Drought-Tolerant Bacteria and Arbuscular Mycorrhizal Fungi Mitigate the Detrimental Effects of Drought Stress Induced by Withholding Irrigation at Critical Growth Stages of Soybean (Glycine max, L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Symbiotic Bacteria
2.2. Isolation of Non-Symbiotic Bacteria
2.3. Screening Drought-Stress Tolerance
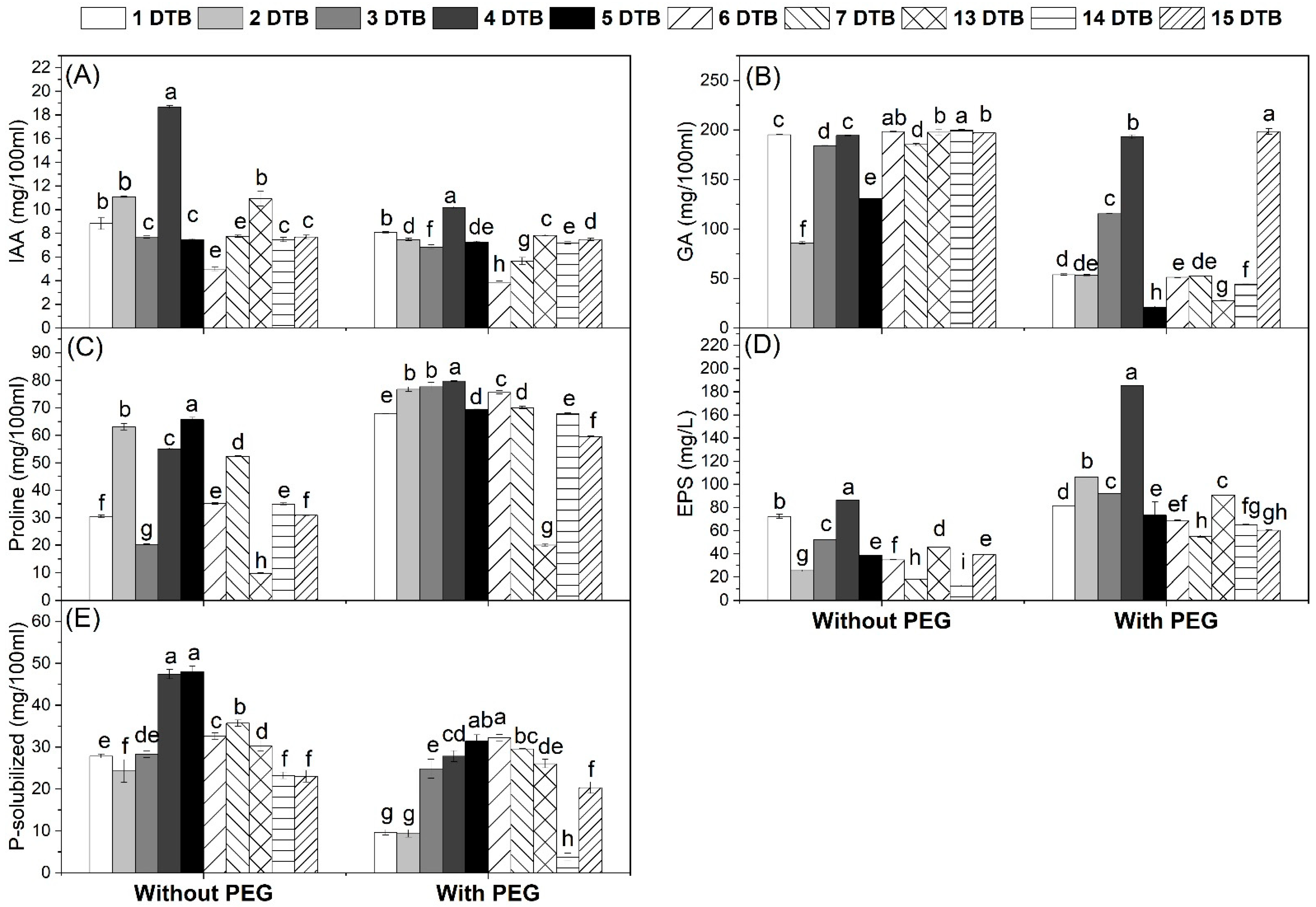
2.4. Estimation of Plant Growth Promotion Traits
2.5. Identification of Bacterial Isolates
2.6. The Field Experiment
2.6.1. Bacterial Inoculant Preparation
2.6.2. Mycorrhizal Inoculum
2.6.3. Agronomic Practices
2.7. Morpho-Physiological, Nodulation, Nutrient Concentrations, and Soil Enzyme Activities
2.8. Microbiological Analysis
2.9. Yield Traits
2.10. Statistical Analysis
3. Results
3.1. Isolation, Screening, and Identification of Drought-Tolerant Bacteria
3.1.1. Bradyrhizobium
3.1.2. Rhizobacteria
3.1.3. Identification of the Most Potent Isolates
3.2. Bacterial Count, Mycorrhizal Colonization, and Enzyme Activities under Drought Stress
3.3. Morphological Traits of Soybean Plants under Drought Stress
3.4. Chlorophyll and Proline Contents in Soybean Leaves under Drought Stress
3.5. Nutrient Contents in Soybean Leaves under Drought Stress
3.6. Nodulation and Yield Traits of Soybean Plants under Drought Stress
3.7. Assessment of Bioinoculants and Their Combinations Effects by Principal Component Analysis, Heatmap of Correlation, and Pearson’s Correlation Analysis under Drought Stress
4. Discussion
4.1. Isolated Drought-Tolerant Bacteria Showed Higher Activity to Produce Plant Growth Substances
4.2. Drought-Tolerant Bacteria and AMF Improve Bacterial Counts, Mycorrhizal Colonization, and Enzyme Activities
4.3. Drought-Tolerant Bacteria and AMF Improve Morpho-Physiological Traits of Soybean Plants
4.4. Drought-Tolerant Bacteria and AMF Improve Nutrient Content in Soybean Plants
4.5. Drought-Tolerant Bacteria and AMF Improve Nodulation and Yield Traits in Soybean Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grassini, P.; Cafaro La Menza, N.; Rattalino Edreira, J.I.; Monzón, J.P.; Tenorio, F.A.; Specht, J.E. Chapter 8—Soybean. In Crop Physiology Case Histories for Major Crops; Sadras, V.O., Calderini, D.F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 282–319. [Google Scholar]
- Liu, S.; Zhang, M.; Feng, F.; Tian, Z. Toward a “Green Revolution” for Soybean. Mol. Plant 2020, 13, 688–697. [Google Scholar] [CrossRef]
- Pareek, A.; Dhankher, O.P.; Foyer, C.H. Mitigating the impact of climate change on plant productivity and ecosystem sustainability. J. Exp. Bot. 2020, 71, 451–456. [Google Scholar] [CrossRef]
- Meckel, L.; Egli, D.B.; Phillips, R.E.; Radcliffe, D.; Leggett, J.E. Effect of Moisture Stress on Seed Growth in Soybeans1. Agron. J. 1984, 76, 647–650. [Google Scholar] [CrossRef]
- Buezo, J.; Sanz-Saez, Á.; Moran, J.F.; Soba, D.; Aranjuelo, I.; Esteban, R. Drought tolerance response of high-yielding soybean varieties to mild drought: Physiological and photochemical adjustments. Physiol. Plant. 2019, 166, 88–104. [Google Scholar] [CrossRef]
