The Influence of Homologous Arm Length on Homologous Recombination Gene Editing Efficiency Mediated by SSB/CRISPR-Cas9 in Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Culture Conditions
2.2. Plasmid Construction
2.3. Construction of Donor DNA
2.4. Genome Editing
3. Results
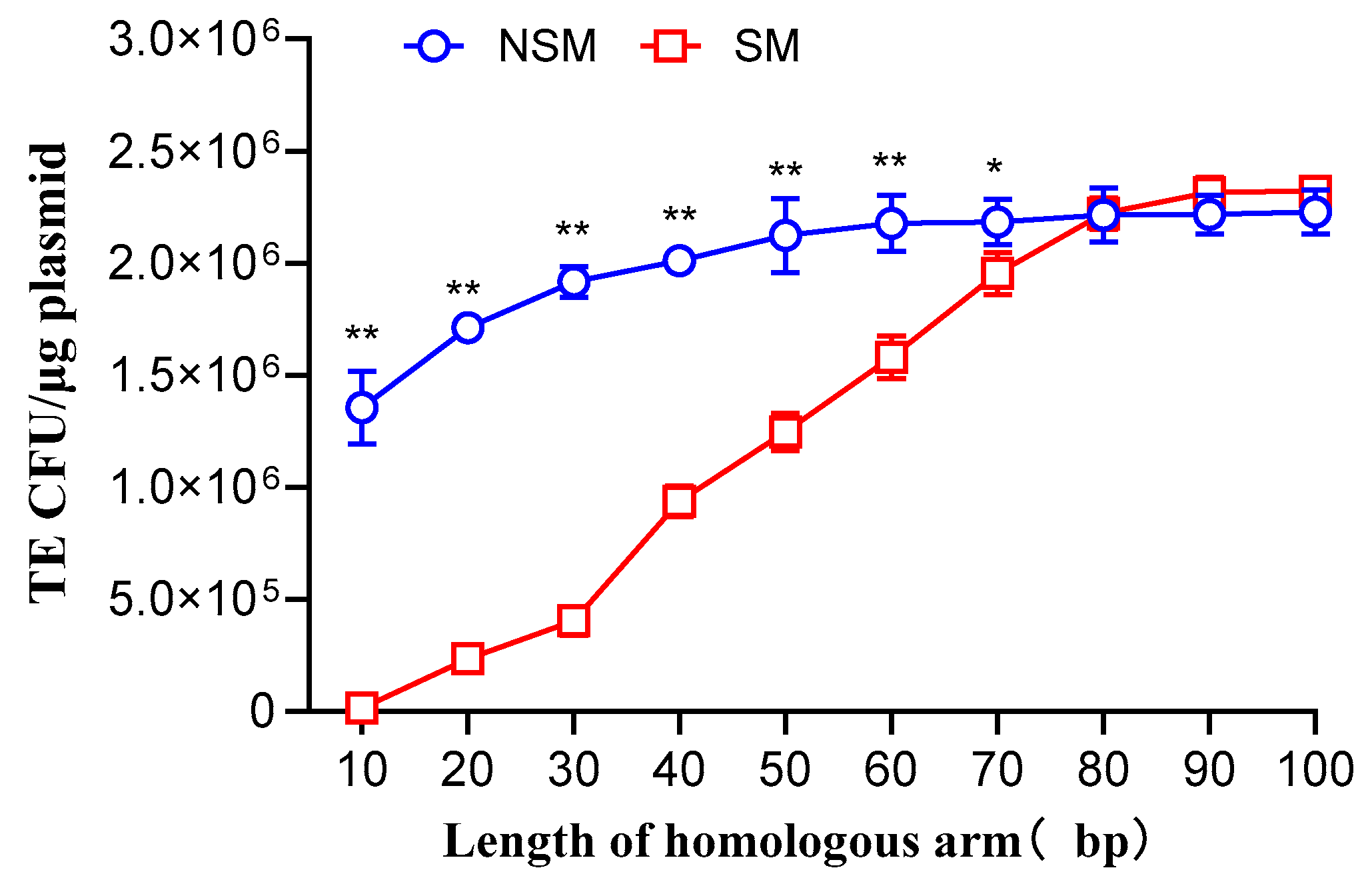
3.1. Impact of Homologous Arm Length of Donor DNA on Transformation Efficiency
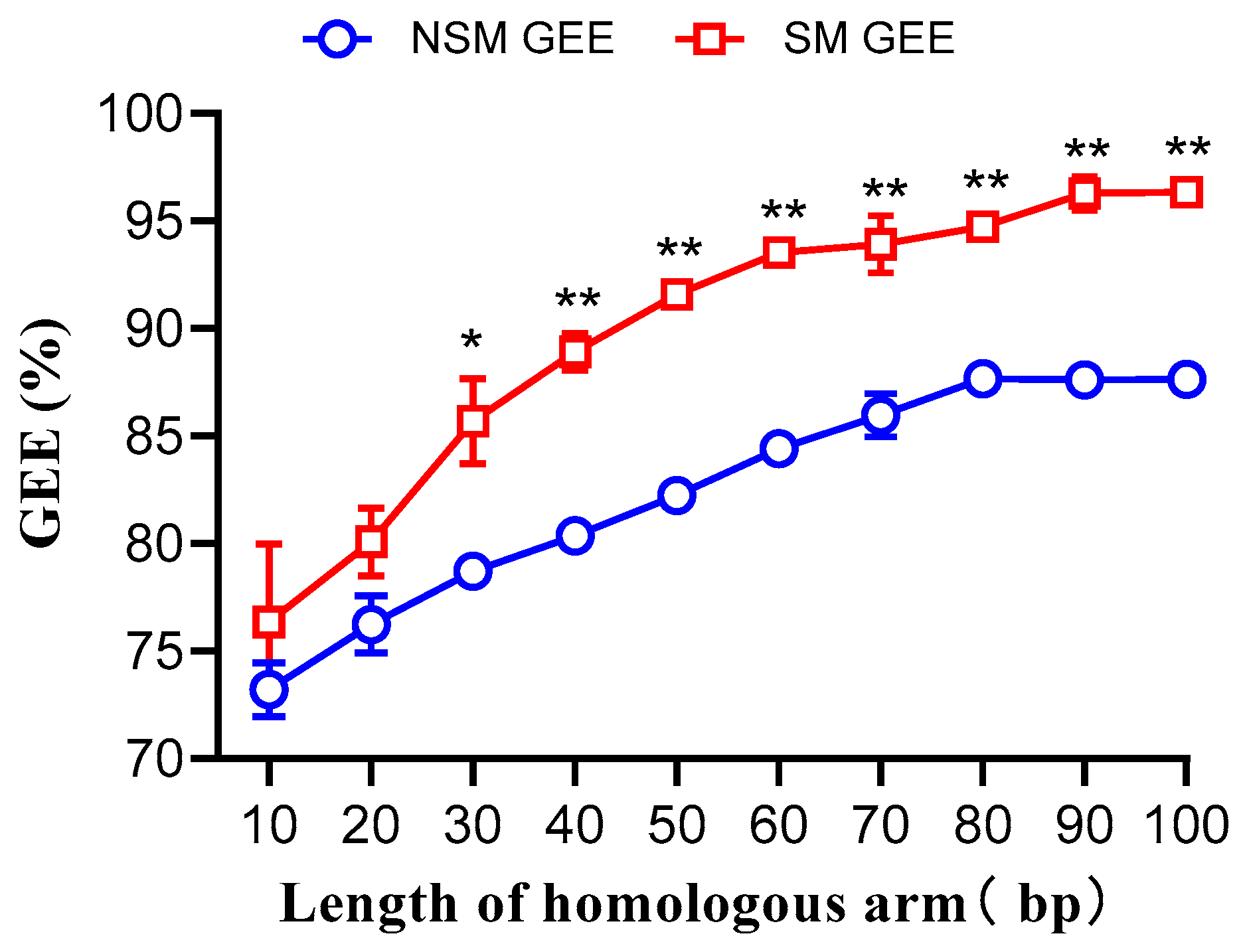
3.2. Impact of Donor DNA Homologous Arm Length on Gene Editing Efficiency
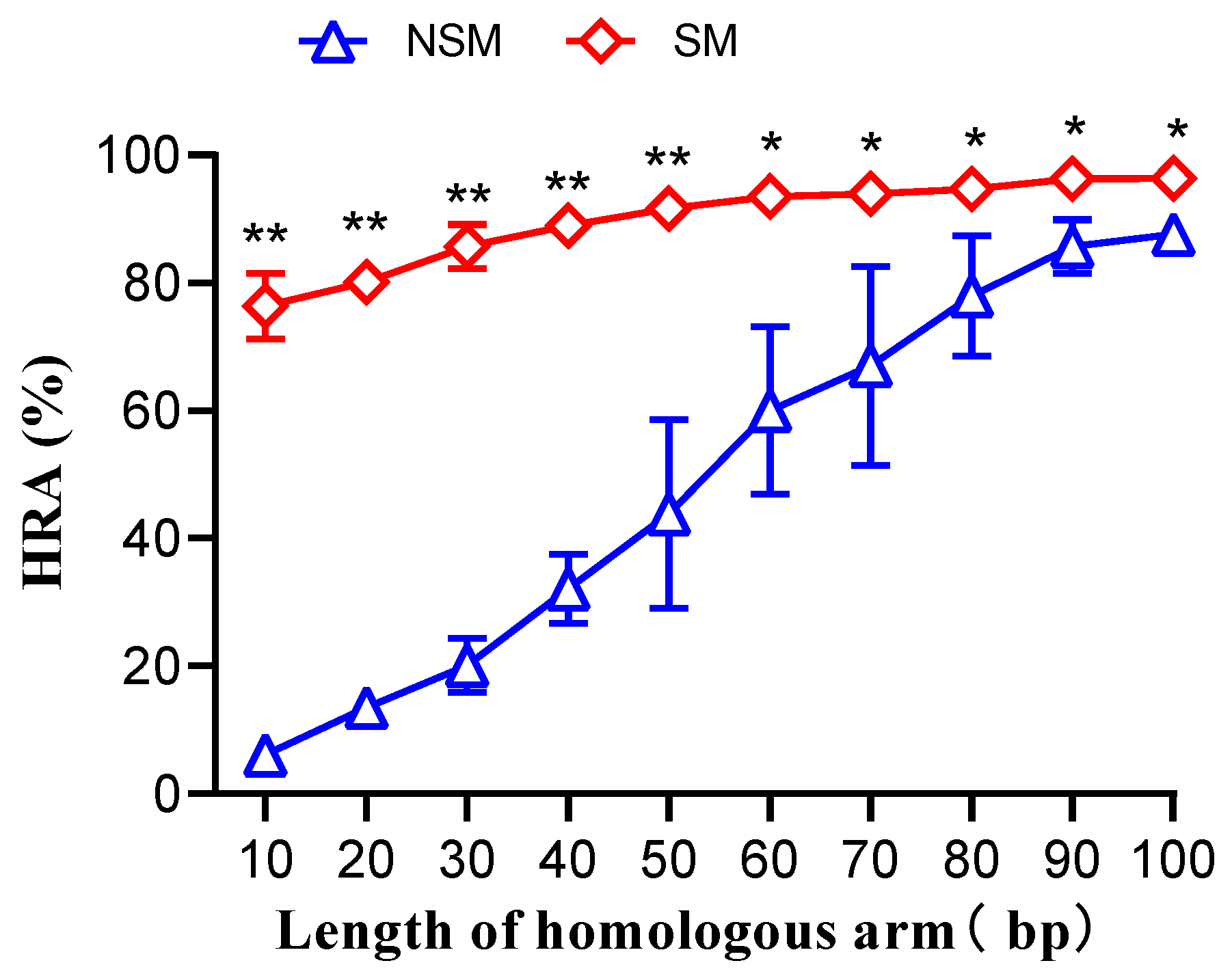
3.3. Impact of Donor DNA Homologous Arm Length on Homologous Recombination Rate
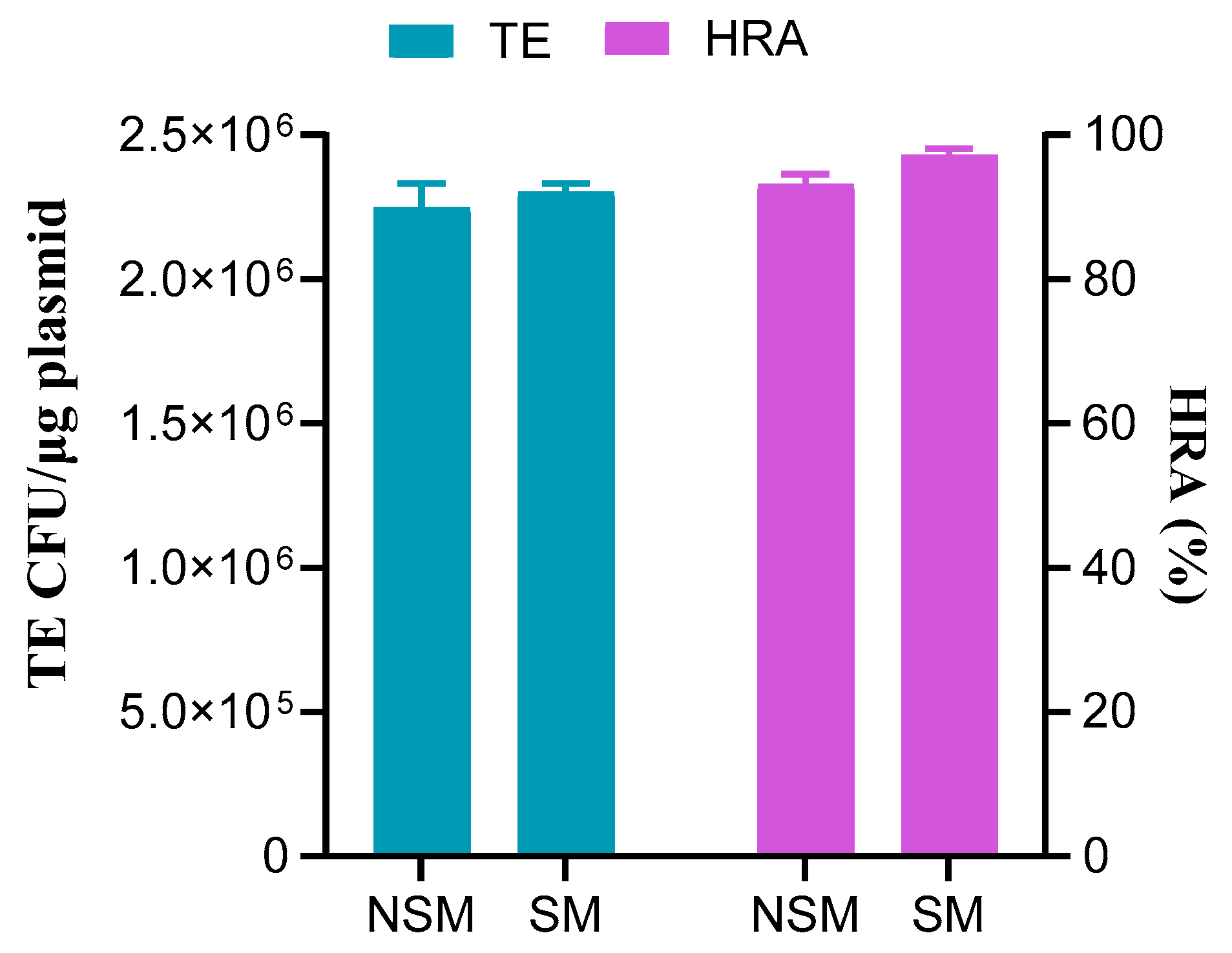
3.4. Impact of Selection Marker on Dual-Site Gene Editing with Donor DNA
3.5. Simultaneous Editing of Three Genes with 100 bp Homologous Arm Length Donor DNA Fragments without Selection Marker
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef] [PubMed]
- Khanzadi, M.N.; Khan, A.A. CRISPR/Cas9: Nature’s gift to prokaryotes and an auspicious tool in genome editing. J. Basic. Microb. 2020, 60, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.; Da Silva, G.J. CRISPR-Cas: Converting A Bacterial Defence Mechanism into A State-of-the-Art Genetic Manipulation Tool. Antibiotics 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Seo, S.; Lynn, P.; Lu, T.; Jin, Y.; Blaschek, H.P. Bacterial Genome Editing with CRISPR-Cas9: Deletion, Integration, Single Nucleotide Modification, and Desirable “Clean” Mutant Selection in Clostridium beijerinckii as an Example. ACS Synth. Biol. 2016, 5, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.M.; Silvis, M.R.; Zhao, D.; Hawkins, J.S.; Gross, C.A.; Qi, L.S. Bacterial CRISPR: Accomplishments and prospects. Curr. Opin. Microbiol. 