A Promising Amphotericin B Derivative Induces Morphological Alterations, Mitochondrial Damage, and Oxidative Stress In Vitro and Prevents Mice from Death Produced by a Virulent Strain of Trypanosoma cruzi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Parasites and Mammalian Cells
2.3. Effect of A21 on the Number and Mobility of T. cruzi
2.4. Metabolic Activity of Trypanosomatids
2.5. Effect of A21 on Intracellular Amastigotes
2.6. Cytotoxic Effect of A21 in Vero Cells and Selectivity Index
2.7. Effect of A21 in Morphology and Ultrastructure of T. cruzi
2.8. Cell Volume Determination
2.9. Change in Cell Membrane Permeability
2.10. Determination of Intracellular Oxidative Stress
2.11. Mitochondrial Potential Assay (ΔΨm)
2.12. Ergosterol Quantification
2.13. Effect of A21 on In Vivo Infection Model
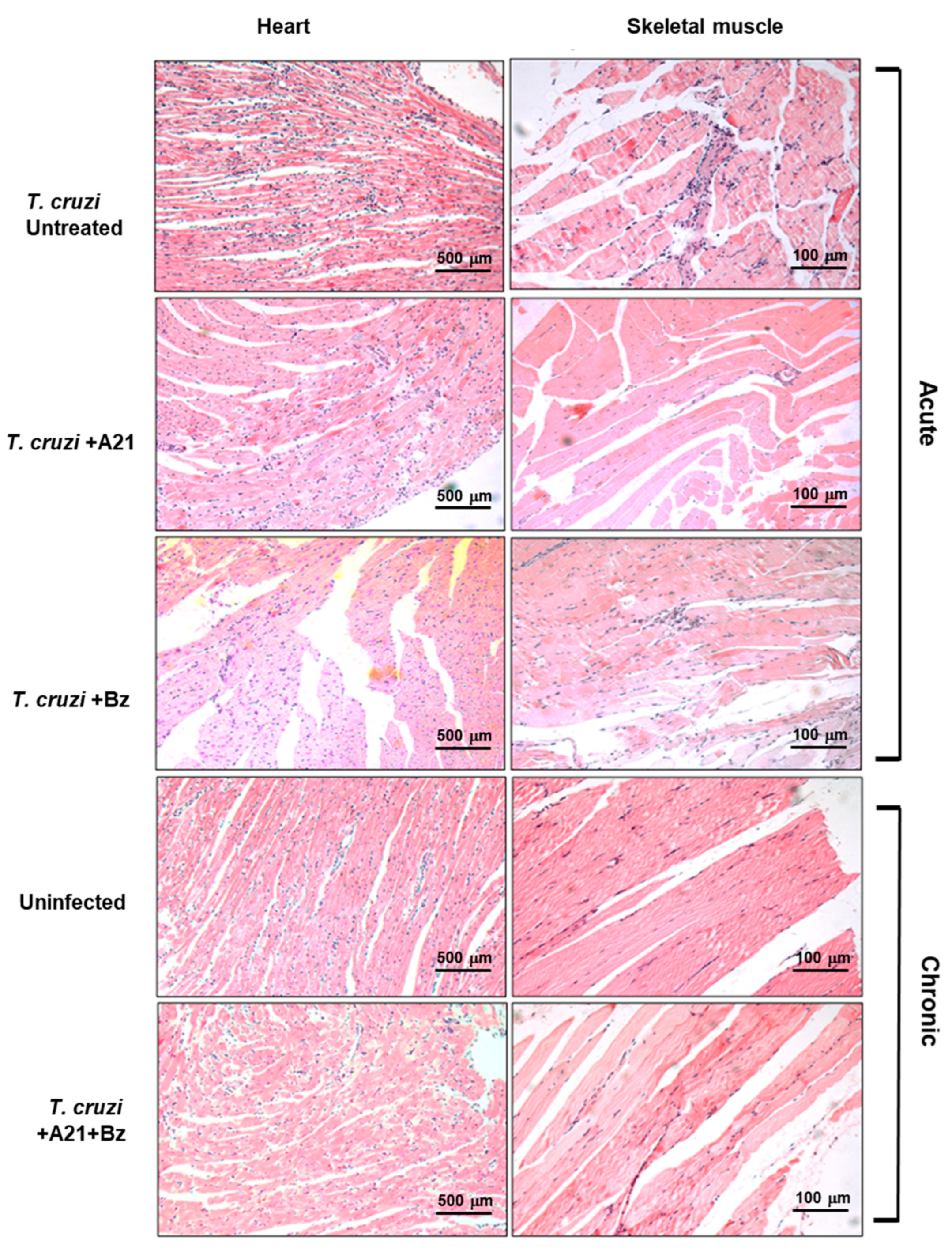
2.14. Histological Analysis
2.15. Mice and Animal Ethical Management
