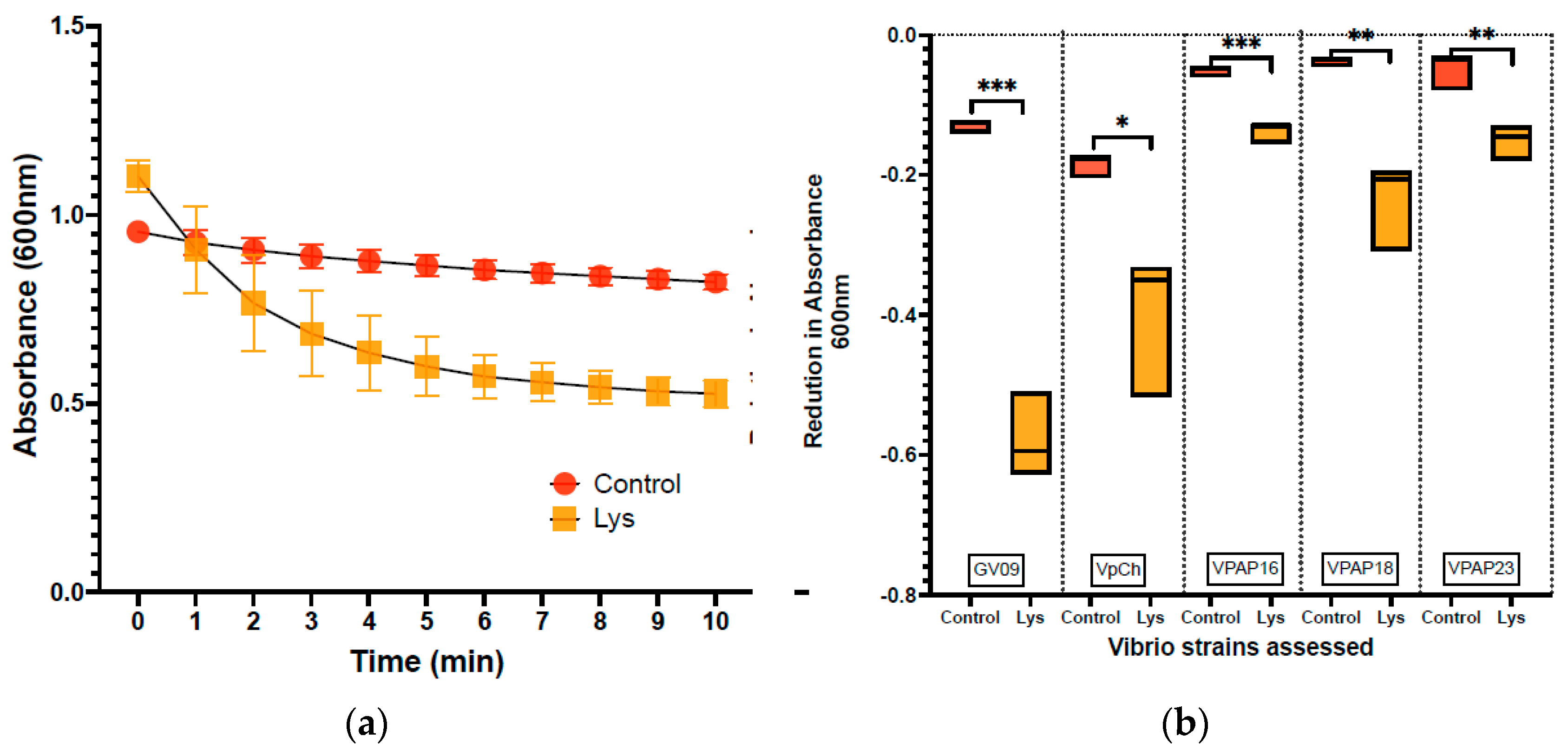
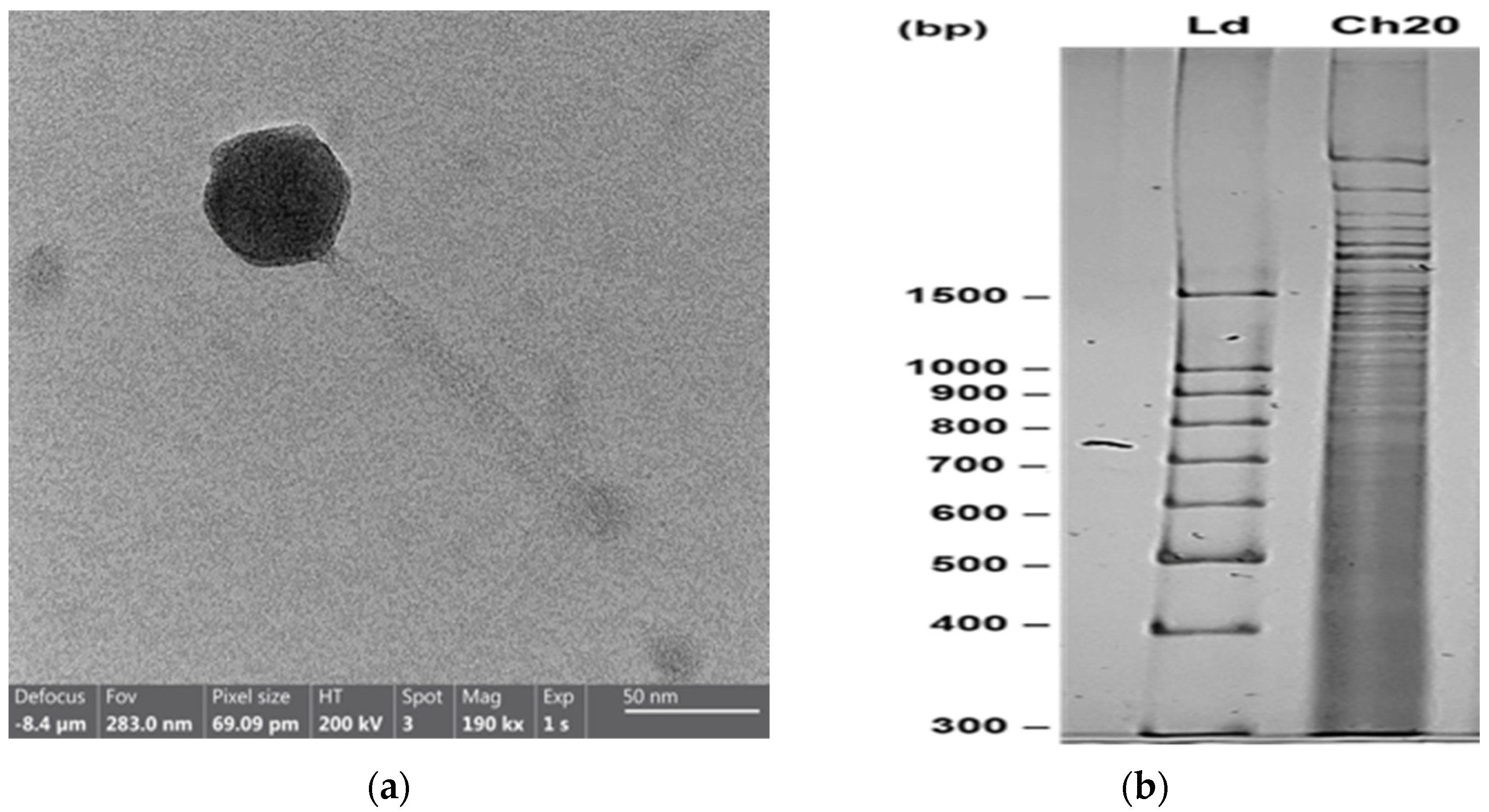
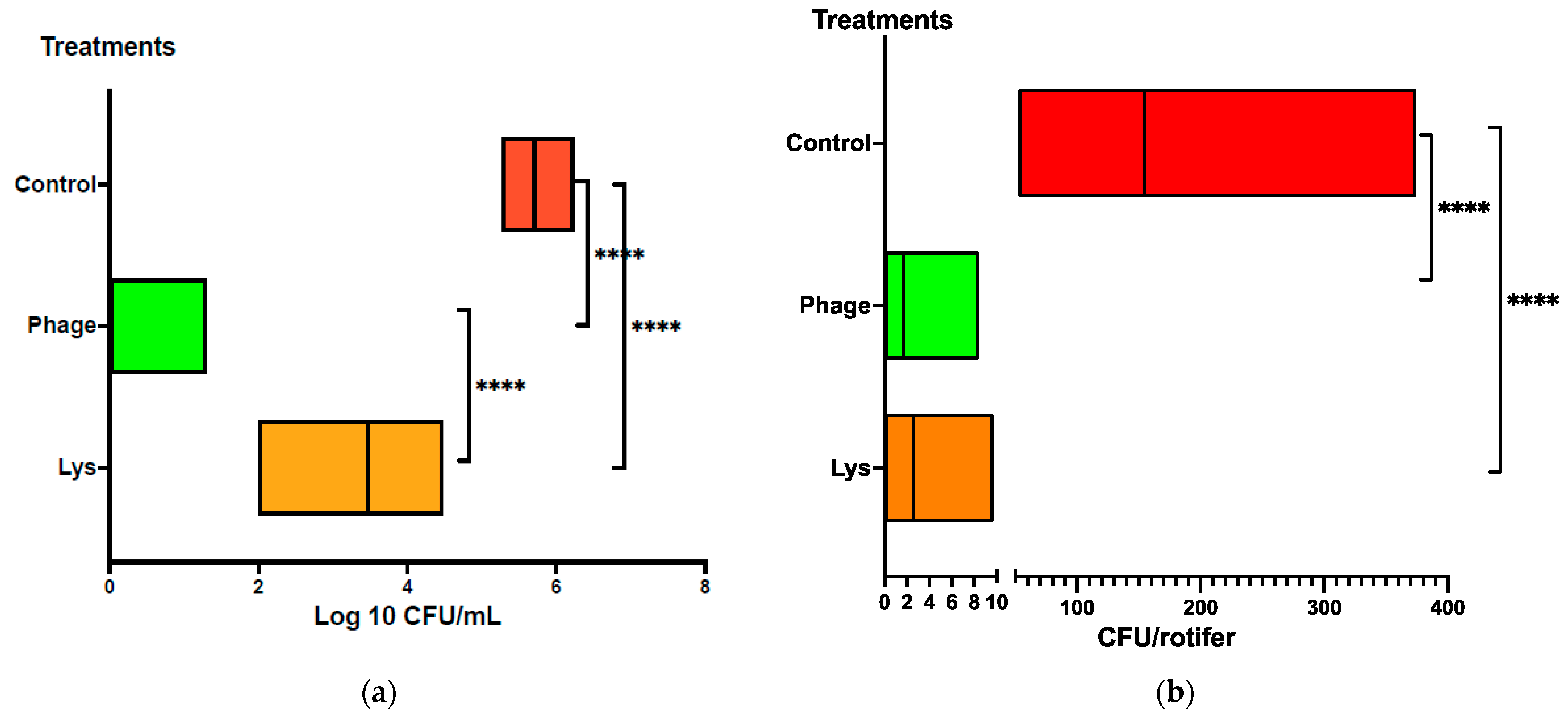
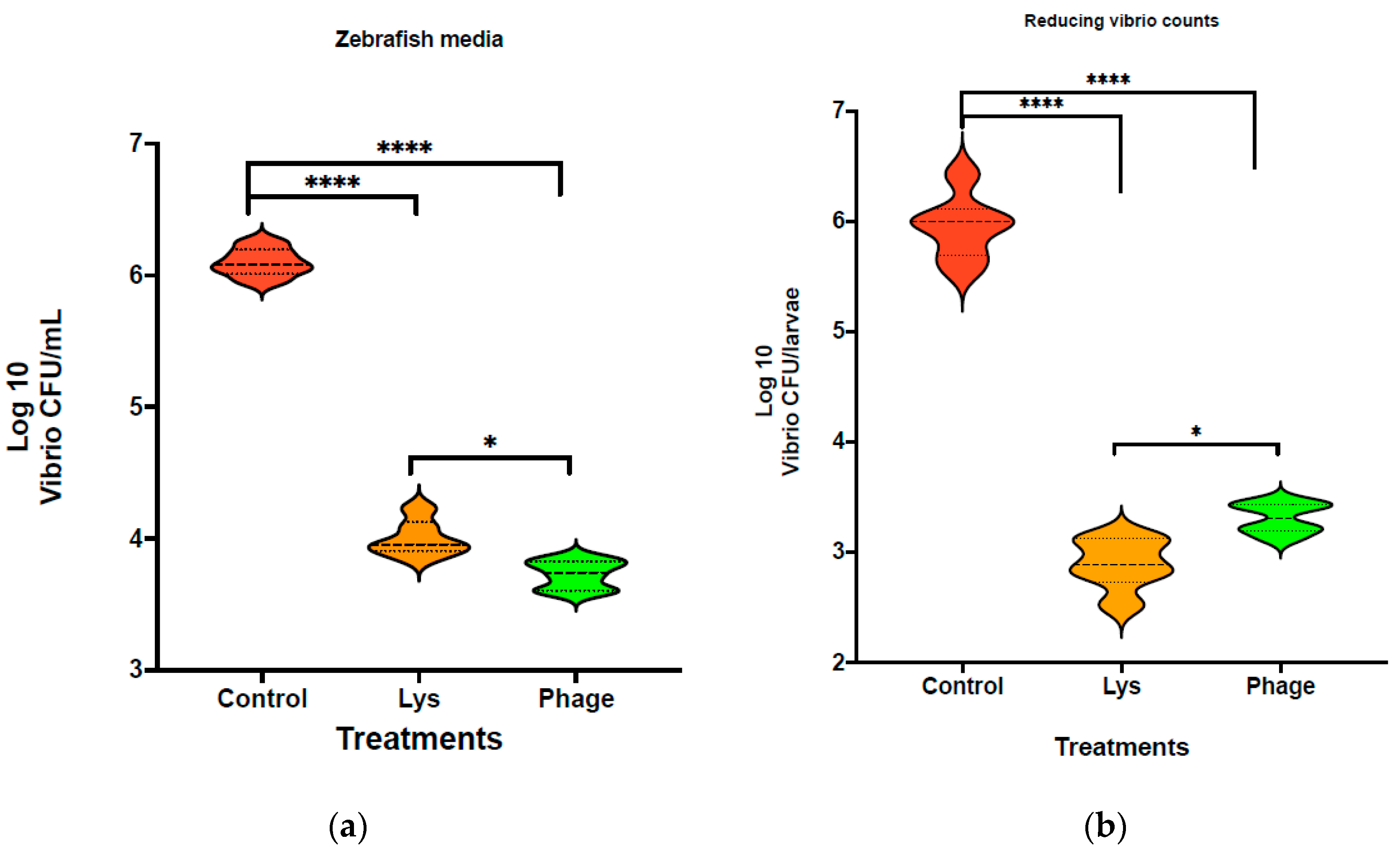
| Vibrio Strain | LysVPp1 Lysis | CH20 Lysis |
|---|---|---|
| V. alginolyticus GV09 | + | + |
| V. parahaemolyticus PMC 57.5 | + | - |
| V. splendidus VPAP16 | + | - |
| V. splendidus VPAP18 | + | - |