2015, 27, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Malyarchuk, S.; Wright, D.; Castore, R.; Klepper, E.; Weiss, B.; Doherty, A.J.; Harrison, L. Expression of Mycobacterium tuberculosis Ku and Ligase D in Escherichia coli results in RecA and RecB-independent DNA end-joining at regions of microhomology. DNA Repair. 2007, 6, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Dev, H.; Chiang, T.W.; Lescale, C.; de Krijger, I.; Martin, A.G.; Pilger, D.; Coates, J.; Sczaniecka-Clift, M.; Wei, W.; Ostermaier, M.; et al. Shieldin complex promotes DNA end-joining and counters homologous recombination in BRCA1-null cells. Nat. Cell Biol. 2018, 20, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Jakočiūnas, T.; Jensen, M.K.; Keasling, J.D. CRISPR/Cas9 advances engineering of microbial cell factories. Metab. Eng. 2016, 34, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Song, D.; Zhu, H. Homology-dependent recombination of large synthetic pathways into E. coli genome via λ-Red and CRISPR/Cas9 dependent selection methodology. Microb. Cell Fact. 2020, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Pyne, M.E.; Moo-Young, M.; Chung, D.A.; Chou, C.P. Coupling the CRISPR/Cas9 System with Lambda Red Recombineering Enables Simplified Chromosomal Gene Replacement in Escherichia coli. Appl. Environ. Microb. 2015, 81, 5103–5114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, B.; Duan, C.; Sun, B.; Yang, J.; Yang, S. Multigene Editing in the Escherichia coli Genome via the CRISPR-Cas9 System. Appl. Environ. Microb. 