Performance of Halo-Alkali-Tolerant Endophytic Bacteria on Hybrid Pennisetum and Bacterial Community under Varying Soil Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hybrid Pennisetum and Endophytic Bacteria
2.2. Bacteria Morphological Characteristics
2.3. Bacteria Physiological Characteristics
2.4. Gnotobiotic Trials
2.5. Greenhouse and Field Trials
2.6. DNA Extraction, Amplification, and 16SrRNA Sequence-Based Metagenomics
2.7. Statistical Analysis
3. Results
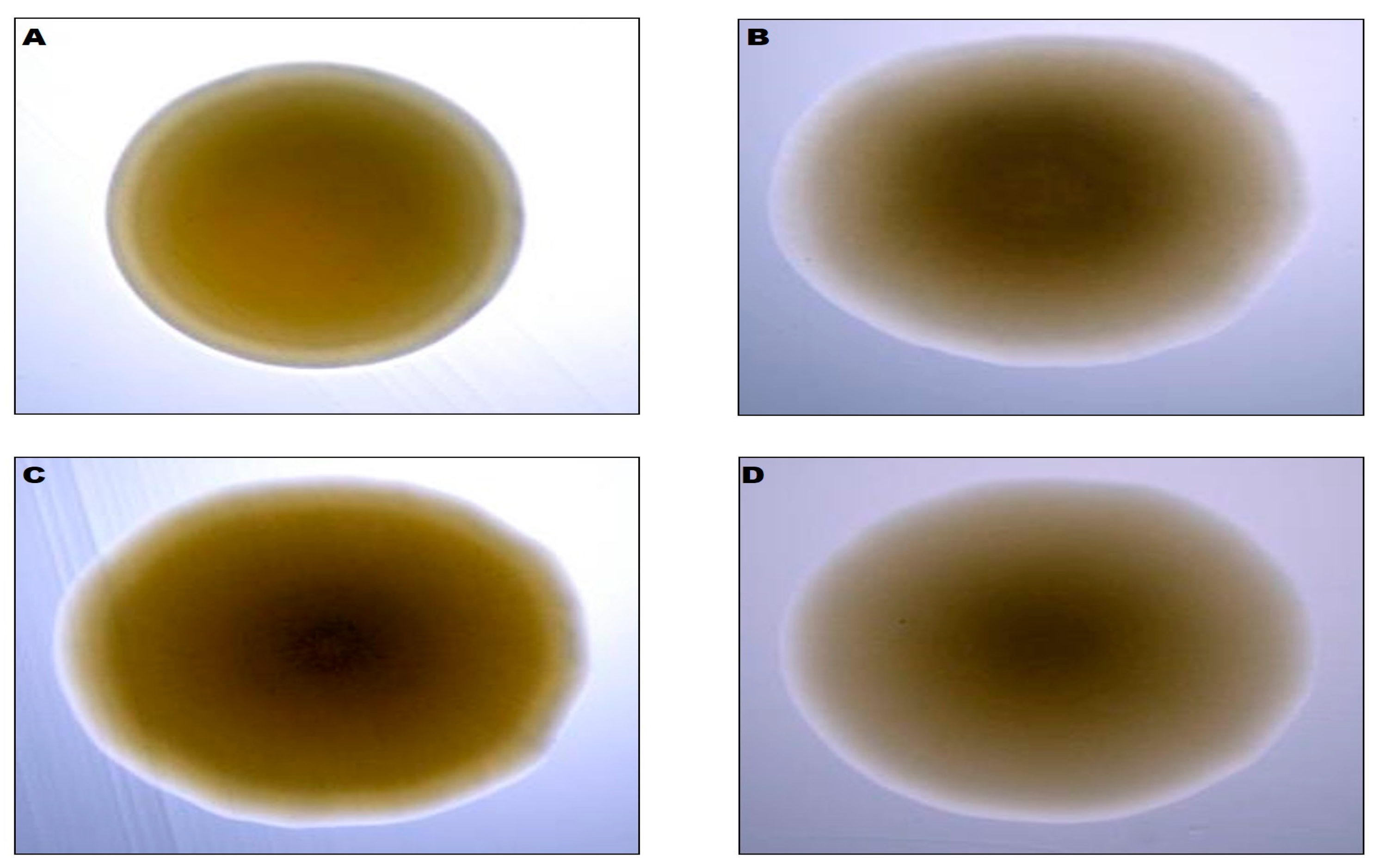
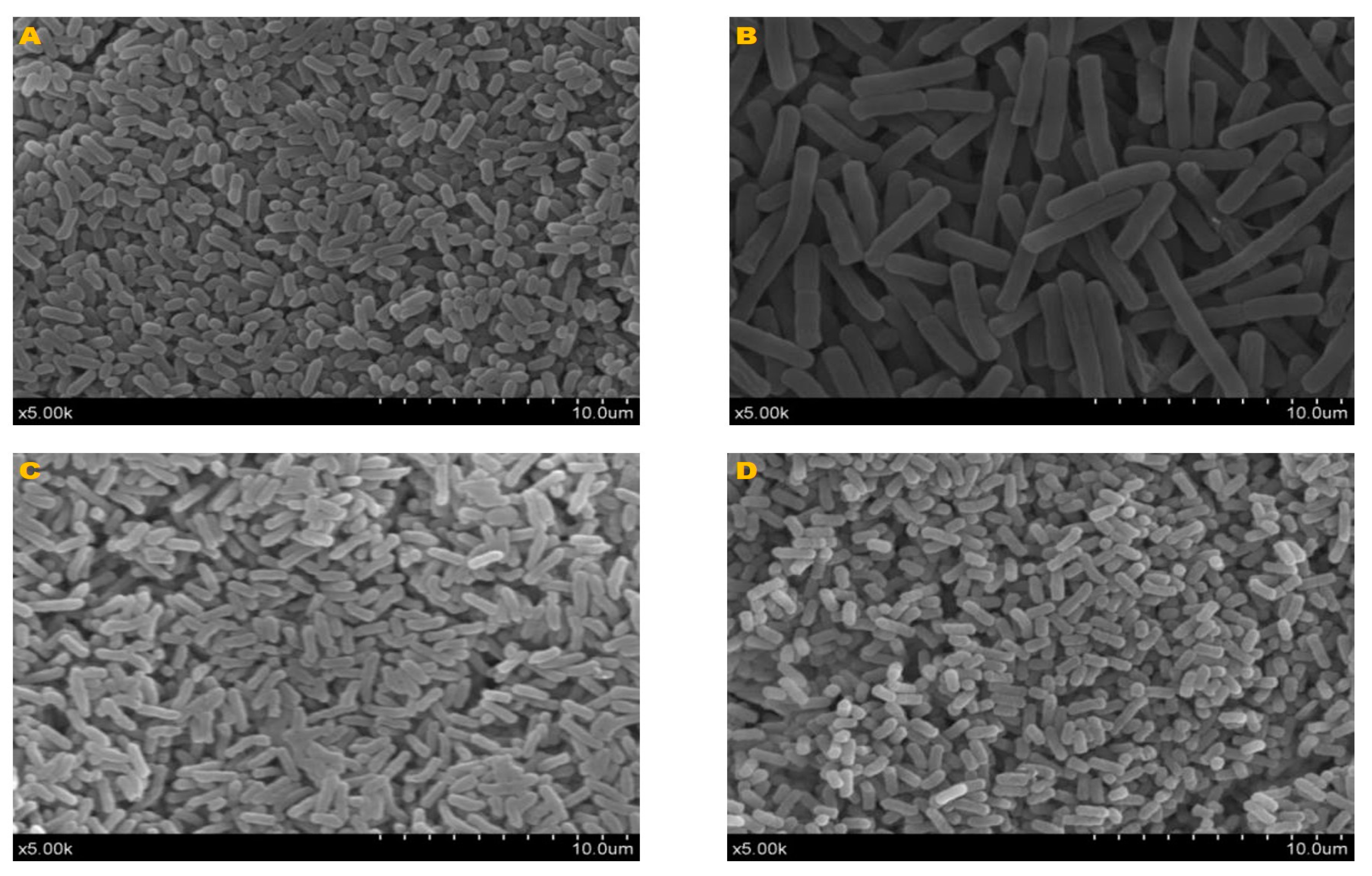
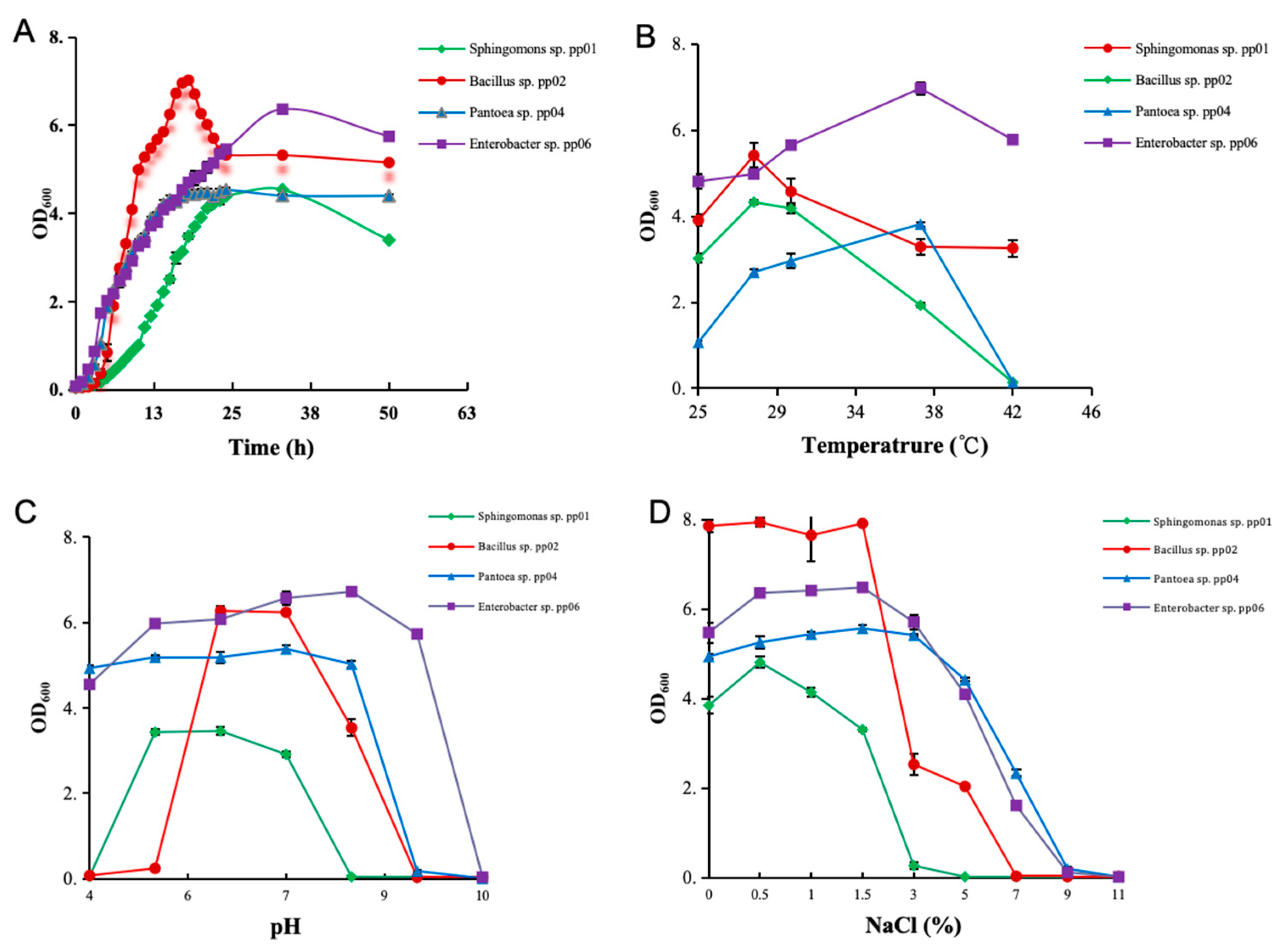
3.1. Bacteria Morphological and Physiological Properties
3.2. PGP Effects of Endophytic Bacteria under Gnotobiotic Conditions
3.3. PGP Effects of Endophytic Bacteria under Coastal Saline Soil Conditions
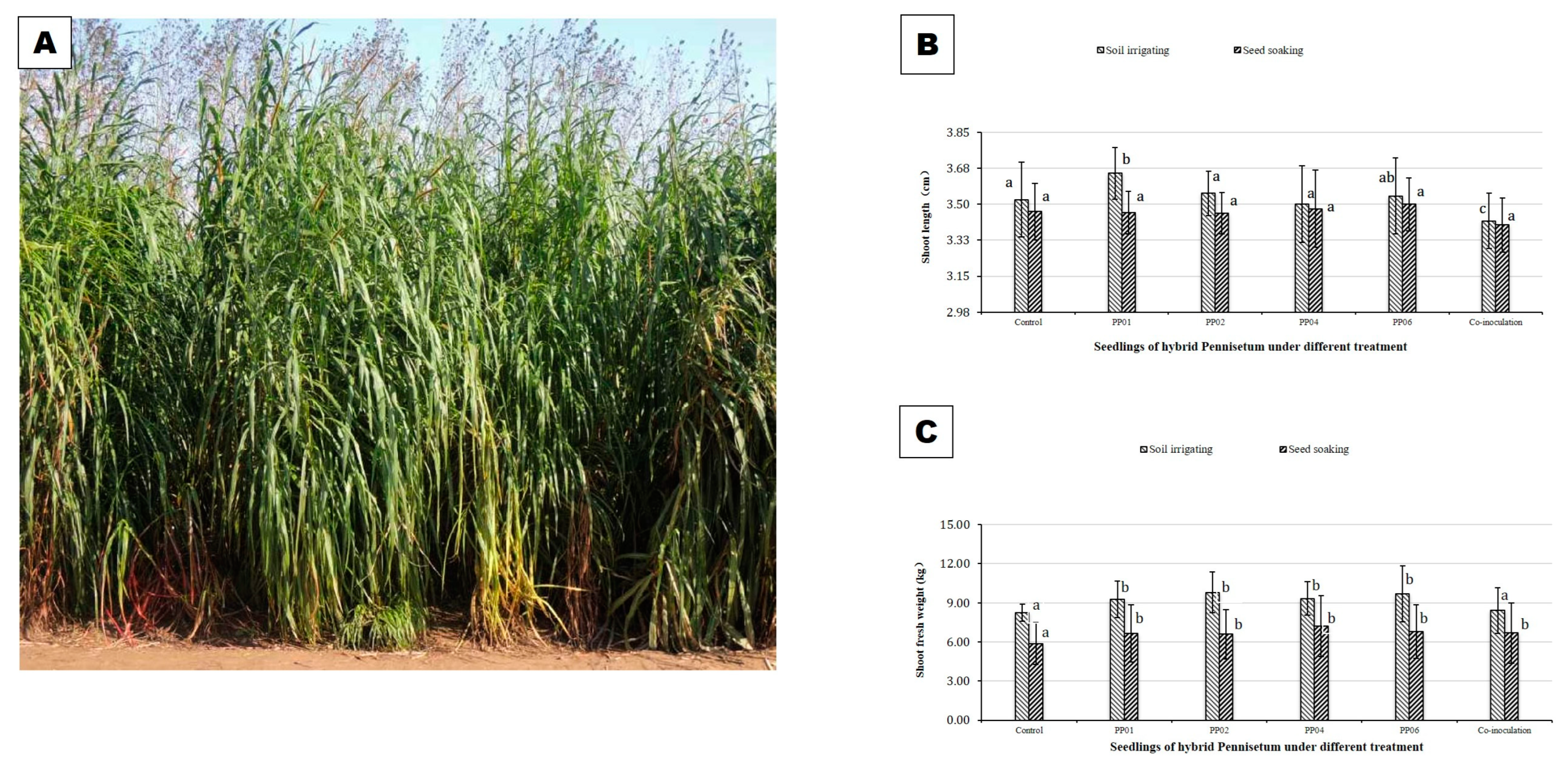
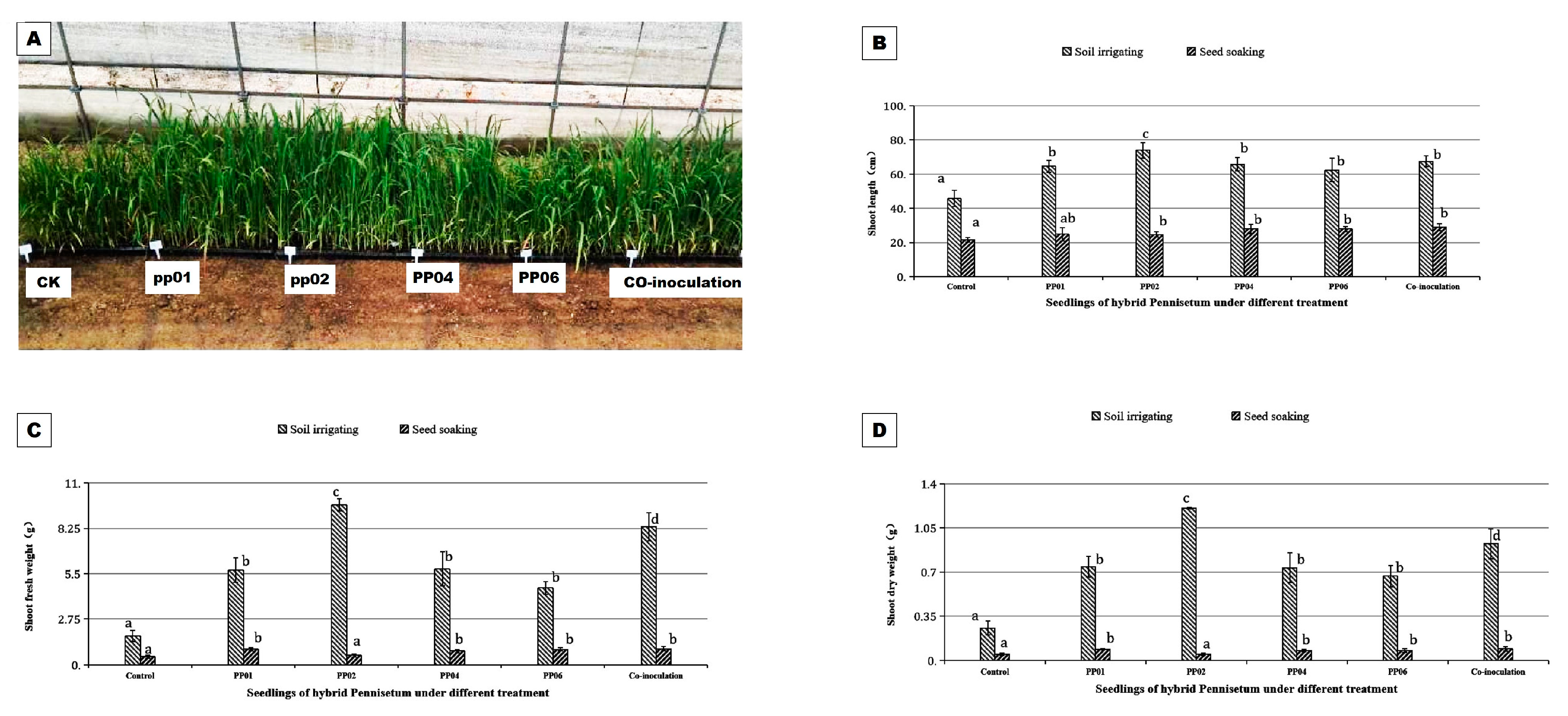
3.4. PGP Effects of Endophytic Bacteria under Greenhouse Conditions
3.5. Bacterial Community Associated with Endophytic Bacteria under Greenhouse Conditions
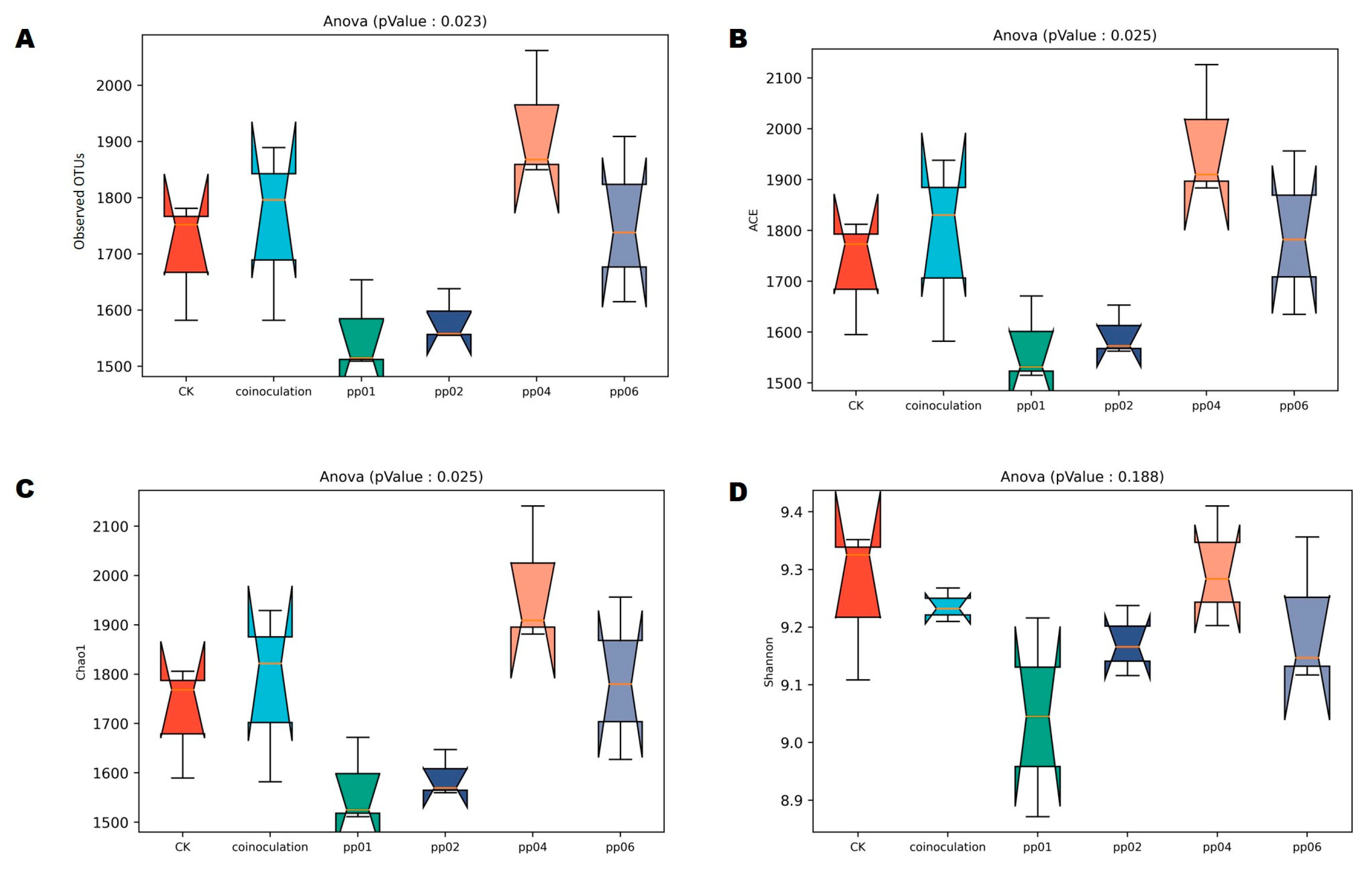
3.5.1. Diversity of Bacterial Community Associated with Endophytic Bacteria
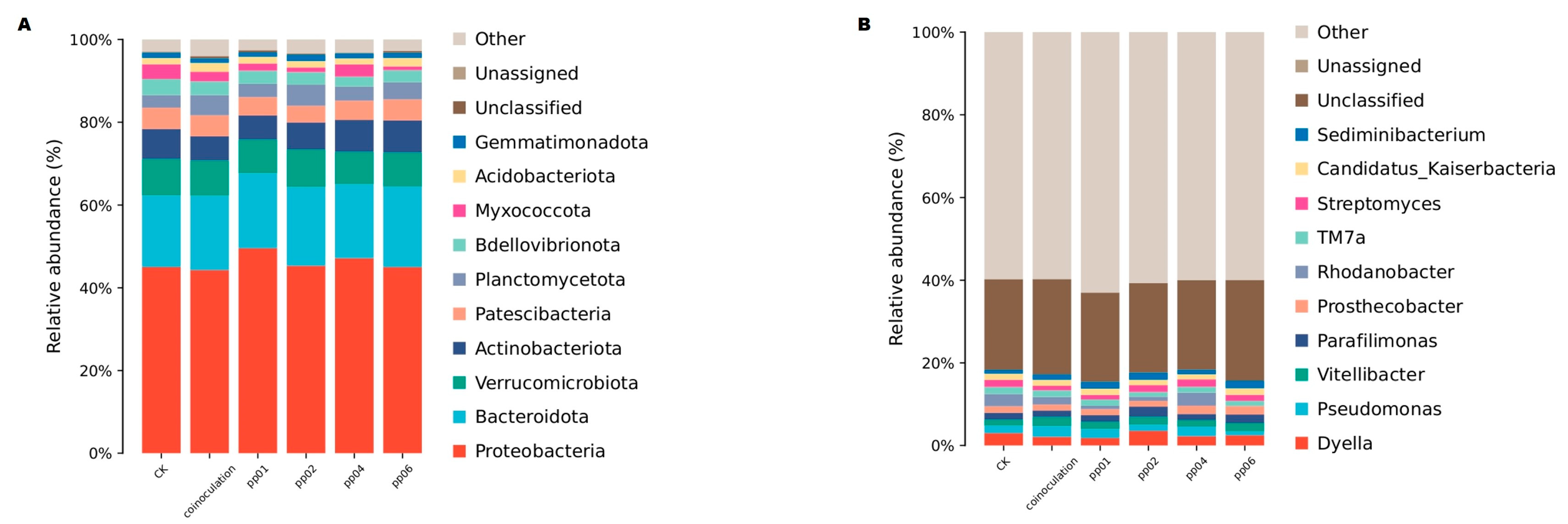
3.5.2. Taxonomic Composition of the Bacterial Community Associated with Endophytic Bacteria
3.5.3. Biomarkers of the Bacterial Community Associated with Endophytic Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Navarro-Torre, S.; Garcia-Caparrós, P.; Nogales, A.; Abreu, M.M.; Santos, E.; Cortinhas, A.L.; Caperta, A.D. Sustainable agricultural management of saline soils in arid and semi-arid Mediterranean regions through halophytes, microbial and soil-based technologies. Environ. Exp. Bot. 2023, 212, 105397. [Google Scholar] [CrossRef]
- Kushwaha, P.; Kashyap, P.L.; Bhardwaj, A.K.; Kuppusamy, P.; Srivastava, A.K.; Tiwari, R.K. Bacterial endophyte mediated plant tolerance to salinity: Growth responses and mechanisms of action. World J. Microbiol. Biotechnol. 2020, 36, 26. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chang, M.; Han, X.; Wang, Q.; Wang, J.; Yang, H.; Guan, Q.; Dai, S. Beneficial effects of endophytic Pantoea ananatis with ability to promote rice growth under saline stress. J. Appl. Microbiol. 