Metallo-Glycodendrimeric Materials against Enterotoxigenic Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
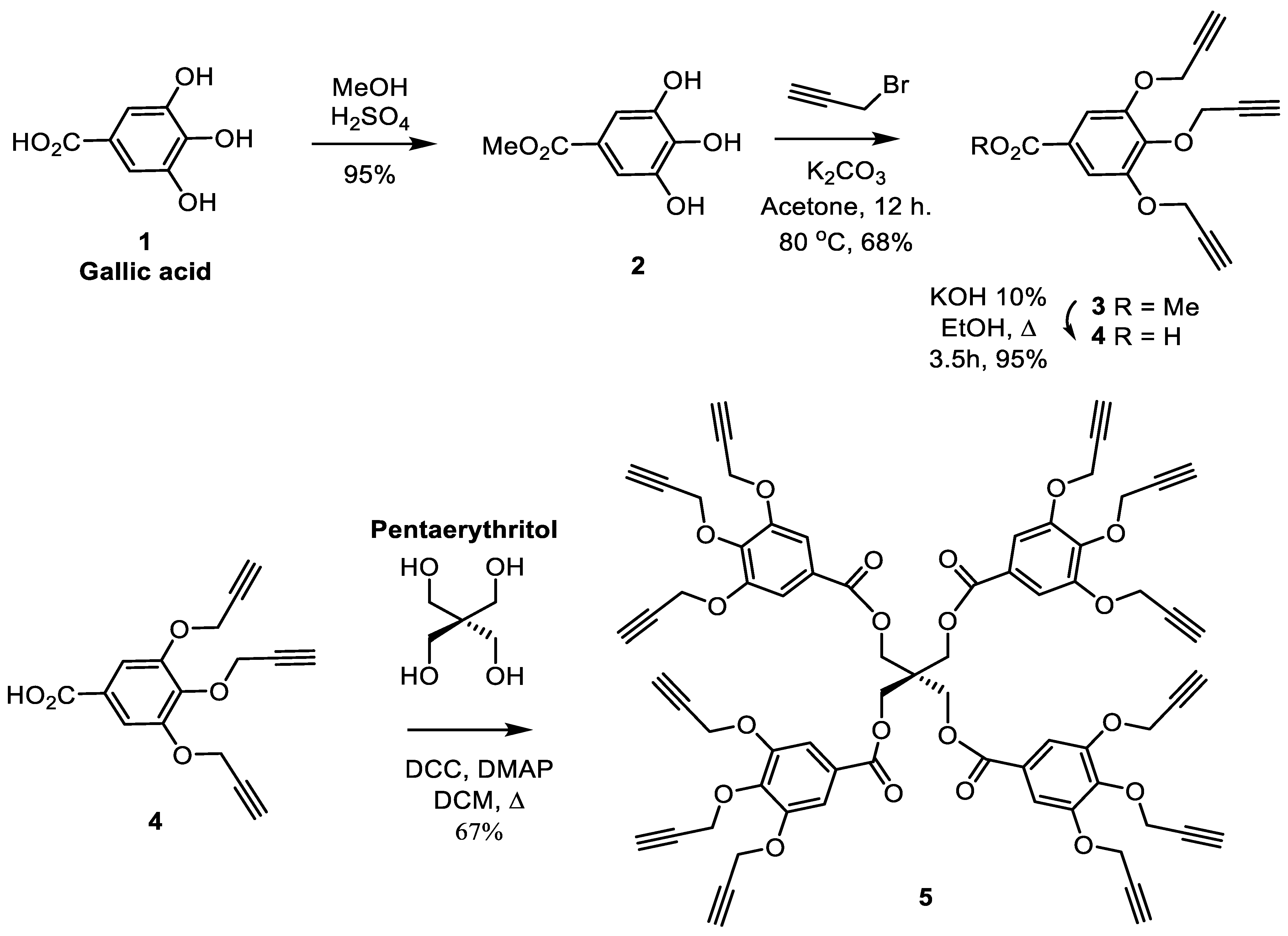
3.1. Synthesis of the Core Structure
3.2. Synthesis of the Carbohydrate for Core Branching
3.3. Synthesis of Mannosylated Glycodendrimer
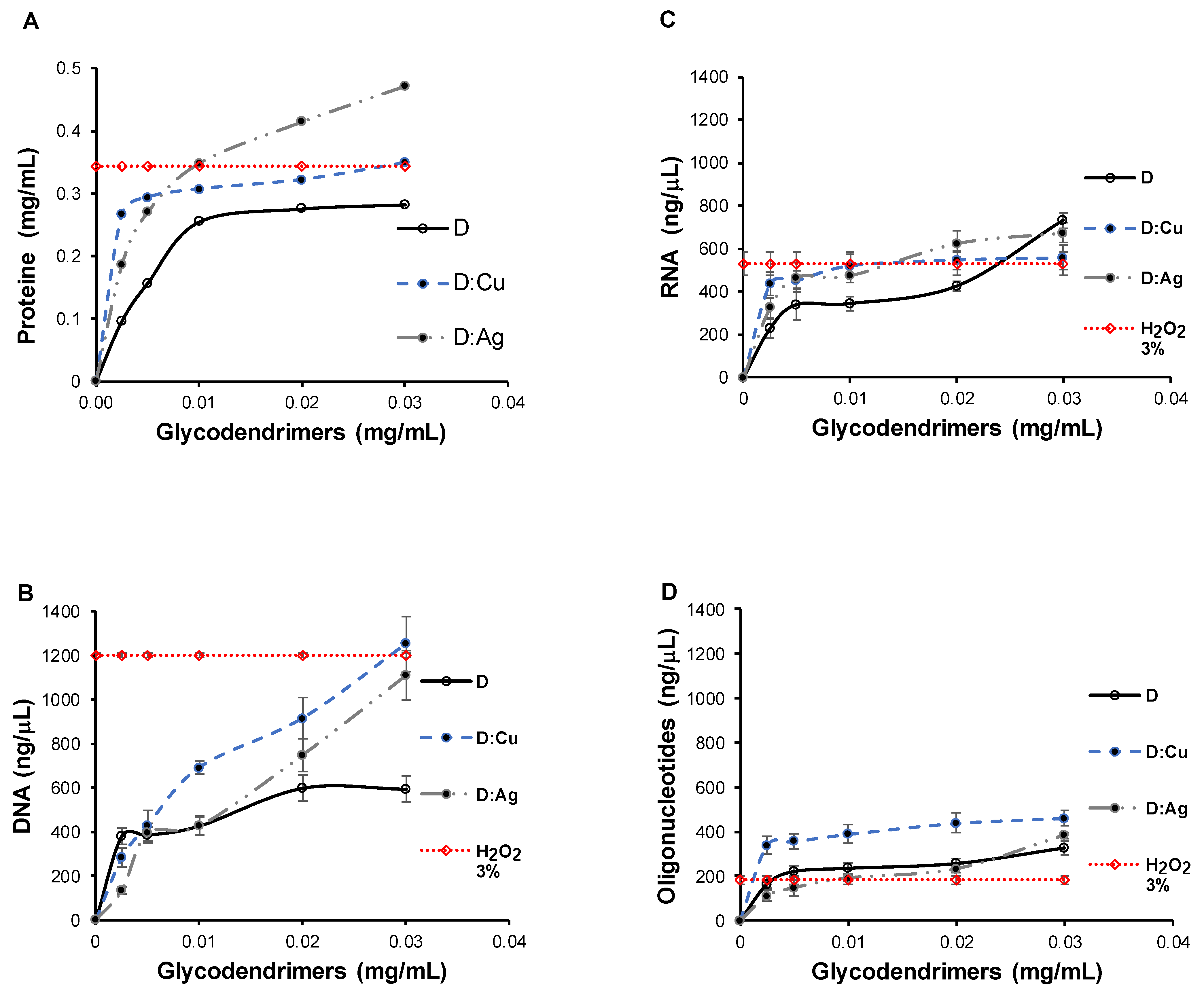
3.4. Characterization of Nanoparticles of Cu- and Ag-Loaded Glycodendrimers
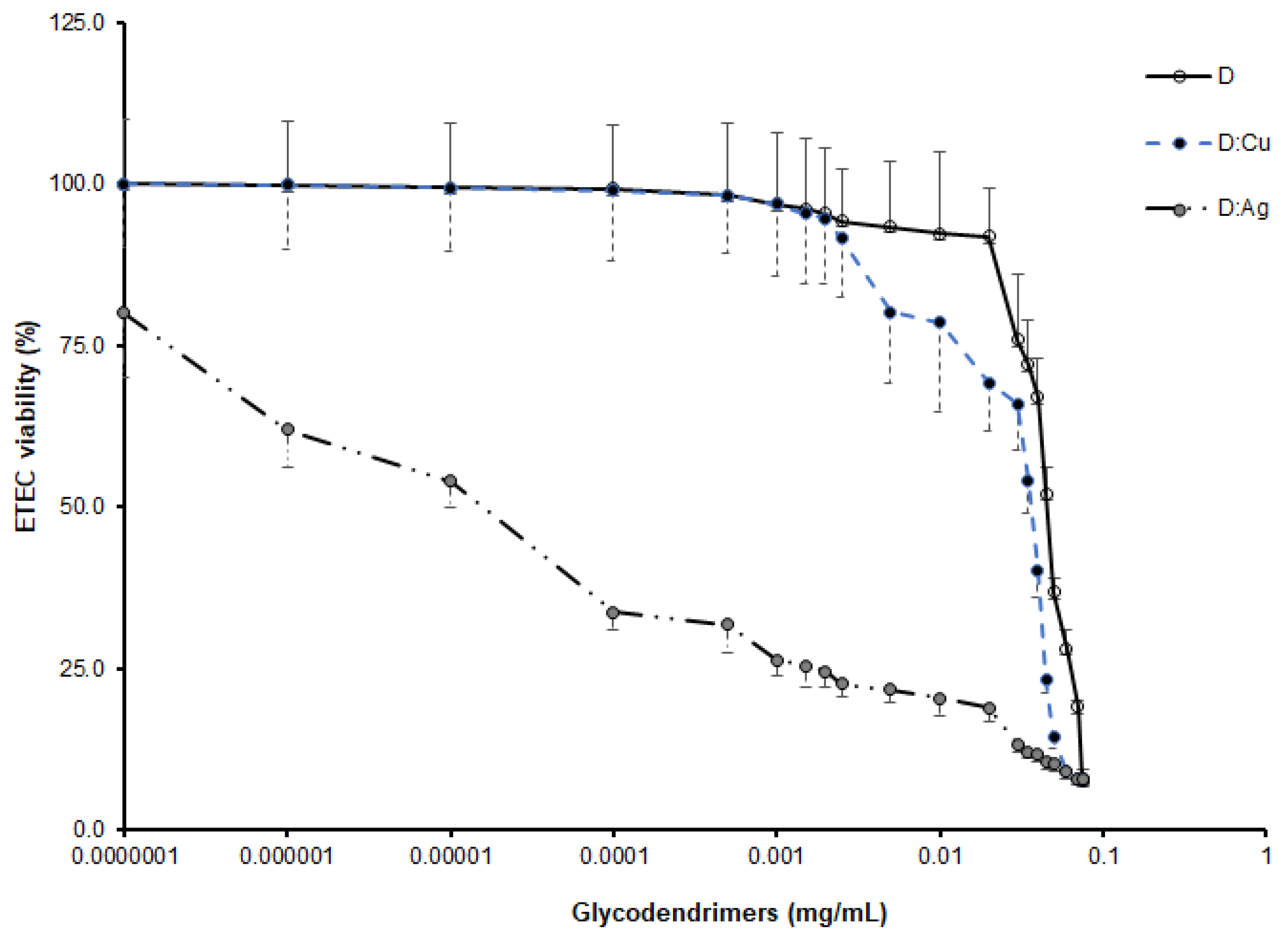
3.5. Bactericidal Activity of Metal-Glycodendrimers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| BSA | Bovine Serum Albumin |
| (CD3)2SO | Deuterated dimethyl sulfoxide |
| CDCl3 | Deuterated chloroform |
| 13C-NMR | Carbon-13 Nuclear Magnetic Resonance |
| COCH3 | Acetyl group |
| CRh6G | Calcozine Red 6G |
| CuAAC | Copper(I) azide–alkyne cycloaddition reaction |
| Cu(OAc)2 | Copper(II) acetate |
| D | Glycodendrimers |
| d | Doublet |
| dd | Doublet of doublet |
| D:Ag | Glycodendrimers loaded with silver |
| D:Cu | Glycodendrimers loaded with copper |
| DCC | N, N′-dicyclohexylcarbodiimide |
| DCM | Dichloromethane |
| DLS | Dynamic Light Scattering |
| DMAP | (Dimethylamino)pyridine |
| DMF | N, N-dimethylformamide |
| DMSO-d6 | Deuterated dimethyl sulfoxide |
| 2D NMR | Two Dimension Nuclear Magnetic Resonance |
| EtOAc | Ethyl acetate |
| EDA | Ethylene diamine |
| EDTA | Ethylenediaminetetraacetic acid |
| ETEC:F4 | Enterotoxigenic Escherichia coli fimbriae 4 |
| IC50 | Half Inhibitory Concentration |
| J | Coupling constants |
| K2CO3, | Potassium carbonate |
| MA | Methyl acrylate |
| MeOD | Deuterated methanol |
| MIC | Minimum Inhibitory Concentration |
| Na2SO4 | Sodium sulfate |
| NPs | Nanoparticles |
| ppm | Parts per million |
| ROS | Reactive Oxygen Species |
| THF | Tetrahydrofuran |
| TLC | Thin-Layer Chromatography |
References
- Fairbrother, J.M.; Nadeau, É.; Gyles, C.L. Escherichia coli in Postweaning Diarrhea in Pigs: An Update on Bacterial Types, Pathogenesis, and Prevention Strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef]
- Eriksen, E.Ø.; Kudirkiene, E.; Barington, K.; Goecke, N.B.; Blirup-Plum, S.A.; Nielsen, J.P.; Olsen, J.E.; Jensen, H.E.; Pankoke, K.; Larsen, L.E. An Observational Field Study of Porcine Post-Weaning Diarrhea: Clinical and Microbiological Findings, and Fecal pH-Measurements as a Potential Diagnostic Tool. Porcine Health Manag. 2023, 9, 33–51. [Google Scholar] [CrossRef]
