Translating Human and Animal Model Studies to Dogs’ and Cats’ Veterinary Care: Beta-Glucans Application for Skin Disease, Osteoarthritis, and Inflammatory Bowel Disease Management
Abstract
:1. Introduction
2. Method of Search
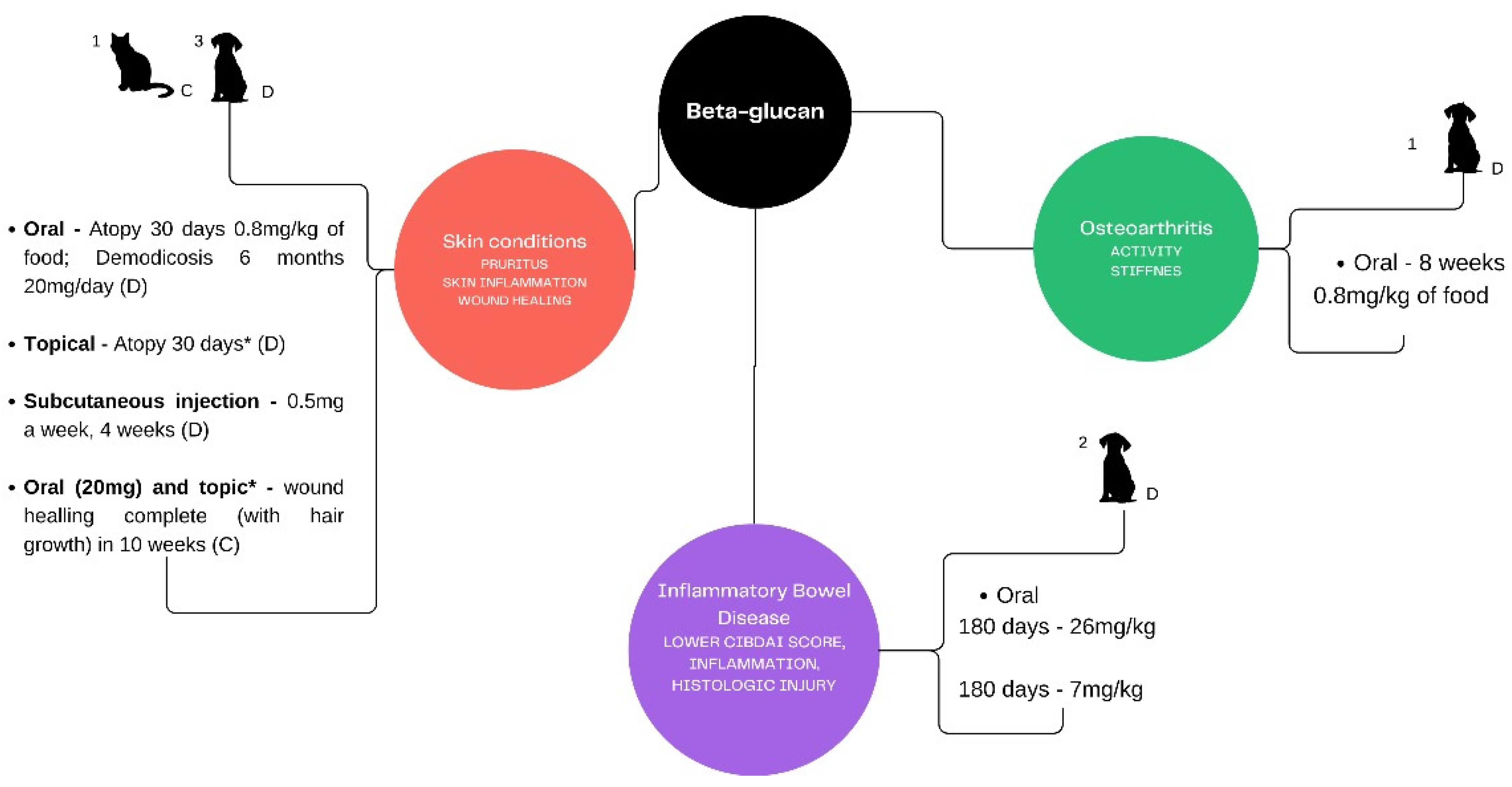
3. Beta-Glucans for the Skin
4. Beta-Glucans and Osteoarthritis
5. Beta-Glucans and Inflammatory Bowel Disease
| Reference | Population | Βeta-Glucan Origin/Dose/Frequency | Main Findings | Limitations |
|---|---|---|---|---|
| Ganda Mall et al. (2018) [92] | Human with CD | Oat-beta-glucan/nonspecific/6 weeks | No significant effects on intestinal permeability, inflammatory/oxidative levels in blood plasma and self-reported health. | Beta-glucan estimated by food frequency questionnaire and 85% of the patients had insufficient dietary fiber intake. |
| Spagnuolo et al. (2017) [93] | Human with IBD | Yeast-beta-glucan/55 mg a day/4 weeks | Reduction in abdominal pain together with reduction in bloating and flatulence after four weeks of treatment. | No individualization of the treatments: mixture of beta-glucan, inositol and digestive enzymes (Biointol®) + mesalamine. |
| Chermesh et al. (2007) [94] | Humans with CD after surgery | Undeclared/2.5 g a day/2 years | No effect on postoperative recurrence of GI signs and markers of inflammation. | No individualization of the treatment: use of a symbiotic (Symbiotic 2000®). |
| Segarra et al. (2016) [95] | Dogs with IBD | Undeclared/26 mg kg−1/180 days | Significant 1.53-fold decrease (p < 0.01) in median overall histologic score. Higher blood concentrations of PON1, and reduced TAC levels. | No individualization of the treatment: use of a mixture of chondroitin sulfate, resistant starch and MOS and small sample size. |
| Hallert et al. (2003) [96] | Humans with quiescent UC | Not purified oat beta-glucan/60 g of oat bran a day/12 weeks | Increased butyrate fecal concentrations and reduced abdominal pain and reflux. | Small sample size. Placebo composition undeclared. |
| Rychlik et al. (2013) [97] | Dogs with IBD | Yeast beta-glucan/7 mg kg−1/6 weeks | Lowering of CIBDAI values to below 3, improved histopathological parameters, decreased IL-6 levels, increasing IL-10 concentrations and remission periods longer than six months. | |
| Lee et al. (2014) [79] | Mice with induced IBD | Bacterial beta-glucan/2.5 or 5 mg kg−1/2 weeks prior IBD DSS induction | Recovery of colonic architecture, disease score and histological score. Expression of IL-1β, IL-6, and IL-17α were markedly decreased in the colon. Induction of Tregs population. Reverse of the functional defects of NK cells and excessive IgA production. | DSS induced IBD. |
| Zyła et al. (2019) [73] | Rats with induced IBD | Oat beta-glucan/1% of low or high molecular weight beta-glucan/21 days | Reduction of the number of T lymphocytes in populations of IELs and LPLs. Reduction of percentage of B cells and IL-12, decreased gene expression and secretion of proinflammatory cytokines and other inflammatory signaling molecules in the tissue of rats with induced colitis.High molecular weight is more effective as a specific internal dressing on the inflamed tissue and low molecular weight modulates the immune cells function on the molecular level. | Beta-glucan intake was not controlled. TBNS induced IBD. |
| Heinsbroek et al. (2015) [80] | Mice with induced IBD | Bacteria, yeast beta-glucan and glucan phosphate/1 mg a day/2 weeks | Increased histopathologic inflammation score, TNF-α and CCL-2 cytokine. Unlike curdlan and zymosan, glucan phosphate–treated mice did not show significant differences with vehicle treatment in any of the measured cytokines and was the only one to increase IL-10 levels. | DSS induced IBD. |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| ACVIM | American College of Veterinary Internal Medicine |
| AD | atopic dermatitis |