2021, 131, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Santoyo, G.; White, J.F.; Mishra, V.K. Special Issue “Microbial Endophytes: Functional biology and applications”: Editorial. Microorganisms 2023, 11, 918. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fu, Q.; Zhu, J.; Sun, Y.; He, H.; Hu, H. Endophytic Bacteria in Ricinus communis L.: Diversity of bacterial community, plant-growth promoting traits of the isolates and its effect on Cu and Cd speciation in soil. Agronomy 2023, 13, 333. [Google Scholar] [CrossRef]
- Lou, F.; Okoye, C.O.; Gao, L.; Jiang, H.; Wu, Y.; Wang, Y.; Li, X.; Jiang, J. Whole-genome sequence analysis reveals phenanthrene and pyrene degradation pathways in newly isolated bacteria Klebsiella michiganensis EF4 and Klebsiella oxytoca ETN19. Microbiol. Res. 2023, 273, 127410. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, M.; Anandham, R.; Madhaiyan, M.; Venkateswaran, V.; Sa, T. Endophytic bacteria: Perspectives and applications in agricultural crop production. In Bacteria in Agrobiology: Crop Ecosystems; Maheshwari, D., Ed.; Springer: Heidelberg, Germany, 2011; pp. 61–96. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Iqrar, I. Selective isolation and characterization of agriculturally beneficial endophytic bacteria from wild hemp using canola. Pak. J. Bot. 2015, 47, 1999–2008. [Google Scholar]
- Durán, P.; Acuña, J.J.; Jorquera, M.A.; Azcón, R.; Paredes, C.; Rengel, Z.; de la Luz Mora, M. Endophytic bacteria from selenium-supplemented wheat plants could be useful for plant-growth promotion, biofortification and Gaeumannomyces graminis biocontrol in wheat production. Biol. Fertil. Soils 2014, 50, 983–990. [Google Scholar] [CrossRef]
- Ji, S.H.; Gururani, M.A.; Chun, S.-C. Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol. Res. 2014, 169, 83–98. [Google Scholar] [CrossRef]
- Mei, C.; Flinn, B.S. The use of beneficial microbial endophytes for plant biomass and stress tolerance improvement. Recent Patents Biotechnol. 2010, 4, 81–95. [Google Scholar] [CrossRef]
- Mohanty, S.R.; Dubey, G.; Kollah, B. Endophytes of Jatropha curcas promote growth of maize. Rhizosphere 2017, 3, 20–28. [Google Scholar] [CrossRef]
- Munif, A.; Hallmann, J.; Sikora, R.A. The influence of endophytic bacteria on meloidogyne incognita infection and tomato plant growth. J. ISSAAS 2013, 19, 68–74. [Google Scholar]
