Abstract
Background: Pneumonia is one of the most common infectious diseases, mostly caused by viruses or bacteria. In response to bacteria or viruses which are different but which also are partly overlapping, innate and adaptive immune responses are induced, which can be quantified using the determination of specific biomarkers. Among these, C-reactive protein (CRP) has been established as a marker of innate immune function, whereas Neopterin, which is mainly produced upon stimulation with interferon-gamma, reflects cellular immune activation. Aim: We investigated inflammation markers in patients with microbiologically confirmed viral or bacterial pneumonia, and studied the potential of CRP, Neopterin, and the CRP/Neopterin ratio to distinguish between viral and bacterial pathogenesis. Furthermore, we examined, how often neuropsychiatric symptoms occur in patients suffering from different kinds of pneumonia. Patients and method: A total of 194 patients diagnosed with either coronavirus disease 2019 (COVID-19) (n = 63), bacterial pneumonia (n = 58), Influenza infection (n = 10), Influenza and a bacterial superinfection (n = 9), and COVID-19 patients with a bacterial superinfection (n = 54) were included in our pilot study. Clinical as well as laboratory parameters were determined shortly after admission. Results: We found significantly higher CRP/Neopterin ratios in patients with bacterial pneumonia (median: 0.34) and lower CRP/Neopterin ratios in patients hospitalized with COVID-19 infection (median: 0.03; p < 0.001). Both in men and in women, the CRP/Neopterin ratio was able to distinguish between viral and bacterial pathogens, but also was able to detect bacterial super-infection (BSI) in subjects with initial viral pneumonia (p < 0.001). Patients with BSI presented with significantly lower CRP/Neopterin ratios (median 0.08) than patients with bacterial infection only (median 0.34; p < 0.001). Interestingly, COVID-19 patients had a decreased physical functioning (as reflected in the ECOG score) and a higher frequency of fatigue (84.1%) and neurological symptoms (54.8%) than patients with pneumonia, due to other underlying pathogens. Patients that reported fatigue during viral and bacterial pneumonia presented with lower CRP concentrations than patients without it. Conclusions: The CRP/Neopterin ratio is useful to differentiate between viral and bacterial pathogenesis. The occurrence of neuropsychiatric symptoms in pneumonia appears to depend on the kind of pathogen causing the infection. Lower CRP concentrations at admission appear to be related to fatigue during acute viral and bacterial infection.
1. Introduction
Pneumonia is the most common infectious disease leading to death in industrialized countries. In Europe, hospitalization is required in up to 50% of cases [1]. Pneumonia can be caused by different pathogens, mainly bacteria and viruses, and can often progress to multi-organ disease. Differentiation between viral and bacterial pneumonia is often challenging. However, it is essential for further adequate treatment. Microbiological cultures are helpful to diagnose underlying pathogens. However, in recent years, as results of microbiological cultures are often available only after several days, underlying pathogens are mostly diagnosed by antigen rapid tests or polymerase chain reaction (PCR).
In addition to microbiological tests, the monitoring of the immune response as reflected by inflammation parameters can help to estimate the extent of disease and differentiate between viral and bacterial pathogens. C-reactive protein is one of the acute phase inflammatory proteins. It is induced by interleukin (IL)-6 and IL-1 in the case of infection or inflammation. CRP is particularly elevated during bacterial infections. In cases of viral or fungal infection, CRP levels are usually not increased or are only slightly elevated [2]. Neopterin, on the other hand, is a marker of cellular immune activation, and is mainly produced by monocytes and macrophages after IFN-y stimulation. In contrast to CRP, Neopterin production is mainly increased in viral infections like HIV infection or measles [3]. In the case of bacterial infections, Neopterin formation plays a minor role due to the predominance of the humoral immune response [4,5]. The ratio of CRP/Neopterin has earlier been shown to be useful to differentiate between bacterial or viral pathogens in acute respiratory tract infections [6]. Also, in patients with chronic obstructive pulmonary disease, a higher CRP/Neopterin ratio was found in patients with community-acquired pneumonia (CAP) and COPD compared to patients with acute exacerbations of COPD without pneumonia [7].
CAP is the most common form of pneumonia caused by bacteria. It is defined by developing outside the hospital or within the first 48 h of hospitalization, and is a leading cause of morbidity and mortality worldwide. The most common pathogen leading to CAP is Streptococcus pneumoniae, followed by Haemophilus influenzae. Atypical pneumonia, on the other hand, can be caused by Mycoplasma or Chlamydia, and usually goes along with a rather slow onset [4].
Influenza infection usually has its peak during the cold season, and annually causes up to 5 million severe infections globally, which can be a burden for public health [8]. Influenza A (IAV) is highly susceptible to antigenic variation, and therefore poses a considerable risk of triggering an epidemic or pandemic.
SARS-CoV-2 virus already challenged health care systems worldwide significantly over the years by spreading very quickly and affecting billions of people worldwide. Interestingly, infection by this virus went along with very different courses of disease, varying from asymptomatic to acute respiratory distress syndrome (ARDS) [9].
Secondary bacterial superinfection can occur in patients infected with viral infections like SARS-CoV-2 and those with Influenza [10,11]. Many mechanisms have been identified for increased bacterial susceptibility after Influenza infection, like, e.g., disruption of the barrier function of the alveolar epithelium [12]. SARS-CoV-2, on the other hand, also seems to impair the function of many different cells of the host. It does not only infect alveolar epithelial cells and endothelial cells, but also harms cells of the extracellular matrix and induces the release of various pro-inflammatory cytokines (including IL-1, IL-6, tumor necrosis-factor alpha (TNF-α), and transforming growth factor beta), thereby destroying the “blood gas barrier” [13]. Additionally, SARS-CoV-2 was observed to disrupt the activity of macrophages through pathways like TLR4 and 5, potentially facilitating bacterial attachment and leading to secondary infections [14].
Apart from the abovementioned pathways, viral infection can also induce mucosal cell death, compromising the body’s ability to clear pathogens and promoting bacterial adhesion [15]. Interactions between the virus and host cells can trigger the production of pro-inflammatory markers like TNF-α, which may harm host cells and increase susceptibility to opportunistic bacterial infections [16]. The most common pathogen for secondary bacterial infection is Staphylococcus aureus, followed by Haemophilus influenzae and Pseudomonas aeruginosa [10,12]. These pathogens interfere with the body’s normal functions by impeding immune responses and generating harmful toxins [17].
Therefore, different respiratory pathogens appear to use different mechanisms to invade host cells and also attack host immune cells. And in fact, also, the integrity of the barriers of the airways, and especially the overall “fitness” of the host’s immune system (i.e., the ability of certain immune cells to fight and eliminate pathogens), play a pivotal role in the course of disease in patients with pneumonia.
Also, the development of certain symptoms might in fact be altered by the interaction of pathogens and the immune system, e.g., the immune system tries to deprive pathogens of essential nutrients like, e.g., tryptophan [18]. Earlier studies showed that inflammation and interferon-gamma mediated biochemical pathways, like Neopterin formation and tryptophan catabolism, are induced in patients with Influenza ([19]), patients with acute exacerbated COPD [7], Epstein–Barr virus infection [20], and also pneumonia [21]. In patients with pneumonia, enhanced immune response and tryptophan catabolism were associated with a worse prognosis of patients [21], while other studies also showed that over-activated inflammation and induction of interferon gamma-related biochemical pathways were associated with symptoms/an impaired performance of patients, e.g., in patients with acute COVID-19 infection [22], persistent symptoms after COVID-19 [6,23], and patients with Epstein–Barr virus infection [20]. As higher Neopterin concentrations and enhanced tryptophan catabolism have also been related with symptoms like anemia, fatigue, and depression in earlier studies [24], we also wanted to investigate whether the symptoms of patients are related to inflammatory markers, and especially the CRP/Neopterin ratio, in patients with different kinds of pneumonia.