| BAPS | biologically active polysaccharides |
| BCFAs | branched-chain fatty acids |
| CADESI | canine atopic dermatitis extent and severity index |
| Ca-LG | calcium lactate-gluconate |
| CD | Chron’s disease |
| DSS | dextran sulfate sodium |
| IBD | inflammatory bowel disease |
| IEL | intraepithelial lymphocyte |
| IL | interleukin |
| INF | interferon |
| LA | lactic acid |
| MMP3 | metalloproteinase 3 |
| NF-κβ | nuclear factor kappa β |
| NK | natural killer cell |
| NO | nitric oxide |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| OA | osteoarthritis |
| OVA | ova-albumin |
| PAMPS | pathogen-associated molecular patterns |
| PG | prostaglandin |
| SCFAs | short-chain fatty acids |
| Th | T helper cell |
| TLR | tool-like receptor |
| TNF-α | tumor necrosis factor |
| Treg | regulatory T cell |
| TSLP | thymic stromal lymphopoietin |
| UC | ulcerative colitis |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis Index |
References
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A concise review on the molecular structure and function relationship of β-glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural features, modification, and functionalities of β-glucan. Fibers 2020, 8, 1. [Google Scholar] [CrossRef]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-Functional Activity Relationship of β-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Daou, C.; Zhang, H. Oat Β-Glucan: Its Role in Health Promotion and Prevention of Diseases. Compr. Rev. Food Sci. Food Saf. 2012, 11, 355–365. [Google Scholar] [CrossRef]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Šimić, G.; Horvat, D.; Lalić, A.; Komlenić, D.K.; Abičić, I.; Zdunić, Z. Distribution of β-glucan, phenolic acids, and proteins as functional phytonutrients of hull-less barley grain. Foods 2019, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhao, J.; Zhang, X.; Liu, S.; Zhao, C. Antitumor effect of soluble β-glucan as an immune stimulant. Int. J. Biol. Macromol. 2021, 179, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Β glucan: Supplement or drug? From laboratory to clinical trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Hong, J.T.; Kim, Y.; Han, S.B. Stimulatory Effect of β-glucans on Immune Cells. Immune Netw. 2011, 11, 191. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. An evaluation of the immunological activities of commercially available β-1,3-glucans. Jana 2007, 10, 25–31. [Google Scholar]
- Vetvicka, V.; Oliveira, C. β(1-3)(1-6)-D-glucans Modulate Immune Status and Blood Glucose Levels in Dogs. Br. J. Pharm. Res. 2014, 4, 981–991. [Google Scholar] [CrossRef]
- Stuyven, E.; Verdonck, F.; Van Hoek, I.; Daminet, S.; Duchateau, L.; Remon, J.P.; Goddeeris, B.M.; Cox, E. Oral administration of β-1,3/1,6-glucan to dogs temporally changes total and antigen-specific IgA and IgM. Clin. Vaccine Immunol. 2010, 17, 281–285. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.A.F.; Vetvicka, V.; Zanuzzo, F.S. β-Glucan successfully stimulated the immune system in different jawed vertebrate species. Comp. Immunol. Microbiol. Infect. Dis. 2019, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Marchi, P.H.; Vendramini, T.H.A.; Zafalon, R.V.A.; Príncipe, L.A.; Cesar, C.G.L.; Perini, M.P.; Putarov, T.C.; Gomes, C.O.M.S.; Balieiro, J.C.C.; Brunetto, M.A. Effects of Increasing Levels of Purified Β-1,3/1,6-Glucans on the Fecal Microbiome, Digestibility, and Immunity Variables of Healthy Adult Dogs. Microorganisms 2024, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.; Vendramini, T.H.A.; Amaral, A.R.; Rentas, M.F.; Ernandes, M.C.; da Silva, F.L.; Oba, P.M.; Filho, F.O.R.; Brunetto, M.A. Metabolic variables of obese dogs with insulin resistance supplemented with yeast β-glucan. BMC Vet. Res. 2022, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Beynen, A.C.; Saris, D.H.; Paap, P.M.; Altena, F.V.; Visser, E.A.; Middelkoop, J.; De Jong, L.; Staats, M. Dietary β-1,3/1,6-glucans reduce clinical signs of canine atopy. Am. J. Anim. Vet. Sci. 2011, 6, 146–152. [Google Scholar]
