Abstract
Protected areas are widely considered an essential strategy for biodiversity conservation. Dictyostelids are unique protists known to have important ecological functions in promoting soil and plant health through their top-down regulation of ecosystem processes, such as decomposition, that involve bacterial populations. But the relationship between dictyostelid diversity within protected areas remains poorly understood, especially on a large scale. Herein, we report data on the distribution of dictyostelids, identified with ITS + SSU rRNA molecular and morphology-based taxonomy, from soil samples collected in the Fanjing Mountain protected area of Guizhou Province, Southwest China. We compared the biodiversity data of dictyostelids in Fanjing Mountain with similar data from previously sampled sites in four other protected areas, including Changbai Mountain (CB), Gushan Mountain (GS), Baiyun Mountain (BY), and Qinghai–Tibet Plateau (QT) in China. We identified four species of dictyostelids belonging to three genera (Dictyostelium, Heterostelium, and Polysphondylium) and herein provide information on the taxonomy of these species. Two species (Heterostelium pallidum and Dictyostelium purpureum) are common and widely distributed throughout the world, but one species (Polysphondylium fuscans) was new to China. Our data indicate that there is no distinguishable significant correlation between the dictyostelid species studied and environmental factors. Overall, the similarity index between Baiyun Mountain in Henan Province and Fanjing Mountain in Guizhou Province, located at approximately the same longitude, is the highest, and the Jaccard similarity coefficients (Jaccard index) of family, genus, and species are 100%, 100%, and 12.5%, respectively. From a species perspective, species in the same climate zone are not closely related, but obvious geographical distributions are evident in different climate zones. This preliminary study provided evidence of the ecological adaptation of dictyostelids to different biological niches.
1. Introduction
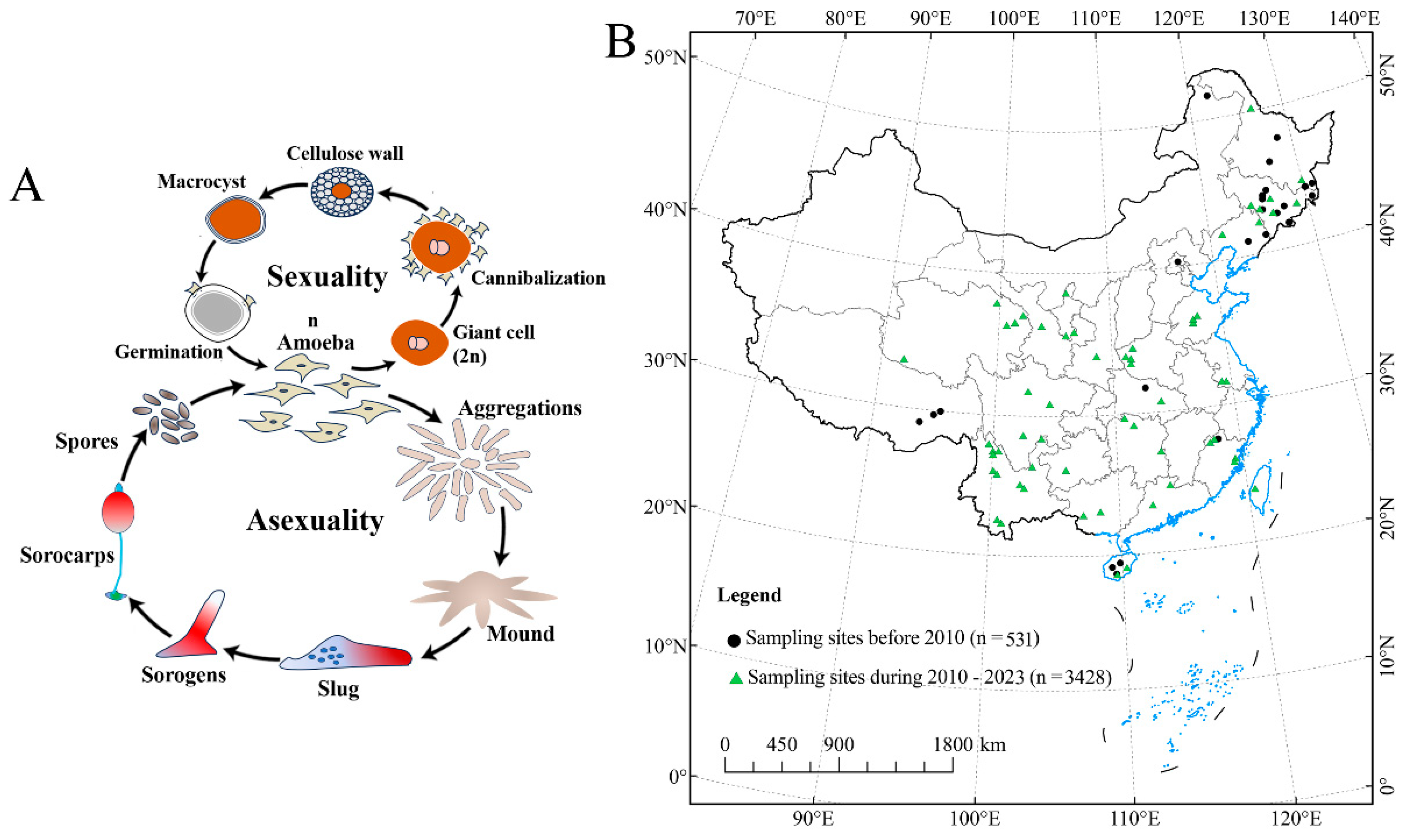
Soil protists are ubiquitous yet remain largely unknown in terrestrial environments, although they have immense morphological and lifestyle diversity [1,2,3]. Dictyostelid social amoebae are highly diverse protists in soil, where they play an important role in bacterial population control and also the turnover of nutrients and minerals in nature [4,5]. They have a unique life cycle that begins with a unicellular (vegetative) stage, but when the food bacteria are consumed, the unicellular forms aggregate to form a multicellular stage in which fruiting bodies (sorocarps) [6,7] are formed (Figure 1A). Therefore, they offer a good system to study the evolution of multicellular complexity, with a well-resolved phylogeny and molecular genetic tools being available, among which Dictyostelium discoideum is now one of the most widely studied eukaryotic microbial model organisms [8,9,10].
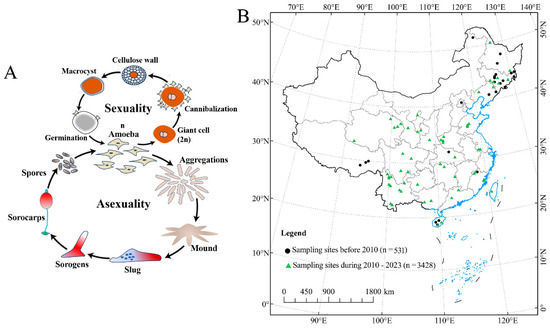
Figure 1.
Changes in the distribution of sampling sites used to assess dictyostelid communities in China and the life cycles of Amoebozoan groups related to the dictyostelids. (A) The dictyostelids show both asexual and sexual cycles. (B) The legend of “sampling sites” is based on a literature analysis (i.e., China National Knowledge Infrastructure, CNKI).
Traditional morphology-based taxonomy of dictyostelids has been replaced by a molecular phylogeny. Their phylogenetic relationships were first reconstructed with molecular data based on SSU rDNA, which established four major groups of dictyostelids, simply named Groups 1, 2, 3, and 4 [11]. But now, the taxonomy of dictyostelids is based on the morphology of their fruiting body and a new classification with strong molecular phylogenetic support [12,13].
Protected areas have been considered a key part of an essential strategy for maintaining habitat integrity and species diversity [14,15], especially in the face of rapid urban expansion that has a profound impact on global biodiversity through habitat conversion, degradation, fragmentation, and species extinction [16,17]. However, the dictyostelids, as permanent inhabitants of the upper layers of soil, occupy a habitat that is suggestive of the primitive ecological environment [18]. Herein, we review the history of Chinese research on dictyostelids in light of the recent wave of research, with an increase in sample collection since 2010 (Figure 1B). There have been 58 species and varieties reported, which makes China one of the world’s biodiversity hotspots [19,20,21,22,23,24]. In fact, most of these species were from nature reserves. Guizhou Province is located in Southwest China, and has a wide range of soil types, vegetation, and climatic conditions along the gradient of elevation [25]. Previous studies of dictyostelid biodiversity have yielded only nine species (Dictyostelium macrocephalum, D. implicatum, D. crassicaul, D. firmibasis, D. purpureum, Heterostelium tenuissimum, H. pallidum, Polysphondylium violaceum, and Cavenderia delicata) from Guizhou Province [26,27].
Herein, using morphology and molecular systematics, we isolated and identified dictyostelids in the soil of the Fanjing Mountain Nature Reserve while also exploring their diversity and the impact of environmental factors on these species. More importantly, we analyzed the distribution characteristics of dictyostelids in current Chinese protected natural areas.
2. Materials and Methods
2.1. Study Area Description
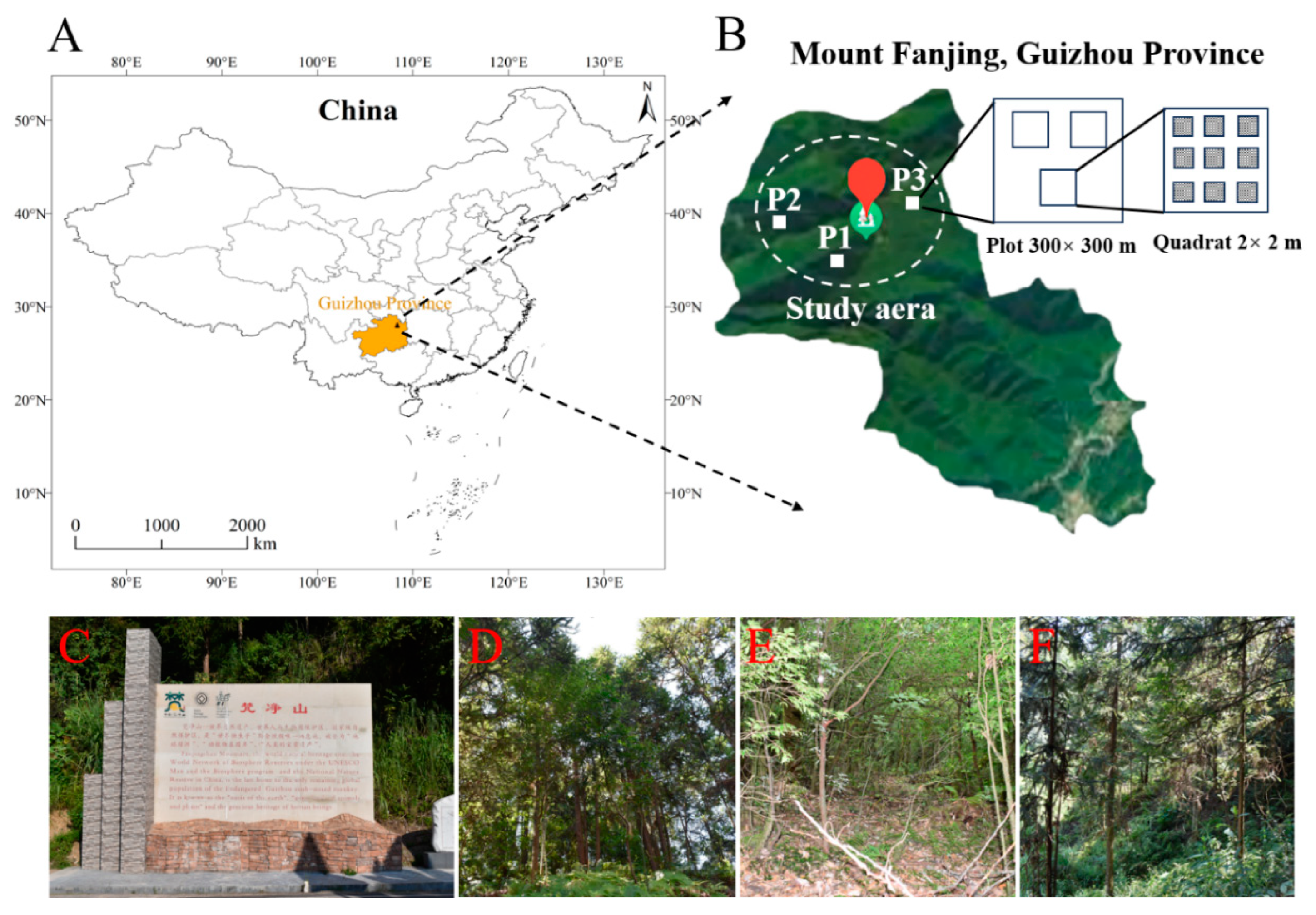
The Fanjing Mountain Nature Reserve (27°49′50″ N to 28°1′30″ N and 108°45′55″ E to 108°48′30″ E) is located in the central portion of the Tongren region in the northeast of Guizhou Province, China, with an elevation of 2572 m and a total area of 567 km2 (Figure 2A). The Fanjing Mountain Nature Reserve is in the subtropical humid monsoon climate zone, with an average temperature of 13.1–14.7 ℃. The frost-free period is 270–278 days, and the average annual precipitation is 1100–2600 mm [28,29].