- Tan, F.; He, L.; Zhu, Q.; Wang, Y.; Chen, C.; He, M. Pennisetum hydridum: A potential energy crop with multiple functions and the current status in China. BioEnergy Res. 2021, 15, 850–862. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Y.; Wang, B.; Zhang, X.; Tan, T. Biomass to bio-ethanol: The evaluation of hybrid Pennisetum used as raw material for bio-ethanol production compared with corn stalk by steam explosion joint use of mild chemicals. Renew. Energy 2016, 88, 164–170. [Google Scholar] [CrossRef]
- He, J.; Su, S.; Han, Y. Determination of energy index of energy plants in rocky desertification area. Modern. Agric. Sci. Technol. 2017, 9, 253. (In Chinese) [Google Scholar] [CrossRef]
- Kang, X.; Sun, Y.; Li, L.; Kong, X.; Yuan, Z. Improving methane production from anaerobic digestion of Pennisetum Hybrid by alkaline pretreatment. Bioresour. Technol. 2018, 255, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Sun, Y.; Yuan, Z.; Lv, P.; Kang, X.; Zhang, Y.; Yang, G. Influence of the feedstock ratio and organic loading rate on the co-digestion performance of Pennisetum hybridum and cow manure. Energy Fuels 2018, 32, 5171–5180. [Google Scholar] [CrossRef]
- Wang, J.; Xin, D.; Hou, X.; Wu, J.; Fan, X.; Li, K.; Zhang, J. Structural properties and hydrolysabilities of Chinese Pennisetum and Hybrid Pennisetum: Effect of aqueous ammonia pretreatment. Bioresour. Technol. 2016, 199, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Liu, J.; Zhuang, X.; Yuan, Z.; Wang, W.; Qi, W.; Wang, Q.; Tan, X.; Kong, X. Liquid hot water pretreatment of energy grasses and its influence of physico-chemical changes on enzymatic digestibility. Bioresour. Technol. 2016, 199, 265–270. [Google Scholar] [CrossRef]
- Li, X.; Geng, X.; Xie, R.; Fu, L.; Jiang, J.; Gao, L.; Sun, J. The endophytic bacteria isolated from elephant grass (Pennisetum purpureum Schumach) promote plant growth and enhance salt tolerance of Hybrid Pennisetum. Biotechnol. Biofuels 2016, 9, 190. [Google Scholar] [CrossRef]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Pramanik, K.; Mitra, S.; Soren, T.; Maiti, T.K. Enhancement of growth and salt tolerance of rice seedlings by ACC deaminase-producing Burkholderia sp. MTCC 12259. J. Plant Physiol. 2018, 231, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Sturz, A. Bacterial endophytes: Potential role in developing sustainable systems of crop production. Crit. Rev. Plant Sci. 2000, 19, 1–30. [Google Scholar] [CrossRef]