- Anderson, K.L.; O’Neill, D.G.; Brodbelt, D.C.; Church, D.B.; Meeson, R.L.; Sargan, D.; Summers, J.F.; Zulch, H.; Collins, L.M. Prevalence, duration and risk factors for appendicular osteoarthritis in a UK dog population under primary veterinary care. Sci. Rep. 2018, 8, 5641. [Google Scholar] [CrossRef] [PubMed]
- Cave, N. Nutritional Management of Gastrointestinal Diseases. In Applied Veterinary Clinical Nutrition, 1st ed.; Fascetti, A.J., Delaney, S.J., Eds.; Wiley-Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2013; pp. 175–219. [Google Scholar]
- Kim, I.S.; Lee, S.H.; Kim, J.A.; Yu, D.Y.; Hong, Y.H.; Kim, J.Y.; Lim, J.M.; Lee, S.S.; Yun, C.H.; Choi, I.S.; et al. Effect of oral administration of β-glucans derived from Aureobasidium pullulans SM-2001 in model mice and rat with atopic dermatitis-like phenotypes. Food Sci. Biotechnol. 2018, 27, 1185–1192. [Google Scholar] [CrossRef]
- Jesenak, M.; Banovcin, P.; Rennerova, Z.; Majtan, J. β-Glucans in the treatment and prevention of allergic diseases. Allergol. Immunopathol. 2014, 42, 149–156. [Google Scholar] [CrossRef]
- Nuttall, T.J.; Marsella, R.; Rosenbaum, M.R.; Gonzalez, A.J.; Fadok, V.A. Update on pathogenesis, diagnosis, and treatment of atopic dermatitis in dogs. J. Am. Vet. Med. Assoc. 2019, 254, 1291–1300. [Google Scholar] [CrossRef]
- Cergan, R.; Berghi, O.N.; Dumitru, M.; Vrinceanu, D.; Manole, F.; Serboiu, C.S. Biologics for Chronic Rhinosinusitis—A Modern Option for Therapy. Life 2023, 13, 2165. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Vetvicka, V. Glucans as Biological Response Modifiers. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 67–75. [Google Scholar] [CrossRef]
- Sarinho, E.; Medeiros, D.; Schor, D.; Silva, A.R.; Sales, V.; Motta, M.E.; Costa, A.; Azoubel, A.; Rizzo, J.A. Production of interleukin-10 in asthmatic children after Β-1-3-glucan. Allergol. Immunopathol. 2009, 37, 188–192. [Google Scholar] [CrossRef]
- Dore, C.M.P.G.; Azevedo, T.C.G.; de Souza, M.C.R.; Rego, L.A.; de Dantas, J.C.M.; Silva, F.R.F.; Rocha, H.A.; Baseia, I.G.; Leite, E.L. Antiinflammatory, antioxidant and cytotoxic actions of β-glucan-rich extract from Geastrum saccatum mushroom. Int. Immunopharmacol. 2007, 7, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yajima, T.; Nishimura, H.; Aiba, K.; Ishimitsu, R.; Matsuguchi, T.; Fushimi, T.; Ohshima, Y.; Tsukamoto, Y.; Yoshikai, Y. Soluble branched β-(1,4)glucans from Acetobacter species show strong activities to induce interleukin-12 in vitro and inhibit T-helper 2 cellular response with immunoglobulin E production in vivo. J. Biol. Chem. 2003, 278, 38571–38578. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vashishta, A.; Saraswat-Ohri, S.; Vetvickova, J. Immunological effects of yeast- and mushroom-derived β-glucans. J. Med. Food 2008, 11, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Shimamura, T.; Tagami, T.; Takatsuki, F.; Hamuro, J. The skewing to Th1 induced by lentinan is directed through the distinctive cytokine production by macrophages with elevated intracellular glutathione content. Int. Immunopharmacol. 2002, 2, 673–689. [Google Scholar] [CrossRef]
- Jesenak, M.; Urbancek, S.; Majtan, J.; Banovcin, P.; Hercogova, J. β-Glucan-based cream (containing pleuran isolated from Pleurotus ostreatus) in supportive treatment of mild-to-moderate atopic dermatitis. J. Dermatol. Treat 2016, 27, 351–354. [Google Scholar] [CrossRef]
- Kim, I.S.; Lee, S.S.S.H.; Kwon, Y.M.; Adhikari, B.; Kim, J.A.; Yu, D.Y.; Kim, G.I.; Lim, J.M.; Kim, S.H.; Lee, S.S.; et al. Oral Administration of β-Glucan and Lactobacillus plantarum Alleviates Atopic Dermatitis-Like Symptoms. J. Microbiol. Biotechnol. 2019, 29, 1693–1706. [Google Scholar] [CrossRef]