In our pilot study, we thus also investigated the frequency of certain common symptoms (fatigue and impaired physical function, depression, sleep disturbance, and neurological symptoms) in patients with different kinds of pneumonia, and retrospectively analyzed whether inflammatory markers were related with those symptoms and were dependent on the involved pathogen. We were especially interested in these symptoms, as many patients after the acute COVID-19 infection also suffer from such symptoms persistently.
2. Patients and Methods
2.1. Patient Recruitment and Data Collection
Our study population included 194 patients who were hospitalized at the University Hospital for Internal Medicine II in Innsbruck between 2016 and 2020. Among them, 63 patients had a COVID-19 infection, 58 had a bacterial pneumonia, 10 suffered from Influenza, 9 had Influenza and an additional bacterial superinfection, and, in addition, 54 COVID-19-infected patients had bacterial superinfections.
Clinical symptoms, and in particular neuropsychiatric symptoms, were noted at the time of admission. Routine laboratory parameters were determined in all patients, including CRP, complete and differential blood count, and Neopterin. Aliquots of the plasma samples were frozen until the analysis of the relationship between the laboratory parameters and the clinical symptoms. The patients gave informed consent for their blood to be used for scientific research, and the study was approved by the ethical board of the Medical University of Innsbruck. Only individuals older than 18 years were included in the study, and all personal data of patients were pseudonymized through consecutive numbering. The study was approved by the Ethics Committee of the Medical University Innsbruck (EK number: 1167/2020).
2.2. Laboratory Examinations
The blood sampling was performed during the first 3 days of their admission to hospital.
Routine laboratory values were analyzed by the Central Institute for Medical and Chemical Laboratory Diagnostics in Innsbruck, Austria.
CRP was analyzed using a Cobas 8000 platform (Roche Diagnostics, Rotkreuz, Switzerland).
Neopterin measurements were performed at the Institute of Medical Biochemistry Biocenter of the Medical University of Innsbruck. Neopterin concentrations were measured using an enzyme-linked immunosorbent assay (BRAHMS GmbH, Hennigsdorf, Germany) following the manufacturer’s protocol (sensitivity, 2 nmol/L).
2.3. Statistical Analysis
The statistical analysis was performed with IBM SPSS Statistics, version 28 (IBM Corporation, Armonk, New York, NY, USA). All statistical tests were two-sided, and p values < 0.05 were considered to be statistically significant.
Since not all data showed normal distribution in the Shapiro–Wilk test, non-parametric tests were applied. To compare two independent samples, the Mann–Whitney test was used. For more than two independent samples, the Kruskal–Wallis test with pairwise post hoc tests and the Bonferroni correction for multiple testing were used. Differences in ECOG performance status were assessed with the Chi-squared test. Figures were created with IBM SPSS Statistics, version 28 (IBM Corporation, Armonk, New York, NY, USA).