2015, 81, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.; Ramirez, J.; Xander, C.; Upreti, C.; Bhatt, S. Lambda Red-mediated Recombineering in the Attaching and Effacing Pathogen Escherichia albertii. Biol. Proced. Online 2016, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Gao, M.; Cheng, X.; Kang, G.; Cao, X.; Huang, H. Engineered butyrate-producing bacteria prevents high fat diet-induced obesity in mice. Microb. Cell Fact. 2020, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.I.; Sun, B.; Chen, J.; Zhang, Y.; Jiang, Y.U.; Yang, S. A modified pCas/pTargetF system for CRISPR-Cas9-assisted genome editing in Escherichia coli. Acta Bioch Bioph Sin. 2021, 53, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Figueredo, E.F.; Cruz, T.A.D.; Almeida, J.R.D.; Batista, B.D.; Marcon, J.; Andrade, P.A.M.D.; Hayashibara, C.A.D.A.; Rosa, M.S.; Azevedo, J.L.; Quecine, M.C. The key role of indole-3-acetic acid biosynthesis by Bacillus thuringiensis RZ2MS9 in promoting maize growth revealed by the ipdC gene knockout mediated by the CRISPR-Cas9 system. Microbiol. Res. 2023, 266, 127218. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tian, J.; Liu, L.; Zhu, T.; Yu, X.; Chu, X.; Yao, B.; Wu, N.; Fan, Y. Identification of an operon involved in fluoride resistance in Enterobacter cloacae FRM. Sci. Rep. 2017, 7, 6786. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.; Zhang, C.; Tian, F.; Li, H.; Yang, Q.; Song, A.; Qiu, L. Recombination function and recombination kinetics of Escherichia coli single-stranded DNA-binding protein. Sci. Bull. 2016, 61, 1594–1604. [Google Scholar] [CrossRef]
- Chai, R.; Zhang, Q.; Wu, J.; Shi, Z.; Li, Y.; Gao, Y.; Qi, Y.; Qiu, L. Single-Stranded DNA-Binding Proteins Mediate DSB Repair and Effectively Improve CRISPR/Cas9 Genome Editing in Escherichia coli and Pseudomonas. Microorganisms 2023, 11, 850. [Google Scholar] [CrossRef]
- Mitsunobu, H.; Teramoto, J.; Nishida, K.; Kondo, A. Beyond Native Cas9: Manipulating Genomic Information and Function. Trends Biotechnol. 2017, 35, 983–996. [Google Scholar] [CrossRef]