| V. splendidus VPAP23 | + | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, J.; Blas-Chumacero, S.; Urzúa, V.; Villasante, A.; Opazo, R.; Gajardo, F.; Miranda, C.D.; Rojas, R. Lysin and Lytic Phages Reduce Vibrio Counts in Live Feed and Fish Larvae. Microorganisms 2024, 12, 904. https://doi.org/10.3390/microorganisms12050904
Romero J, Blas-Chumacero S, Urzúa V, Villasante A, Opazo R, Gajardo F, Miranda CD, Rojas R. Lysin and Lytic Phages Reduce Vibrio Counts in Live Feed and Fish Larvae. Microorganisms. 2024; 12(5):904. https://doi.org/10.3390/microorganisms12050904
Chicago/Turabian StyleRomero, Jaime, Sergueia Blas-Chumacero, Victoria Urzúa, Alejandro Villasante, Rafael Opazo, Felipe Gajardo, Claudio D. Miranda, and Rodrigo Rojas. 2024. "Lysin and Lytic Phages Reduce Vibrio Counts in Live Feed and Fish Larvae" Microorganisms 12, no. 5: 904. https://doi.org/10.3390/microorganisms12050904
APA StyleRomero, J., Blas-Chumacero, S., Urzúa, V., Villasante, A., Opazo, R., Gajardo, F., Miranda, C. D., & Rojas, R. (2024). Lysin and Lytic Phages Reduce Vibrio Counts in Live Feed and Fish Larvae. Microorganisms, 12(5), 904. https://doi.org/10.3390/microorganisms12050904