3. Results
3.1. Baseline Characteristics and Symptoms of the Study Population
A total of 194 patients admitted to the inpatient ward of the University Hospital of Internal Medicine II between 2016 and 2020 were recruited. In total, 62.4% of the patients (n = 121) were males, and 37.6% were females (n = 73). The age at the time of admission ranged from 19 to 95 years (median: 70).
The cohort was subcategorized according to the different pathogens causing pneumonia, namely, SARS-CoV-2 (n = 63; 32.5%), bacteria (n = 58; 29.9%), Influenza virus (n = 10; 5.2%), Influenza and bacterial superinfection (n = 9; 4.6%), and COVID-19 and superinfection (n = 54; 27.8%).
Interestingly, especially in patients with COVID-19, the proportion of males predominated (73% vs. 27%). This was also found in COVID-19 plus bacterial superinfection (75.9% vs. 24.1%). Patients with bacterial pneumonia, on the other hand, were mostly women (59% vs. 41%). Thus, gender comparisons were also performed. Men and women did not differ significantly regarding CRP and Neopterin concentrations. However, the CRP/Neopterin ratio was tendentially higher in women in the whole population (p = 0.055).
In terms of origin, among the patients with bacterial pneumonia and Influenza pneumonia, nearly all were living in Austria. Among the COVID-19 patients, 24 were from foreign countries (but all were from Europe, and thus were Caucasian).
Smoking status was different in patients with pneumonia. While only seven COVID-19 infected patients were smokers (4.4%), the percentage of smokers in Influenza patients (40%) and in patients with bacterial superinfection was higher (10.3%). Out of 58 patients with bacterial pneumonia, 10 were smokers (17.2%).
3.2. Laboratory Parameters in Patients with Infection
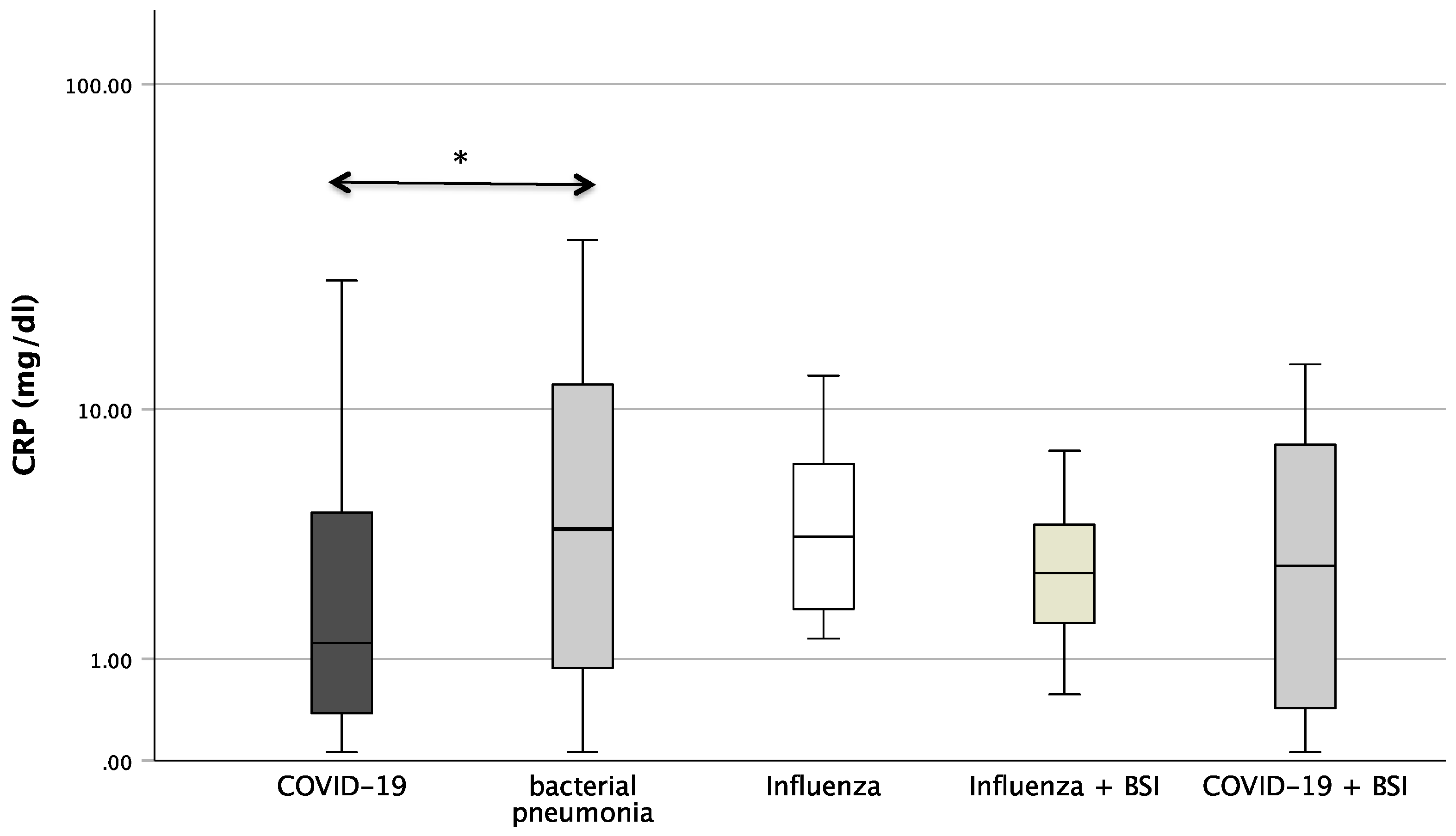
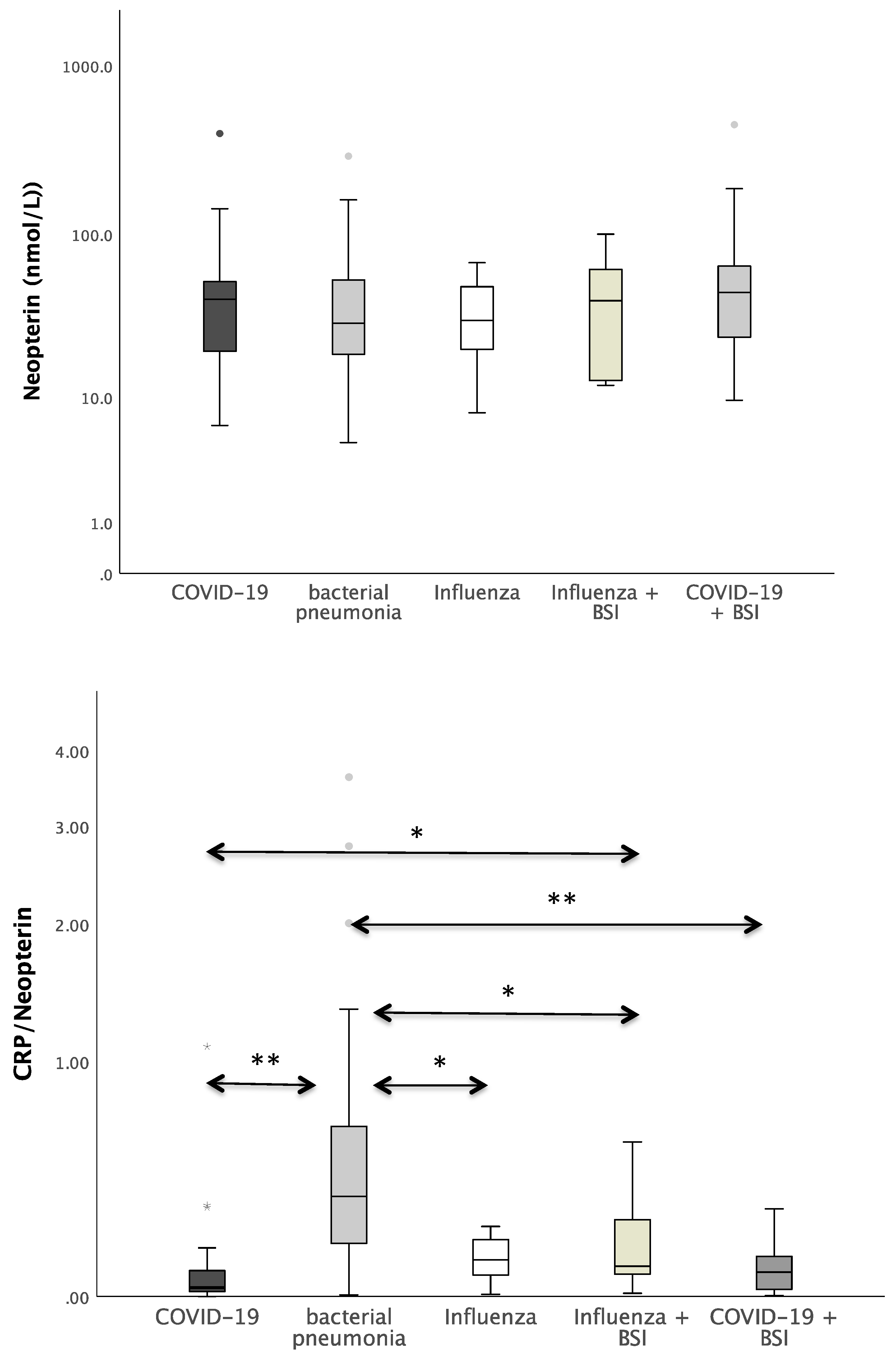
Figure 1 shows the boxplots of the investigated laboratory parameters (CRP, Neopterin, CRP/Neopterin) at admission for the whole cohort of patients with pneumonia divided by the pathogen subgroups. Regarding CRP, a significant difference was found between the pathogens (p = 0.034). In the pairwise post hoc tests, COVID-19 patients presented with significantly lower CRP levels than patients with a bacterial infection (p = 0.003). In addition, significant differences were also found with regard to CRP/Neopterin (p < 0.001). COVID-19 patients had lower CRP/Neopterin ratios compared to bacterial pathogens (p < 0.001) and compared to Influenza patients with superinfection (p = 0.035). Furthermore, patients with bacterial pneumonia showed higher CRP/Neopterin ratios than patients with COVID-19 superinfection (p < 0.001) and than patients with Influenza (p = 0.034) and Influenza superinfection (p = 0.038), as shown in Figure 1.
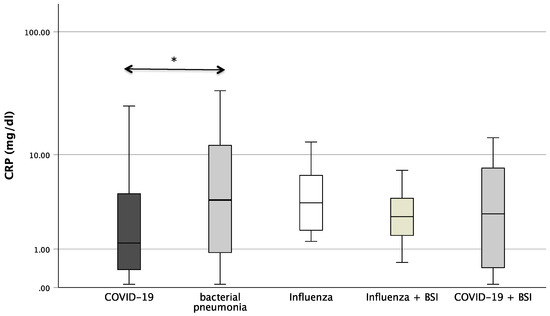
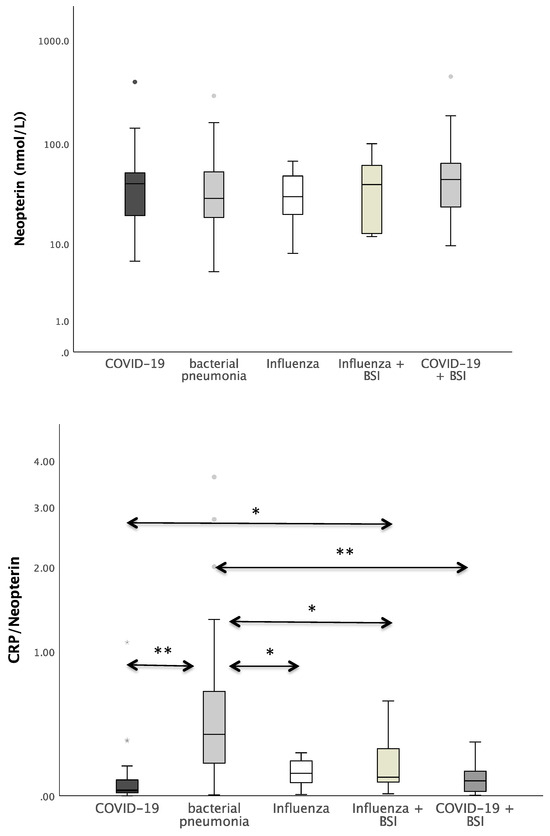
Figure 1.