- Mathenge, C.; Thuita, M.; Masso, C.; Gweyi-Onyango, J.; Vanlauwe, B. Variability of soybean response to rhizobia inoculant, vermicompost, and a legume-specific fertilizer blend in Siaya County of Kenya. Soil Tillage Res. 2019, 194, 104290. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Yousaf, S.; Reichenauer, T.G.; Sessitsch, A. The inoculation method affects colonization and performance of bacterial inoculant strains in the phytoremediation of soil contaminated with diesel oil. Int. J. Phytoremediat. 2012, 14, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Nowell, J.A.; Parule, J.B. Preparation of Experimental Tissue for Scanning Electron Microscopy; AMSO-HARE: Chicago, IL, USA, 1980. [Google Scholar]
- Wang, W.W.; Shi, S.Z.; Sui, Z.D. Effects of soil improvement measures on total salt content and pH of coastal saline alkali soil. J. Jiangsu Sci. Technol. 2017, 44, 17–20. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Niu, L.Y.; Wang, W.W.; Wang, L.P.; Lu, L.; Wang, F.Z.; Yu, L. Characteristics of soil salinity in plough layer of coastal saline alkali area of Cangzhou. Shaanxi J. Agric. Sci. 2021, 67, 62–66. [Google Scholar] [CrossRef]
- Chang, P.; Gerhardt, K.E.; Huang, X.-D.; Yu, X.-M.; Glick, B.R.; Gerwing, P.D.; Greenberg, B.M. Plant growth-promoting bacteria facilitate the growth of barley and oats in salt-impacted soil: Implications for phytoremediation of saline soils. Int. J. Phytoremediat. 2014, 16, 1133–1147. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Zhang, Y.; Bi, Y.; Sun, Z. Responses of saline soil properties and cotton growth to different organic amendments. Pedosphere 2018, 28, 521–529. [Google Scholar] [CrossRef]
- Schultz, N.; Pereira, W.; Silva, P.d.A.; Baldani, J.I.; Boddey, R.M.; Alves, B.J.R.; Urquiaga, S.; Reis, V.M. Yield of sugarcane varieties and their sugar quality grown in different soil types and inoculated with a diazotrophic bacteria consortium. Plant Prod. Sci. 2017, 20, 366–374. [Google Scholar] [CrossRef]