- Castro, J.; Barros, M.M.; Araújo, D.; Campos, A.M.; Oliveira, R.; Silva, S.; Almeida, C. Swine Enteric Colibacillosis: Current Treatment Avenues and Future Directions. Front. Vet. Sci. 2022, 9, 981207. [Google Scholar] [CrossRef]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post Weaning Diarrhea in Pigs: Risk Factors and Non-Colistin-Based Control Strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar]
- Ercumen, A.; Pickering, A.J.; Kwong, L.H.; Arnold, B.F.; Parvez, S.M.; Alam, M.; Sen, D.; Islam, S.; Kullmann, C.; Chase, C. Animal Feces Contribute to Domestic Fecal Contamination. Environ. Sci. Technol. 2017, 51, 8725–8734. [Google Scholar] [CrossRef]
- Penakalapati, G.; Swarthout, J.; Delahoy, M.J.; McAliley, L.; Wodnik, B.; Levy, K.; Freeman, M.C. Exposure to Animal Feces and Human Health: A Systematic Review and Proposed Research Priorities. Environ. Sci. Technol. 2017, 51, 11537–11552. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.D. Transport of Copper. In Proceedings of the Trace Elements in Clinical Medicine: Proceedings of the Second Meeting of the International Society for Trace Element Research in Humans (ISTERH), Tokyo, Japan, 28 August–1 September 1989; Springer: Berlin/Heidelberg, Germany, 1990; pp. 421–424. [Google Scholar]
- Ferreira, V.; Magalhães, R.; Teixeira, P.; Castro, P.M.L.; Calheiros, C.S.C. Occurrence of Fecal Bacteria and Zoonotic Pathogens in Different Water Bodies: Supporting Water Quality Management. Water 2022, 14, 780. [Google Scholar] [CrossRef]
- Sentamu, D.N.; Kungu, J.; Dione, M.; Thomas, L.F. Prevention of Human Exposure to Livestock Faecal Waste in the Household: A Scoping Study of Interventions Conducted in Sub-Saharan Africa. BMC Public Health 2023, 23, 1613–1624. [Google Scholar] [CrossRef]
- Calderon Toledo, C.; von Mentzer, A.; Agramont, J.; Thorell, K.; Zhou, Y.; Szabó, M.; Colque, P.; Kuhn, I.; Gutiérrez-Cortez, S.; Joffré, E. Circulation of Enterotoxigenic Escherichia coli (ETEC) Isolates Expressing CS23 from the Environment to Clinical Settings. Msystems 2023, 8, e00141-23. [Google Scholar] [CrossRef]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia Coli. EcoSal Plus 2016, 7, ESP-0006-2016. [Google Scholar] [CrossRef]