- Li, S.; Xie, Y.; Liu, G.; Wang, J.; Lin, H.; Xin, Y.; Zhai, J. Water Use Efficiency of Soybean under Water Stress in Different Eroded Soils. Water 2020, 12, 373. [Google Scholar] [CrossRef]
- Specht, J.E.; Hume, D.J.; Kumudini, S.V. Soybean Yield Potential—A Genetic and Physiological Perspective. Crop Sci. 1999, 39, 1560–1570. [Google Scholar] [CrossRef]
- Gui, Y.-W.; Sheteiwy, M.S.; Zhu, S.-G.; Batool, A.; Xiong, Y.-C. Differentiate effects of non-hydraulic and hydraulic root signaling on yield and water use efficiency in diploid and tetraploid wheat under drought stress. Environ. Exp. Bot. 2021, 181, 104287. [Google Scholar] [CrossRef]
- Ning, L.-H.; Du, W.-K.; Song, H.-N.; Shao, H.-B.; Qi, W.-C.; Sheteiwy, M.S.A.; Yu, D.-Y. Identification of responsive miRNAs involved in combination stresses of phosphate starvation and salt stress in soybean root. Environ. Exp. Bot. 2019, 167, 103823. [Google Scholar] [CrossRef]
- He, F.; Shen, H.; Lin, C.; Fu, H.; Sheteiwy, M.S.; Guan, Y.; Huang, Y.; Hu, J. Transcriptome Analysis of Chilling-Imbibed Embryo Revealed Membrane Recovery Related Genes in Maize. Front. Plant Sci. 2017, 7, 1978. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ Response to Abiotic Stress: Mechanisms and Strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; El-Sawah, A.M. The Mode of Integration Between Azotobacter and Rhizobium Affect Plant Growth, Yield, and Physiological Responses of Pea (Pisum sativum L.). J. Soil Sci. Plant Nutr. 2022, 22, 1238–1251. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; El-Sawah, A.M.; Kobae, Y.; Basit, F.; Holford, P.; Yang, H.; El-Keblawy, A.; Abdel-Fattah, G.G.; Wang, S.; Araus, J.L.; et al. The effects of microbial fertilizers application on growth, yield and some biochemical changes in the leaves and seeds of guar (Cyamopsis tetragonoloba L.). Food Res. Int. 2023, 172, 113122. [Google Scholar] [CrossRef]
- Mun, B.-G.; Hussain, A.; Park, Y.-G.; Kang, S.-M.; Lee, I.-J.; Yun, B.-W. The PGPR Bacillus aryabhattai promotes soybean growth via nutrient and chlorophyll maintenance and the production of butanoic acid. Front. Plant Sci. 2024, 15, 1341993. [Google Scholar] [CrossRef]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant Growth Promoting Rhizobacteria in Amelioration of Salinity Stress: A Systems Biology Perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Ashry, N.M.; Alaidaroos, B.A.; Mohamed, S.A.; Badr, O.A.M.; El-Saadony, M.T.; Esmael, A. Utilization of drought-tolerant bacterial strains isolated from harsh soils as a plant growth-promoting rhizobacteria (PGPR). Saudi J. Biol. Sci. 2022, 29, 1760–1769. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Venkateswar Rao, L. Isolation and characterization of drought-tolerant ACC deaminase and exopolysaccharide-producing fluorescent Pseudomonas sp. Ann. Microbiol. 2014, 64, 493–502. [Google Scholar] [CrossRef]