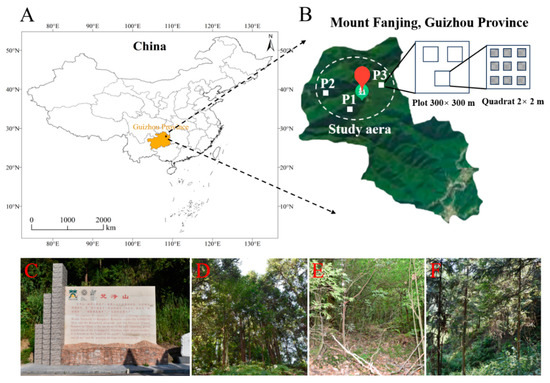
Figure 2.
Location of the Fanjing Mountain Nature Reserve in China. (A) Map of the Fanjing Mountain Nature Reserve in the northeast of Guizhou Province, China. (B) Study area (nine-point sampling method) used in the Fanjing Mountain Nature Reserve. (C) Image of the Fanjing Mountain Nature Reserve. (D) Mixed forest. (E) Broadleaf forest. (F) Coniferous forest.
2.2. Experimental Design and Sampling
This survey was conducted in late August 2022, which falls within the midsummer season that occurs throughout the year. During this period, the topsoil has completely thawed and the vegetation and biomass of the forest are at their peak. The reserve has the largest distribution area of yellow mountain soil and dark yellow-brown soil, which are mainly forest soils and largely undisturbed by human activities.
We set up a single plot for each forest type (coniferous forest = P1, broadleaf forest = P2, mixed forest = P3) in the study areas, and the plot was randomly chosen on a 300 × 300 m grid (Figure 2B) with respect to three representative forest types (Figure 2C–F) that were selected, with three replicates (2 × 2 m quadrats) prepared for each plot. To avoid the effects of distance on the microbial community, the interval between each quadrat was controlled to be less than 100 m, and nine points in each quadrat were randomly selected for sampling.
In each quadrat, we used sterile drills to collect soil after the litter layer above the soil had been removed. Afterwards, the mixed samples from nine sampling points were combined into one sample, and about 100 g of this was placed into disposable sterile ziplock bags, marked with number, latitude and longitude, elevation, vegetation type, air humidity, temperature, and weather conditions (Table 1). All these samples were numbered and recorded in the laboratory soil sample database and then stored at 4 °C in a refrigerator.

Table 1.
List of localities sampled in the Fanjing Mountain Nature Reserve in Guizhou Province.
2.3. Sample Processing
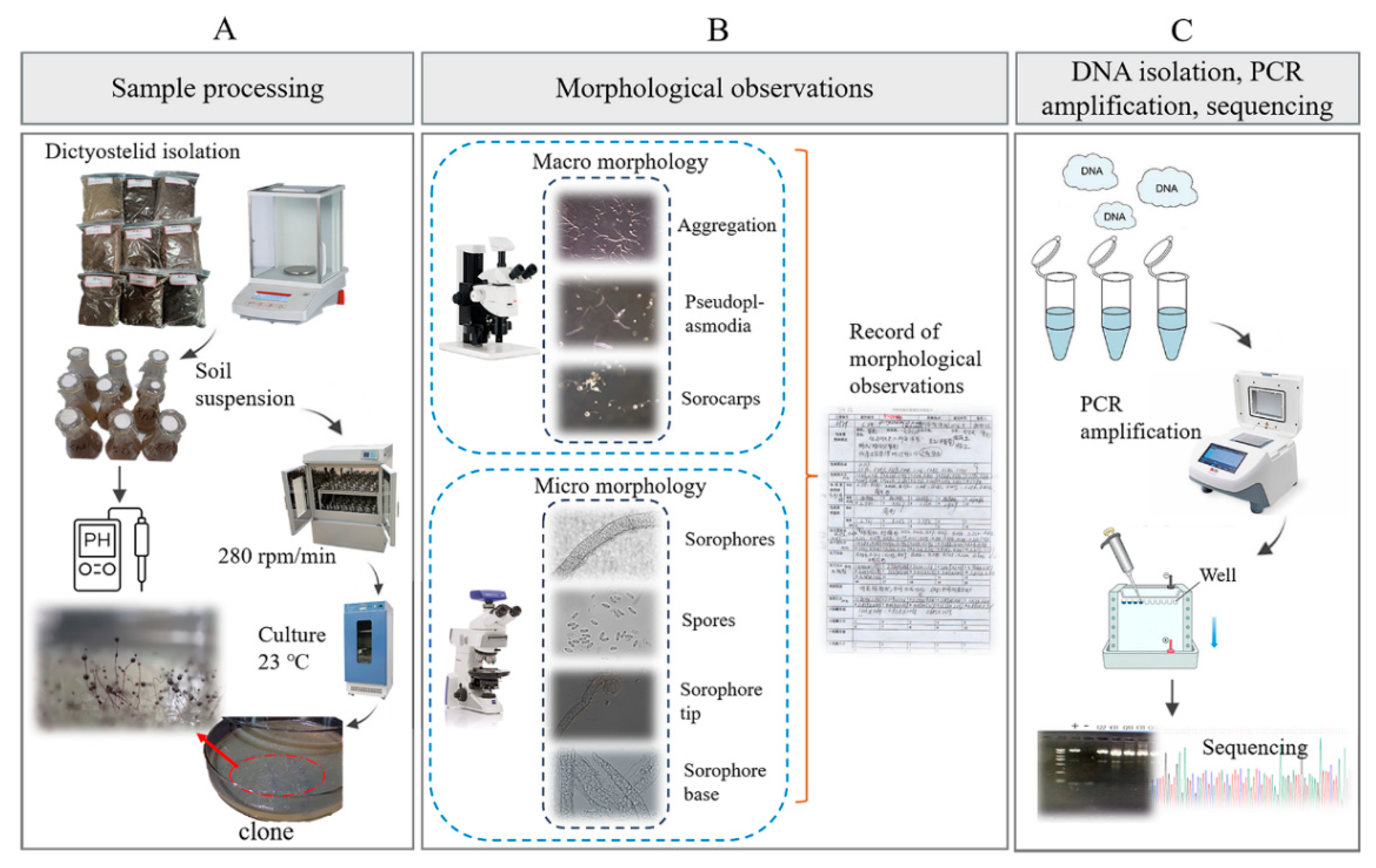
(1) Dictyostelid isolation. The isolation methods used in the present study followed those described by Cavender and Raper [30]. A portion (10 g) of each soil sample was mixed with 90 mL of distilled water and shaken at 280 rpm/min at 23 ℃ for 2 min to prepare an initial soil suspension of 1:10. Soil water moisture was determined using gravimetric analysis [31]. The pH values of the soil dilutions were determined with a PHS-3C pH meter. Afterwards, 5 mL of the soil suspension was mixed with 7.5 mL of sterile water to make a soil suspension of 1:25. Then, 0.5 mL of the soil suspension and 0.4 mL of a suspension of Escherichia coli (food) were uniformly spread over the surface of a hay infusion agar medium using an applicator.
Each soil sample was set in up in six parallels; each plate contained 0.02 g soil, and the total soil of the six plates was 0.12 g. Each inoculated plate was examined carefully at least once a day following the appearance of initial aggregations of amoebae or fruiting bodies that had developed from the propagules present in the sample material, and each aggregation/fruiting (“clone” [30]) was marked (Figure 3A). The formulae used to evaluate the data were calculated as follows [30,32]:
where SJ is the similarity coefficient of the corresponding classification units in the two comparison areas, a is the number of shared classification units, b and c are the numbers of classification units that only appear in one place, respectively.
Jaccard similarity coefficient (SJ) (%) = [a/(a + b + c)] × 100%
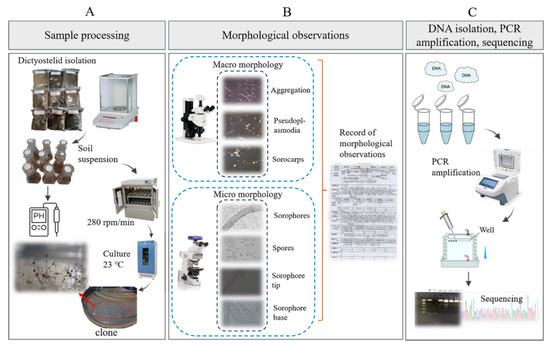
Figure 3.
Outline of the experimental workflow used to isolate dictyostelids from the collected soil samples. (A) Sample processing. (B) Morphological observations. (C) DNA isolation, PCR amplification, sequencing.
(2) Dictyostelid purification. This was followed by adding 0.4 mL of a suspension of E. coli, which was spread evenly over the surface of the agar. Five duplicate plates were prepared for each sample and placed in an incubator at 23 °C with a 12 h light and dark cycle. After two to three days, the plates were examined and any dictyostelids observed were recorded. The isolates appearing in the plates were purified and cultivated for taxonomic studies on non-nutrient water agar plates with E. coli pregrown for 12 to 24 h.
(3) Dictyostelid preservation. Spores from these isolates were frozen in HL5 [33] media and stored at −80 °C in the Herbarium of the Mycological Institute of Jilin Agricultural University (HMJAU), Changchun, China.
2.4. Morphological Observations
In the non-nutrient water agar plates, the location of each early sorocarp(s) that developed was marked. The characteristic stages in the life cycle, including cell aggregation and the formation of pseudoplasmodia and sorocarps, were observed under a Zeiss dissecting microscope (Axio Zoom V16; Zeiss, Oberkochen, Germany) with a 1.5× objective and 10× ocular. Slides with sorocarps were prepared with water as the mounting medium. Features of spores, sorophores, and sorocarps were observed and measured on the slides by using a Zeiss light microscope (Axio Imager A2; Zeiss, Oberkochen, Germany), with a 10× ocular and 10, 40, and 100× (oil) objectives (Figure 3B). Photographs were obtained with a (Zeiss Axiocam 506; Zeiss, Oberkochen, Germany) color microscope camera.
2.5. DNA Isolation, PCR Amplification, Sequencing, and Phylogenetic Analysis
The molecular methods used in the present study followed those described by Liu et al. [22]. The genomic DNA solution was used directly for the PCR amplification using the primers 18SF-A (5′AACCTGGTTGATCCTGCCAG3′), 18SR-B (5′TGATCCTTCTGCAGGTTCAC3′) [34] and ITS1 (5′TCCGTAGGTGAACCTGCGG3′), ITS4 (5′TCCTCCGCTTATTGATATGC3′) [35]. PCR products were sent to Sangon Biotech Co., Ltd. (Shanghai, China), for sequencing (Figure 3C). Sequences obtained were deposited in the GenBank database. The eight newly generated sequences were checked and then submitted to GenBank. All the sequences of closely related species were downloaded from GenBank for phylogenetic analysis to determine their phylogenetic relationships with other taxa in the group (Supplementary Table S1). The ITS and SSU sequences were aligned and compared separately by using the program MAFFT v7.505 [36], then manually adjusted in BioEdit version 7.0.9.0 [37]. Maximum-likelihood analyses (ML analyses) were performed using IQTREE v.1.6.12 [38], with 10,000 replicates of ultrafast-likelihood bootstrapping to obtain node support values with the “-bb 10,000” option, and further optimized using a hill-climbing nearest-neighbor interchange (NNI) with the “-bnni” option [39]. We also used the SH-aLRT test to obtain the confidence limit of the topology with the “-alrt 1000” option [40]. The “-nt AUTO” option was used to automatically determine the best number of cores given the current data. We used ModelFinder as implemented within IQ-TREE to determine the best substitution model based on Bayesian information criteria (BIC) [41]. We used a myxomycete sequence (Physarum polycephalum, accession no. X13160.1/NC_002508.1) as the outgroup with the “-o X13160.1”/ “-o NC_002508.1” option.
2.6. Data Collection of Dictyostelids in Four Other Nature Reserves
In order to clarify the geographical distribution of dictyostelids in the nature reserves, four other representative protected areas in China were selected along with Fanjing Mountain Nature Reserve in this study; the data sources for the four representative protected areas included seven species from Changbai Mountain (CB) [23] (Dictyostelium discoideum, D. mucoroides, D. robusticaule, Cavenderia fasciculata, Heterostelium pallidum, H. recretum, H. candidum), three species from Gushan Mountain (GS) [42] (Dictyostelium brefeldianum, D. clavatum, D. purpureum), five species from Baiyun Mountain (BY) [43] (Dictyostelium sphaerocephalum, D. purpureum, Heterostelium candidum, H. tenuissium, Polysphondylium violaceum), and 12 species from the Qinghai–Tibet Plateau (QT) [22] (Dictyostelium brevicaule, D. vermiforme, D. brefeldianum, D. sphaerocephalum, D. minimum, D. crassicaule, D. multiforme, Cavenderia fasciculata, C. antarctica, C. aureostipes, C. exigua, Heterostelium tikalense).