2.16. Statistical Analysis
3. Results
3.1. Compound A21 Reduces the Number and Mobility of T. cruzi
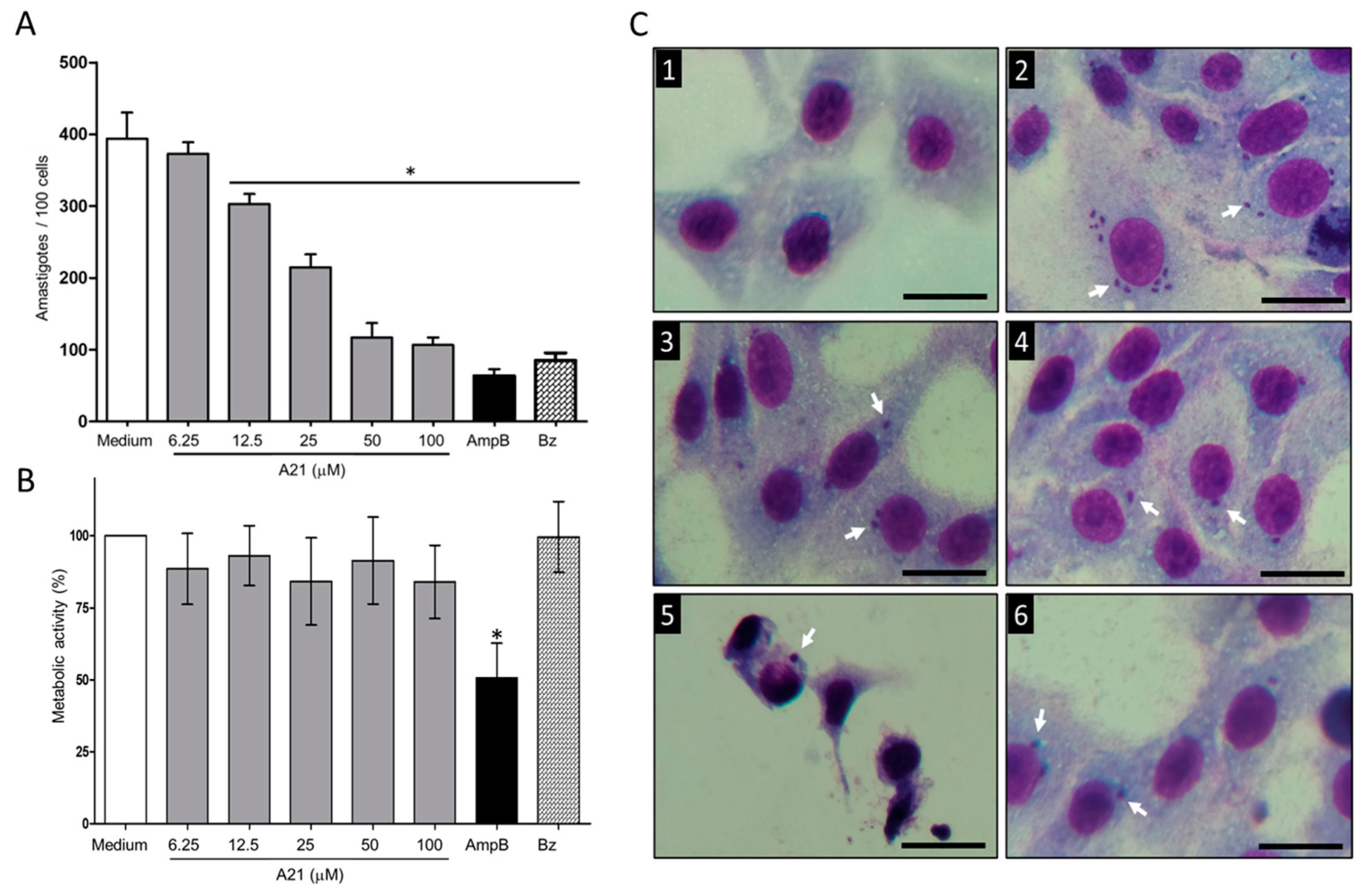
3.2. A21 Reduces the Number of Intracellular Amastigotes
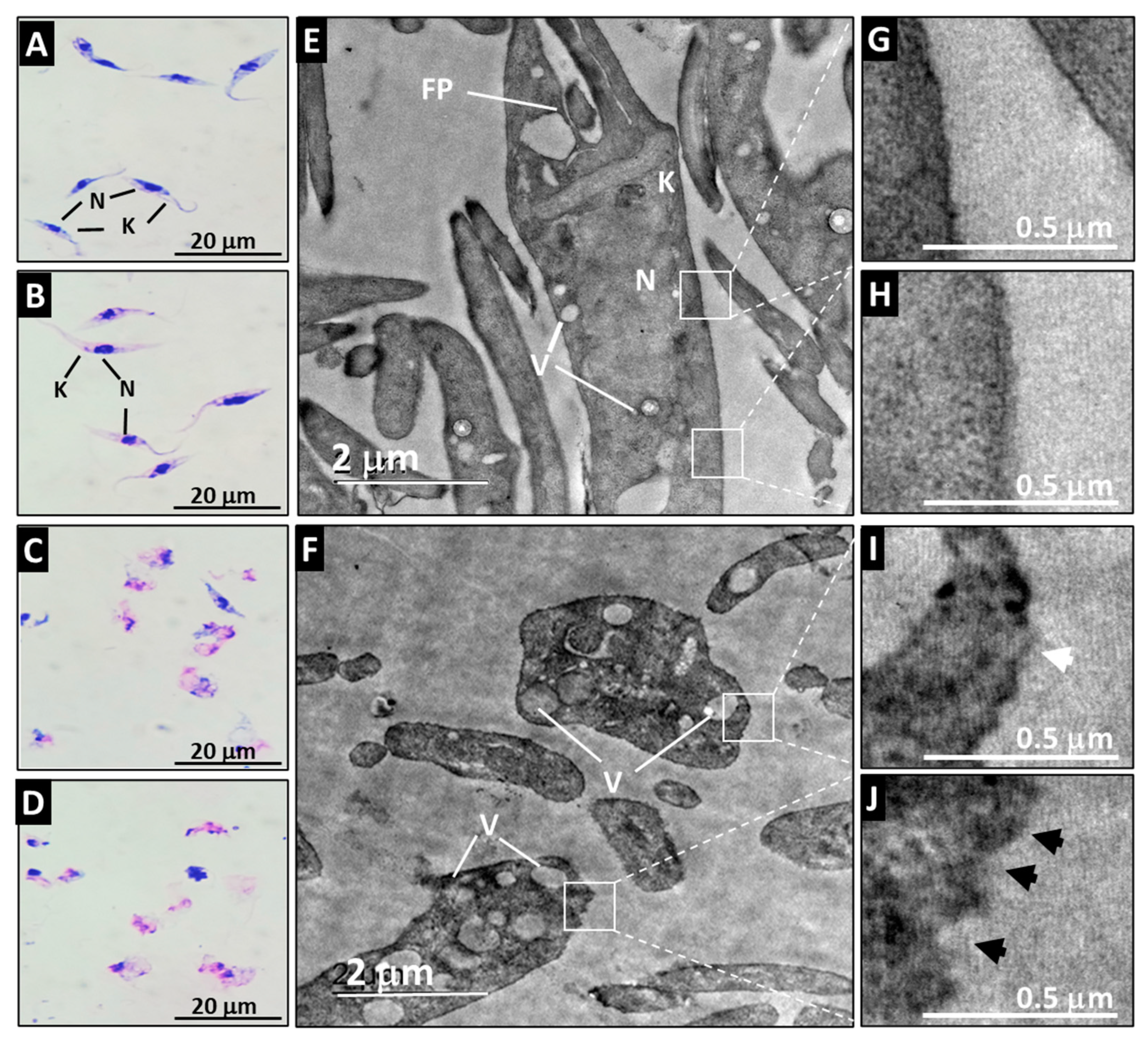
3.3. A21 Alters the Morphology and Ultrastructure of T. cruzi
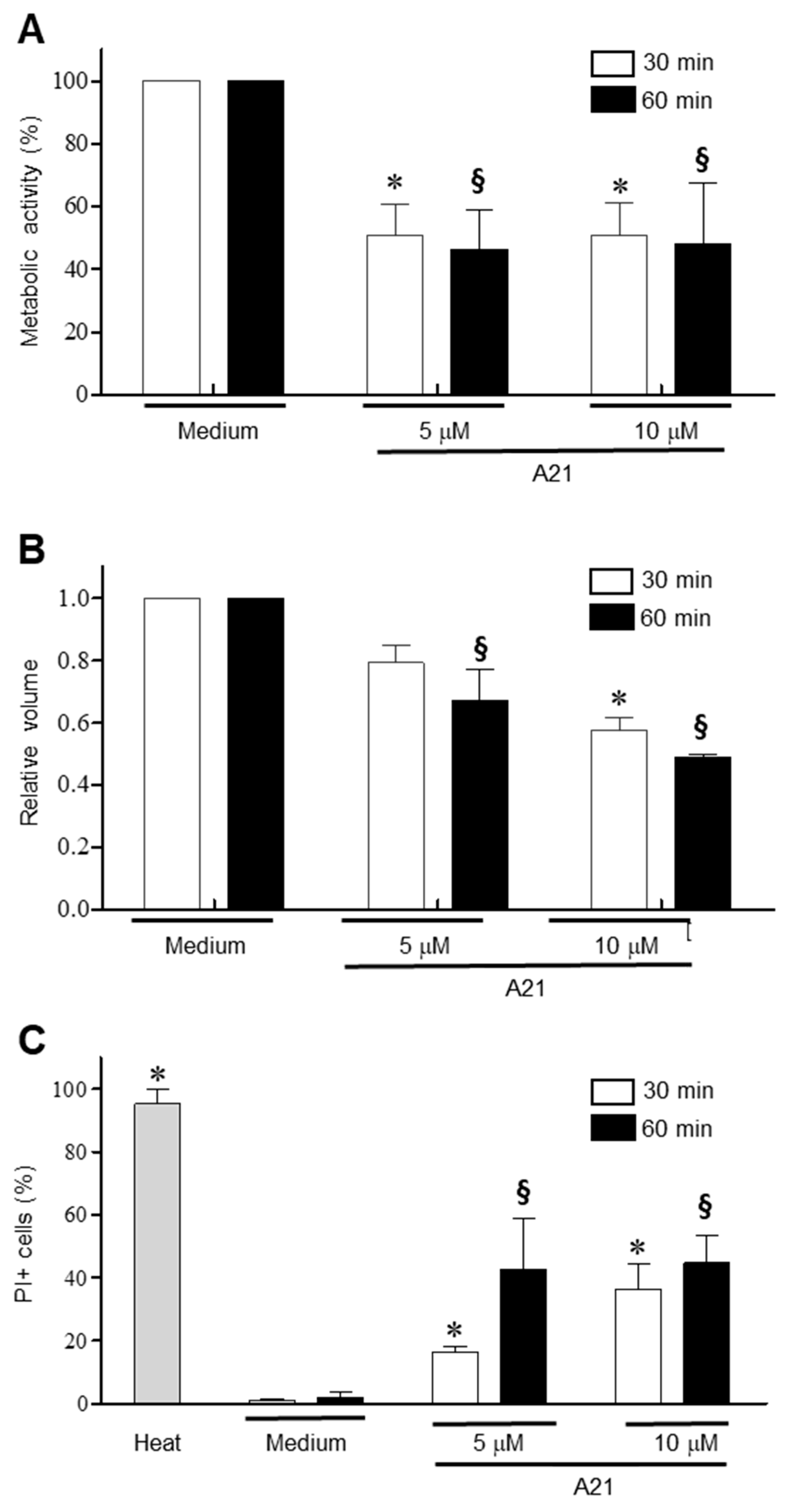
3.4. A21 Induces Early Changes in Metabolic Activity, Cell Volume and Membrane Permeability in T. cruzi
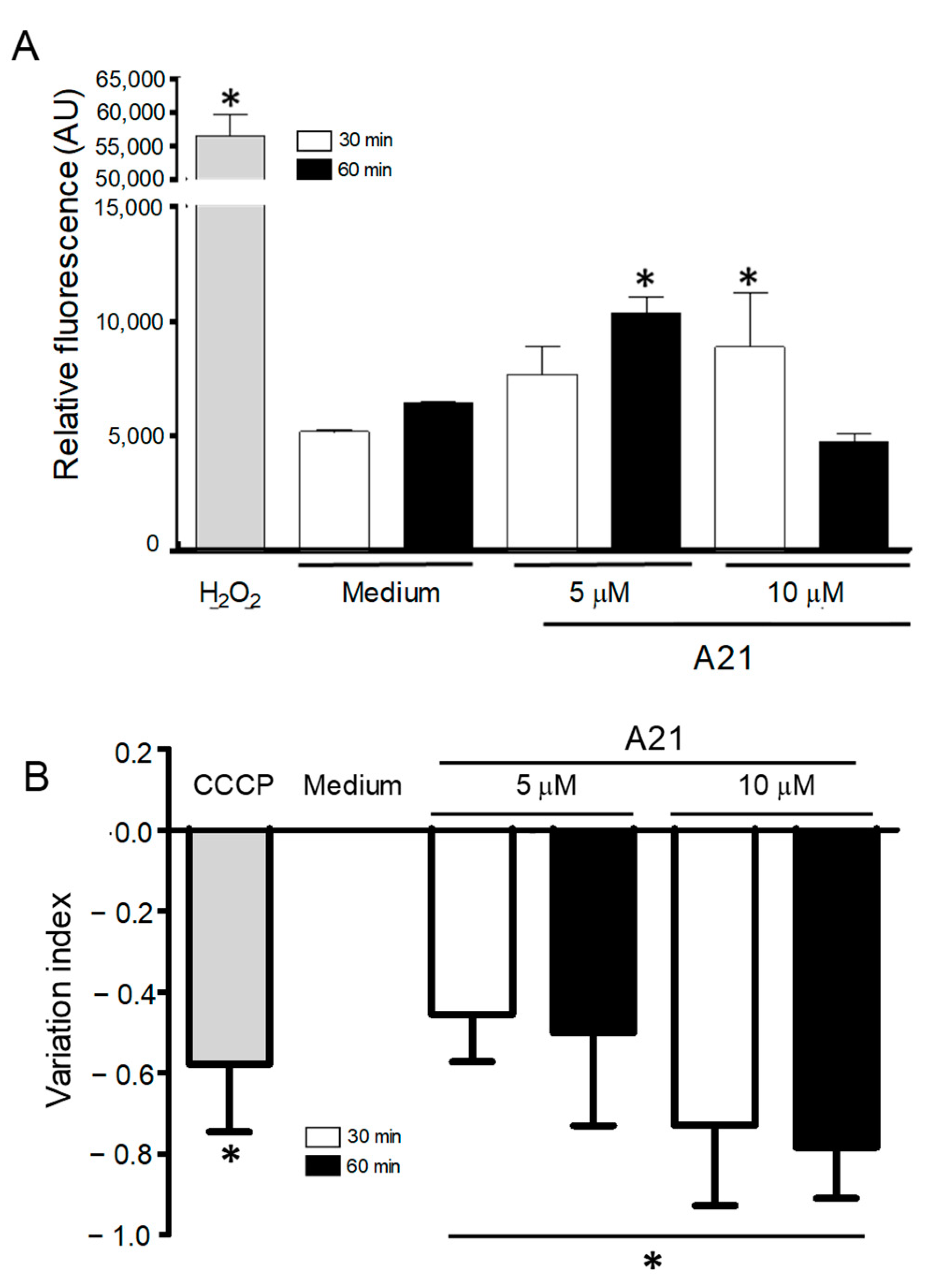