- Kim, K.; Song, M.; Liu, Y.; Ji, P. Enterotoxigenic Escherichia coli Infection of Weaned Pigs: Intestinal Challenges and Nutritional Intervention to Enhance Disease Resistance. Front. Immunol. 2022, 13, 885253. [Google Scholar] [CrossRef] [PubMed]
- Hrala, M.; Bosák, J.; Micenková, L.; Křenová, J.; Lexa, M.; Pirková, V.; Tomáštíková, Z.; Koláčková, I.; Šmajs, D. Escherichia coli Strains Producing Selected Bacteriocins Inhibit Porcine Enterotoxigenic Escherichia coli (ETEC) under Both in Vitro and in Vivo Conditions. Appl. Environ. Microbiol. 2021, 87, e03121-20. [Google Scholar] [CrossRef]
- Navez, M.; Antoine, C.; Laforêt, F.; Goya-Jorge, E.; Douny, C.; Scippo, M.-L.; Vermeersch, M.; Duprez, J.-N.; Daube, G.; Mainil, J. In Vitro Effect on Piglet Gut Microbiota and In Vivo Assessment of Newly Isolated Bacteriophages against F18 Enterotoxigenic Escherichia coli (ETEC). Viruses 2023, 15, 1053. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, J.M.; Nadeau, É. Chapter 52: Colibacillosis. In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 807–834. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Pig Vaccination Strategies Based on Enterotoxigenic Escherichia coli Toxins. Braz. J. Microbiol. 2021, 52, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Matías, J.; Berzosa, M.; Pastor, Y.; Irache, J.M.; Gamazo, C. Maternal Vaccination. Immunization of Sows during Pregnancy against ETEC Infections. Vaccines 2017, 5, 48. [Google Scholar] [CrossRef]
- Matías, J.; Pastor, Y.; Irache, J.M.; Gamazo, C. Protective Passive Immunity in Escherichia coli ETEC-Challenged Neonatal Mice Conferred by Orally Immunized Dams with Nanoparticles Containing Homologous Outer Membrane Vesicles. Vaccines 2020, 8, 286. [Google Scholar] [CrossRef]
- Melkebeek, V.; Goddeeris, B.M.; Cox, E. ETEC Vaccination in Pigs. Vet. Immunol. Immunopathol. 2013, 152, 37–42. [Google Scholar] [CrossRef]
- Rhouma, M.; Beaudry, F.; Thériault, W.; Letellier, A. Colistin in Pig Production: Chemistry, Mechanism of Antibacterial Action, Microbial Resistance Emergence, and One Health Perspectives. Front. Microbiol. 2016, 7, 1789–1811. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Antibacterial and Antidiarrheal Activities of Plant Products against Enterotoxinogenic Escherichia coli. Toxins 2013, 5, 2009–2041. [Google Scholar] [CrossRef]