Boxplots of CRP, Neopterin, and CRP/Neopterin in patients with different kinds of pneumonia (* p-value < 0.05, ** p-value < 0.001). Values that have more than three IQR values from the end of a box are marked with an asterisk (*). Values above 1.5 IQR values but less than 3 IQR values at the end of the box are labelled as outliers (o). The graphs were displayed in different colours to make the different pathogens more visible.
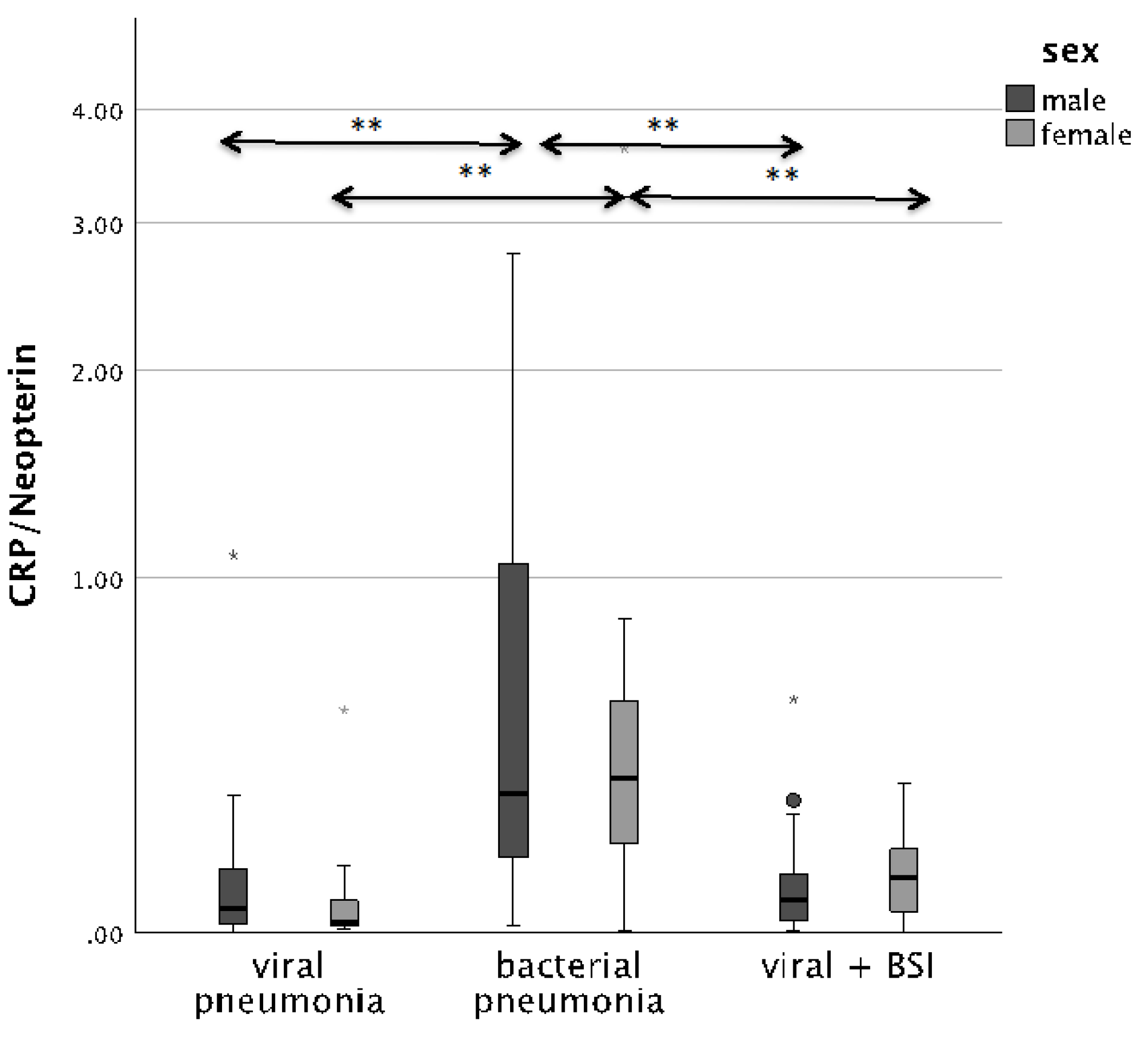
The CRP/Neopterin ratio was able to differentiate between viral and bacterial pathogenesis at admission, as well as superinfections, both in men and in women. Patients with BSI presented with significantly lower CRP/Neopterin ratios than patients with bacterial infection only, as shown in Figure 2 (p < 0.001).
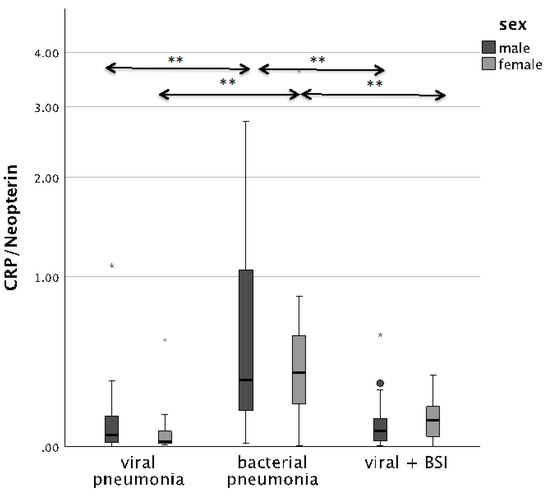
Figure 2.
Boxplots comparing the CRP/Neopterin ratio in male patients (left, dark-grey boxplots) and female patients (right, light-grey boxplots) with different pneumonia pathogens (** p-value < 0.001). Values that have more than three IQR values from the end of a box are marked with an asterisk (*). Values above 1.5 IQR values but less than 3 IQR values at the end of the box are labelled as outliers (o).
3.3. Physical Functioning and Clinical Symptoms
As shown in Table 1, there were significant differences regarding the physical functioning of patients. ECOG Performance status > 4 (ECOG 4: completely disabled, incapable of any selfcare, and totally confined to bed or chair; or ECOG 5: consecutive death) was encountered in patients with COVID-19 infection more than twice as often as in other pathogen subgroups.

Table 1.
Number and percentage of patients in the ECOG score categories at admission, divided by pathogens. Significant differences were found between different kinds of pneumonia.
Interestingly, we found differences in the frequency of symptoms between the pathogen groups (Table 2). COVID-19 infected patients presented significantly more often with fatigue than Influenza-infected patients (84.1% vs. 0%; p < 0.001) and significantly more often with neurological symptoms than in Influenza-infected patients (54.8% vs. 0%; p = 0.003). Patients with bacterial pneumonia likewise suffered from fatigue more often than Influenza patients (58.6% vs. 0%; p < 0.001). Moreover, male patients suffered more often from fatigue than women during acute infection (74.4% vs. 58.9%; p = 0.025). Regarding the age groups, patients older than 65 years had fatigue (78.6% vs. 62.9%; p = 0.020) and neurological symptoms (53.6% vs. 36.3%; p = 0.020) significantly more often.

Table 2.
Comparison between the pathogen groups regarding their clinical symptoms at admission. % of total (n = 194) patients. Significant differences were found regarding neurological symptoms and fatigue.
3.4. Laboratory Parameters and Clinical Symptoms
We also investigated whether patients with/without different symptoms (fatigue, neurological symptoms, depression, and sleep disorder) differed regarding inflammatory parameters. Patients who reported fatigue had unexpectedly lower CRP concentrations at admission than patients without fatigue (see Table 3). Regarding the other observed neuropsychiatric symptoms, no significant differences were found.

Table 3.
Median concentrations and interquartile range of the investigated laboratory parameters at admission, divided by neuropsychiatric symptoms. CRP differed significantly between patients with fatigue.