- Savic, N.; Ringnalda, F.C.; Lindsay, H.; Berk, C.; Bargsten, K.; Li, Y.; Neri, D.; Robinson, M.D.; Ciaudo, C.; Hall, J.; et al. Covalent linkage of the DNA repair template to the CRISPR-Cas9 nuclease enhances homology-directed repair. eLife 2018, 7, e33761. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Villamañán, A.; Ng, J.W.; Planel, R.; Ménager, H.; Chen, A.; Cui, L.; Bikard, D. On-target activity predictions enable improved CRISPR–dCas9 screens in bacteria. Nucleic Acids Res. Acids Res. 2020, 48, e64. [Google Scholar] [CrossRef] [PubMed]
- Haeussler, M.; Concordet, J. Genome Editing with CRISPR-Cas9: Can It Get Any Better? J. Genet. Genom. 2016, 43, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Gelsinger, D.R.; Vo, P.L.H.; Klompe, S.E.; Ronda, C.; Wang, H.H.; Sternberg, S.H. Bacterial genome engineering using CRISPR-associated transposases. Nat. Protoc. 2024, 19, 752–790. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O.; et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Costantino, N.; Court, D.L. Enhanced levels of λ Red-mediated recombinants in mismatch repair mutants. Proc. Natl. Acad. Sci. USA 2003, 100, 15748–15753. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Bikard, D.; Cox, D.; Zhang, F.; Marraffini, L.A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, C.; Guo, J.; Zhang, P.; Chen, T.; Wang, Z.; Zhao, X. Development and characterization of a CRISPR/Cas9n-based multiplex genome editing system for Bacillus subtilis. Biotechnol. Biofuels 2019, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Watzlawick, H.; Altenbuchner, J. Multiple integration of the gene ganA into the Bacillus subtilis chromosome for enhanced β-galactosidase production using the CRISPR/Cas9 system. AMB Express 2019, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Choi, S.K. Programmed gRNA Removal System for CRISPR-Cas9-Mediated Multi-Round Genome Editing in Bacillus subtilis. Front. Microbiol. 