- Bin, P.; Tang, Z.; Liu, S.; Chen, S.; Xia, Y.; Liu, J.; Wu, H.; Zhu, G. Intestinal Microbiota Mediates Enterotoxigenic Escherichia coli-Induced Diarrhea in Piglets. BMC Vet. Res. 2018, 14, 385. [Google Scholar] [CrossRef]
- Laird, T.J.; Abraham, S.; Jordan, D.; Pluske, J.R.; Hampson, D.J.; Trott, D.J.; O’Dea, M. Porcine Enterotoxigenic Escherichia coli: Antimicrobial Resistance and Development of Microbial-Based Alternative Control Strategies. Vet. Microbiol. 2021, 258, 109117. [Google Scholar] [CrossRef] [PubMed]
- Chabre, Y.M.; Roy, R. Design and Creativity in Synthesis of Multivalent Neoglycoconjugates. Adv. Carbohydr. Chem. Biochem. 2010, 63, 165–393. [Google Scholar] [PubMed]
- Fernandes, G.; Pandey, A.; Kulkarni, S.; Mutalik, S.P.; Nikam, A.N.; Seetharam, R.N.; Kulkarni, S.S.; Mutalik, S. Supramolecular Dendrimers Based Novel Platforms for Effective Oral Delivery of Therapeutic Moieties. J. Drug Deliv. Sci. Technol. 2021, 64, 102647. [Google Scholar] [CrossRef]
- Kiessling, L.L.; Gestwicki, J.E.; Strong, L.E. Synthetic Multivalent Ligands as Probes of Signal Transduction. Angew. Chem. Int. Ed. 2006, 45, 2348–2368. [Google Scholar] [CrossRef]
- Pieters, R.J. Intervention with Bacterial Adhesion by Multivalent Carbohydrates. Med. Res. Rev. 2007, 27, 796–816. [Google Scholar] [CrossRef]
- Sleiman, M.; Varrot, A.; Raimundo, J.-M.; Gingras, M.; Goekjian, P.G. Glycosylated Asterisks Are among the Most Potent Low Valency Inducers of Concanavalin A Aggregation. Chem. Comm. 2008, 48, 6507–6509. [Google Scholar] [CrossRef]
- Mousavifar, L.; Touaibia, M.; Roy, R. Development of Mannopyranoside Therapeutics against Adherent-Invasive Escherichia coli Infections. Acc. Chem. Res. 2018, 51, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Appeldoorn, C.C.; Joosten, J.A.; el Maate, F.A.; Dobrindt, U.; Hacker, J.; Liskamp, R.M.; Khan, A.S.; Pieters, R.J. Novel Multivalent Mannose Compounds and Their Inhibition of the Adhesion of Type 1 Fimbriated Uropathogenic E. coli. Tetrahedron Asymmetry 2005, 16, 361–372. [Google Scholar] [CrossRef]
- Roy, R.; Touaibia, M. Application of Multivalent Mannosylated Dendrimers in Glycobiology. Compr. Glycosci. 2007, 3, 821–870. [Google Scholar]