- Shaffique, S.; Imran, M.; adhikari, A.; Aaqil khan, M.; Rahim, W.; Alomrani, S.O.; Yun, B.-W.; kang, S.-M.; Lee, I.-J. A newly isolated Bacillus pumilus strain SH-9 modulates response to drought stress in soybean via endogenous phytohormones and gene expression (Daegu, South Korea). Plant Stress 2023, 10, 100279. [Google Scholar] [CrossRef]
- Liu, J.; Fimognari, L.; de Almeida, J.; Jensen, C.N.G.; Compant, S.; Oliveira, T.; Baelum, J.; Pastar, M.; Sessitsch, A.; Moelbak, L.; et al. Effect of Bacillus paralicheniformis on soybean (Glycine max) roots colonization, nutrient uptake and water use efficiency under drought stress. J. Agron. Crop Sci. 2023, 209, 547–565. [Google Scholar] [CrossRef]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A Promising Source of Plant Growth-Promoting Molecules and Their Non-Legume Interactions: Examining Applications and Mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Jabborova, D.; Kannepalli, A.; Davranov, K.; Narimanov, A.; Enakiev, Y.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Wirth, S.; Sayyed, R.Z.; et al. Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci. Rep. 2021, 11, 22081. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.-C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M.; et al. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of Arbuscular Mycorrhizal Fungi in Regulating Growth, Enhancing Productivity, and Potentially Influencing Ecosystems under Abiotic and Biotic Stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Begum, N.; Xiao, Y.; Wang, L.; Li, D.; Irshad, A.; Zhao, T. Arbuscular mycorrhizal fungus Rhizophagus irregularis alleviates drought stress in soybean with overexpressing the GmSPL9d gene by promoting photosynthetic apparatus and regulating the antioxidant system. Microbiol. Res. 2023, 273, 127398. [Google Scholar] [CrossRef]
- Oliveira, T.C.; Cabral, J.S.R.; Santana, L.R.; Tavares, G.G.; Santos, L.D.S.; Paim, T.P.; Müller, C.; Silva, F.G.; Costa, A.C.; Souchie, E.L.; et al. The arbuscular mycorrhizal fungus Rhizophagus clarus improves physiological tolerance to drought stress in soybean plants. Sci. Rep. 2022, 12, 9044. [Google Scholar] [CrossRef]
- El-Sawah, A.M.; Abdel-Fattah, G.G.; Holford, P.; Korany, S.M.; Alsherif, E.A.; AbdElgawad, H.; Ulhassan, Z.; Jośko, I.; Ali, B.; Sheteiwy, M.S. Funneliformis constrictum modulates polyamine metabolism to enhance tolerance of Zea mays L. to salinity. Microbiol. Res. 2023, 266, 127254. [Google Scholar] [CrossRef]
- AbdElgawad, H.; El-Sawah, A.M.; Mohammed, A.E.; Alotaibi, M.O.; Yehia, R.S.; Selim, S.; Saleh, A.M.; Beemster, G.T.S.; Sheteiwy, M.S. Increasing atmospheric CO2 differentially supports arsenite stress mitigating impact of arbuscular mycorrhizal fungi in wheat and soybean plants. Chemosphere 2022, 296, 134044. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; El-Sawah, A.M.; Korany, S.M.; Alsherif, E.A.; Mowafy, A.M.; Chen, J.; Jośko, I.; Selim, S.; AbdElgawad, H. Arbuscular Mycorrhizal Fungus “Rhizophagus irregularis” impacts on physiological and biochemical responses of ryegrass and chickpea plants under beryllium stress. Environ. Pollut. 2022, 315, 120356. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.-M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Yang, H.; Fang, C.; Li, Y.; Wu, Y.; Fransson, P.; Rillig, M.C.; Zhai, S.; Xie, J.; Tong, Z.; Zhang, Q.; et al. Temporal complementarity between roots and mycorrhizal fungi drives wheat nitrogen use efficiency. New Phytol. 2022, 236, 1168–1181. [Google Scholar] [CrossRef]
- Igiehon, O.N.; Babalola, O.O. Rhizobium and Mycorrhizal Fungal Species Improved Soybean Yield Under Drought Stress Conditions. Curr. Microbiol. 2021, 78, 1615–1627. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Abd Elgawad, H.; Xiong, Y.-C.; Macovei, A.; Brestic, M.; Skalicky, M.; Shaghaleh, H.; Alhaj Hamoud, Y.; El-Sawah, A.M. Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiol. Plant. 2021, 172, 2153–2169. [Google Scholar] [CrossRef]
- Ashwin, R.; Bagyaraj, D.J.; Mohan Raju, B. Ameliorating the drought stress tolerance of a susceptible soybean cultivar, MAUS 2 through dual inoculation with selected rhizobia and AM fungus. Fungal Biol. Biotechnol. 2023, 10, 10. [Google Scholar] [CrossRef]
- Chhetri, T.K.; Subedee, B.R.; Pant, B. Isolation, Identification and Production of Encapsulated Bradyrhizobium japonicum and Study on their Viability. Nepal J. Biotechnol. 2019, 7, 39–49. [Google Scholar] [CrossRef]
- Sharath, S.; Triveni, S.; Nagaraju, Y.; Latha, P.C.; Vidyasagar, B. The Role of Phyllosphere Bacteria in Improving Cotton Growth and Yield Under Drought Conditions. Front. Agron. 2021, 3, 680466. [Google Scholar] [CrossRef]
- Susilowati, A.; Puspita, A.A.; Yunus, A. Drought resistant of bacteria producing exopolysaccharide and IAA in rhizosphere of soybean plant (Glycine max) in Wonogiri Regency Central Java Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 142, 012058. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; KHAN, M.S. Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk. J. Biol. 2005, 29, 29–34. [Google Scholar]
- Abou-Aly, H.E.; Youssef, A.M.; El-Meihy, R.M.; Tawfik, T.A.; El-Akshar, E.A. Evaluation of heavy metals tolerant bacterial strains as antioxidant agents and plant growth promoters. Biocatal. Agric. Biotechnol. 2019, 19, 101110. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Pikovskaya, R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17: 362–370. Plant Soil 1948, 287, 77–84. [Google Scholar]
- Boltz, D.F.; Mellon, M.G. Spectrophotometric Determination of Phosphorus as Molybdiphosphoric Acid. Anal. Chem. 1948, 20, 749–751. [Google Scholar] [CrossRef]
- Hemalatha, N.; Raja, N.; Jayachitra, A.; Rajalakshmi, A.; Valarmathi, N. Isolation and characterization of phosphate solubilizing bacteria and analyzing their effect on Capsicum annum L. Inter. J. Biol. Pharm. Res 2013, 4, 159–167. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course: A Manual of Methods Useful for Instruction and Research in Soil Chemistry, Physical Chemistry of Soils, Soil Fertility, and Soil Genesis; UW-Madison Libraries Parallel Press: Madison, WI, USA, 2005. [Google Scholar]