2019, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Price, M.A.; Cruz, R.; Baxter, S.; Escalettes, F.; Rosser, S.J. CRISPR-Cas9 In Situ engineering of subtilisin E in Bacillus subtilis. PLoS ONE 2019, 14, e210121. [Google Scholar] [CrossRef] [PubMed]
- Ao, X.; Yao, Y.; Li, T.; Yang, T.T.; Dong, X.; Zheng, Z.T.; Chen, G.Q.; Wu, Q.; Guo, Y. A Multiplex Genome Editing Method for Escherichia coli Based on CRISPR-Cas12a. Front. Microbiol. 2018, 9, 2307. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Liu, W.; Wang, J.J.; Tan, L.; Hong, B.; Guo, L.; Liu, H.; Pan, Y.; Zhao, Y. CRISPR-Cas9 knockout of qseB induced asynchrony between motility and biofilm formation in Escherichia coli. Can. J. Microbiol. 2019, 65, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, A.S.; Jawed, K.; Yazdani, S.S. CRISPR/Cas9-mediated engineering of Escherichia coli for n-butanol production from xylose in defined medium. J. Ind. Microbiol. Biotechnol. 2019, 46, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, F.; Wu, H.; Li, G.; Han, Y.; Ma, Q.; Fan, X.; Zhang, C.; Xu, Q.; Xie, X.; et al. Multiple-step chromosomal integration of divided segments from a large DNA fragment via CRISPR/Cas9 in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2019, 46, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, L.; Mao, X.; Fei, M.; Chen, Y.; Shen, M.; Qiu, J. Chemical transformation mediated CRISPR/Cas9 genome editing in Escherichia coli. Biotechnol. Lett. 2019, 41, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhao, D.; Zhang, X.; Ding, X.; Bi, C. CRISPR/Cas9 Assisted Multiplex Genome Editing Technique in Escherichia coli. Biotechnol. J. 2018, 13, e1700604. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Z.; Huang, C.; Zhang, Y.; Wang, Z.; Tang, Y.J.; Chen, T.; Zhao, X. Metabolic engineering of Escherichia coli using CRISPR-Cas9 meditated genome editing. Metab. Eng. 2015, 31, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.R.; Lee, H.J.; Kim, H.J.; Lee, S.J. Multiplex Single-Nucleotide Microbial Genome Editing Achieved by CRISPR-Cas9 Using 5′-End-Truncated sgRNAs. ACS Synth. Biol. 2023, 12, 2203–2207. [Google Scholar] [CrossRef] [PubMed]
- Blattner, F.R.; Plunkett, G.; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The Complete Genome Sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T.T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989; pp. 16–688. [Google Scholar]
- Zhang, H.; Cheng, Q.X.; Liu, A.M.; Zhao, G.P.; Wang, J. A Novel and Efficient Method for Bacteria Genome Editing Employing both CRISPR/Cas9 and an Antibiotic Resistance Cassette. Front. Microbiol. 2017, 8, 812. [Google Scholar] [CrossRef] [PubMed]
- Adiego-Pérez, B.; Randazzo, P.; Daran, J.M.; Verwaal, R.; Roubos, J.A.; Daran-Lapujade, P.; van der Oost, J. Multiplex genome editing of microorganisms using CRISPR-Cas. FEMS Microbiol. Lett. 2019, 366, fnz86. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yuan, S.; Xiong, B.; Sun, H.; Ye, L.; Li, J.; Zhang, X.; Bi, C. Development of a fast and easy method for Escherichia coli genome editing with CRISPR/Cas9. Microb. Cell Fact. 2016, 15, 205. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Y.; Yeo, W.; Bae, T.; Ji, Q. Rapid and Efficient Genome Editing in Staphylococcus aureus by Using an Engineered CRISPR/Cas9 System. J. Am. Chem. Soc. 2017, 139, 3790–3795. [Google Scholar] [CrossRef] [PubMed]