- Pellegrini, M.; Pagnani, G.; Rossi, M.; D’egidio, S.; Del Gallo, M.; Forni, C. Daucus carota L. seed inoculation with a consortium of bacteria improves plant growth, soil fertility status and microbial community. Appl. Sci. 2021, 11, 3274. [Google Scholar] [CrossRef]
- Gorai, P.S.; Ghosh, R.; Mandal, S.; Ghosh, S.; Chatterjee, S.; Gond, S.K.; Mandal, N.C. Bacillus siamensis CNE6-a multifaceted plant growth promoting endophyte of Cicer arietinum L. having broad spectrum antifungal activities and host colonizing potential. Microbiol. Res. 2021, 252, 126859. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, Z.; Xie, J.; Hesselberg-Thomsen, V.; Tan, T.; Zheng, D.; Strube, M.L.; Dragoš, A.; Shen, Q.; Zhang, R.; et al. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2022, 16, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Prasad, V.; Lata, C. Bacillus: Plant growth promoting bacteria for sustainable agriculture and environment. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J.S., Singh, D.P., Eds.; Elsevier: Lucknow, India, 2019; pp. 43–55. [Google Scholar] [CrossRef]
- Yarzábal, L.A. Agricultural development in tropical acidic soils: Potential and limits of phosphate-solubilizing bacteria. In Soil Biology and Agriculture in the Tropics, Soil Biology; Dion, P., Ed.; Springer: Heidelberg, Germany, 2010; pp. 209–233. [Google Scholar] [CrossRef]
- Bradshaw, M.J.; Pane, A.M. Field inoculations of nitrogen fixing endophytes on turfgrass. Physiol. Mol. Plant Pathol. 2020, 112, 101557. [Google Scholar] [CrossRef]
- Martínez-Viveros, O.; Jorquera, M.; Crowley, D.; Gajardo, G.; Mora, M. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J. Soil Sci. Plant Nutr. 2010, 10, 293–319. [Google Scholar] [CrossRef]
- Afzal, M.; Khan, S.; Iqbal, S.; Mirza, M.S.; Khan, Q.M. Inoculation method affects colonization and activity of Burkholderia phytofirmans PsJN during phytoremediation of diesel-contaminated soil. Int. Biodeterior. Biodegrad. 2013, 85, 331–336. [Google Scholar] [CrossRef]