2.7. Statistical Analyses
2.7.1. Dictyostelid Diversity Analyses
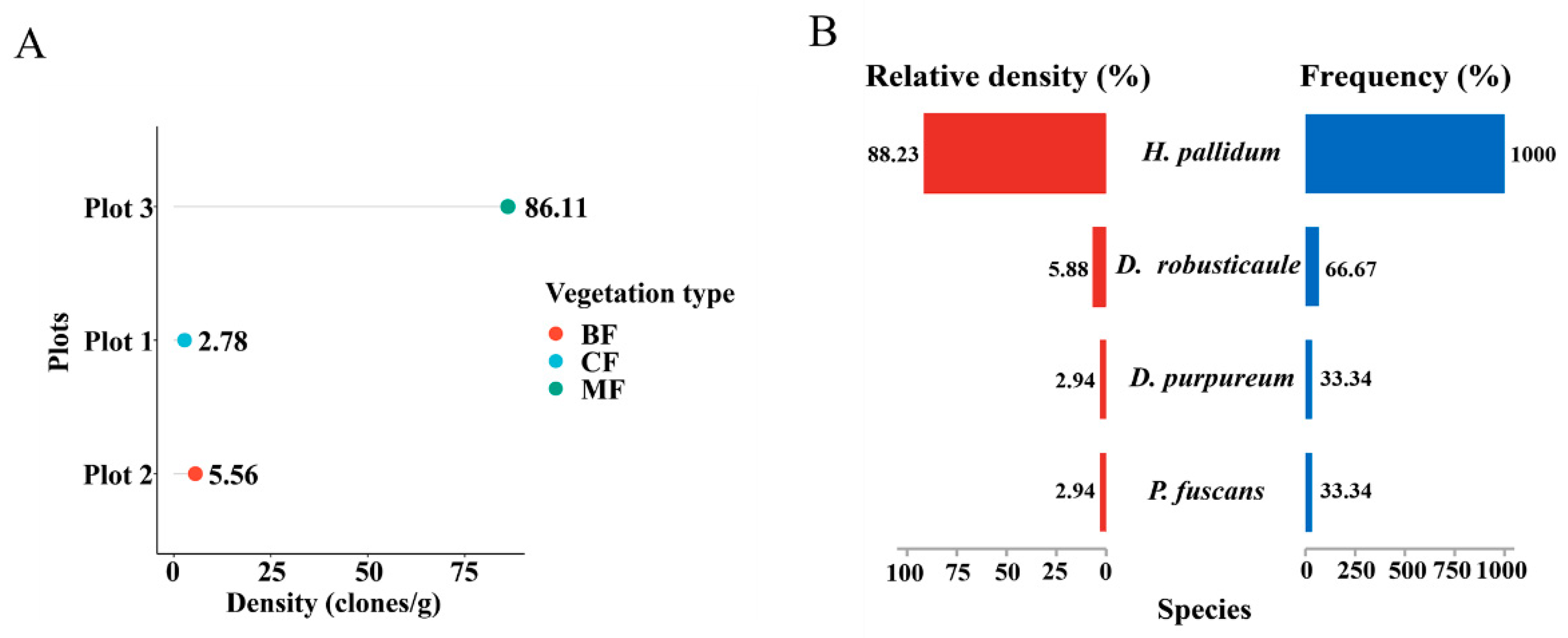
All dictyostelid diversity analyses were generated using the Hiplot Pro Biomedical Visualization Platform (available online: https://hiplot.com.cn/home/index.html, accessed on 12 April 2023). The data on community calculation—density (clones/g), frequency (%), and relative density (%)—were analyzed based on the Excel table. The DotChart Plot (Figure 4A) of dictyostelid clones from all soil samples was used to evaluate dictyostelid density in three plots; the Pyramid Chart (Figure 4B) of relative abundance and frequency of dictyostelid species was used to evaluate the dominant species.
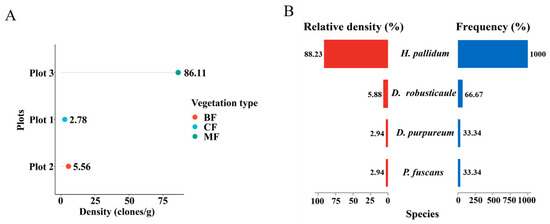
Figure 4.
Comparison of the community diversity of dictyostelids along the different dimensional diversity levels. (A) Density in the plots. Abbreviations: broadleaf forest (BF), coniferous forest (CF), mixed forest (MF). (B) The relative abundance and frequency of species.
2.7.2. Environmental Impacts on Dictyostelid Communities
The influence of environmental factors on the dictyostelid communities of the collecting locality was analyzed by redundancy analysis (RDA, which is based on a linear model) (Section 3.4) using the cloud platform (available online: http://www.cloud.biomicroclass.com/, accessed on 18 April 2023). First, we sorted out the species abundance table, the list of environmental factors, and the grouping files in Excel. Then, these three files were respectively converted to the “txt” format. Environmental factors include edaphic factors (e.g., water moisture, pH), biotic factors (e.g., vegetation), spatial factors (e.g., elevation, longitude, and latitude), and climatic factors (e.g., temperature, air humidity).
2.7.3. Geographic Distribution of Dictyostelids in Nature Reserves
In order to compare the relevance of dictyostelid species in the nature reserves, the Jaccard similarity coefficient (SJ) was applied to calculate the value of similarity between species classification units (family, genus, species) (Section 3.5), the cloud platform (http://www.cloud.biomicroclass.com/, accessed on 23 April 2023) was used to perform a principal component analysis (PCA) (Section 3.5), and the Stacked Column Chart with a clustering tree (Section 3.5) based on Bray–Curtis distances was used to detect the geographical distribution of dictyostelids in the nature reserves.
3. Results
3.1. Dictyostelid Community Composition and Diversity
Nine mixed soil samples from three plots in the Fanjing Mountain Nature Reserve were screened for clones using pure cultivation. A total of 34 clones were isolated from the soil samples in this study; only in a single plot (plot 3) did the number of clones recorded exceed 30, and in two others the number of clones was less than five (Table 1). Moreover, the number of clones in quadrat 3 was 31, accounting for 91% of the total number of clones (Table 1). Numbers of clones per gram of sample material varied widely, and the highest density for a particular locality (86.11 clones/g) was recorded for a mixed forest, whereas the lowest density (2.78 clones/g) was obtained for samples from a coniferous forest in the Fanjing Mountain Nature Reserve (Figure 4A). Heterostelium pallidum was the most widespread species and was recorded in mixed forests of quadrat C2 and quadrat C3 in the Fanjing Mountain Nature Reserve, which had the highest relative density and frequency (Figure 4B).
3.2. Dictyostelid Species
A total of four species were obtained from nine quadrats in three plots from which soil samples were collected in the Fanjing Mountain Nature Reserve, Guizhou Province, during August 2022. Among these, the species recovered were classified as follows: Polysphondylium fuscans (clone no. = 1), Dictyostelium purpureum (clone no. = 1), D. robusticaule (clone no. = 2), and Heterostelium pallidum (clone no. = 30) (Table 1). These four species recorded in the entire study were present in only four quadrats. In contrast, no isolates were recovered from the other five quadrats processed. Soil samples from quadrat C2 of the Fanjing Mountain Nature Reserve (collected from a mixed forest at 27°50′39″ N, 108°47′13″ E) yielded the largest number of species, a total of two species. P. fuscans (Figure 5) and D. robusticaule (Figure 6) were not previously known from Guizhou Province, and P. fuscans is a new record for China. Two species were D. purpureum (Figure 7) and H. pallidum (Figure 8), which were common in Guizhou Province. The morphological characteristics of these species vary widely in shape, size, and coloration among species, and most of the characteristics used for species identification are described below (Table 2).
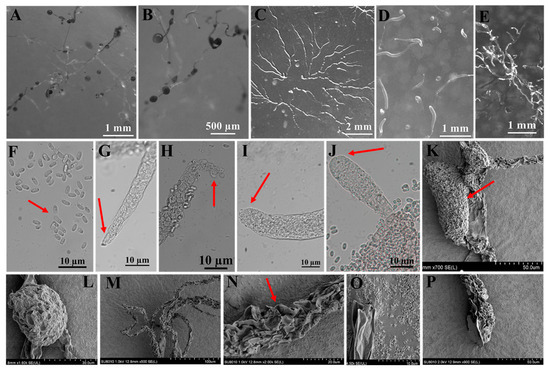
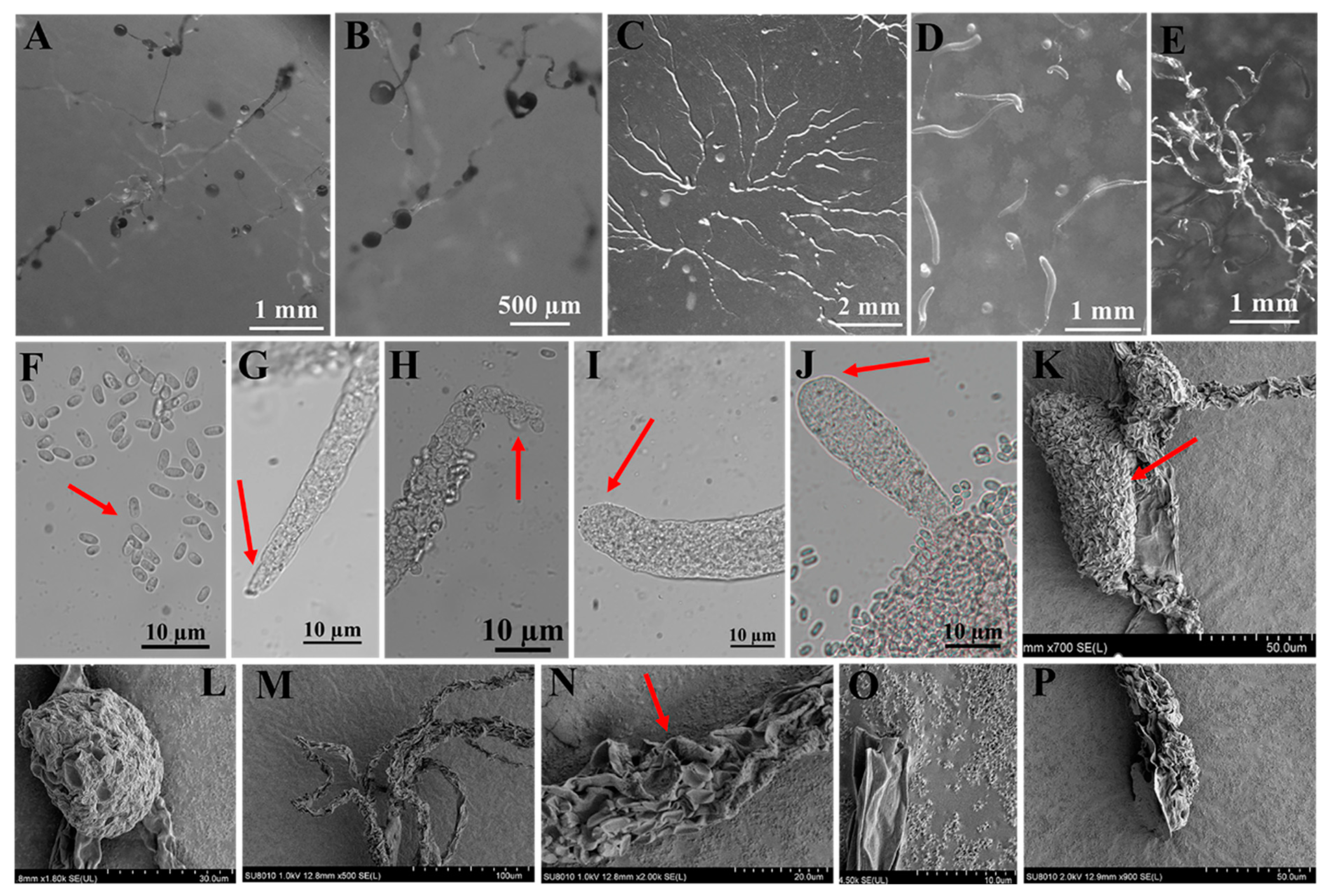
Figure 5.
Morphological features of Polysphondylium fuscans Perrigo and Romeralo (HMJAU A241). (A,B) Sorocarps. (C) Aggregations. (D) Pseudoplasmodia. (E) Clustered sorogens. (F) Spores. (G,H) Sorophore tips. (I,J) Sorophore bases. (K,L) Sorus (SEM). (M–O) Sorophores (SEM). (P) Sorophore base (SEM). The red arrows refer to the key distinguishing characteristics.