- Touaibia, M.; Wellens, A.; Shiao, T.C.; Wang, Q.; Sirois, S.; Bouckaert, J.; Roy, R. Mannosylated G (0) Dendrimers with Nanomolar Affinities to Escherichia coli FimH. ChemMedChem: Chem. Enabling Drug Discov. 2007, 2, 1190–1201. [Google Scholar] [CrossRef]
- Touaibia, M.; Roy, R. Glycodendrimers as Anti-Adhesion Drugs against Type 1 Fimbriated E. coli Uropathogenic Infections. Mini-Rev. Med. Chem. 2007, 7, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sayed, P.; Kaeppeli, A.; Siriwardena, T.; Darbre, T.; Perron, K.; Jafari, P.; Reymond, J.-L.; Pioletti, D.P.; Applegate, L.A. Anti-Microbial Dendrimers against Multidrug-Resistant P. aeruginosa Enhance the Angiogenic Effect of Biological Burn-Wound Bandages. Sci. Rep. 2016, 6, 22020. [Google Scholar] [CrossRef] [PubMed]
- Noori, F.; Neree, A.T.; Megoura, M.; Mateescu, M.A.; Azzouz, A. Insights into the Metal Retention Role in the Antibacterial Behavior of Montmorillonite and Cellulose Tissue-Supported Copper and Silver Nanoparticles. RSC Adv. 2021, 11, 24156–24171. [Google Scholar] [CrossRef] [PubMed]
- Noori, F.; Megoura, M.; Labelle, M.-A.; Mateescu, M.A.; Azzouz, A. Synthesis of Metal-Loaded Carboxylated Biopolymers with Antibacterial Activity through Metal Subnanoparticle Incorporation. Antibiotics 2022, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM Dendrimer-Cell Membrane Interactions. Adv. Colloid Interface Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E.A. The Effect of PAMAM Dendrimers on the Antibacterial Activity of Antibiotics with Different Water Solubility. Molecules 2013, 18, 8607–8617. [Google Scholar] [CrossRef] [PubMed]
- Căta, A.; Ienașcu, I.M.C.; Ştefănuț, M.N.; Roșu, D.; Pop, O.-R. Properties and Bioapplications of Amphiphilic Janus Dendrimers: A Review. Pharmaceutics 2023, 15, 589. [Google Scholar] [CrossRef]
- Shiabiev, I.; Pysin, D.; Akhmedov, A.; Babaeva, O.; Babaev, V.; Lyubina, A.; Voloshina, A.; Petrov, K.; Padnya, P.; Stoikov, I. Towards Antibacterial Agents: Synthesis and Biological Activity of Multivalent Amide Derivatives of Thiacalix [4] Arene with Hydroxyl and Amine Groups. Pharmaceutics 2023, 15, 2731. [Google Scholar] [CrossRef] [PubMed]
- Pricl, S. The Spicy Science of Dendrimers in the Realm of Cancer Nanomedicine: A Report from the COST Action CA17140 Nano2Clinic. Pharmaceutics 2023, 15, 2013. [Google Scholar] [CrossRef]