- Furue, M.; Tsuji, G.; Mitoma, C.; Nakahara, T.; Chiba, T.; Morino-Koga, S.; Uchi, H. Gene regulation of filaggrin and other skin barrier proteins via aryl hydrocarbon receptor. J. Dermatol. Sci. 2015, 80, 83–88. [Google Scholar] [CrossRef]
- Palomares, O.; Yaman, G.; Azkur, A.K.; Akkoc, T.; Akdis, M.; Akdis, C.A. Role of Treg in immune regulation of allergic diseases. Eur. J. Immunol. 2010, 40, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Kaur, H.; Mande, S.S. Comparative in silico analysis of butyrate production pathways in gut commensals and pathogens. Front. Microbiol. 2016, 7, 1945. [Google Scholar] [CrossRef] [PubMed]
- Thornton, B.P.; Vĕtvicka, V.; Pitman, M.; Goldman, R.C.; Ross, G.D. Analysis of the sugar specificity and molecular location of the β-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. 1996, 156, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Gantner, B.N.; Simmons, R.M.; Canavera, S.J.; Akira, S.; Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and toll-like receptor 2. J. Exp. Med. 2003, 197, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sumiyoshi, M.; Suzuki, T.; Suzuki, T.; Sakanaka, M. Inhibitory effects of water-soluble low-molecular-weight β-(1,3-1,6) d-glucan purified from Aureobasidium pullulans GM-NH-1A1 strain on food allergic reactions in mice. Int. Immunopharmacol. 2007, 7, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Beeck, F.A.L.; Hoekstra, H.; Brunekreef, B.; Willemse, T. Inverse association between endotoxin exposure and canine atopic dermatitis. Vet. J. 2011, 190, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Beyazit, A.; Inceboz, T.; Over, L. Contribution to one world, one health: A dog with demodicosis. Turk. Parazitol. Derg. 2010, 34, 68–71. [Google Scholar]
- Guterres, K.A.; de Matos, C.B.; Osório, L.G.; Schuch, I.D.; Cleff, M.B. The Use of (1–3) β-Glucan Along with Itraconazole Against Canine Refractory Sporotrichosis. Mycopathologia 2014, 177, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Jeong, Y.; An, J.H.; Ahn, J.O.; Chung, J.Y. Application of a Synbio-Glucan Functional Spray for Canine Atopic Dermatitis. J. Vet. Clin. 2023, 40, 8–15. [Google Scholar] [CrossRef]
- Micháľová, A.; Micháľ, M.; Fialkovičová, M. Combination of Β Glucan, Honey and Chlorhexidine in the Wound Management in a Cat a Case Report. Folia Vet 2019, 63, 70–77. [Google Scholar] [CrossRef]
- Ferrari, M.; Leandro, D.; Conti, J.; Carneiro, P.; Sontag, S. Terapêutica da osteoartrite em pequenos animais: Métodos farmacológicos, não-farmacológicos e novas medidas terapêuticas. Enciclopédia Biosf. 2018, 15, 74–89. [Google Scholar] [CrossRef]
- Erbas, M.; Simsek, T.; Kiraz, H.A.; Sahin, H.; Toman, H. Comparação da eficácia de tenoxicam administrado por via oral e intra-articular a pacientes com osteoartrite de joelhos. Braz. J. Anesthesiol. 2015, 65, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.G.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Prevalence of disorders recorded in dogs attending primary-care veterinary practices in England. PLoS ONE 2014, 9, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, R.A.; German, A.J. Investigation and management of canine osteoarthritis. Practice 2016, 38, 1–8. [Google Scholar] [CrossRef]
- Fernández-Torres, J.; Martínez-Nava, G.A.; Gutiérrez-Ruíz, M.C.; Gomez-Quiroz, L.E.; Gutiérrez, M. Papel da via de sinalização do HIF-1α na osteoartrite: Revisão sistemática. Rev. Bras. Reumatol. 2017, 57, 162–173. [Google Scholar] [CrossRef]
- Henrotin, Y.; Sanchez, C.; Balligand, M. Pharmaceutical and nutraceutical management of canine osteoarthritis: Present and future perspectives. Vet. J. 2005, 170, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Aragon, C.L.; Hofmeister, E.H.; Budsberg, S.C. Systematic review of clinical trials of treatments for osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 2007, 230, 514–521. [Google Scholar] [CrossRef]