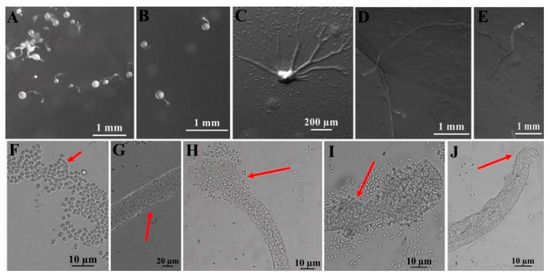
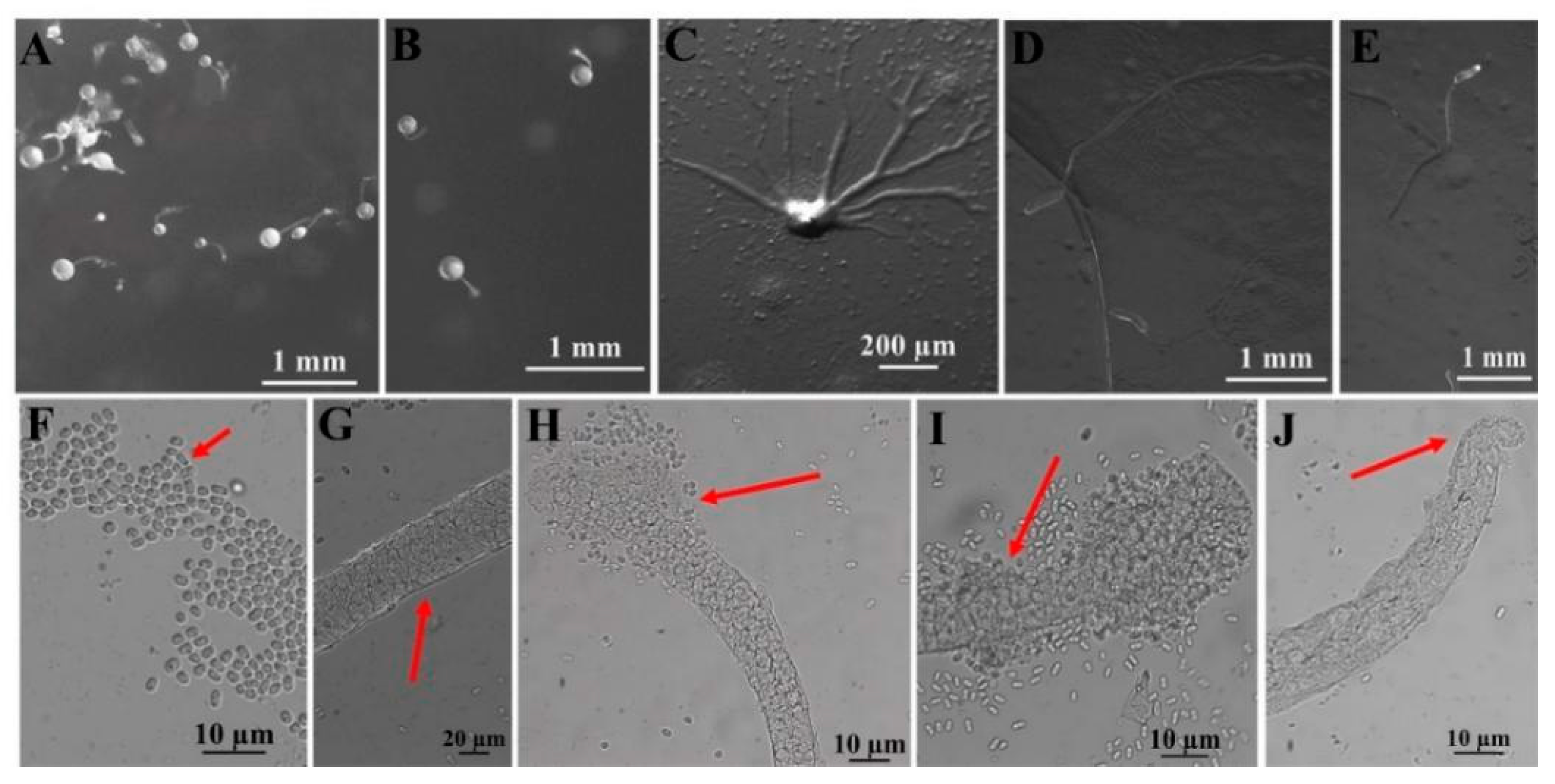
Figure 6.
Morphological features of Dictyostelium robusticaule Y. Li, P. Liu, Y. Zou (HMJAU B341, B321). (A,B) Sorocarps. (C) Aggregations. (D,E) Pseudoplasmodia. (F) Spores. (G) Sorophore. (H,I) Sorophore tips. (J) Sorophore base. The red arrows refer to the key distinguishing characteristics.
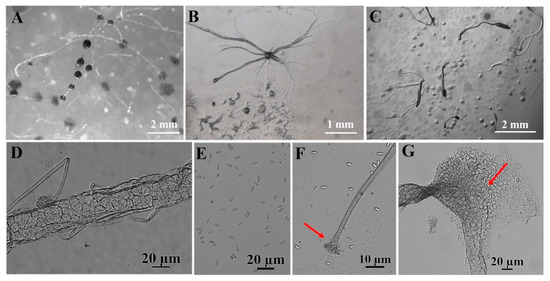
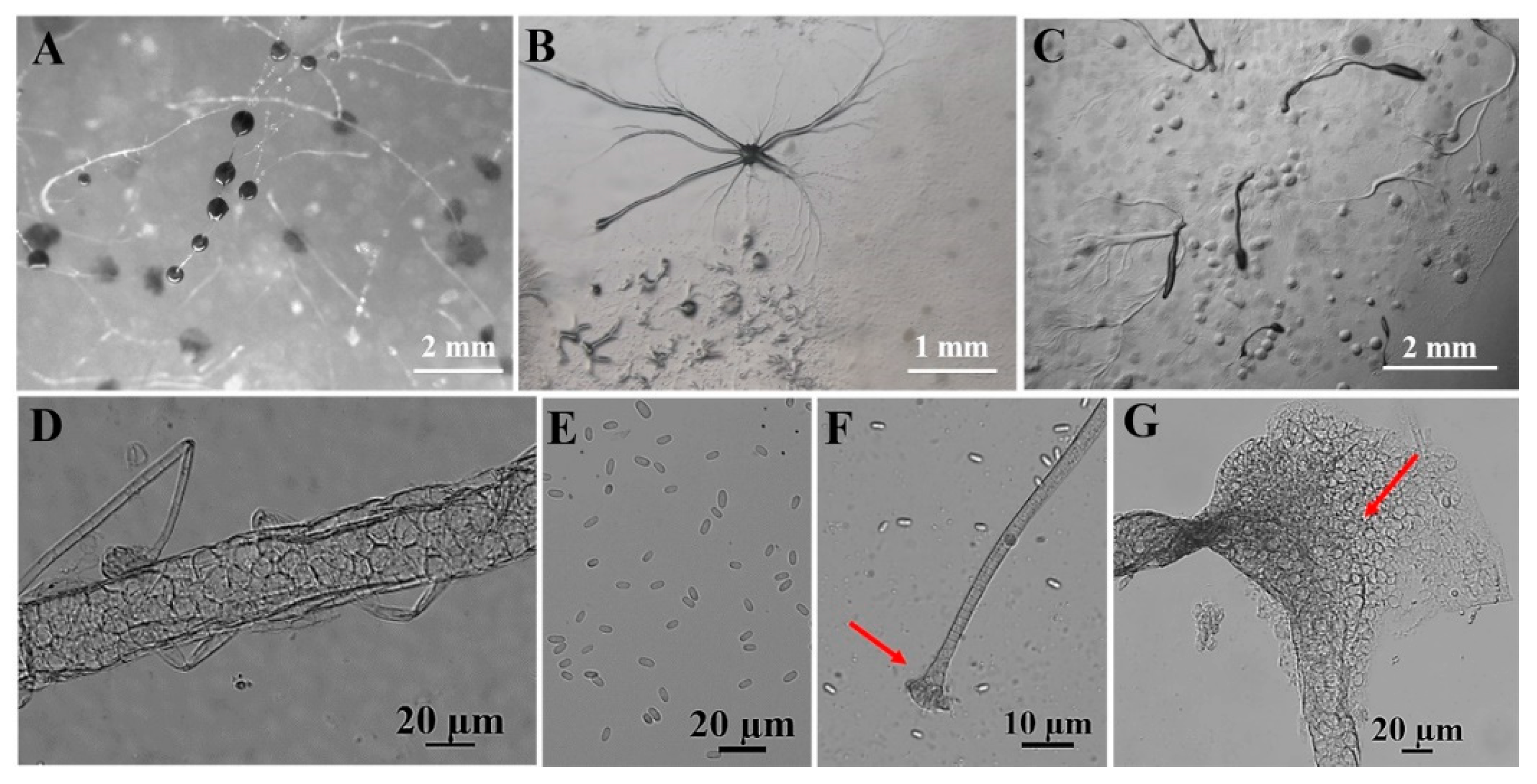
Figure 7.
Morphological features of Dictyostelium purpureum Olive (HMJAU C211). (A) Sorocarps. (B) Aggregations. (C) Pseudoplasmodia. (D) Sorophore. (E) Spores. (F) Sorophore tip. (G) Sorophore base. The red arrows refer to the key distinguishing characteristics.
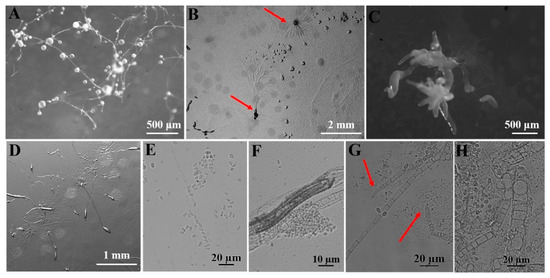
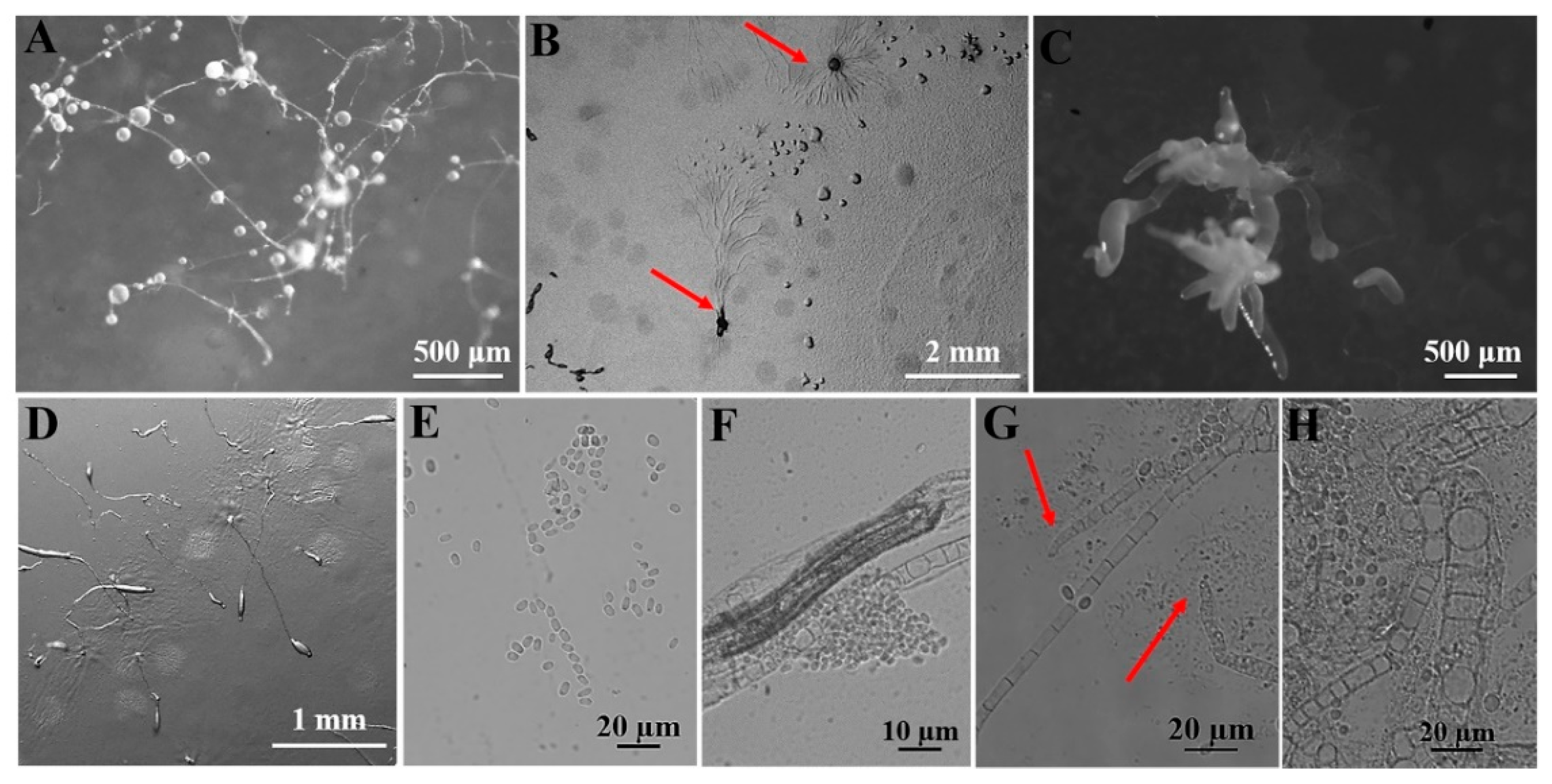
Figure 8.