- De la Mata, F.J.; Gómez, R.; Cano, J.; Sánchez-Nieves, J.; Ortega, P.; Gallego, S.G. Carbosilane Dendritic Nanostructures, Highly Versatile Platforms for Pharmaceutical Applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1871. [Google Scholar] [CrossRef]
- Dhumal, D.; Maron, B.; Malach, E.; Lyu, Z.; Ding, L.; Marson, D.; Laurini, E.; Tintaru, A.; Ralahy, B.; Giorgio, S. Dynamic Self-Assembling Supramolecular Dendrimer Nanosystems as Potent Antibacterial Candidates against Drug-Resistant Bacteria and Biofilms. Nanoscale 2022, 14, 9286–9296. [Google Scholar] [CrossRef]
- Viswanath, V.; Santhakumar, K. Perspectives on Dendritic Architectures and Their Biological Applications: From Core to Cell. J. Photochem. Photobiol. B Biol. 2017, 173, 61–83. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, D.; Liu, X.; Peng, L. Amphiphilic Dendrimer Vectors for RNA Delivery: State-of-the-Art and Future Perspective. Acc. Mater. Res. 2022, 3, 484–497. [Google Scholar] [CrossRef]
- Edr, A.; Wrobel, D.; Krupková, A.; Šťastná, L.Č.; Cuřínová, P.; Novák, A.; Malý, J.; Kalasová, J.; Malý, J.; Malý, M. Adaptive Synthesis of Functional Amphiphilic Dendrons as a Novel Approach to Artificial Supramolecular Objects. Int. J. Mol. Sci. 2022, 23, 2114. [Google Scholar] [CrossRef]
- Kaur, M.; Khatkar, S.; Singh, B.; Kumar, A.; Dubey, S.K. Recent Advancements in Sensing of Silver Ions by Different Host Molecules: An Overview (2018–2023). J. Fluoresc. 2023, 10, 1–23. [Google Scholar] [CrossRef]
- Pardeshi, S.; Gholap, A.; More, M.; Togre, N.; Rebello, N.; Giram, P. Chapter 5: Dendrimers Based Antibacterial and Antiviral Materials. In Antibacterial and Antiviral Functional Materials; Deshmukh, K., Hussain, C.M., Eds.; ACS Symposium Series; eBook: Plzeň, Czech Republic; Newark, NJ, USA, 2023; pp. 139–169. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y. Controlled Release from Cleavable Polymerized Liposomes upon Redox and pH Stimulation. Bioconjugate Chem. 2011, 22, 523–528. [Google Scholar] [CrossRef]
- Van Ameijde, J.; Liskamp, R.M. Synthesis of Novel Trivalent Amino Acid Glycoconjugates Based on the Cyclotriveratrylene (‘CTV’) Scaffold. Org. Biomol. Chem. 2003, 1, 2661–2669. [Google Scholar] [CrossRef]