As our cohort included more men (especially with COVID-19 infection) and the CRP/Neopterin ratio tended to differ between women and men, we also examined whether there were possible gender differences. In fact, only men with fatigue presented with significantly lower CRP concentrations at admission (p = 0.035).
To further clarify whether inflammatory markers differed between patients with/without fatigue depending on pathogens, we analyzed the parameters for the different subgroups (except for influenza, as patients did not report fatigue; see Table 4).

Table 4.
Median concentrations and interquartile range of the investigated laboratory parameters at admission, divided by fatigue. CRP differed significantly in patients with bacterial pneumonia. Mann–Whitney-U tests were performed.
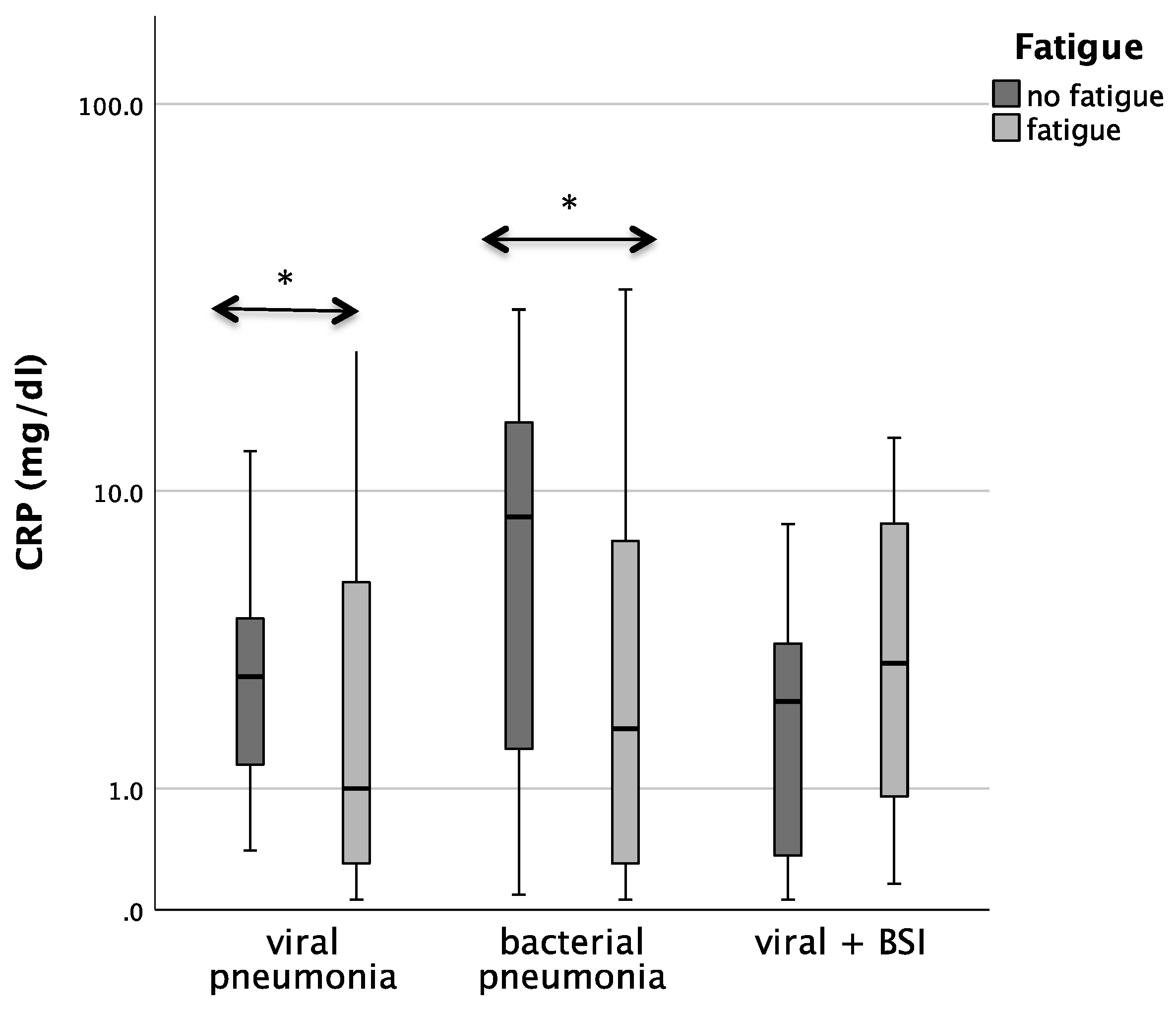
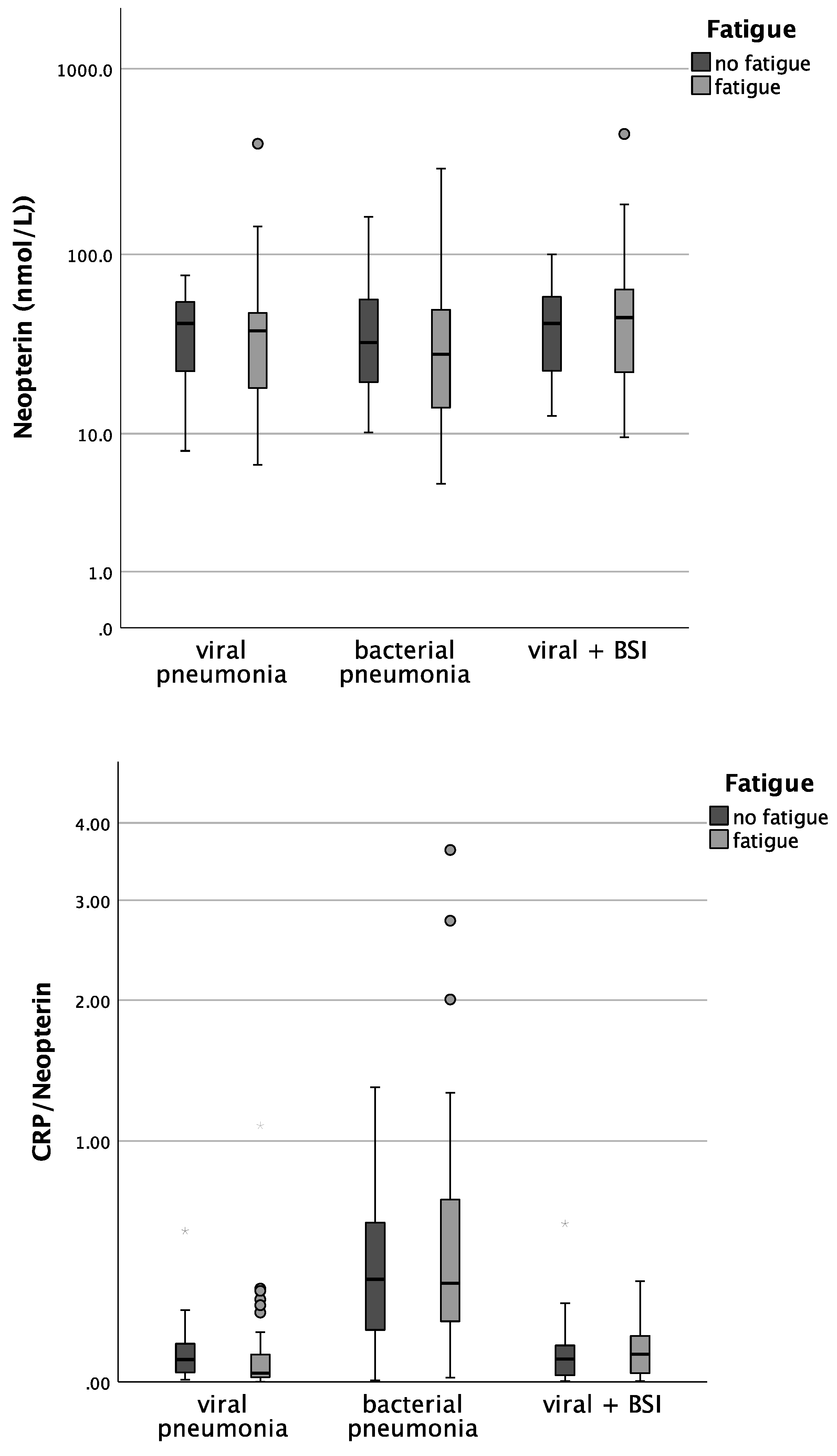
Patients with bacterial pneumonia and fatigue had significantly lower CRP concentrations than patients without fatigue (median 1.8 vs. 8.5; p = 0.029). In patients with COVID-19 pneumonia. CRP/Neopterin was tendentially lower in patients with fatigue (p = 0.071, see Figure 3).