- Comblain, F.; Serisier, S.; Barthelemy, N.; Balligand, M.; Henrotin, Y. Review of dietary supplements for the management of osteoarthritis in dogs in studies from 2004 to 2014. J. Vet. Pharmacol. Ther. 2016, 39, 1–15. [Google Scholar] [CrossRef]
- Du, B.; Lin, C.; Bian, Z.; Xu, B. An insight into anti-inflammatory effects of fungal β-glucans. Trends Food Sci. Technol. 2015, 41, 49–59. [Google Scholar] [CrossRef]
- Yoshitomi, H.; Sakaguchi, N.; Kobayashi, K.; Brown, G.D.; Tagami, T.; Sakihama, T.; Hirota, K.; Tanaka, S.; Nomura, T.; Miki, I. A role for fungal β-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 2005, 201, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Shiota, E.; Maekawa, M.; Kono, T. Analysis of the levels of endotoxin and β-d-glucan in the synovial fluid of hemodialysis patients. Mod. Rheumatol. 2001, 11, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.R.; Komarow, L.; Zolopa, A.R.; Finkelman, M.A.; Powderly, W.G.; Sax, P.E. Test performance of blood β-glucan for Pneumocystis jirovecii pneumonia in patients with AIDS and respiratory symptoms. Aids 2013, 27, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Cho, H.R.; Ku, S.K. Efficacy test of polycan, a β-glucan originated from aureobasidium pullulans sm-2001, on anterior cruciate ligament transection and partial medial meniscectomy-induced-osteoarthritis rats. J. Microbiol. Biotechnol. 2012, 22, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Shin, H.S.; Kim, K.Y.; Ku, S.K.; Choi, I.S.; Kim, J.W. Effect of Polycalcium, a mixture of Polycan and calcium lactate-gluconate in a 1:9 weight ratio, on rats with surgery-induced osteoarthritis. Exp. Ther. Med. 2015, 9, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Shin, H.S.; Ha, Y.M.; Kim, K.Y.; Ku, S.K.; Choi, I.S.; Kim, K.Y.; Cho, H.R.; Rha, C.H.; Ku, S.K. A 2-week repeated-dose oral toxicity test of Polycalcium, a mixed composition of Polycan and calcium lactate-gluconate 1: 9 (g/g). Toxicol. Environ. Health Sci. 2014, 6, 176–191. [Google Scholar] [CrossRef]
- Truong, T.T.T.; Lim, J.M.; Cho, H.R.; Kim, Y.S.; Dao, D.G.; Tran, Q.H.; Choi, J.S. A Double-Blind, Randomized Controlled 12-Week Follow-Up Trial to Evaluate the Efficacy and Safety of Polycan in Combination with Glucosamine for the Treatment of Knee Osteoarthritis. Evid. Based Complement. Alternat. Med. 2019, 2019, 9750531. [Google Scholar] [CrossRef] [PubMed]
- Beynen, A.C.; Legerstee, E. Influence of dietary β-1,3/1,6-glucans on clinical signs of canine osteoarthritis in a double-blind, placebo-controlled trial. Am. J. Anim. Vet. Sci. 2010, 5, 90–94. [Google Scholar] [CrossRef]
- Li, J.; Li, D.F.; Xing, J.J.; Cheng, Z.B.; Lai, C.H. Effects of β-glucan extracted from Saccharomyces cerevisiae on growth performance, and immunological and somatotropic responses of pigs challenged with Escherichia coli lipopolysaccharide. J. Anim. Sci. 2006, 84, 2374–2381. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Fitz Gerald, M.X. Matrix metalloproteases and lung disease. Thorax 1994, 49, 602–609. [Google Scholar] [CrossRef]
- Wen, Z.; Fiocchi, C. Inflammatory bowel disease: Autoimmune or immune-mediated pathogenesis? Clin. Dev. Immunol. 2004, 11, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Cerquetella, M.; Spaterna, A.; Laus, F.; Tesei, B.; Rossi, G.; Antonelli, E.; Villanacci, V.; Bassotti, G. Inflammatory bowel disease in the dog: Differences and similarities with humans. World J. Gastroenterol. 2010, 16, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Washabau, R.J.; Day, M.J. Canine and Feline Gastroenterology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–996. [Google Scholar]
- Gálvez, J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, R.M.; Suchodolski, J.S. Is inflammatory bowel disease in dogs and cats associated with a Th1 or Th2 polarization? Vet. Immunol. Immunopathol. 2015, 168, 131–134. [Google Scholar] [CrossRef]