- Snell&Snell. Colorimetric Methods of Analysis; D. von. Nastrand Company, Inc.: New York NY, USA, 1967. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and waters. Soil Sci. 1962, 93, 68. [Google Scholar] [CrossRef]
- Casida, L.E.J.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA, SSSA, Publisher: Madison, WI, USA, 1982. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161, IN16–IN18. [Google Scholar] [CrossRef]
- Trouvelot, A. Measure du taux de mycorrhization d’un systeme radiculaire. Recherche de methods d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Skerman, V.B.D. A Guide to Identification of the Genera of Bacteria, 2nd ed.; The Williams & Wilkins Co.: Baltimore, MD, USA, 1967. [Google Scholar]
- Iovieno, P.; Bååth, E. Effect of drying and rewetting on bacterial growth rates in soil. FEMS Microbiol. Ecol. 2008, 65, 400–407. [Google Scholar] [CrossRef]
- Gao, C.; El-Sawah, A.M.; Ali, D.F.I.; Alhaj Hamoud, Y.; Shaghaleh, H.; Sheteiwy, M.S. The Integration of Bio and Organic Fertilizers Improve Plant Growth, Grain Yield, Quality and Metabolism of Hybrid Maize (Zea mays L.). Agronomy 2020, 10, 319. [Google Scholar] [CrossRef]
- El-Sawah, A.M.; El-Keblawy, A.; Ali, D.F.I.; Ibrahim, H.M.; El-Sheikh, M.A.; Sharma, A.; Alhaj Hamoud, Y.; Shaghaleh, H.; Brestic, M.; Skalicky, M.; et al. Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Enhance Soil Key Enzymes, Plant Growth, Seed Yield, and Qualitative Attributes of Guar. Agriculture 2021, 11, 194. [Google Scholar] [CrossRef]
- Anli, M.; Baslam, M.; Tahiri, A.; Raklami, A.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Toubali, S.; Ait Rahou, Y.; et al. Biofertilizers as Strategies to Improve Photosynthetic Apparatus, Growth, and Drought Stress Tolerance in the Date Palm. Front. Plant Sci. 2020, 11, 516818. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Van Staden, J. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998, 21, 535–553. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Zarik, L.; Meddich, A.; Hijri, M.; Hafidi, M.; Ouhammou, A.; Ouahmane, L.; Duponnois, R.; Boumezzough, A. Use of arbuscular mycorrhizal fungi to improve the drought tolerance of Cupressus atlantica G. Comptes Rendus Biol. 2016, 339, 185–196. [Google Scholar] [CrossRef]
- Ortiz, N.; Armada, E.; Duque, E.; Roldán, A.; Azcón, R. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: Effectiveness of autochthonous or allochthonous strains. J. Plant Physiol. 2015, 174, 87–96. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Ngosong, C.; Tatah, B.N.; Olougou, M.N.E.; Suh, C.; Nkongho, R.N.; Ngone, M.A.; Achiri, D.T.; Tchakounté, G.V.T.; Ruppel, S. Inoculating plant growth-promoting bacteria and arbuscular mycorrhiza fungi modulates rhizosphere acid phosphatase and nodulation activities and enhance the productivity of soybean (Glycine max). Front. Plant Sci. 2022, 13, 934339. [Google Scholar] [CrossRef]