| Strain or Plasmid | Description | Source or Reference |
|---|---|---|
| E. coli | ||
| DH5α | F− endA1 glnV44 thi-1 recA1 relA1 gyrA96 deoR nupG φ80dlacZ∆M15 ∆(lacZYA-argF) U169 hsdR17 (rK− mK+) λ− | TaKaRa |
| MG1655 | K-12; F−, λ−, ilvG−, rfb-50, rph-1 | [40] |
| Plasmids | ||
| pCas-SSB | repA101(Ts) kan Pcas-cas9 ParaB-SSB lacIq Ptrc-sgRNA-pMB1 | [19] |
| pTargetF | pMB1 aadA sgRNA | [12] |
| pTargetF-lacZ | pMB1 aadA sgRNA-lacZ | [19] |
| pTargetF-lacZ-2 | pMB1 aadA sgRNA-lacZ sgRNA-lacZ2 | This study |
| pTargetF-3 | pMB1 aadA sgRNA-RecA1 sgRNA-RecA2 sgRNA-RecBCD1 sgRNA-RecBCD2 sgRNA-SSB1 sgRNA-SSB2 | This study |
| Primer | Sequence (5′-3′) |
|---|---|
| pC01 | GGAAACAGCTAATTCTCATGTTTGA |
| pC02 | CCGGTTATTATATCTATATCGAGAT |
| pC03 | ATTTCACACAGGAAACAGCTAATTCTCATGTTTGA |
| pC04 | CATGGCCTGCCCGGTTATTATATCTATATC GAGAT |
| pC05 | GCGGATAACAATTTCACACAGGAAACAGCTAATTCTCATGTTTGA |
| pC06 | TACGGGCAGACATGGCCTGCCCGGTTATTATATCTATATC GAGAT |
| pC07 | GGAATTGTGAGCGGATAACAATTTCACACAGGAAACAGCTAATTCTCATGTTTGA |
| pC08 | TTACGCGAAATACGGGCAGACATGGCCTGCCCGGTTATTATATCTATATC GAGAT |
| pC09 | TATGTTGTGTGGAATTGTGAGCGGATAACAATTTCACACAGGAAACAGCTAATTCTCATGTTTGA |
| pC10 | ATGGATTTCCTTACGCGAAATACGGGCAGACATGGCCTGCCCGGTTATTATATCTATATC GAGAT |
| pC11 | TTCCGGCTCGTATGTTGTGTGGAATTGTGAGCGGATAACAATTTCACACAGGAAACAGCTAATTCTCATGTTTGA |
| pC12 | ATAGTACATAATGGATTTCCTTACGCGAAATACGGGCAGACATGGCCTGCCCGGTTATTATATCTATATCGAGAT |
| pC13 | CACTTTATGCTTCCGGCTCGTATGTTGTGTGGAATTGTGAGCGGATAACAATTTCACACAGGAAACAGCTAATTCTCATGTTTGA |
| pC14 | TGTTTTTTAAATAGTACATAATGGATTTCCTTACGCGAAATACGGGCAGACATGGCCTGCCCGGTTATTATATCTATATCGAGAT |
| pC15 | CCAGGCTTTACACTTTATGCTTCCGGCTCGTATGTTGTGTGGAATTGTGAGCGGATAACAATTTCACACAGGAAACAGCTAATTCTCATGTTTGA |