Morphological features of Heterostelium pallidum (Olive) S. Baldauf, S. Sheikh, and Thulin (HMJAU C231; C311-C315; C321-C324; C331-C336; C341-C347; C351-C357). (A) Sorocarps. (B) Aggregations. (C) Clustered sorogens. (D) Pseudoplasmodia. (E) Spores. (F) Sorophores. (G) Sorophore tips. (H) Sorophore base. The red arrows refer to the key distinguishing characteristics.

Table 2.
Morphological characteristics of dictyostelids in the Fanjing Mountain Nature Reserve in Guizhou Province.
3.3. Complete Sequencing and Phylogenetic Analysis
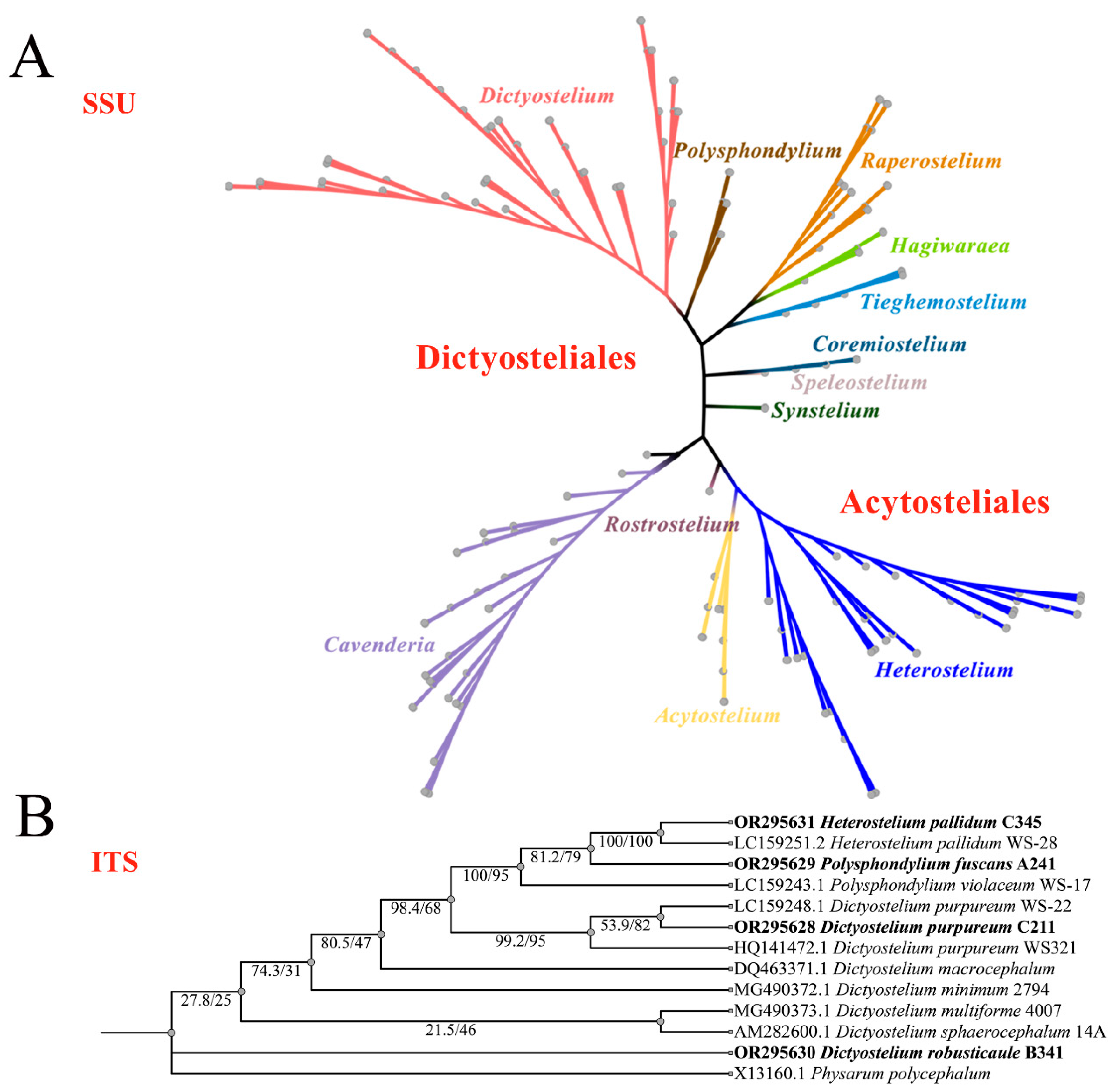
Among the eight new SSU rDNA and ITS rDNA sequences obtained in this study, the lengths of these sequences in dictyostelids ranged from 1600 to 1800 bp (SSU) and 700 to 900 bp (ITS), respectively. They shared 99% to 100% similarity with each other and 99% to 100% similarity with the closest strain in GenBank. A maximum-likelihood phylogenetic tree of the complete SSUrDNA sequences was constructed based on the strains from this study as well as all other known dictyostelids (Figure 9A). A maximum-likelihood phylogenetic tree of the partial ITS rDNA sequences was constructed based on the strains from this study as well as representative strains from GenBank with high similarity to our strains (Figure 9B).
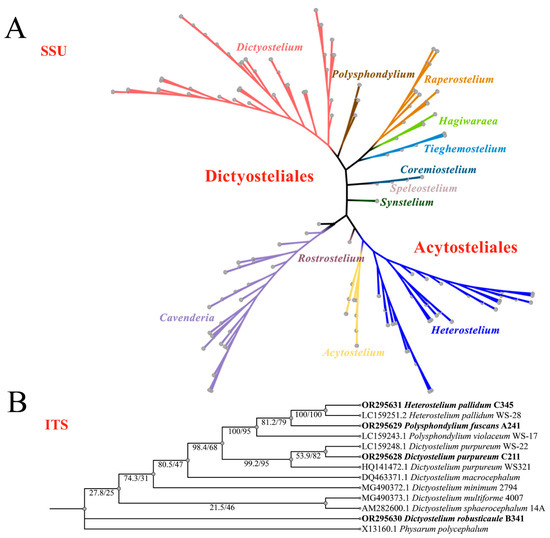
Figure 9.
Phylogeny of all known dictyostelids based on SSU rDNA (A) and closely related species of dictyostelids based on ITS rDNA, newly generated sequences are indicated in bold (B).
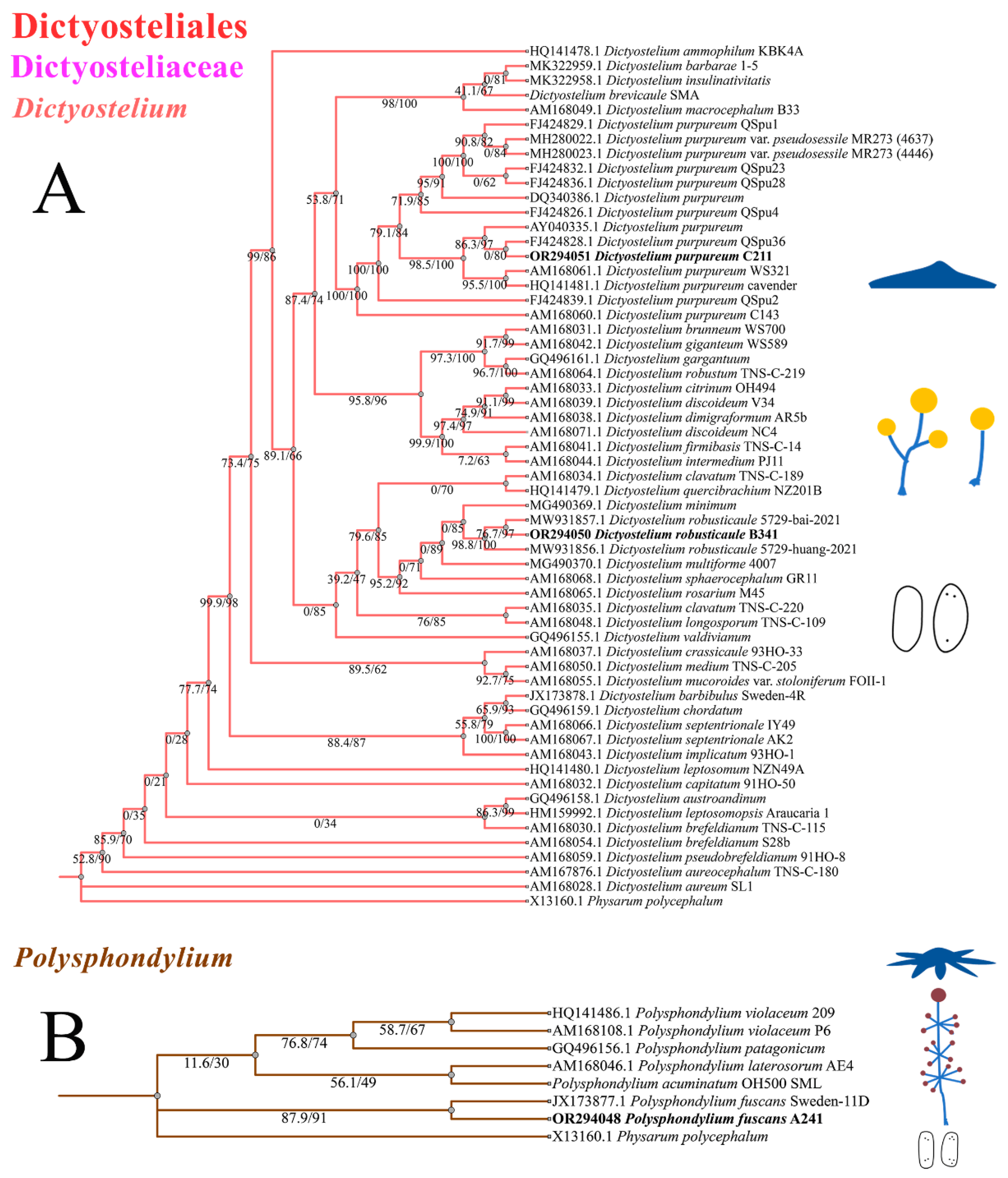
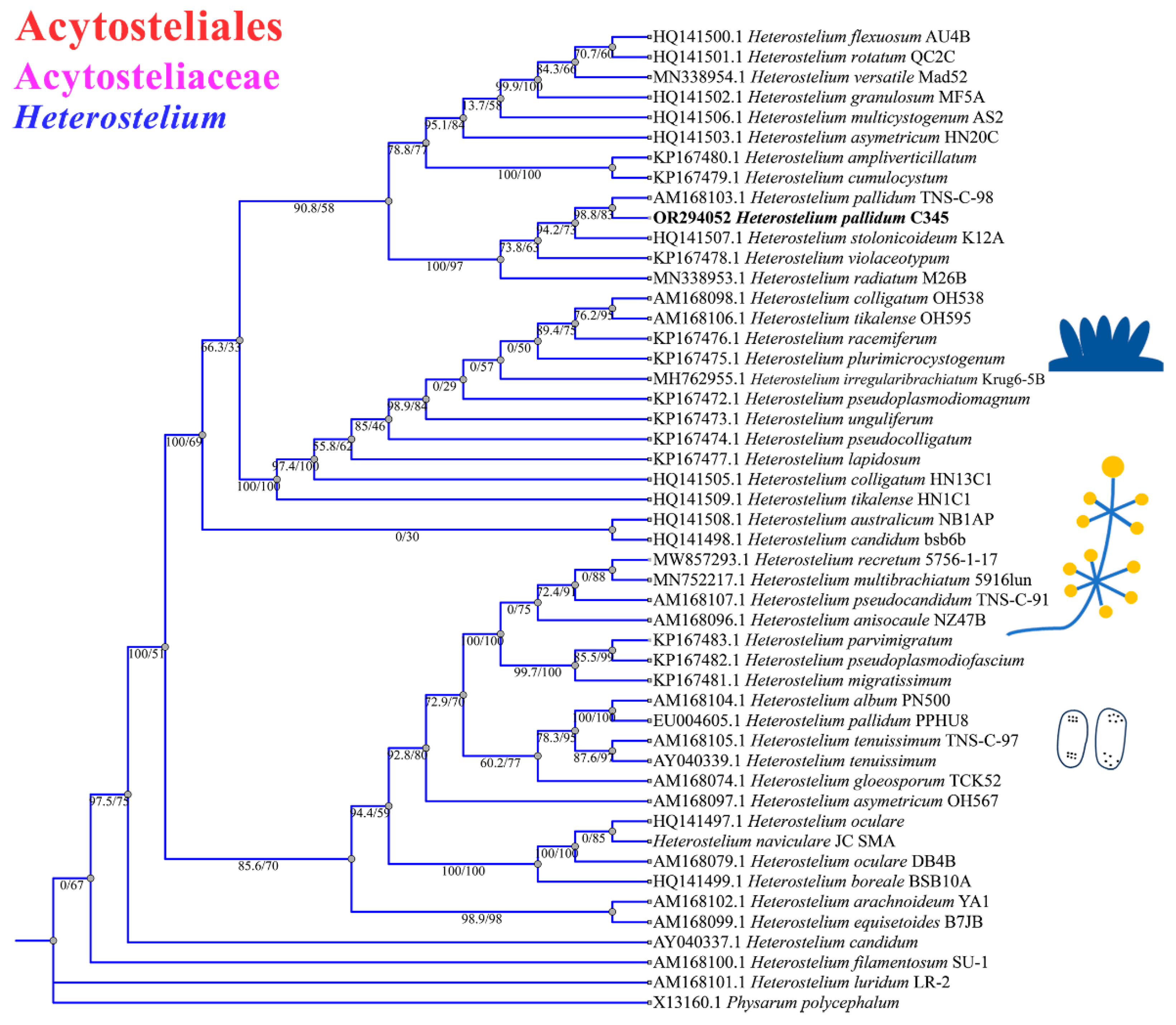
The phylogenetic studies of the SSU rDNA data revealed the four species are members of the orders Dictyosteliales and Acytosteliales, which can be divided into 3 distinct branches within 12 genus branches. Two strains (HMJAU C211 and HMJAU B341) belong to Dictyostelium (sorocarps unbranched or irregular branches, spores without polar granules and polar granules unconsolidated) and cluster together with high support, and are closely related to Dictyostelium purpureum QSpu36 (GenBank accession no. FJ424828.1) and Dictyostelium robusticaule 5729-bai-2021 (GenBank accession no. MW931857.1), respectively. The SH-aLRT support/ultrafast bootstrap support values = 0/80 and 76.7/97 (Figure 10A). One strain (HMJAU A241) belongs to Polysphondylium (sorocarps with violet/lavender/purple sori, branches irregular or regularly spaced whorls, spores with consolidated polar granules) and cluster together with high support, which indicates that they are closely related to Polysphondylium fuscans Sweden-11D (GenBank accession no. JX173877.1), with an SH-aLRT support/ultrafast bootstrap support value of 87.9/91 (Figure 10B). One strain (HMJAU C345) belongs to Heterostelium (sorocarps sparsely branched, spores with granules present/absent, consolidated/unconsolidated) and clusters together with high support, which indicates a close relationship to Heterostelium pallidum TNS-C-98 (GenBank accession no. AM168103.1 and an SH-aLRT support/ultrafast bootstrap value of 98.8/83, Figure 11).