- Cely, M.V.T.; Siviero, M.A.; Emiliano, J.; Spago, F.R.; Freitas, V.F.; Barazetti, A.R.; Goya, E.T.; Lamberti, G.d.S.; dos Santos, I.M.O.; De Oliveira, A.G.; et al. Inoculation of Schizolobium parahyba with mycorrhizal fungi and Plant growth-promoting rhizobacteria increases wood yield under field conditions. Front. Plant Sci. 2016, 7, 1708. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.A.; Mahmood, I.A.; Ali, A.; Nawaz, Q.; Sultan, T.; Zaman, B.U. Effect of inoculation methods of biozote-max (plant growth promoting rhizobacteria-pgpr) on growth and yield of rice under naturally salt-affected soil. Res. Plant Biol. 2017, 7, 24–26. [Google Scholar] [CrossRef]
- Lopes, M.J.d.S.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful plant growth-promoting microbes: Inoculation methods and abiotic factors. Front. Sustain. Food Syst. 2021, 5, 48. [Google Scholar] [CrossRef]
- Wang, G.-H.; Jin, J.; Xu, M.-N.; Pan, X.-W.; Tang, C. Inoculation with phosphate-solubilizing fungi diversifies the bacterial community in rhizospheres of maize and soybean. Pedosphere 2007, 17, 191–199. [Google Scholar] [CrossRef]
- Yasuda, M.; Dastogeer, K.M.G.; Sarkodee-Addo, E.; Tokiwa, C.; Isawa, T.; Shinozaki, S.; Okazaki, S. Impact of Azospirillum sp. B510 on the rhizosphere microbiome of rice under field conditions. Agronomy 2022, 12, 1367. [Google Scholar] [CrossRef]
- Gadhave, K.R.; Devlin, P.F.; Ebertz, A.; Ross, A.; Gange, A.C. Soil inoculation with Bacillus spp. modifies root endophytic bacterial diversity, evenness, and community composition in a context-specific manner. Microb. Ecol. 2018, 76, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, F.; Novello, G.; Bona, E.; Gorrasi, S.; Gamalero, E. Impact of plant-beneficial bacterial inocula on the resident bacteriome: Current knowledge and future perspectives. Microorganisms 2022, 10, 2462. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Huang, Y.; Liu, Y.; Mohamed, O.A.A.; Fan, X.; Wang, L.; Li, L.; Ma, J. Bacterial community structure and potential microbial coexistence mechanism associated with three halophytes adapting to the extremely hypersaline environment. Microorganisms 2022, 10, 1124. [Google Scholar] [CrossRef]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J.; Schenk, P.M. Inner plant values: Diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Matsutani, M.; Shiwa, Y.; Ishige, T.; Sakamoto, H.; Saitoh, H.; Tsushima, S. Comparative analysis of bacterial diversity and community structure in the rhizosphere and root endosphere of two halophytes, Salicornia europaea and Glaux maritima, collected from two brackish lakes in Japan. Microbes Environ. 