- Nguyen Van, N.; Taglinger, K.; Helps, C.R.; Tasker, S.; Gruffydd-Jones, T.J.; Day, M.J. Measurement of cytokine mRNA expression in intestinal biopsies of cats with inflammatory enteropathy using quantitative real-time RT-PCR. Vet. Immunol. Immunopathol. 2006, 113, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, S.; Atwater, D.; Bogel, E.; Greiter-Wilke, A.; Gerold, A.; Baumgart, M.; Bender, H.; McDonough, P.L.; McDonough, S.P.; Goldstein, R.E.; et al. The relationship of mucosal bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet. Microbiol. 2008, 128, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Waly, N.E.; Peters, I.R.; Day, M.J.; Stokes, C.R.; Bailey, M.; Gruffydd-Jones, T.J. Measurement of IL-12 (p40, p35), IL-23p19, and IFN-γ mRNA in Duodenal Biopsies of Cats with Inflammatory Enteropathy. J. Vet. Intern. Med. 2014, 28, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, B.; Allenspach, K. Canine inflammatory bowel disease: Does innate immunity fail to discriminate between friend and foe? Vet. J. 2012, 194, 7–8. [Google Scholar] [CrossRef]
- Bollrath, J.; Powrie, F. Feed your Tregs more fiber. Science 2013, 341, 463–464. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef]
- Zyła, E.; Dziendzikowska, K.; Gajewska, M.; Wilczak, J.; Harasym, J.; Gromadzka-Ostrowska, J. Beneficial effects of oat β-glucan dietary supplementation in colitis depend on its molecular weight. Molecules 2019, 24, 3591. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M. Prebiotics: The concept revisited. J. Nutr. 2007, 137, 830S–837S. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Shu, D.; Zheng, M.; Wang, J.; Luo, C.; Wang, Y.; Guo, F.; Zou, X.; Lv, X.; Li, Y.; et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 2016, 6, 24838. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Kotlo, K.U.; Dudeja, P.K.; Layden, B.T. Role of short chain fatty acid receptors in intestinal physiology and pathophysiology. Compr. Physiol. 2018, 8, 1065–1090. [Google Scholar]
- Satyaraj, E. Emerging Paradigms in Immunonutrition. Top. Companion. Anim. Med. 2011, 26, 25–32. [Google Scholar] [CrossRef]
- Municio, C.; Alvarez, Y.; Montero, O.; Hugo, E.; Rodríguez, M.; Domingo, E.; Alonso, S.; Fernández, N.; Crespo, M.S. The Response of Human Macrophages to β-Glucans Depends on the Inflammatory Milieu. PLoS ONE 2013, 8, e62016. [Google Scholar] [CrossRef]
- Lee, K.H.; Park, M.; Ji, K.Y.; Lee, H.Y.; Jang, J.H.; Yoon, I.J.; Oh, S.S.; Kim, S.M.; Jeong, Y.H.; Yun, C.H.; et al. Bacterial β-(1,3)-glucan prevents DSS-induced IBD by restoring the reduced population of regulatory T cells. Immunobiology 2014, 219, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Heinsbroek, S.E.M.; Williams, D.L.; Welting, O.; Meijer, S.L.; Gordon, S.; de Jonge, W.J. Orally delivered β-glucans aggravate dextran sulfate sodium (DSS)-induced intestinal inflammation. Nutr. Res. 2015, 35, 1106–1112. [Google Scholar] [CrossRef]
- Orel, R.; Trop, T.K. Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 11505–11524. [Google Scholar] [CrossRef]
- Mazmanian, S.K. Gut Immune Balance Is as Easy as S-F-B. Immunity 2009, 31, 536–538. [Google Scholar] [CrossRef]
- Turunen, K.; Tsouvelakidou, E.; Nomikos, T.; Mountzouris, K.C.; Karamanolis, D.; Triantafillidis, J.; Kyriacou, A. Impact of β-glucan on the faecal microbiota of polypectomized patients: A pilot study. Anaerobe 2011, 17, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ames, N.P.; Tun, H.M.; Tosh, S.M.; Jones, P.J.; Khafipour, E. High molecular weight barley β-glucan alters gut microbiota toward reduced cardiovascular disease risk. Front. Microbiol. 2016, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Velikonja, A.; Lipoglavšek, L.; Zorec, M.; Orel, R.; Avguštin, G. Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley β glucans in patients with high risk for metabolic syndrome development. Anaerobe 2019, 55, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chen, D.; Mao, X.; He, J.; Yu, B.; Cheng, L.; Zeng, D. Purified β-glucans of different molecular weights enhance growth performance of LPS-challenged piglets via improved gut barrier function and microbiota. Animals 2019, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Drzikova, B.; Dongowski, G.; Gebhardt, E. Dietary fibre-rich oat-based products affect serum lipids, microbiota, formation of short-chain fatty acids and steroids in rats. Br. J. Nutr. 2005, 94, 1012–1025. [Google Scholar] [CrossRef] [PubMed]
- Snart, J.; Bibiloni, R.; Grayson, T.; Lay, C.; Zhang, H.; Allison, G.E.; Laverdiere, J.K.; Temelli, F.; Vasanthan, T.; Bell, R.; et al. Supplementation of the diet with high-viscosity β-glucan results in enrichment for lactobacilli in the rat cecum. Appl. Environ. Microbiol. 2006, 72, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Aoe, S.; Yamanaka, C.; Fuwa, M.; Tamiya, T.; Nakayama, Y.; Miyoshi, T.; Kitazono, E. Effects of BARLEYmax and high-β-glucan barley line on short-chain fatty acids production and microbiota from the cecum to the distal colon in rats. PLoS ONE 2019, 14, e0218118. [Google Scholar] [CrossRef] [PubMed]
- Jaskari, J.; Kontula, P.; Siitonen, A.; Jousimies-Somer, H.; Mattila-Sandholm, T.; Poutanen, K. Oat β-glucan and xylan hydrolysates as selective substrates for Bifidobacterium and Lactobacillus strains. Appl. Microbiol. Biotechnol. 1998, 49, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, T.; Kealy, T.; Mishra, V.K. Effects of β-glucan addition to a probiotic containing yogurt. J. Food Sci. 2007, 72, C405–C411. [Google Scholar] [CrossRef]
- Ganda Mall, J.P.; Fart, F.; Sabet, J.; Lindqvist, C.M.; Keita, A.V.; Brummer, R.J.; Schoultz, I. Effects of dietary fibres on indomethacininduced intestinal permeability in elderly: A randomised placebo controlled parallel clinical trial. United Eur. Gastroenterol. J. 2018, 6, A100–A101. [Google Scholar]
- Spagnuolo, R.; Cosco, C.; Mancina, R.M.M.; Ruggiero, G.; Garieri, P.; Cosco, V.; Doldo, P. Β-glucan, inositol and digestive enzymes improve quality of life of patients with inflammatory bowel disease and irritable bowel syndrome. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 102–107. [Google Scholar] [PubMed]
- Chermesh, I.; Tamir, A.; Reshef, R.; Chowers, Y.; Suissa, A.; Katz, D.; Gelber, M.; Halpern, Z.; Bengmark, S.; Eliakim, R. Failure of synbiotic 2000 to prevent postoperative recurrence of Crohn’s disease. Dig. Dis. Sci. 2007, 52, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Segarra, S.; Martínez-Subiela, S.; Cerdà-Cuéllar, M.; Martínez-Puig, D.; Muñoz-Prieto, A.; Rodríguez-Franco, F.; Rodríguez-Bertos, A.; Allenspach, K.; Velasco, A.; Cerón, J. Oral chondroitin sulfate and prebiotics for the treatment of canine Inflammatory Bowel Disease: A randomized, controlled clinical trial. BMC Vet. Res. 2016, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Hallert, C.; Björck, I.; Nyman, M.; Pousette, A.; Grännö, C.; Svensson, H. Increasing fecal butyrate in ulcerative colitis patients by diet: Controlled pilot study. Inflamm. Bowel. Dis. 2003, 9, 116–121. [Google Scholar] [CrossRef]
- Rychlik, A.; Nieradka, R.; Kander, M.; Nowicki, M.; Wdowiak, M.; Kołodziejska-Sawerska, A. The effectiveness of natural and synthetic immunomodulators in the treatment of inflammatory bowel disease in dogs. Acta Vet. Hung. 2013, 61, 297–308. [Google Scholar] [CrossRef]
| Subject | Search Terms |
|---|---|