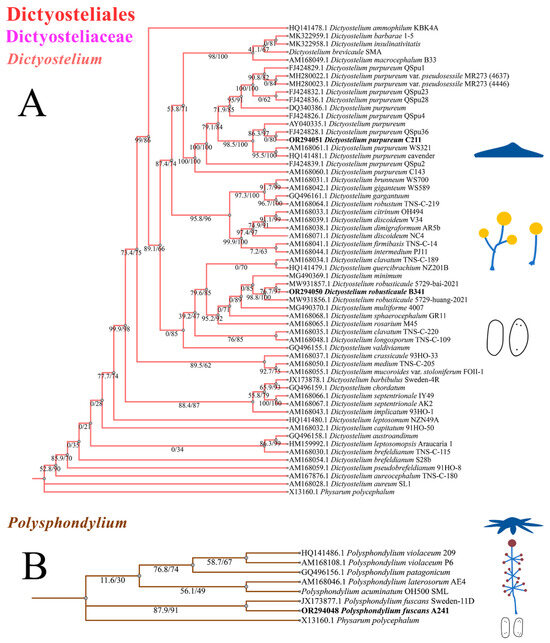
Figure 10.
SSU phylogeny of Dictyostelium (A) and Polysphondylium (B) sequences in the order Dictyosteliales and the family Dictyosteliaceae. Numbers in parentheses are SH-aLRT support (%)/ultrafast bootstrap support (%). Newly generated sequences are indicated in bold.
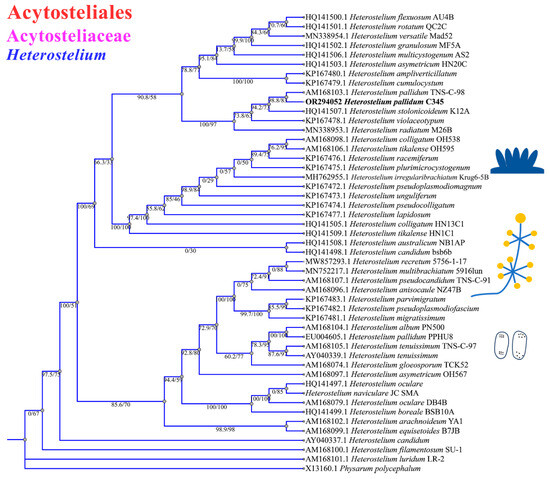
Figure 11.
SSU phylogeny of Heterostelium sequences in the order Acytosteliales, family Acytosteliaceae. Numbers in parentheses are SH-aLRT support (%)/ultrafast bootstrap support (%). Newly generated sequences are indicated in bold.
3.4. Environmental Responses of Dictyostelid Communities in Different Habitats
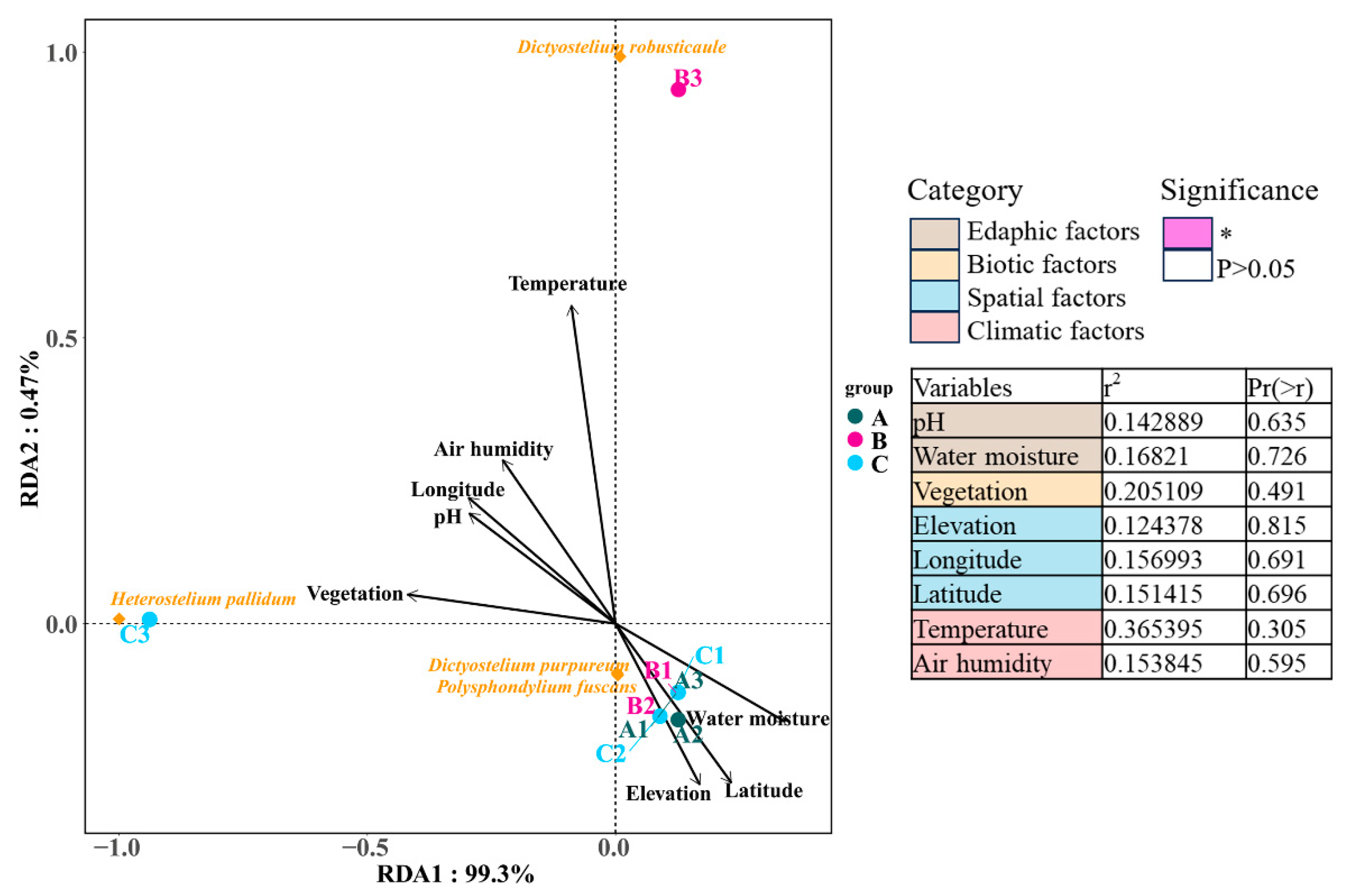
Redundancy analysis was applied to determine correlations between eight environmental factors in four categories and dictyostelid communities. The RDA results showed that environmental factors explained 99.3% and 0.47% of the variation in soil dictyostelid communities among the environmental factors in the Fanjing Mountain Nature Reserve in this study. Among the environmental data, the environmental factors had no distinguishable significant correlation with variations in dictyostelid communities (p > 0.05, Figure 12), as they accounted for 20.51% and 36.54% of the total variation, respectively.
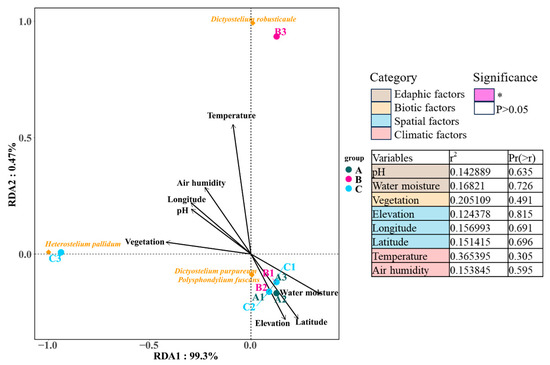
Figure 12.
The edaphic, biotic, spatial, and climatic factors explaining the diversity of dictyostelid communities with the variables analyzed by RDA. Groups are as follows: Fanjing Mountain Nature Reserve Plot A, Plot B, and Plot C. The significance of variables was tested using ANOVA. The variation partitioning analysis was computed using the significant variables identified within each category. Significance levels are * p < 0.05.