2020, 35, ME20072. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gu, C.; Liu, X.; Yang, C.; Li, W.; Wang, S. Impact of soybean nodulation phenotypes and nitrogen fertilizer levels on the rhizosphere bacterial community. Front. Microbiol. 2020, 11, 750. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Garcia, E.Y.; Hernandez-Trejo, V.; Guevara-Luna, J.; Rojas-Rojas, F.U.; Arroyo-Herrera, I.; Meza-Radilla, G.; Vasquez-Murrieta, M.S.; Estrada-de Los Santos, P. Plant growth-promoting bacteria isolated from wild legume nodules and nodules of Phaseolus vulgaris L. trap plants in central and southern Mexico. Microbiol. Res. 2020, 239, 126522. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-C.; Peng, H.-M.; Wollney, S.; Shen, C.-H. Rhizosphere microbiome regulates the growth of mustard under organic greenhouse cultivation. Agriculture 2021, 11, 987. [Google Scholar] [CrossRef]
- Bhat, B.A.; Tariq, L.; Nissar, S.; Islam, S.T.; Islam, S.U.; Mangral, Z.; Ilyas, N.; Sayyed, R.Z.; Muthusamy, G.; Kim, W.; et al. The role of plant-associated rhizobacteria in plant growth, biocontrol and abiotic stress management. J. Appl. Microbiol. 2022, 133, 2717–2741. [Google Scholar] [CrossRef]
- Georgieva, G.; Nedeva, T.; Badalova, M.; Deleva, V.; Savov, V. Study of the plant growth-promoting capacity of Pseudomonas putida 1046 in a model plant system. BioRisk 2023, 20, 115–128. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd Allah, E. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Zulueta-Rodriguez, R.; Cordoba-Matson, M.V.; Hernandez-Montiel, L.G.; Murillo-Amador, B.; Rueda-Puente, E.; Lara, L. Effect of Pseudomonas putida on growth and anthocyanin pigment in two poinsettia (Euphorbia pulcherrima) cultivars. Sci. World J. 2014, 2014, 810192. [Google Scholar] [CrossRef] [PubMed]
- Comeau, D.; Balthazar, C.; Novinscak, A.; Bouhamdani, N.; Joly, D.L.; Filion, M. Interactions between Bacillus spp., Pseudomonas spp. and Cannabis sativa Promote Plant Growth. Front. Microbiol. 2021, 12, 715758. [Google Scholar] [CrossRef]