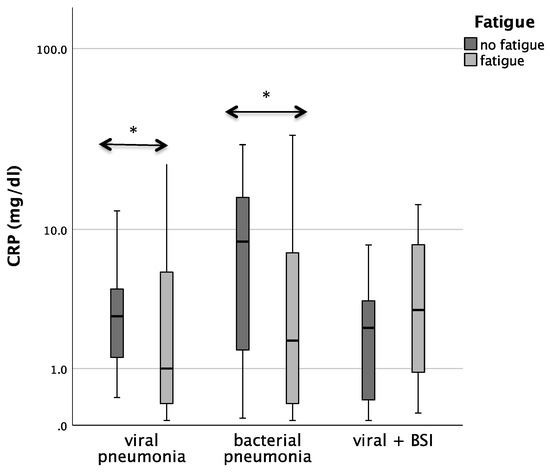
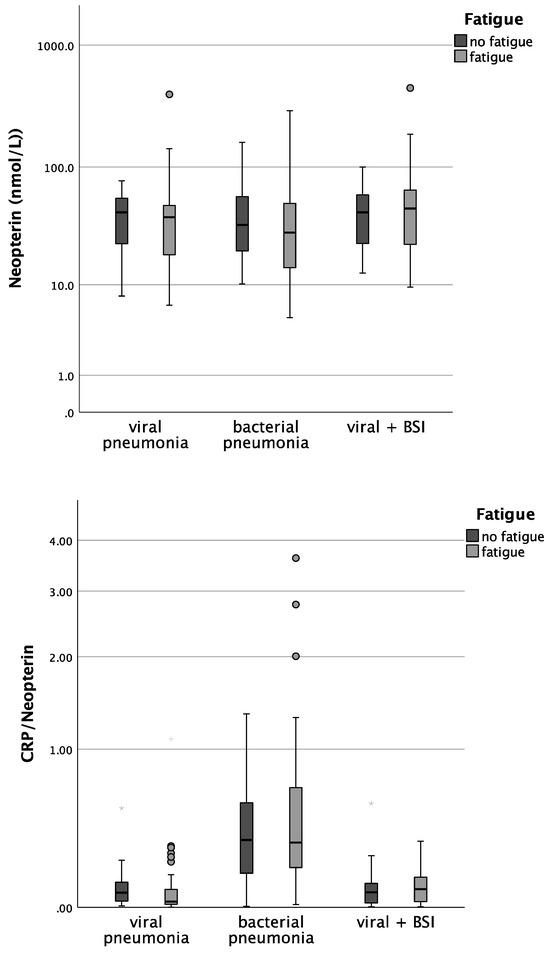
Figure 3.
Boxplots comparing CRP, Neopterin, and CRP/Neopterin in patients without fatigue (left, light-grey boxplots) and in patients with fatigue (right, dark-grey boxplots) with different pneumonia pathogens (* p-value < 0.05). Values that have more than three IQR values from the end of a box are marked with an asterisk (*). Values above 1.5 IQR values but less than 3 IQR values at the end of the box are labelled as outliers (o).
Also, the analysis in the whole cohort confirmed that CRP concentrations in both viral as well as bacterial pneumonia were lower in patients with fatigue during acute infection (p = 0.029 for bacterial pneumonia, p = 0.048 for viral pneumonia).
Interestingly, we found differences in the frequency of symptoms between the pathogen groups (Table 5). COVID-19-infected patients presented significantly more often with fatigue than Influenza-infected patients (84.1% vs. 0%; p < 0.001) and presented significantly more often with neurological symptoms than Influenza-infected patients (54.8% vs. 0%; p = 0.003). Patients with bacterial pneumonia likewise suffered from fatigue more often than Influenza patients (58.6% vs. 0%; p < 0.001). Moreover, male patients suffered more often from fatigue than women during acute infection (74.4%vs. 58.9%; p = 0.025). Regarding the age groups, patients older than 65 years had significantly fatigue (78.6% vs. 62.9%; p = 0.020) and neurological symptoms (53.6% vs. 36.3%; p = 0.020) more often.

Table 5.
Comparison between the pathogen groups regarding their clinical symptoms at admission. % of total (n = 194) patients. Significant differences were found regarding neurological symptoms and fatigue.
As our cohort included more men (especially with COVID-19 infection) and the CRP/Neopterin ratio tended to differ between women and men, we also examined whether there were possible gender differences. In fact, only men with fatigue presented with significantly lower CRP concentrations at admission (p = 0.035).
To further clarify whether inflammatory markers differed between patients with/without fatigue depending on pathogens, we analyzed the parameters for the different subgroups (except for influenza, as patients did not report fatigue; see Table 6).

Table 6.
Median concentrations and interquartile range of the investigated laboratory parameters at admission, divided by fatigue. CRP differed significantly in patients with bacterial pneumonia. Mann–Whitney-U tests were performed.
Patients with bacterial pneumonia and fatigue had significantly lower CRP concentrations than patients without fatigue (Table 6, median 1.8 vs. 8.5; p = 0.029). In patients with COVID-19, pneumonia CRP/Neopterin was tendentially lower in patients with fatigue (p = 0.071).
Also, the analysis in the whole cohort confirmed that CRP concentrations in both viral as well as bacterial pneumonia were lower in patients with fatigue during acute infection (p = 0.029 for bacterial pneumonia, p = 0.048 for viral pneumonia).
4. Discussion
Viral and bacterial respiratory diseases have been a major threat for the global health system in recent decades, even before COVID-19. Within this pilot study, we wanted to investigate the potential of the CRP/Neopterin ratio to distinguish between viral and bacterial pneumonia.
In patients admitted to hospital with pneumonia, a routine lab test was performed to guide therapy and choose whether antibiotics were necessary or not. In cases of bacterial infection, CRP and leukocytes are usually increased, whereas increased lymphocyte counts and Neopterin levels are observed in viral infections [25]. This was also the case in our study population. CRP levels were highest in bacterial pneumonia, followed by Influenza. Moreover, CRP levels were higher in patients older than 65 years. The CRP/Neopterin ratio differed significantly between patients with viral and bacterial pneumonia in our cohort, and was lowest in patients hospitalized with COVID-19 infection and highest in patients with bacterial pneumonia. The ratio was, as expected, age-dependent, being higher in the >65 years age group than in the <65 years group. This is probably, amongst other reasons, due to the higher release of cytokines in elderly people, as well as due to the decrease in innate and adaptive immune responses [26].
As in our study population, gender distribution was different depending on the underlying pathogens. We also performed additional analyses to examine whether gender-related differences might play a role. COVID-19 patients were mostly men (73%), while patients with bacterial pneumonia were mostly women (59%). This finding is well in line with clinical observations; in fact, men have a higher risk of a severe course of COVID-19 and a 61% higher mortality than women [27].