- Percec, V.; Leowanawat, P.; Sun, H.-J.; Kulikov, O.; Nusbaum, C.D.; Tran, T.M.; Bertin, A.; Wilson, D.A.; Peterca, M.; Zhang, S. Modular Synthesis of Amphiphilic Janus Glycodendrimers and Their Self-Assembly into Glycodendrimersomes and Other Complex Architectures with Bioactivity to Biomedically Relevant Lectins. J. Am. Chem. Soc. 2013, 135, 9055–9077. [Google Scholar] [CrossRef]
- Li, J.; Zacharek, S.; Chen, X.; Wang, J.; Zhang, W.; Janczuk, A.; Wang, P.G. Bacteria Targeted by Human Natural Antibodies Using α-Gal Conjugated Receptor-Specific Glycopolymers. Bioorg. Med. Chem. 1999, 7, 1549–1558. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Dane, E.L.; O’Toole, G.A.; Grinstaff, M.W. Exploiting Dendrimer Multivalency to Combat Emerging and Re-Emerging Infectious Diseases. Mol. Pharm. 2012, 9, 342–354. [Google Scholar] [CrossRef]
- Li, K.; Guan, G.; Zhu, J.; Wu, H.; Sun, Q. Antibacterial Activity and Mechanism of a Laccase-Catalyzed Chitosan–Gallic Acid Derivative against Escherichia coli and Staphylococcus aureus. Food Control 2019, 96, 234–243. [Google Scholar] [CrossRef]
- Sharma, R.; Naresh, K.; Chabre, Y.M.; Rej, R.; Saadeh, N.K.; Roy, R. “Onion Peel” Dendrimers: A Straightforward Synthetic Approach towards Highly Diversified Architectures. Polym. Chem. 2014, 5, 4321–4331. [Google Scholar] [CrossRef]
- Sharma, R.; Porterfield, J.E.; An, H.-T.; Jimenez, A.S.; Lee, S.; Kannan, S.; Sharma, A.; Kannan, R.M. Rationally Designed Galactose Dendrimer for Hepatocyte-Specific Targeting and Intracellular Drug Delivery for the Treatment of Liver Disorders. Biomacromolecules 2021, 22, 3574–3589. [Google Scholar] [CrossRef]
- Neises, B.; Steglich, W. Simple Method for the Esterification of Carboxylic Acids. Angew. Chem. Int. Ed. Engl. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. 2002, 114, 2708–2711. [Google Scholar] [CrossRef]
- Uhl, L.; Dukan, S. Hydrogen Peroxide Induced Cell Death: The Major Defences Relative Roles and Consequences in E. coli. PLoS ONE 2016, 11, e0159706. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization of Prion-Contaminated Medical Instruments. Infect. Control Hosp. Epidemiol. 2010, 31, 107–117. [Google Scholar] [CrossRef]