- Huang, Y. Illumina-based analysis of endophytic bacterial diversity of four Allium species. Sci. Rep. 2019, 9, 15271. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, S.; Mehnaz, S.; Malik, K.A. Comparative study of the rhizosphere and root endosphere microbiomes of cholistan desert plants. Front. Microbiol. 2021, 12, 618742. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qi, Y.; Liu, J.; Wu, Z.; Gao, P.; Chen, Z.; Huang, F.; Yu, L. Dynamic changes in the endophytic bacterial community during maturation of Amorphophallus muelleri seeds. Front. Microbiol. 2022, 13, 996854. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Shi, L.; Xie, Z.; Lian, L.; Zhang, J.; Jiang, Z.; Wu, C. Shifts in the diversity of root endophytic microorganisms across the life cycle of the ratooning rice Jiafuzhan. Front. Microbiol. 2023, 14, 1161263. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; Van Der Heijden, M.G. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; de Bruin, S.; Luckerhoff, L.; Van Logtestijn, R.S.P.; Schlaeppi, K. A widespread plant–fungal–bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J. 2016, 10, 389–399. [Google Scholar] [CrossRef]
- Duran, P.; Thiergart, T.; Garrido-Oter, R.; Agler, M.; Kemen, E.; Schulze-Lefert, P.; Hacquard, S. Microbial interkingdom interactions in roots promote Arabidopsis survival. Cell 2018, 175, 973–983. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Ding, Y.; Okoye, C.O.; Geng, X.; Jiang, H.; Wang, Y.; Wu, Y.; Gao, L.; Fu, L.; Jiang, J.; et al. Performance of Halo-Alkali-Tolerant Endophytic Bacteria on Hybrid Pennisetum and Bacterial Community under Varying Soil Conditions. Microorganisms 2024, 12, 1062. https://doi.org/10.3390/microorganisms12061062
Li X, Ding Y, Okoye CO, Geng X, Jiang H, Wang Y, Wu Y, Gao L, Fu L, Jiang J, et al. Performance of Halo-Alkali-Tolerant Endophytic Bacteria on Hybrid Pennisetum and Bacterial Community under Varying Soil Conditions. Microorganisms. 2024; 12(6):1062. https://doi.org/10.3390/microorganisms12061062
Chicago/Turabian StyleLi, Xia, Yiming Ding, Charles Obinwanne Okoye, Xiaoyan Geng, Huifang Jiang, Yongli Wang, Yanfang Wu, Lu Gao, Lei Fu, Jianxiong Jiang, and et al. 2024. "Performance of Halo-Alkali-Tolerant Endophytic Bacteria on Hybrid Pennisetum and Bacterial Community under Varying Soil Conditions" Microorganisms 12, no. 6: 1062. https://doi.org/10.3390/microorganisms12061062