| Skin disease Articles retrieved: Embase—442 PubMed—1110 Duplicated: 12 Removed (type of paper): 82 Removed (title and abstract): 668 Total: 7 | (dog OR canis canis OR canis domesticus OR canis familiaris OR canis lupus familiaris OR dog OR dogs OR cat OR human OR homo sapiens OR human OR human being OR human body OR human race OR human subject OR humans OR man (homo sapiens) OR animal model OR animal disease model OR animal model OR animal models OR model, animal OR models, animal) AND (skin disease OR cutaneous disease OR dermal disease OR dermal disorder OR dermatopathology OR dermatopathy OR dermatosis OR dermopathy OR disease, cutaneous OR disease, dermal OR facial dermatoses OR foot dermatoses OR leg dermatoses OR scalp dermatoses OR skin and connective tissue diseases OR skin disease OR skin diseases OR skin diseases, genetic OR skin disorder) AND (beta-glucan OR beta-dextroglucan OR beta-glucan OR beta-glucans OR beta-glucans OR macrogard) |
| Osteoarthrites Articles retrieved: Embase—13 PubMed—325 Duplicated: 0 Removed (type of paper): 32 Removed (title and abstract): 298 Total: 8 | (dog OR canis canis OR canis domesticus OR canis familiaris OR canis lupus familiaris OR dog OR dogs OR cat OR human OR homo sapiens OR human OR human being OR human body OR human race OR human subject OR humans OR man (homo sapiens) OR animal model/exp OR animal disease model OR animal model OR animal models OR model, animal OR models, animal) AND (osteoarthritis/exp OR arthritis, degenerative OR arthritis, noninflammatory OR arthrosis OR degenerative arthritis OR degenerative joint disease OR noninflammatory arthritis OR osteo-arthritis OR osteo-arthrosis OR osteoarthritis OR osteoarthrosis OR primary osteoarthritis OR rheumatoid arthrosis) AND (beta-glucan/exp OR beta-dextroglucan OR beta-glucan OR beta-glucans OR beta-glucans OR macrogard) |
| Inflammatory Bowel Disease Articles retrieved: Embase—156 PubMed—72 Duplicated: 6 Removed (type of paper): 13 Removed (title and abstract): 200 Total: 9 | (dog OR canis canis OR canis domesticus OR canis familiaris OR canis lupus familiaris OR dog OR dogs OR cat OR felis OR cat OR cats OR feline OR felines OR human OR homo sapiens OR human OR human being OR human body OR human race OR human subject OR humans OR man (homo sapiens) OR animal model OR animal disease model OR animal model OR animal models OR model, animal OR models, animal) AND (beta-glucan OR beta-dextroglucan OR beta-glucan OR beta-glucans OR beta-glucans OR macrogard) AND (inflammatory bowel disease OR inflammatory bowel disease OR inflammatory bowel diseases) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, A.R.; Risolia, L.W.; Rentas, M.F.; Marchi, P.H.; Balieiro, J.C.d.C.; Vendramini, T.H.A.; Brunetto, M.A. Translating Human and Animal Model Studies to Dogs’ and Cats’ Veterinary Care: Beta-Glucans Application for Skin Disease, Osteoarthritis, and Inflammatory Bowel Disease Management. Microorganisms 2024, 12, 1071. https://doi.org/10.3390/microorganisms12061071
Amaral AR, Risolia LW, Rentas MF, Marchi PH, Balieiro JCdC, Vendramini THA, Brunetto MA. Translating Human and Animal Model Studies to Dogs’ and Cats’ Veterinary Care: Beta-Glucans Application for Skin Disease, Osteoarthritis, and Inflammatory Bowel Disease Management. Microorganisms. 2024; 12(6):1071. https://doi.org/10.3390/microorganisms12061071
Chicago/Turabian StyleAmaral, Andressa Rodrigues, Larissa Wünsche Risolia, Mariana Fragoso Rentas, Pedro Henrique Marchi, Júlio Cesar de Carvalho Balieiro, Thiago Henrique Annibale Vendramini, and Marcio Antonio Brunetto. 2024. "Translating Human and Animal Model Studies to Dogs’ and Cats’ Veterinary Care: Beta-Glucans Application for Skin Disease, Osteoarthritis, and Inflammatory Bowel Disease Management" Microorganisms 12, no. 6: 1071. https://doi.org/10.3390/microorganisms12061071
APA StyleAmaral, A. R., Risolia, L. W., Rentas, M. F., Marchi, P. H., Balieiro, J. C. d. C., Vendramini, T. H. A., & Brunetto, M. A. (2024). Translating Human and Animal Model Studies to Dogs’ and Cats’ Veterinary Care: Beta-Glucans Application for Skin Disease, Osteoarthritis, and Inflammatory Bowel Disease Management. Microorganisms, 12(6), 1071. https://doi.org/10.3390/microorganisms12061071