3.5. A21 Increases the Reactive Oxygen Species
3.6. A21 Induce Depolarization of Mitochondrial Membrane Potential (ΔΨm)
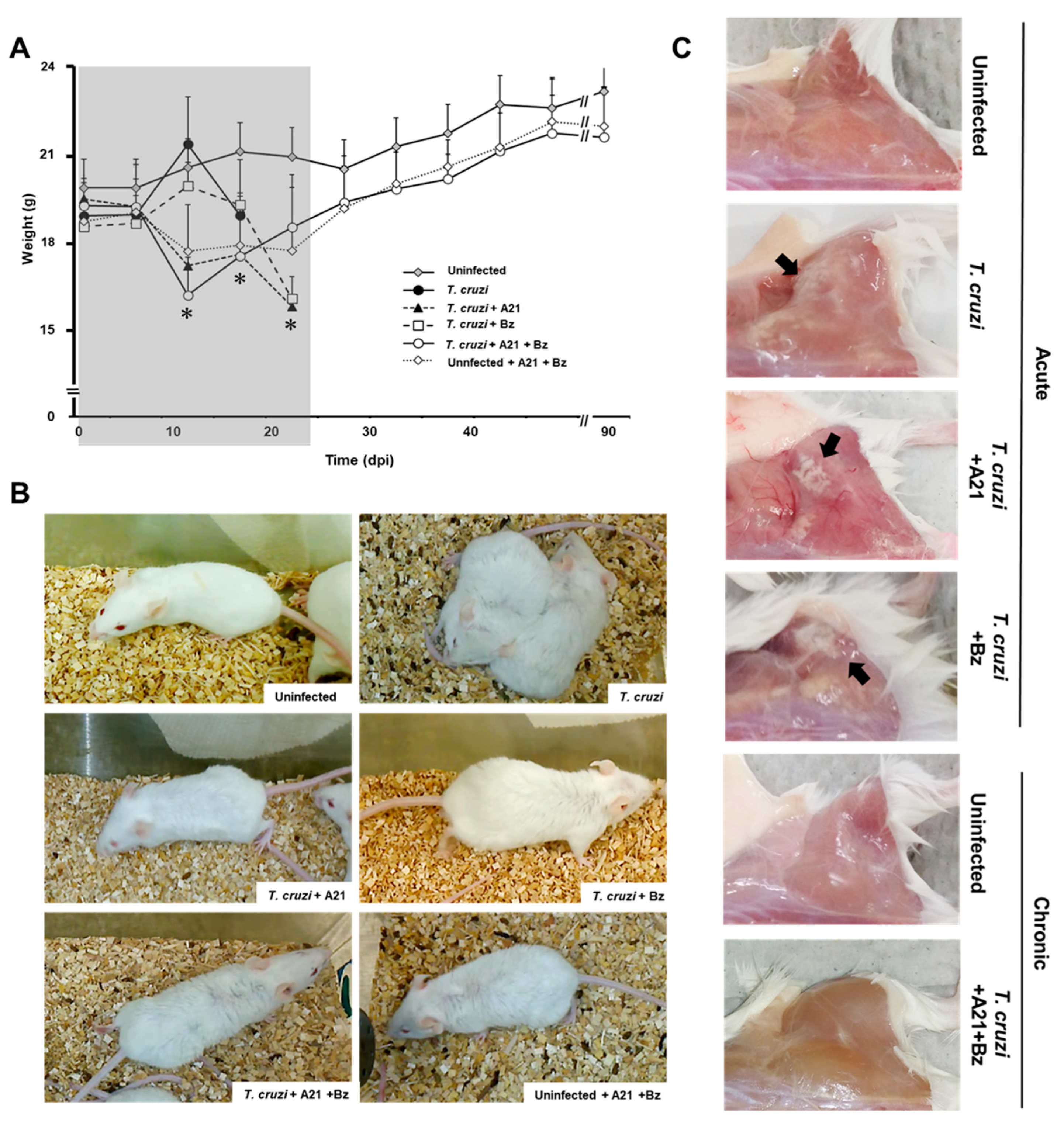
3.7. A21 Affects the Mobility and Reduces Weight in T. cruzi Infected Mice
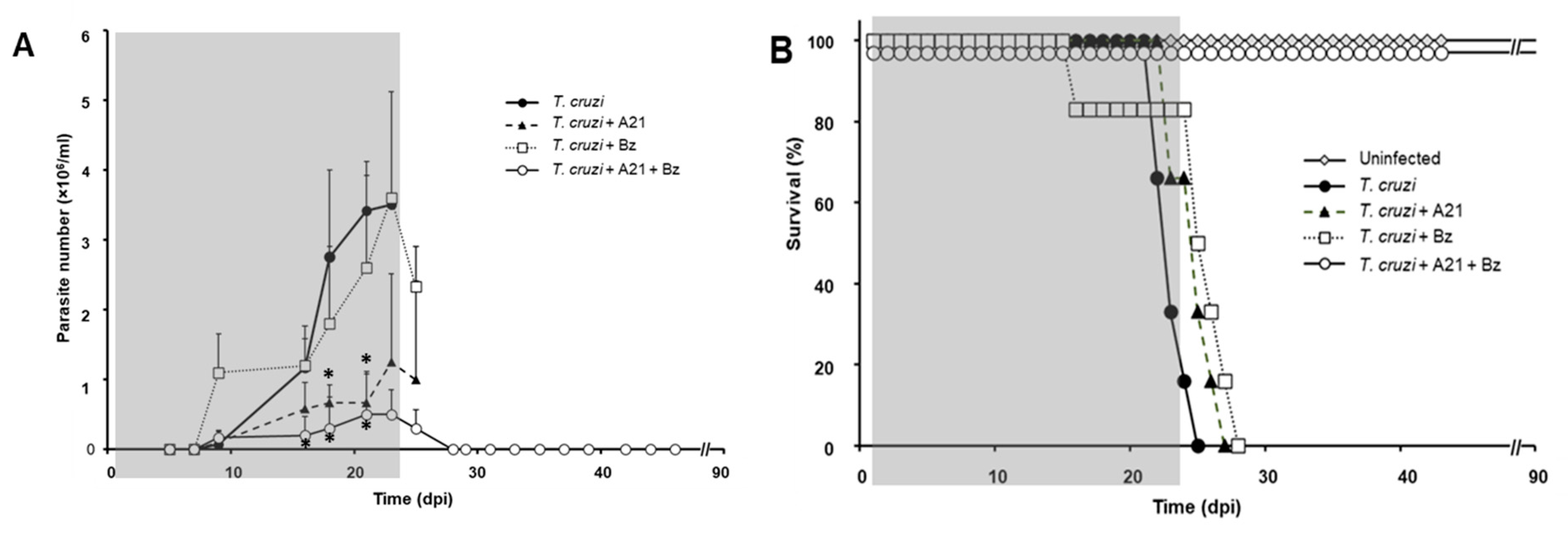
3.8. A21 in Combination with Benznidazole Reduce Parasitemia and Prevents Death in Mice
4. Discussion
5. Conclusions
6. Mexican Patente
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vázquez, C.; García-Vázquez, E.; Carrilero, B.; Simón, M.; Franco, F.; Iborra, M.A.; Gil-Gallardo, L.J.; Segovia, M. Tolerance and adherence of patients with chronic Chagas disease treated with Benznidazole. Rev. Soc. Bras. Med. Trop. 2023, 56, e0384. [Google Scholar] [CrossRef] [PubMed]
- WHO Chagas Disease (Also Known as American Trypanosomiasis). World Health Organization, Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 25 January 2024).