Both in men and in women, the CRP/Neopterin ratio was useful to distinguish between bacterial and viral infections in patients with pneumonia, and was also suited better to differentiate bacterial superinfection compared to CRP alone. To our knowledge, our pilot study shows, for the first time, that the CRP/Neopterin ratio can also distinguish between bacterial superinfection and viral or bacterial pneumonia.
Due to the increase in antibiotic resistance worldwide, it is essential to have biomarkers to guide whether antibiotic treatment is necessary or not, and the CRP/Neopterin ratio might in fact be a very useful biomarker to assist this decision in the early phase of infection.
In general, there are also only a few studies comparing all three groups of pathogens in regard to the abovementioned inflammatory parameters, and also the frequency of extrapulmonary symptoms has—at least to our knowledge—never been compared for different pathogens.
Data of this pilot study confirm our clinical observation, namely, that COVID-19 patients had significantly more neuropsychiatric symptoms such as fatigue or neurological symptoms in comparison to other pneumonia patients, alongside had a worse physical functioning. Interestingly, there were also differences regarding inflammatory marker CRP between patients with and without fatigue, indicating that inflammatory processes could be involved in the development of symptoms in patients with acute infection. However, rather unexpectedly, we found lower CRP concentrations at admission in patients reporting fatigue. In fact, we would rather have expected the opposite: namely, higher inflammatory markers in patients with fatigue. Earlier data had shown higher CRP and Interleukin-6 levels in 31 patients with sleep disturbance during acute COVID-19 infection, and higher CRP concentrations in patients with persistent fatigue 60 days after acute COVID-19 infection [6]. However, in that earlier study, only COVID-19 patients were included, while in this study we compared patients with different kinds of pneumonia in a cohort comprising significantly more patients. Another explanation for our finding could be that those patients, who had very low energy levels (and thus probably impaired mitochondrial function of many cells in the body, also including immune and liver cells) also had reduced capacities to produce CRP and fight the pathogens adequately.
Irrespective of the cause, longitudinal studies investigating the dynamics of inflammatory parameters, and especially the CRP/Neopterin ratio during the course of acute infections and reconvalescence depending on underlying pathogens, would certainly be useful to monitor the immune–host interaction even more precisely, especially in patients with COVID-19 infection, as these patients often suffer from persistent symptoms for weeks or even years—a syndrome also called Long COVID [28]. Following their recovery from the initial illness, approximately 10 to 20% of COVID-19-infected patients, as estimated by the World Health Organization (WHO), are anticipated to encounter mid- to long-term effects like fatigue/impaired physical function, neurological symptoms, sleep disorder, and depression [29,30,31].
An increased incidence of cognitive disabilities, as well as depressed mood and anxiety, has been reported during acute infection and thereafter [32]. However, other pathogens can also induce neuropsychiatric symptoms in pneumonia patients [33]. Neurological complications have been linked to both Influenza type A and Influenza type B. Additionally, individuals diagnosed with Influenza also appear to be at a higher risk of developing depression later in life [34].
The frequency of depression during acute infection did not differ significantly between pathogens in our population. On the other hand, COVID-19 patients were affected by fatigue significantly more often, especially in comparison to Influenza patients. Also, the physical functioning of patients with COVID-19 was more strongly impaired compared to infection with other respiratory pathogens, which fits well with our clinical observations. Also, neurological symptoms, such as headaches and smell or taste disorders, were observed significantly more often in COVID-19 patients compared to Influenza-infected patients. Several studies have proposed/shown that SARS-CoV-2 also affects the nervous system [31,35,36] and might affect brain function by inducing neuroinflammatory cascades.
In a comparison of mice with mild respiratory infection with H1N1 Influenza or COVID-19, a similar pathology was observed in the hippocampus. For example, 7 days after infection, in both groups, various neuro-inflammatory cytokines were still elevated in the cerebrospinal fluid, as well as white matter-selective microglial/macrophage reactivity in the subcortical and hippocampal white matter. However, in contrast to COVID-19, the Influenza-infected mice showed no lasting effects on subcortical white matter integrity [30]. These findings in mice might also explain why patients suffering from Long COVID might suffer from ongoing deficits of memory, focus, and concentration.
There are limitations of our pilot study which have to be taken into account. The relatively small number of patients, especially Influenza and Influenza patients, with a superinfection compared to COVID-19 patients, and the retrospective study design, may limit our general conclusions. Furthermore, repeated measurements of inflammation parameter concentrations and CRP/Neopterin ratio, as well as an assessment of the neuropsychiatric dynamics of the patients, are unfortunately missing. In future studies, it might also be useful to explore the symptoms via standardized questionnaires so that they can be better quantified. Another limitation of our pilot study is certainly that many multimorbid patients were included, and that the study population was rather heterogeneous, which can pose a challenge for comparison and general conclusions.
On the other hand, our study showed interesting new data which merit further investigation in larger longitudinal studies with more homogenous patient populations.
Author Contributions
Conceptualization, R.B.-W., G.W., K.K. and K.K.L.W.; methodology K.K.L.W., S.M., J.G., L.P., D.F., S.H., C.I., A.G. and G.W.; software, I.G. and S.M.; validation, K.K., R.B.-W. and G.W.; formal analysis, K.K.L.W., S.M. and K.K.; investigation, D.C., I.G., A.S., F.B., K.K.L.W., R.B.-W. and K.K.; resources, R.B.-W., J.G. and G.W.; data curation, S.M. and A.G.; writing—original draft preparation, K.K.L.W. and K.K.; writing—review and editing, K.K.L.W., D.C., I.G., A.S., F.B., K.K.L.W., S.M., J.G., L.P., D.F., S.H., C.I., A.G., G.W., R.B.-W. and K.K.; visualization, K.K.L.W.; supervision, R.B.-W., G.W. and K.K.; project administration, K.K.; funding acquisition: none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsoumani, E.; Carter, J.A.; Salomonsson, S.; Stephens, J.M.; Bencina, G. Clinical, economic, and humanistic burden of community acquired pneumonia in Europe: A systematic literature review. Expert. Rev. Vaccines 2023, 22, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Calabró, P.; Willerson, J.T.; Yeh, E.T.H. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation 2003, 108, 1930–1932. [Google Scholar] [CrossRef] [PubMed]