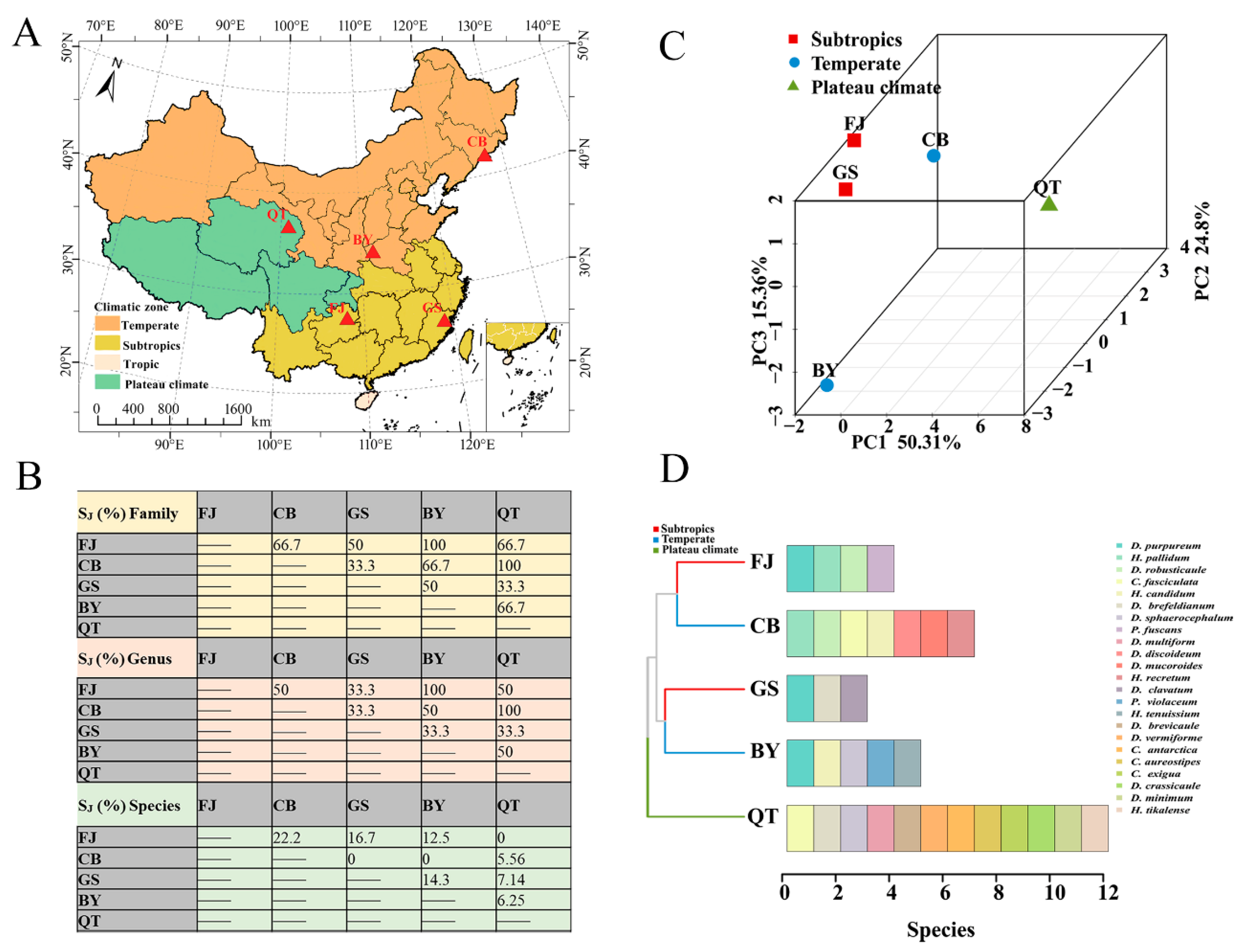
3.5. Comparison of Similarity and Geographical Distribution of Dictyostelids in Representative Natural Reserves
The Jaccard similarity coefficient (SJ %) of the family, genus, and species was calculated using data obtained from Changbai Mountain (CB) [23] in Jilin Province, Gushan Mountain (GS) [42] in Fujian Province, Baiyun Mountain (BY) [43] in Henan Province, Qinghai–Tibet Plateau in Tibet (QT) [22], and the Fanjing Mountain Nature Reserve (FJ) in Guizhou in the present study (Figure 13A). At the family level, the majority for each other (SJ %) was higher than 50%, reaching moderate similarity. At the family level, the values (SJ %) for FJ and BY, CB, and QT were 100%. A total of only one family of the Dictyosteliaceae was the same in five reserves. At the genus level, the comparison was generally similar. The difference was mainly manifested in the abundance of dictyostelids in CB and QT, where dictyostelid distribution was obviously unbranched or had irregular branches, including Dictyostelium, Heterostelium, and Cavenderia. At the species level, FJ and CB had the highest species similarity coefficient (SJ %), which was 22.2%, while others were 5.56%–16.7%. Although the family similarity coefficient (SJ %) was relatively high, the interspecies similarity values were all low in these five reserves. We found that the dictyostelids have different environmental requirements, which also reflects their unique ecological adaptability.
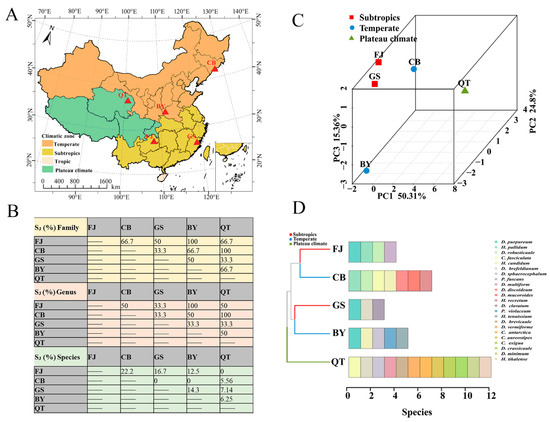
Figure 13.
Analysis of dictyostelid diversity comparisons and geographical characteristics in different protected areas. (A) Map of the protected area. (B) Jaccard similarity coefficients (SJ %) of dictyostelids in family, genus, and species within Changbai Mountain in Jilin Province (CB), Gushan Mountain in Fujian Province (GS), Baiyun Mountain in Henan Province (BY), Qinghai–Tibet Plateau in Tibet (QT), and Fanjing Mountain in Guizhou Province (FJ). (C,D) Beta diversity was analyzed using PCA; bar chart clustered by Bray–Curtis similarities calculated based on the dictyostelid species of the three climate zones.
Using the dictyostelid species from those five protected areas noted above, the distribution of dictyostelids in three climate zone protected areas were elucidated using principal component analysis (PCA) and a clustered bar chart (Figure 13B,C). Dictyostelids were mainly assigned to subtropical (FJ and GS), temperate (CB and BY), and plateau (QT) climatic zones, with proportions of 40%, 40%, and 20%, respectively. The PCA results demonstrated that resultant principal components (PCs) explained 90.47% of the variance. There was some similarity between the dictyostelids (FJ) and the dictyostelids (GS) in the subtropical climatic zone. However, the most distinct differences in community composition were observed between the dictyostelids (CB) and dictyostelids (BY) in the temperate climatic zone. In addition, the dictyostelid (QT) community structure in the plateau climate zone was quite distinct from those of both the subtropical and the temperate climatic zones (Figure 13B). It is worth noting that D. purpureum appeared in all three (FJ, GS, BY) protected areas (Figure 13D).
4. Discussion
4.1. Taxonomy and Molecular Phylogeny
In this study, we isolated 34 strains which could be assigned to four species based on morphology and as confirmed by molecular sequence data (Table 2; Figure 9, Figure 10 and Figure 11). They belong to two families in the Dictyosteliaceae and Acytosteliaceae, with three genera (Dictyostelium, Heterostelium, and Polysphondylium) from Guizhou Province, Southwest China, as determined by laboratory cultivation (Figure 2; Table 1). Among these, P. fuscans was new to China; its sori (Figure 5A,B) start out colorless and develop violet coloration that darkens with age. This places the species in a highly supported clade together with P. fuscans (GenBank accession no. JX173877.1) in an SSU rRNA phylogeny (Figure 10B). Morphologically, P. fuscans is most similar to Polysphondylium violaceum but differs primarily in the lighter initial pigmentation of the sori, the smaller number of whorls, and the larger spore size. This species was first reported from mildly acidic soils (pH = 5.5) in a coniferous forest in central Sweden [44]. Surprisingly, the species isolated in this study also came from coniferous forests with mildly acidic soils (pH = 5.7), indicating the species’ preference for this type of soil habitat. Dictyostelium purpureum (Figure 7), with large, robust sorocarps and deep purplish sori, is a cosmopolitan species reported from Africa, across North America, and in many localities in Asia [45]. Dictyostelium robusticaule, with the species name referring to its robust sorophores, was first reported by Zou et al. [23] from a mixed forest on Changbai Mountain, China. In this study, the species was isolated from a broadleaf forest, and SSU rRNA phylogeny places it in a highly supported clade together with D. robusticaule (GenBank accession no. MW931857.1) (Figure 10A). Heterostelium pallidum is characterized by whirls of sori, which places this species in a highly supported clade together with H. pallidum (GenBank accession no. AM168103.1) in an SSU rRNA phylogeny (Figure 11). Stephenson and Landolt [46] studied canopy soil in Puerto Rico and showed that H. pallidum tends to be found in regions with a tropical or subtropical climate. Furthermore, Cavender [47] suggested that H. pallidum is one of the few cellular slime molds that seem to be indifferent to climatic conditions.
4.2. Environmental Factors Drive the Effects of Dictyostelid Diversity
At present, numerous regions in China (Figure 1A) have carried out extensive sampling to investigate dictyostelids, with identification mostly based on purely descriptive species information. However, the ecology of these extremely unique populations is still poorly understood, and ecological factors are rarely associated with the diversity distribution of this group’s organisms. Previously, some researchers reported ecological information from China, North America, Central America, and East Africa [24,48,49,50,51] along with Madagascar [50] and Christmas Island [52], thus providing information that deepened our interest in this particular group.
In general, the overall diversity and abundance of dictyostelids in the Fanjing Mountain Nature Reserve appear to be low. As noted above, five (A1, A3, B1, B2, and C1) of the nine quadrats yielded no clones, and a total of only 34 clones were recovered from four samples (Table 1). Many factors affect the distribution and density of dictyostelids, such as abiotic factors (moisture, organics, pH, temperature, soil quality, and elevation) and biotic factors (prey–predator and other interspecies interactions) [53,54]. The growth and reproduction of soil protozoa largely rely on the habitat provided by vegetation, which not only changes the local microclimate but affects the nutrients, pH, water content, oxygen content, and temperature through litter or root exudates [55,56]. In this study, the environmental factors had no distinguishable significant correlation with variations in dictyostelid communities (p > 0.05, Figure 12). Our results found that the mixed forest with a total of 31 clones isolated had the highest density (86.11 clones/g), including two species, D. purpureum (clone no. = 1) and H. pallidum (clone no. = 30) (Table 1), which is consistent with a previous report indicating that the highest species abundance occurs in mixed forests in Changbai Mountain [23]. The reason is that through complementary interactions, mixed forests are more resistant to relatively small-scale and selective natural disturbances; therefore, productivity may be higher than in a single forest type [57,58].
4.3. Comparative Analysis of Geographical Distribution and Species Similarity in Natural Reserves
All the current research on protected areas in China summarized above makes the facilitation of cooperation among scientists from different countries and continents particularly important. For dictyostelid distribution, the similarity between the families and genera of dictyostelids in different regions ranges from 33.3% to 100%; the similarity of species ranges from 0 to 22.2% (Figure 13A). Based on the available information on types of dictyostelids and the literature records, the geographical distribution of dictyostelids in five protected areas was divided into three types: subtropical distribution (6 species), temperate distribution (11 species), and plateau distribution (12 species). The proportions of distributional types were 26.09%, 47.83%, and 52.17%, respectively (Figure 13C). The frequency of D. purpureum was the highest, with it occurring in three (FJ, GS, BY) protected areas. Swanson et al. [54] reported earlier on the known distributional data of dictyostelids found in forest soils around the world, based on data available at that time. The distribution of these species can be divided into four categories: cosmopolitan, intermittent, limited, and subtropical. Drawing inspiration from these distribution categories, it seems that in addition to the cosmopolitan species (e.g., D. purpureum and H. pallidum), other species (e.g., P. fuscans and D. minimum) in different regions have certain geographical limits based on the overall data from this study.
In addition, from an overall perspective, the species distribution in the subtropical climatic zone (FJ and GS) is characterized by small spatial distances, while that in the temperate climatic zone (CB and BY) is more distant. Of course, this is also influenced by the number of samples and various environmental factors [47], so more researchers need to be involved in the discovery of species. This indicates that more repetitive samplings across broader temporal and spatial scales are needed to accurately describe the true geographical distribution of this group of organisms, based on big data and interdisciplinary approaches.
5. Conclusions
In this study, 34 isolates representing four species of dictyostelids were obtained from soil samples collected from Fanjing Mountain in Guizhou Province, China. One species (Polysphondylium fuscans) was new to China, which provides a new starting point for the study of this species. Our data indicate that the distribution patterns of dictyostelids are most influenced by vegetation and temperature, which provides important information for understanding the ecological characteristics of these organisms. These obvious geographical distribution patterns of dictyostelids in different climate zones have great significance for developing a better understanding of their ecological adaptability and evolutionary history.
However, in future studies, we should (1) consider the limitations of the research area, such as by increasing the collection of samples and adopting new technologies such as high-throughput sequencing; (2) consider the complexity of environmental factors, such as soil conditions and the influence of biological factors; and (3) propose specific protection measures or recommendations to help protect these precious biological resources.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12061061/s1. Supplementary Table S1 Species and sequences used in the phylogenetic analyses. Newly generated sequences are indicated in bold.