- Mazzeti, A.L.; Capelari-Oliveira, P.; Bahia, M.T.; Mosqueira, V.C.F. Review on experimental treatment strategies against Trypanosoma cruzi. J. Exp. Pharmacol. 2021, 13, 409–432. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Abolmaali, S.S.; Tamaddon, A.M.; Zomorodian, K.; Sarkari, B.S. Nanotechnology approaches for delivery and targeting of Amphotericin B in fungal and parasitic diseases. Nanomedicine 2021, 16, 857–877. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 2012, 3, 286. [Google Scholar] [CrossRef] [PubMed]
- de-Castro, S.L.; Soeiro, M.N.; Higashi, K.O.; Meirelles, M.N. Differential effect of amphotericin B on the three evolutive stages of Trypanosoma cruzi and on the host cell-parasite interaction. Braz. J. Med. Biol. Res. 1993, 26, 1219–1229. [Google Scholar] [PubMed]
- Rolón, M.; Serrano, D.R.; Lalatsa, A.; de Pablo, E.; Torrado, J.J.; Ballesteros, M.P.; Healy, A.M.; Vega, C.; Coronel, C.; Bolás-Fernández, F.; et al. Engineering oral and parenteral amorphous amphotericin B formulations against experimental Trypanosoma cruzi infections. Mol. Pharm. 2017, 14, 1095–1106. [Google Scholar] [CrossRef]
- Antillón, A.; de Vries, A.H.; Espinosa-Caballero, M.; Falcón-González, J.M.; Flores Romero, D.; González-Damián, J.; Jiménez-Montejo, F.E.; León-Buitimea, A.; López-Ortiz, M.; Magaña, R.; et al. An amphotericin B derivative equally potent to amphotericin B and with increased safety. PLoS ONE 2016, 11, e0162171. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Blake, I.; Fernández-Zertuche, M.; Regla, I.; Sánchez-Peña, W.; Gómez-Solis, A.; Jaimes-Chavez, P.; Galván-Hernández, A.; Tovar-Garduño, E.; Rodríguez-Fragoso, L. Preclinical safety evaluation of amphotericin A21: A novel antifungal. Basic Clin. Pharmacol. Toxicol. 2021, 129, 72–81. [Google Scholar] [CrossRef]
- Lazardi, K.; Urbina, J.A.; de Souza, W. Ultrastructural alterations induced by two ergosterol biosynthesis inhibitors, ketoconazole and terbinafine, on epimastigotes and amastigotes of Trypanosoma (Schizotrypanum) cruzi. Antimicrob. Agents Chemother. 1990, 34, 2097–2105. [Google Scholar] [CrossRef]
- García, M.C.; Manzo, R.H.; Jimenez-Kairuz, A.F. Extemporaneous benznidazole oral suspension prepared from commercially available tablets for treatment of Chagas disease in pediatric patients. Trop. Med. Int. Health 2015, 20, 864–870. [Google Scholar] [CrossRef]
- Barbeira, P.J.; Silva, G.M.; Beatriz, M.L.; Stradiotto, N.R. Polarographic determination of benznidazole in DMSO. J. Pharm. Biomed. Anal. 1999, 20, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Flores-Romero, J.D.; Rodríguez-Lozada, J.; López-Ortiz, M.; Magaña, R.; Ortega-Blake, I.; Regla, I.; Fernández-Zertuche, M. Multigram scale synthesis of, A21., a new antibiotic equally effective and less toxic than amphotericin, B. Org. Process Res. Dev. 2016, 20, 1529–1532. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, K.D.; Martínez, I.; Reyes-Chilpa, R.; Espinoza, B. Mammea type coumarins isolated from Calophyllum brasiliense induced apoptotic cell death of Trypanosoma cruzi through mitochondrial dysfunction, ROS production and cell cycle alterations. Bioorganic Chem. 2020, 100, 103894. [Google Scholar] [CrossRef] [PubMed]
- Romero-Meza, G.; Vélez-Ramírez, D.E.; Florencio-Martínez, L.E.; Román-Carraro, F.C.; Manning-Cela, R.; Hernández-Rivas, R.; Martínez-Calvillo, S. Maf1 is a negative regulator of transcription in Trypanosoma brucei. Mol. Microbiol. 2017, 103, 452–468. [Google Scholar] [CrossRef]