- Egli, D.B.; Yu, Z.-W. Crop Growth Rate and Seeds per Unit Area in Soybean. Crop Sci. 1991, 31, 439–442. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O.; Cheseto, X.; Torto, B. Effects of rhizobia and arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress. Microbiol. Res. 2021, 242, 126640. [Google Scholar] [CrossRef] [PubMed]





| Bradyrhizobium spp. | Rhizobacteria | ||
|---|---|---|---|
| No. of Isolate | OD at 600 nm | No. of Isolate | OD at 600 nm |
| 1 DTB | 0.560 ± 0.174 bc | 1 DTR | 0.373 ± 0.082 c–h |
| 2 DTB | 0.610 ± 0.039 bc | 2 DTR | 0.449 ± 0.120 a–e |
| 3 DTB | 0.965 ± 0.026 a | 3 DTR | 0.531 ± 0.072 a–d |
| 4 DTB | 0.930 ± 0.021 a | 4 DTR | 0.111 ± 0.046 hi |
| 5 DTB | 1.000 ± 0.036 a | 5 DTR | 0.070 ± 0.026 i |
| 6 DTB | 0.973 ± 0.028 a | 6 DTR | 0.072 ± 0.029 i |
| 7 DTB | 0.971 ± 0.052 a | 7 DTR | 0.080 ± 0.102 i |
| 8 DTB | 0.183 ± 0.146 e–g | 8 DTR | 0.086 ± 0.044 i |
| 9 DTB | 0.251 ± 0.117 ef | 9 DTR | 0.154 ± 0.040 g–i |
| 10 DTB | 0.169 ± 0.024 e–g | 10 DTR | 0.117 ± 0.019 hi |
| 11 DTB | 0.444 ± 0.043 cd | 11 DTR | 0.061 ± 0.044 i |
| 12 DTB | 0.452 ± 0.084 cd | 12 DTR | 0.076 ± 0.074 i |
| 13 DTB | 0.606 ± 0.064 bc | 13 DTR | 0.044 ± 0.030 i |
| 14 DTB | 0.568 ± 0.123 bc | 14 DTR | 0.173 ± 0.053 f–i |
| 15 DTB | 0.617 ± 0.110 bc | 15 DTR | 0.065 ± 0.039 i |
| 16 DTB | 0.466 ± 0.063 cd | 16 DTR | 0.262 ± 0.107 e–i |
| 17 DTB | 0.477 ± 0.027 cd | 17 DTR | 0.409 ± 0.135 b–g |
| 18 DTB | 0.177 ± 0.139 e–g | 18 DTR | 0.691 ± 0.509 a |
| 19 DTB | 0.185 ± 0.045 e–g | 19 DTR | 0.256 ± 0.088 e–i |
| 20 DTB | 0.218 ± 0.093 e–g | 20 DTR | 0.643 ± 0.171 ab |
| 21 DTB | 0.054 ± 0.053 g | 21 DTR | 0.161 ± 0.016 g–i |
| 22 DTB | 0.453 ± 0.109 cd | 22 DTR | 0.211 ± 0.068 e–i |
| 23 DTB | 0.101 ± 0.038 fg | 23 DTR | 0.320 ± 0.355 d–i |
| 24 DTB | 0.675 ± 0.261 b | 24 DTR | 0.435 ± 0.067 a–f |
| 25 DTB | 0.347 ± 0.070 de | 25 DTR | 0.189 ± 0.046 e–i |
| 26 DTB | 0.558 ± 0.148 bc | 26 DTR | 0.245 ± 0.060 e–i |
| 27 DTB | 0.052 ± 0.024 g | 27 DTR | 0.211 ± 0.015 e–i |
| 28 DTB | 0.465 ± 0.172 cd | 28 DTR | 0.222 ± 0.133 e–i |
| 29 DTB | 0.458 ± 0.099 cd | 29 DTR | 0.584 ± 0.075 a–c |
| 30 DTB | 0.136 ± 0.024 fg | 30 DTR | 0.643 ± 0.170 ab |
| Treatments | Bacterial Counts | Mycorrhizal Colonization % | ||||
|---|---|---|---|---|---|---|
| Total | P-Dissolvers | F | M | A | ||
| CK | 100% NPK | 7.062 + 0.054 f | 5.911 + 0.031 m | – | – | – |
| 50% NPK | 6.018 + 0.023 h | 5.698 + 0.044 n | – | – | – | |
| Bradyrhiz. + 50% NPK | 8.133 + 0.154 e | 6.088 + 0.105 g–j | – | – | – | |
| B. subtilis + 50% NPK | 8.223 + 0.162 de | 6.093 + 0.127 g–i | – | – | – | |
| AMF + 50% NPK | 8.380 + 0.598 a–e | 5.994 + 0.023 i–l | 89.91 ± 1.10 c | 51.00 ± 2.13 a | 43.80 ± 2.57 a | |
| Bradyrhiz.+ B. subtilis + 50% NPK | 8.319 + 0.047 b–e | 6.193 + 0.089 e–g | – | – | – | |