- Mahaseth, T.; Kuzminov, A. Potentiation of Hydrogen Peroxide Toxicity: From Catalase Inhibition to Stable DNA-Iron Complexes. Mutat. Res. Rev. Mutat. Res. 2017, 773, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.J.; Brandi, R.J.; Cassano, A.E.; Labas, M.D. Chemical Disinfection with H2O2− The Proposal of a Reaction Kinetic Model. J. Chem. Eng. 2012, 198, 388–396. [Google Scholar] [CrossRef]
- Uhl, L.; Gerstel, A.; Chabalier, M.; Dukan, S. Hydrogen Peroxide Induced Cell Death: One or Two Modes of Action? Heliyon 2015, 1, e00049. [Google Scholar] [CrossRef]
- Sneideris, T.; Erkamp, N.A.; Ausserwöger, H.; Saar, K.L.; Welsh, T.J.; Qian, D.; Katsuya-Gaviria, K.; Johncock, M.L.; Krainer, G.; Borodavka, A. Targeting Nucleic Acid Phase Transitions as a Mechanism of Action for Antimicrobial Peptides. Nat. Commun. 2023, 14, 7170–7186. [Google Scholar] [CrossRef]
- Zając, M.; Kotyńska, J.; Zambrowski, G.; Breczko, J.; Deptuła, P.; Cieśluk, M.; Zambrzycka, M.; Święcicka, I.; Bucki, R.; Naumowicz, M. Exposure to Polystyrene Nanoparticles Leads to Changes in the Zeta Potential of Bacterial Cells. Sci. Rep. 2023, 13, 9552. [Google Scholar] [CrossRef]
- Ficerman, W.; Wiśniewski, M.; Roszek, K. Interactions of Nanomaterials with Cell Signalling Systems–Focus on Purines-Mediated Pathways. Colloids Surf. B Biointerfaces 2022, 220, 112919. [Google Scholar] [CrossRef]
- Villanueva-Flores, F.; Castro-Lugo, A.; Ramírez, O.T.; Palomares, L.A. Understanding Cellular Interactions with Nanomaterials: Towards a Rational Design of Medical Nanodevices. J. Nanotechnol. 2020, 31, 132002. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Riz, A.; Tchoumi Neree, A.; Mousavifar, L.; Roy, R.; Chorfi, Y.; Mateescu, M.A. Metallo-Glycodendrimeric Materials against Enterotoxigenic Escherichia coli. Microorganisms 2024, 12, 966. https://doi.org/10.3390/microorganisms12050966
El Riz A, Tchoumi Neree A, Mousavifar L, Roy R, Chorfi Y, Mateescu MA. Metallo-Glycodendrimeric Materials against Enterotoxigenic Escherichia coli. Microorganisms. 2024; 12(5):966. https://doi.org/10.3390/microorganisms12050966
Chicago/Turabian StyleEl Riz, Aly, Armelle Tchoumi Neree, Leila Mousavifar, René Roy, Younes Chorfi, and Mircea Alexandru Mateescu. 2024. "Metallo-Glycodendrimeric Materials against Enterotoxigenic Escherichia coli" Microorganisms 12, no. 5: 966. https://doi.org/10.3390/microorganisms12050966