- Martínez, I.; Nogueda, B.; Martínez-Hernández, F.; Espinoza, B. Microsatellite and mini-exon analysis of Mexican human DTU I Trypanosoma cruzi strains and their susceptibility to nifurtimox and benznidazole. Vector-Borne Zoonotic Dis. 2013, 13, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, K.D.; Martínez, I.; Agredano-Moreno, L.T.; Jiménez-García, L.F.; Reyes-Chilpa, R.; Espinoza, B. Coumarins isolated from Calophyllum brasiliense produce ultrastructural alterations and affect in vitro infectivity of Trypanosoma cruzi. Phytomedicine 2019, 61, 152827. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D.; Sexton, D.W. Trypanocidal activity of salinomycin is due to sodium influx followed by cell swelling. Parasites Vectors 2013, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Morales, D.; Hernández, K.D.; Martínez, I.; Agredano-Moreno, L.T.; Jiménez-García, L.F.; Espinoza, B. Ultrastructural and physiological changes induced by different stress conditions on the human parasite Trypanosoma cruzi. Cell Stress Chaperones 2017, 22, 15–27. [Google Scholar] [CrossRef]
- Arthington-Skaggs, B.A.; Jradi, H.; Desai, T.; Morrison, C.J. Quantitation of ergosterol content: Novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 1999, 37, 3332–3337. [Google Scholar] [CrossRef] [PubMed]
- Vizcaíno-Castillo, A.; Jiménez-Marín, A.; Espinoza, B. Exacerbated skeletal muscle inflammation and calcification in the acute phase of infection by Mexican Trypanosoma cruzi DTUI strain. Biomed. Res. Int. 2014, 2014, 450389. [Google Scholar] [CrossRef]
- Van Meer, P.; Raber, J. Mouse behavioural analysis in systems biology. Biochem. J. 2005, 389, 593–610. [Google Scholar] [CrossRef]
- Ros-Lucas, A.; Martinez-Peinado, N.; Bastida, J.; Gascón, J.; Alonso-Padilla, J. The use of alphafold for in silico exploration of drug targets in the parasite Trypanosoma cruzi. Front. Cell. Infect. Microbiol. 2022, 12, 944748. [Google Scholar] [CrossRef]
- Umegawa, Y.; Yamamoto, T.; Dixit, M.; Funahashi, K.; Seo, S.; Nakagawa, Y.; Suzuki, T.; Matsuoka, S.; Tsuchikawa, H.; Hanashima, S.; et al. Amphotericin B assembles into seven-molecule ion channels: An NMR and molecular dynamics study. Sci. Adv. 2022, 8, eabo2658. [Google Scholar] [CrossRef]
- Battista, T.; Colotti, G.; Ilari, A.; Fiorillo, A. Targeting trypanothione reductase, a key enzyme in the redox trypanosomatid metabolism, to develop new drugs against leishmaniasis and trypanosomiases. Molecules 2020, 25, 1924. [Google Scholar] [CrossRef]
- Barreiro-Costa, O.; Quiroga Lozano, C.; Muñoz, E.; Rojas-Silva, P.; Medeiros, A.; Comini, M.A.; Heredia-Moya, J. Evaluation of the anti-Leishmania mexicana and -Trypanosoma brucei activity and mode of action of 4,4’-(Arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol). Biomedicines 2022, 10, 1913. [Google Scholar] [CrossRef]
- Yardley, V.; Croft, S.L. In vitro and in vivo activity of amphotericin B-lipid formulations against experimental Trypanosoma cruzi infections. Am. J. Trop. Med. Hyg. 1999, 61, 193–197. [Google Scholar] [CrossRef]
- Moraes-Souza, H.; Pianetti, G.M.; Barretto, O.C.; Nonoyama, K.; Grolg, M.; Chiari, E. Aminoquinolone WR6026 as a feasible substitute for gentian violet in Chagas’ disease prophylaxis in preserved blood for transfusional purposes. Rev. Soc. Bras. Med. Trop. 2002, 35, 563–569. [Google Scholar] [CrossRef]
- Villamizar, L.H.; Cardoso, M.D.; Andrade, J.; Teixeira, M.L.; Soares, M.J. Linalool, a Piper aduncum essential oil component, has selective activity against Trypanosoma cruzi trypomastigote forms at 4 °C. Memórias Inst. Oswaldo Cruz 2017, 112, 131–139. [Google Scholar] [CrossRef]
- Ramos, H.; Valdivieso, E.; Gamargo, M.; Dagger, F.; Cohen, B.E. Amphotericin B kills unicellular leishmanias by forming aqueous pores permeable to small cations and anions. J. Membr. Biol. 1996, 152, 65–75. [Google Scholar] [CrossRef]
- Yamamoto, E.S.; de Jesus, J.A.; Bezerra-Souza, A.; Brito, J.R.; Lago, J.H.G.; Laurenti, M.D.; Passero, L.F.D. Tolnaftate inhibits ergosterol production and impacts cell viability of Leishmania sp. Bioorganic Chem. 2020, 102, 104056. [Google Scholar] [CrossRef]