| Bradyrhiz. + AMF + 50% NPK | 8.324 + 0.082 b–e | 6.096 + 0.149 g–i | 92.33 ± 1.72 b | 41.09 ± 1.90 c | 35.36 ± 2.44 c | |
| B. subtilis +AMF + 50% NPK | 8.261 + 0.051 c–e | 6.019 + 0.053 h–l | 90.62 ± 1.28 bc | 40.20 ± 0.40 c | 36.16 ± 1.68 c | |
| Mixture + 50% NPK | 8.467 + 0.004 a–d | 6.108 + 0.056 gh | 95.67 ± 2.56 a | 46.64 ± 1.13 b | 38.16 ± 2.73 b | |
| D1 | 100% NPK | 7.248 + 0.015 f | 6.054 + 0.006 h–k | – | – | – |
| 50% NPK | 6.259 + 0.048 g | 5.982 + 0.016 j–m | – | – | – | |
| Bradyrhiz. + 50% NPK | 8.379 + 0.043 a–e | 6.267 + 0.024 b–e | – | – | – | |
| B. subtilis + 50% NPK | 8.49 + 0.022 a–d | 6.271 + 0.014 b–e | – | – | – | |
| AMF + 50% NPK | 8.479 + 0.124 a–d | 6.175 + 0.029 e–g | 71.43 ± 0.99 h | 32.54 ± 0.79 hj | 24.80 ± 0.41 ef | |
| Bradyrhiz.+ B. subtilis + 50% NPK | 8.560 + 0.037 ab | 6.322 + 0.021 a–d | – | – | – | |
| Bradyrhiz. + AMF + 50% NPK | 8.531 + 0.017 ab | 6.355 + 0.029 a–c | 76.92 ± 2.70 f | 30.14 ± 0.24 j | 23.46 ± 2.58 f | |
| B. subtilis +AMF + 50% NPK | 8.505 + 0.024 a–c | 6.372 + 0.015 ab | 73.33 ± 2.14 gh | 31.45 ± 0.20 i | 21.41 ± 0.20 g | |
| Mixture + 50% NPK | 8.611 + 0.028 a | 6.391 + 0.012 a | 81.82 ± 3.18 e | 36.77 ± 0.58 e | 26.44 ± 1.35 de | |
| D2 | 100% NPK | 7.138 + 0.033 f | 5.954 + 0.024 k–m | – | – | – |
| 50% NPK | 6.069 + 0.052 gh | 5.883 + 0.018 m | – | – | – | |
| Bradyrhiz. + 50% NPK | 8.315 + 0.042 b–e | 6.222 + 0.007 d–f | – | – | – | |
| B. subtilis + 50% NPK | 8.373 + 0.103 a–e | 6.117 + 0.129 f–h | – | – | – | |
| AMF + 50% NPK | 8.24 + 0.124 c–e | 6.014 + 0.024 h–l | 75.00 ± 1.83 fg | 35.54 ± 1.32 ef | 26.57 ± 0.19 de | |
| Bradyrhiz.+ B. subtilis + 50% NPK | 8.428 + 0.081 a–d | 6.258 + 0.047 c–e | – | – | – | |
| Bradyrhiz. + AMF + 50% NPK | 8.398 + 0.081 a–d | 6.278 + 0.023 e–b | 82.91 ± 2.16 e | 34.54 ± 1.30 fg | 26.91 ± 1.21 d | |
| B. subtilis +AMF + 50% NPK | 8.397 + 0.021 a–d | 6.22 + 0.053 d–f | 83.33 ± 1.74 e | 33.55 ± 0.50 gh | 23.05 ± 0.63 fg | |
| Mixture + 50% NPK | 8.471 + 0.078 a–d | 6.258 + 0.025 c–e | 85.92 ± 0.45 d | 38.67 ± 1.01 d | 28.50 ± 2.04 d | |
| Fertilization | *** | *** | *** | *** | *** | |
| Drought | *** | *** | *** | *** | *** | |
| Fertilization × Drought | *** | *** | *** | *** | *** | |
| Treatments | Shoot Length (cm) | Root Length (cm) | Leaf Area (cm2) | Dry Weight (g/Plant) | |
|---|---|---|---|---|---|
| CK | 100% NPK | 114.43 ± 1.33 e | 29.57 ± 1.55 c–f | 66.13 ± 4.50 d–f | 60.67 ± 1.64 de |
| 50% NPK | 100.27 ± 1.05 fg | 19.97 ± 1.24 jk | 45.40 ± 3.10 gh | 52.82 ± 2.17 f–h | |
| Bradyrhiz. + 50% NPK | 115.60 ± 4.57 e | 32.17 ± 3.00 b–d | 88.41 ± 9.65 c | 59.97 ± 6.07 de | |
| B. subtilis + 50% NPK | 122.27 ± 1.01 d | 31.27 ± 1.95 b–e | 62.85 ± 2.18 ef | 57.91 ± 3.08 ef | |
| AMF + 50% NPK | 139.97 ± 4.93 b | 35.00 ± 1.51 b | 97.00 ± 5.71 b | 78.42 ± 2.57 a | |
| Bradyrhiz.+ B. subtilis + 50% NPK | 122.63 ± 8.28 d | 30.10 ± 1.57 c–f | 66.30 ± 2.65 d–f | 59.47 ± 5.16 de | |