- Gostner, J.M.; Becker, K.; Kurz, K.; Fuchs, D. Disturbed Amino Acid Metabolism in HIV: Association with Neuropsychiatric Symptoms. Front. Psychiatry 2015, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Eshwara, V.; Mukhopadhyay, C.; Rello, J. Community-acquired bacterial pneumonia in adults: An update. Indian J. Med. Res. 2020, 151, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Schroecksnadel, S.; Sucher, R.; Kurz, K.; Fuchs, D.; Brandacher, G. Influence of immunosuppressive agents on tryptophan degradation and neopterin production in human peripheral blood mononuclear cells. Transpl. Immunol. 2011, 25, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Piater, T.; Gietl, M.; Hofer, S.; Gostner, J.M.; Sahanic, S.; Tancevski, I.; Sonnweber, T.; Pizzini, A.; Egger, A.; Schennach, H.; et al. Persistent Symptoms and IFN-γ-Mediated Pathways after COVID-19. J. Pers. Med. 2023, 13, 1055. [Google Scholar] [CrossRef]
- Pizzini, A.; Lunger, F.; Sahanic, A.; Nemati, N.; Fuchs, D.; Weiss, G.; Kurz, K.; Bellmann-Weiler, R. Diagnostic and Prognostic Value of Inflammatory Parameters Including Neopterin in the Setting of Pneumonia, COPD, and Acute Exacerbations, COPD. J. Chronic Obstr. Pulm. Dis. 2017, 14, 298–303. [Google Scholar] [CrossRef]
- Lafond, K.E.; Porter, R.M.; Whaley, M.J.; Suizan, Z.; Ran, Z.; Aleem, M.A.; Thapa, B.; Sar, B.; Proschle, V.S.; Peng, Z.; et al. Global burden of influenza-associated lower respiratory tract infections and hospitalizations among adults: A systematic review and meta-analysis. PLoS Med. 2021, 18, e1003550. [Google Scholar] [CrossRef]
- Ghosn, J.; Piroth, L.; Epaulard, O.; Le Turnier, P.; Mentré, F.; Bachelet, D.; Laouénan, C. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: Results from a large prospective cohort. Clin. Microbiol. Infect. 2021, 27, 1041.e1–1041.e4. [Google Scholar] [CrossRef]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I.; et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, 354–365. [Google Scholar] [CrossRef]
- Rothberg, M.B.; Haessler, S.D.; Brown, R.B. Complications of viral influenza. Am. J. Med. 2008, 121, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Almond, M.H.; McAuley, D.F.; Wise, M.P.; Griffiths, M.J.D. Influenza-related pneumonia. Clin. Med. Lond. Engl. 2012, 12, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Shirvaliloo, M. The blood-gas barrier in COVID-19: An overview of the effects of SARS-CoV-2 infection on the alveolar epithelial and endothelial cells of the lung. Tissue Barriers 2021, 9, 1937013. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.; Ojha, V.; Fricke, I.; Al-Sheboul, S.A.; Imarogbe, C.; Gravier, T.; Green, M.; Peterson, L.; Koutsaroff, I.P.; Demir, A.; et al. Innate and Adaptive Immunity during SARS-CoV-2 Infection: Biomolecular Cellular Markers and Mechanisms. Vaccines 2023, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Futosi, K.; Fodor, S.; Mócsai, A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Khan, M.; Saleem, W.; Karobari, M.I.; Mohamed, R.N.; Heboyan, A.; Rabaan, A.A.; Mutair, A.A.; Alhumaid, S.; Alsadiq, S.A.; et al. Evaluation of Bi-Lateral Co-Infections and Antibiotic Resistance Rates among COVID-19 Patients. Antibiotics 2022, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Brooks, L.R.K.; Mias, G.I. Streptococcus pneumoniae’s Virulence and Host Immunity: Aging, Diagnostics, and Prevention. Front. Immunol. 2018, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Mehraj, V.; Routy, J.P. Tryptophan Catabolism in Chronic Viral Infections: Handling Uninvited Guests. Int. J. Tryptophan Res. 2015, 8, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Pizzini, A.; Kurz, K.; Santifaller, J.; Tschurtschenthaler, C.; Theurl, I.; Fuchs, D.; Weiss, G.; Bellmann-Weiler, R. Assessment of neopterin and indoleamine 2,3-dioxygenase activity in patients with seasonal influenza: A pilot study. Influenza Other Respir. Viruses 2019, 13, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Bellmann-Weiler, R.; Kurz, K.; Holzer, C.; Larcher, C.; Fuchs, D.; Weiss, G. IFN-gamma mediated pathways in patients with fatigue and chronic active Epstein Barr virus-infection. J. Affect. Disord. 2008, 108, 171–176. [Google Scholar] [CrossRef]
- Meier, M.; Ottiger, M.; Vögeli, A.; Steuer, C.; Bernasconi, L.; Thomann, R.; Christ-Crain, M.; Henzen, C.; Hoess, C.; Zimmerli, W.; et al. Activation of the tryptophan/serotonin pathway is associated with severity and predicts outcomes in pneumonia: Results of a long-term cohort study. Clin. Chem. Lab. Med. (CCLM) 2017, 55, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Gietl, M.; Burkert, F.; Seiwald, S.; Böhm, A.; Hofer, S.; Gostner, J.M.; Piater, T.; Geisler, S.; Weiss, G.; Loeffler-Ragg, J.; et al. Interferon-gamma Mediated Metabolic Pathways in Hospitalized Patients During Acute and Reconvalescent COVID-19. Int. J. Tryptophan Res. IJTR 2023, 16, 11786469231154244. [Google Scholar] [CrossRef] [PubMed]
- Taenzer, M.; Löffler-Ragg, J.; Schroll, A.; Monfort-Lanzas, P.; Engl, S.; Weiss, G.; Brigo, N.; Kurz, K. Urine Metabolite Analysis to Identify Pathomechanisms of Long COVID: A Pilot Study. Int. J. Tryptophan Res. 2023, 16, 11786469231220781. [Google Scholar] [CrossRef] [PubMed]
- Lanser, L.; Kink, P.; Egger, E.M.; Willenbacher, W.; Fuchs, D.; Weiss, G.; Kurz, K. Inflammation-Induced Tryptophan Breakdown is Related with Anemia, Fatigue, and Depression in Cancer. Front. Immunol. 2020, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Rainer, T.H.; Chan, C.P.Y.; Leung, M.F.; Leung, W.; Ip, M.; Lee, N.; Cautherley, G.W.; Graham, C.A.; Fuchs, D.; Renneberg, R. Diagnostic utility of CRP to neopterin ratio in patients with acute respiratory tract infections. J. Infect. 2009, 58, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Pietrobon, A.J.; Teixeira, F.M.E.; Sato, M.N. Immunosenescence and Inflammaging: Risk Factors of Severe COVID-19 in Older People. Front. Immunol. 2020, 11, 579220. [Google Scholar] [CrossRef] [PubMed]
- Ortolan, A.; Lorenzin, M.; Felicetti, M.; Doria, A.; Ramonda, R. Does gender influence clinical expression and disease outcomes in COVID-19? A systematic review and meta-analysis. Int. J. Tryptophan Res. IJTR 2020, 99, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Lechner-Scott, J.; Levy, M.; Hawkes, C.; Yeh, A.; Giovannoni, G. Long COVID or post COVID-19 syndrome. Mult. Scler. Relat. Disord. 2021, 55, 103268. [Google Scholar] [CrossRef] [PubMed]
- Løkke, F.B.; Hansen, K.S.; Dalgaard, L.S.; Öbrink-Hansen, K.; Schiøttz-Christensen, B.; Leth, S. Long-term complications after infection with SARS-CoV-1, influenza and MERS-CoV—Lessons to learn in long COVID? Infect. Dis. Now 2023, 53, 104779. [Google Scholar] [CrossRef]
- Fernández-Castañeda, A.; Lu, P.; Geraghty, A.C.; Song, E.; Lee, M.H.; Wood, J.; O’Dea, M.R.; Dutton, S.; Shamardani, K.; Nwangwu, K.; et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 2022, 185, 2452–2468. [Google Scholar] [CrossRef] [PubMed]
- Schou, T.M.; Joca, S.; Wegener, G.; Bay-Richter, C. Psychiatric and neuropsychiatric sequelae of COVID-19—A systematic review. Brain Behav. Immun. 2021, 97, 328–348. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Davydow, D.S.; Hough, C.L.; Levine, D.A.; Langa, K.M.; Iwashyna, T.J. Functional disability, cognitive impairment, and depression after hospitalization for pneumonia. Am. J. Med. 2013, 126, 615–624. [Google Scholar] [CrossRef]
- Bornand, D.; Toovey, S.; Jick, S.S.; Meier, C.R. The risk of new onset depression in association with influenza—A population-based observational study. Brain Behav. Immun. 2016, 53, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, A.; Bhandarkar, B.S.; Lodha, L.; Marate, S. SARS-CoV-2 and the nervous system: Current perspectives. Arch. Virol. 2023, 168, 171. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, D.; Xu, X.; Liu, X.; Zhang, J.; Huang, K.; Wang, F.; Liu, J.; Zhang, Z.; Tao, E. Central nervous system complications in SARS-CoV-2-infected patients. J. Neurol. 2023, 270, 4617–4631. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).