- Clemons, K.V.; Sobel, R.A.; Martinez, M.; Correa-Oliveira, R.; Stevens, D.A. Lack of efficacy of liposomal amphotericin B against acute and chronic Trypanosoma cruzi infection in mice. Am. J. Trop. Med. Hyg. 2017, 97, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Silva, J.; Menezes, D.S.; Fernandes, J.M.P.; Almeida, M.C.; Vasco-Dos-Santos, D.R.; Saraiva, R.M.; Viçosa, A.L.; Perez, S.A.C.; Andrade, S.G.; Suarez-Fontes, A.M.; et al. The repositioned drugs disulfiram/diethyldithiocarbamate combined to benznidazole: Searching for Chagas disease selective therapy, preventing toxicity and drug resistance. Front. Cell. Infect. Microbiol. 2022, 12, 926699. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.M.C.; Pedra Rezende, Y.; de Melo, T.G.; de Oliveira, G.; Cascabulho, C.M.; Pereira, E.N.G.D.S.; Daliry, A.; Salem, K.S. Experimental Combination Therapy with Amiodarone and Low-Dose Benznidazole in a Mouse Model of Trypanosoma cruzi Acute Infection. Microbiol. Spectr. 2022, 10, e0185221. [Google Scholar] [CrossRef] [PubMed]
- Valdez, R.H.; Tonin, L.T.; Ueda-Nakamura, T.; Silva, S.O.; Dias Filho, B.P.; Kaneshima, E.N.; Yamada-Ogatta, S.F.; Yamauchi, L.M.; Sarragiotto, M.H.; Nakamura, C.V. In vitro and in vivo trypanocidal synergistic activity of N-butyl-1-(4-dimethylamino)phenyl-1,2,3,4-tetrahydro-β-carboline-3-carboxamide associated with benznidazole. Antimicrob. Agents Chemother. 2012, 56, 507–512. [Google Scholar] [CrossRef] [PubMed]

| Parasite | Strain | Stage | DTU * | A21 IC50 (μM) at 6 h ** | Selectivity Index |
|---|---|---|---|---|---|
| Qro | Trypomastigotes | Tc I | <0.3 | >333 | |
| Qro | Epimastigotes | TcI | 1.26 ± 1.0 | >79.36 | |
| Qro | Amastigotes | Tc I | 27.56 ± 2.32 | >3.62 | |
| T. cruzi | Ninoa | Epimastigotes | TcI | 2.03 ± 1.03 | >49.26 |
| Silvio | Epimastigotes | TcI | 0.86 ± 0.1 | >116 | |
| Cl Brener | Epimastigotes | TcVI | >10 | ND | |
| Ver 6 | Epimastigotes | TcVI | 2.86 ± 0.97 | >34.96 | |
| L. mexicana | Bricaire | Promastigotes | ---- | 0.8 ± 0.4 | >125 |
| T. brucei | 29-13 | Epimastigotes | ---- | 3.2 ± 0.5 | >31.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, I.; Rivera-Santiago, L.; Rodríguez-Hernández, K.D.; Galván-Hernández, A.; Rodríguez-Fragoso, L.; Díaz-Peralta, L.; Torres-Martínez, L.; Agredano-Moreno, L.T.; Jiménez-García, L.F.; Ortega-Blake, I.; et al. A Promising Amphotericin B Derivative Induces Morphological Alterations, Mitochondrial Damage, and Oxidative Stress In Vitro and Prevents Mice from Death Produced by a Virulent Strain of Trypanosoma cruzi. Microorganisms 2024, 12, 1064. https://doi.org/10.3390/microorganisms12061064
Martínez I, Rivera-Santiago L, Rodríguez-Hernández KD, Galván-Hernández A, Rodríguez-Fragoso L, Díaz-Peralta L, Torres-Martínez L, Agredano-Moreno LT, Jiménez-García LF, Ortega-Blake I, et al. A Promising Amphotericin B Derivative Induces Morphological Alterations, Mitochondrial Damage, and Oxidative Stress In Vitro and Prevents Mice from Death Produced by a Virulent Strain of Trypanosoma cruzi. Microorganisms. 2024; 12(6):1064. https://doi.org/10.3390/microorganisms12061064
Chicago/Turabian StyleMartínez, Ignacio, Lucio Rivera-Santiago, Karla Daniela Rodríguez-Hernández, Arturo Galván-Hernández, Lourdes Rodríguez-Fragoso, Lucero Díaz-Peralta, Lisset Torres-Martínez, Lourdes Teresa Agredano-Moreno, Luis Felipe Jiménez-García, Iván Ortega-Blake, and et al. 2024. "A Promising Amphotericin B Derivative Induces Morphological Alterations, Mitochondrial Damage, and Oxidative Stress In Vitro and Prevents Mice from Death Produced by a Virulent Strain of Trypanosoma cruzi" Microorganisms 12, no. 6: 1064. https://doi.org/10.3390/microorganisms12061064
APA StyleMartínez, I., Rivera-Santiago, L., Rodríguez-Hernández, K. D., Galván-Hernández, A., Rodríguez-Fragoso, L., Díaz-Peralta, L., Torres-Martínez, L., Agredano-Moreno, L. T., Jiménez-García, L. F., Ortega-Blake, I., & Espinoza, B. (2024). A Promising Amphotericin B Derivative Induces Morphological Alterations, Mitochondrial Damage, and Oxidative Stress In Vitro and Prevents Mice from Death Produced by a Virulent Strain of Trypanosoma cruzi. Microorganisms, 12(6), 1064. https://doi.org/10.3390/microorganisms12061064