Author Contributions
Z.Z.: writing—original draft, data curation, software, conceptualization. M.L.: methodology, formal analysis. S.Z.: validation, data curation. Y.Q.: resources. J.Z.: software. Y.L.: supervision, project administration. S.L.S.: writing—review and editing. J.Q.: project administration, supervision. P.L.: writing—review and editing, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by funding supplied by the National Natural Science Foundation of China (Nos. 32370020, 32070009, and 31870015), the Natural Science Foundation of Jilin Province (No. 20220101187JC), and the Program of Creation and Utilization of Germplasm of Mushroom Crop of the “111” Project (No. D17014).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Geisen, S.; Mitchell, E.A.D.; Adl, S.; Bonkowski, M.; Dunthorn, M.; Ekelund, F.; Fernandez, L.D.; Jousset, A.; Krashevska, V.; Singer, D.; et al. Soil protists: A fertile frontier in soil biology research. FEMS Microbiol. Rev. 2018, 42, 293–323. [Google Scholar] [CrossRef]
- Zhao, Z.B.; He, J.Z.; Geisen, S.; Han, L.L.; Wang, J.T.; Shen, J.P.; Wei, W.X.; Fang, Y.T.; Li, P.P.; Zhang, L.M. Protist communities are more sensitive to nitrogen fertilization than other microorganisms in diverse agricultural soils. Microbiome 2019, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.T.; Clemente, J.C.; Flores, G.E.; Walters, W.A.; Parfrey, L.W.; Knight, R.; Fierer, N. Global biogeography of highly diverse protistan communities in soil. ISME J. 2013, 7, 652–659. [Google Scholar] [CrossRef]
- Chen, X.; Koellner, T.G.; Shaulskye, G.; Jia, Q.; Dickschat, J.S.; Gershenzon, J.; Chen, F. Diversity and Functional Evolution of Terpene Synthases in Dictyostelid Social Amoebae. Sci. Rep. 2018, 8, 14361. [Google Scholar] [CrossRef] [PubMed]
- Rukseree, K.; Wonganun, K.; Palittapongarnpim, P.; Ajawatanawong, P. Phylogenetic diversity of 18S rDNA sequences of dictyostelids from Amnat Charoen Province, Thailand. Mycosphere 2018, 9, 202–214. [Google Scholar] [CrossRef]
- Basu, S.; Fey, P.; Pandit, Y.; Dodson, R.; Kibbe, W.A.; Chisholm, R.L. dictyBase 2013: Integrating multiple Dictyostelid species. Nucleic Acids Res. 2013, 41, D676–D683. [Google Scholar] [CrossRef]
- Romeralo, M.; Escalante, R.; Baldauf, S.L. Evolution and Diversity of Dictyostelid Social Amoebae. Protist 2012, 163, 327–343. [Google Scholar] [CrossRef]
- Heidel, A.J.; Gloeckner, G. Mitochondrial genome evolution in the social amoebae. Mol. Biol. Evol. 2008, 25, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.; Kronforst, M.R.; Queller, D.C.; Strassmann, J.E. Genetic diversity in the social amoeba Dictyostelium discoideum: Population differentiation and cryptic species. Mol. Phylogenet. Evol. 2011, 60, 455–462. [Google Scholar] [CrossRef]
- Schaap, P. Evolution of developmental signalling in dictyostelid social amoebas. Curr. Opin. Genet. Dev. 2016, 39, 29–34. [Google Scholar] [CrossRef]
- Schaap, P.; Winckler, T.; Nelson, M.; Alvarez-Curto, E.; Elgie, B.; Hagiwara, H.; Cavender, J.; Milano-Curto, A.; Rozen, D.E.; Dingermann, T.; et al. Molecular phylogeny and evolution of morphology in the social amoebas. Science 2006, 314, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Raper, K.B. The Dictyostelids; Princeton University Press: Princeton, NJ, USA, 1984. [Google Scholar] [CrossRef]
- Sheikh, S.; Thulin, M.; Cavender, J.C.; Escalante, R.; Kawakami, S.-i.; Lado, C.; Landolt, J.C.; Nanjundiah, V.; Queller, D.C.; Strassmann, J.E.; et al. A new classification of the dictyostelids. Protist 2018, 169, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Venter, O.; Fuller, R.A.; Segan, D.B.; Carwardine, J.; Brooks, T.; Butchart, S.H.M.; Di Marco, M.; Iwamura, T.; Joseph, L.; O’Grady, D.; et al. Targeting global protected area expansion for imperiled biodiversity. PLoS Biol. 2014, 12, e1001891. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.L.; Hill, S.L.L.; Newbold, T.; Hudson, L.N.; Borger, L.; Contu, S.; Hoskins, A.J.; Ferrier, S.; Purvis, A.; Scharlemann, J.P.W. Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Nat. Commun. 2016, 7, 12306. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fang, C.; Li, Y.; Wang, Z.; Sun, S.; He, S.; Qi, W.; Bao, C.; Ma, H.; Fan, Y.; et al. Global impacts of future urban expansion on terrestrial vertebrate diversity. Nat. Commun. 2022, 13, 1628. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.P.; Clemants, S.E.; Corlett, R.T.; Hahs, A.K.; McCarthy, M.A.; McDonnell, M.J.; Schwartz, M.W.; Thompson, K.; Vesk, P.A.; Williams, N.S.G. Plant traits and extinction in urban areas: A meta-analysis of 11 cities. Glob. Ecol. Biogeogr. 2011, 20, 509–519. [Google Scholar] [CrossRef]
- Stephenson, S.L.; Feest, A. Ecology of soil eumycetozoans. Acta Protozool. 2012, 51, 201–208. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, P.; An, Y.; Yao, Y.; Li, Y. Dictyostelium annularibasimum (Dictyosteliaceae, Dictyostelida), a new purple species from China. Nova Hedwig. 2017, 104, 351–358. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, S.; Zou, Y.; Kang, X.; Li, Y. Dictyostelids from Jilin Province, China 3: New Cavenderia and Dictyostelium records. Mycotaxon 2019, 134, 613–618. [Google Scholar] [CrossRef]
- Liu, P.; Zou, Y.; Hou, J.; Stephenson, S.L.; Li, Y. Dictyostelium purpureum var. pseudosessile, a new variant of dictyostelid from tropical China. BMC Evol. Biol. 2019, 19, 78. [Google Scholar] [CrossRef]
- Liu, P.; Zou, Y.; Li, S.; Stephenson, S.L.; Wang, Q.; Li, Y. Two new species of dictyostelid cellular slime molds in high-elevation habitats on the Qinghai-Tibet Plateau, China. Sci. Rep. 2019, 9, 5. [Google Scholar] [CrossRef]
- Zou, Y.; Hou, J.; Guo, S.; Li, C.; Li, Z.; Stephenson, S.L.; Pavlov, I.N.; Liu, P.; Li, Y. Diversity of dictyostelid cellular slime molds, including two species new to science, in forest soils of Changbai Mountain, China. Microbiol. Spectr. 2022, 10, e0240222. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, S.; Zou, Y.; Li, Z.; Stephenson, S.L.; Li, Y. Distribution and ecology of dictyostelids in China. Fungal Biol. Rev. 2020, 34, 170–177. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, Q.; Luo, Y.; Cai, M.; Zhou, X.; Yan, W.; Peng, D.; Zhang, J. Vegetation dynamics and its response to driving factors in typical karst regions, Guizhou Province, China. Front. Earth Sci. 2021, 15, 167–183. [Google Scholar] [CrossRef]
- Yuan, H.Y.; Liu, P.; Li, Y. Two new records of Dictyostelium for China and two new records of Dictyostelium for mainland China. J. Fungal Res. 2012, 10, 66–71. (In Chinese) [Google Scholar]
- Zhang, Z.; Yang, Y.; Zhao, J.; Li, Y.; Stephenson, S.L.; Qiu, J.; Liu, P. Environmental factors influencing the diversity and distribution of dictyostelid cellular slime molds in forest and farmland soils of western China. Microbiol. Spectr. 2023, 11, e0173223. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, W.; Mu, G.; Wu, X.; Su, S.; Zhang, Z. C:N:P stoichiometric characteristics of the soil-vegetation system of three rare tree species growing on Mount Fanjing in Southwest China. Glob. Ecol. Conserv. 2021, 32, e01893. [Google Scholar] [CrossRef]
- Chen, J.; Schmelz, R.M.; Zhang, Z.; Xie, Z. The enchytraeid fauna (Enchytraeidae: Clitellata) of the Fanjing Mountain National Nature Reserve (China) with description of two new species. J. Nat. Hist. 2022, 56, 1957–1996. [Google Scholar] [CrossRef]
- Cavender, J.C.; Raper, K.B. The Acrasieae in nature. I. Isolation. Am. J. Bot. 1965, 52, 294–296. [Google Scholar] [CrossRef]
- Routledge, D.B.; Sabey, B.R. Use of a microwave oven for moisture determination in a soil science laboratory 1. J. Agron. Educ. 1976, 5, 25–27. [Google Scholar] [CrossRef]
- Wang, H.S. Floristic Geography; Science Press: Beijing, China, 1992. [Google Scholar]
- Cocucci, S.M.; Sussman, M. RNA in cytoplasmic and nuclear fractions of cellular slime mold amebas. J. Cell Biol. 1970, 45, 399–407. [Google Scholar] [CrossRef]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 1990, 31, 315–322. [Google Scholar]
- Zhang, D.; Gao, F.; Jakovlic, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Tippmann, H.-F. Analysis for free: Comparing programs for sequence analysis. Brief. Bioinform. 2004, 5, 82–87. [Google Scholar] [CrossRef]
- Lam-Tung, N.; Schmidt, H.A.; von Haeseler, A.; Bui Quang, M. IQ-TREE: A fast and effective stochastic algorithm for estimating Maximum-Likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Diep Thi, H.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Le Sy, V. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Shimodaira, H.; Hasegawa, M. Multiple comparisons of Log-Likelihoods with applications to phylogenetic inference. Mol. Biol. Evol. 1999, 16, 1114–1116. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Bui Quang, M.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Zhao, M.J.; Liu, P.; Tang, X.M.; Li, Y. A Preliminary Report on Dictyostelium from Fujian Province. 2014. Available online: http://www.paper.edu.cn (accessed on 4 March 2023). (In Chinese).
- Li, C.; Liu, P.; Li, Y. Studies on dictyostelid cellular slime molds in Henan Province. J. Fungal Res. 2014, 12, 148–153. [Google Scholar]
- Perrigo, A.L.; Baldauf, S.L.; Romeralo, M. Diversity of dictyostelid social amoebae in high latitude habitats of Northern Sweden. Fungal Divers. 2013, 58, 185–198. [Google Scholar] [CrossRef]
- Hagiwara, H. The Taxonomic Study of Japanese Dictyostelid Cellular Slime Molds; National Science Museum: Tokyo, Japan, 1989.
- Stephenson, S.L.; Landolt, J.C. Dictyostelid cellular slime molds in canopy soils of tropical forests. Biotropica 1998, 30, 657–661. [Google Scholar] [CrossRef]
- Cavender, J.C. Geographical distribution of Acrasiae. Mycologia 1973, 65, 1044–1054. [Google Scholar] [CrossRef]
- Cavender, J.C. The occurrence and distribution of Acrasieae in forest soils. II. East Africa. Am. J. Bot. 1969, 56, 993–998. [Google Scholar] [CrossRef]
- Landolt, J.C.; Stephenson, S.L.; Slay, M.E. Dictyostelid cellular slime molds from caves. J. Cave Karst Stud. 2006, 68, 22–26. [Google Scholar]
- Cavender, J.C.; Landolt, J.C.; Vadell, E.M.; Perrigo, A.L.; Stephenson, S.L.; De Basanta, D.W.; Lado, C.; Liu, P. Distribution and ecology of dictyostelids in Madagascar. Phytotaxa 2021, 505, 176–186. [Google Scholar] [CrossRef]
- Romeralo, M.; Moya-Larano, J.; Lado, C. Social Amoebae: Environmental factors influencing their distribution and diversity across South-Western Europe. Microb. Ecol. 2011, 61, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zou, Y.; Li, W.; Li, Y.; Li, X.; Che, S.; Stephenson, S.L. Dictyostelid cellular slime molds from Christmas Island, Indian Ocean. Msphere 2019, 4, e00133-19. [Google Scholar] [CrossRef] [PubMed]
- Paillet, Y.; Satre, M. The biodiversity of dictyostelids in mountain forests: A case study in the French Alps. Pedobiologia 2010, 53, 337–341. [Google Scholar] [CrossRef]
- Swanson, A.R.; Vadell, E.M.; Cavender, J.C. Global distribution of forest soil dictyostelids. J. Biogeogr. 1999, 26, 133–148. [Google Scholar] [CrossRef]
- Acosta-Mercado, D.; Lynn, D.H. Soil ciliate species richness and abundance associated with the rhizosphere of different subtropical plant species. J. Eukaryot. Microbiol. 2004, 51, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Araujo, A.S.; Mendes, L.W.; Lemos, L.N.; Lopes Antunes, J.E.; Aguiar Beserra, J.E., Jr.; Catanho Pereira de Lyra, M.d.C.; Barreto Figueiredo, M.d.V.; de Almeida Lopes, A.C.; Ferreira Gomes, R.L.; Bezerra, W.M.; et al. Protist species richness and soil microbiome complexity increase towards climax vegetation in the Brazilian Cerrado. Commun. Biol. 2018, 1, 135. [Google Scholar] [CrossRef] [PubMed]
- Jactel, H.; Bauhus, J.; Boberg, J.; Bonal, D.; Castagneyrol, B.; Gardiner, B.; Ramon Gonzalez-Olabarria, J.; Koricheva, J.; Meurisse, N.; Brockerhoff, E.G. Tree diversity drives forest stand resistance to natural disturbances. Curr. For. Rep. 2017, 3, 223–243. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J. A review of processes behind diversity-productivity relationships in forests. Curr. For. Rep. 2016, 2, 45–61. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).