| pC16 | CAAAAGTTTGTGTTTTTTAAATAGTACATAATGGATTTCCTTACGCGAAATACGGGCAGACATGGCCTGC CCGGTTATTATATCTATATCGAGAT |
| pC17 | ATTAGGCACCCCAGGCTTTACACTTTATGCTTCCGGCTCGTATGTTGTGTGGAATTGTGAGCGGATAACAATTTCACACAGGAAACAGCTAATTCTCATGTTTGA |
| pC18 | ACCGAACATCCAAAAGTTTGTGTTTTTTAAATAGTACATAATGGATTTCCTTACGCGAAATACGGGCAGA CATGGCCTGC CCGGTTATTATATCTATATCGAGAT |
| pC19 | TAGCTCACTCATTAGGCACCCCAGGCTTTACACTTTATGCTTCCGGCTCGTATGTTGTGTGGAATTGTGAGCGGATAACAATTTCACACAGGAAACAGCTAATTCTCATGTTTGA |
| pC20 | AAAAGAATAAACCGAACATCCAAAAGTTTGTGTTTTTTAAATAGTACATAATGGATTTCCTTACGCGAAATACGGGCAGACATGGCCTGCCCGGTTATTATATCTATATCGAGAT |
| pC21 | CGGTAGTGGGATACGACGAT |
| pC22 | CGGTTGGAATAATAGCGAGA |
| pC23 | TCGTCAGGCTACTGCGTAT |
| pC24 | ATCCAACAGGCGAGCAT |
| pC25 | CGCAACAAGGGATTTACAC |
| pC26 | GATGGCGGATTTAGGGT |
| pC27 | GAACCGAGGTCACAACATA |
| pC28 | TCACAATGGCGGACAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, R.; Guo, J.; Geng, Y.; Huang, S.; Wang, H.; Yao, X.; Li, T.; Qiu, L. The Influence of Homologous Arm Length on Homologous Recombination Gene Editing Efficiency Mediated by SSB/CRISPR-Cas9 in Escherichia coli. Microorganisms 2024, 12, 1102. https://doi.org/10.3390/microorganisms12061102
Chai R, Guo J, Geng Y, Huang S, Wang H, Yao X, Li T, Qiu L. The Influence of Homologous Arm Length on Homologous Recombination Gene Editing Efficiency Mediated by SSB/CRISPR-Cas9 in Escherichia coli. Microorganisms. 2024; 12(6):1102. https://doi.org/10.3390/microorganisms12061102
Chicago/Turabian StyleChai, Ran, Jiaxiang Guo, Yue Geng, Shuai Huang, Haifeng Wang, Xinding Yao, Tao Li, and Liyou Qiu. 2024. "The Influence of Homologous Arm Length on Homologous Recombination Gene Editing Efficiency Mediated by SSB/CRISPR-Cas9 in Escherichia coli" Microorganisms 12, no. 6: 1102. https://doi.org/10.3390/microorganisms12061102