| Bradyrhiz. + AMF + 50% NPK | 133.40 ± 2.36 c | 31.27 ± 2.97 b–e | 61.91 ± 3.03 f | 65.93 ± 3.67 bc | |
| B. subtilis +AMF + 50% NPK | 152.83 ± 4.65 a | 44.37 ± 1.07 a | 104.33 ± 5.13 b | 74.36 ± 5.25 a | |
| Mixture + 50% NPK | 152.30 ± 0.95 a | 41.60 ± 2.44 a | 113.00 ± 8.19 a | 78.63 ± 1.89 a | |
| D1 | 100% NPK | 96.01 ± 5.12 g | 15.87 ± 0.49 k | 40.13 ± 0.12 gh | 47.26 ± 1.00 ij |
| 50% NPK | 71.20 ± 0.90 h | 11.57 ± 1.68 l | 16.57 ± 4.56 j | 39.95 ± 1.35 k | |
| Bradyrhiz. + 50% NPK | 101.53 ± 4.85 fg | 15.97 ± 0.76 k | 38.47 ± 5.02 h | 49.67 ± 1.83 hi | |
| B. subtilis + 50% NPK | 100.90 ± 4.97 fg | 15.93 ± 0.50 k | 48.13 ± 1.01 g | 50.26 ± 1.02 hi | |
| AMF + 50% NPK | 100.30 ± 0.20 fg | 16.67 ± 1.19 k | 84.17 ± 4.38 c | 54.43 ± 1.11 f–h | |
| Bradyrhiz.+ B. subtilis + 50% NPK | 102.83 ± 3.29 fg | 19.67 ± 3.13 jk | 41.53 ± 2.16 gh | 49.99 ± 1.41 hi | |
| Bradyrhiz. + AMF + 50% NPK | 112.40 ± 1.05 e | 23.80 ± 0.70 g–j | 45.13 ± 1.53 gh | 52.93 ± 0.34 f–h | |
| B. subtilis +AMF + 50% NPK | 112.67 ± 1.68 e | 30.93 ± 8.55 b–e | 70.93 ± 1.21 de | 50.98 ± 5.34 hi | |
| Mixture + 50% NPK | 112.23 ± 3.40 e | 30.20 ± 0.46 c–f | 72.30 ± 4.56 d | 61.33 ± 0.02 c–e | |
| D2 | 100% NPK | 111.57 ± 1.108 e | 22.13 ± 0.74 ij | 42.33 ± 6.60 gh | 51.94 ± 3.31 hi |
| 50% NPK | 98.20 ± 0.85 fg | 16.07 ± 0.42 k | 29.63 ± 8.30 i | 43.62 ± 1.88 jk | |
| Bradyrhiz. + 50% NPK | 113.57 ± 5.82 e | 23.33 ± 0.06 h–j | 61.33 ± 1.53 f | 57.29 ± 1.14 e–g | |
| B. subtilis + 50% NPK | 114.27 ± 0.90 e | 27.23 ± 2.89 e–h | 46.00 ± 3.48 gh | 52.27 ± 1.53 g–i | |
| AMF + 50% NPK | 124.93 ± 2.00 d | 26.07 ± 3.66 f–i | 84.74 ± 5.00 c | 66.63 ± 3.68 b | |
| Bradyrhiz.+ B. subtilis + 50% NPK | 114.90 ± 1.47 e | 23.73 ± 0.32 g–j | 42.75 ± 2.15 gh | 52.97 ± 1.27 f–h | |
| Bradyrhiz. + AMF + 50% NPK | 121.90 ± 7.02 d | 27.90 ± 2.07 d–g | 47.33 ± 2.31 gh | 61.30 ± 1.16 c–e | |
| B. subtilis +AMF + 50% NPK | 131.47 ± 1.18 c | 41.10 ± 0.69 a | 80.43 ± 9.87 c | 53.22 ± 2.08 f–h | |
| Mixture + 50% NPK | 134.83 ± 3.56 bc | 33.60 ± 2.20 bc | 81.60 ± 1.21 c | 64.53 ± 2.05 b–d | |
| Fertilization | *** | *** | *** | *** | |
| Drought | *** | *** | *** | *** | |
| Fertilization × Drought | *** | *** | *** | *** | |
| Treatments | Nodules/Plant | Pods/Plant | 100 Seeds Weight (g) | Grain Yield (t/ha) | |
|---|---|---|---|---|---|
| CK | 100% NPK | 12.67 ± 2.08 jk | 52.67 ± 2.89 de | 15.17 ± 0.39 cd | 2.06 ± 0.10 c–f |
| 50% NPK | 9.67 ± 0.58 l–o | 43.33 ± 2.89 e–h | 10.81 ± 0.68 gh | 1.23 ± 0.12 k–m | |
| Bradyrhiz. + 50% NPK | 22.67 ± 2.08 a–c | 54.00 ± 4.00 d | 15.71 ± 0.51 c | 2.41 ± 0.45 ab | |
| B. subtilis + 50% NPK | 13.67 ± 0.58 ij | 82.33 ± 3.06 ab | 15.80 ± 0.50 c | 2.04 ± 0.05 d–g | |
| AMF + 50% NPK | 12.00 ± 1.00 j–l | 63.67 ± 9.02 c | 17.57 ± 0.46 ab | 2.10 ± 0.04 b–f | |
| Bradyrhiz.+ B. subtilis + 50% NPK | 20.33 ± 0.58 c–e | 63.33 ± 9.07 c | 15.16 ± 0.93 cd | 2.37 ± 0.23 a–d | |
| Bradyrhiz. + AMF + 50% NPK | 22.67 ± 1.53 a–c | 76.00 ± 6.08 ab | 16.48 ± 0.72 a–c | 2.16 ± 0.04 a–e | |
| B. subtilis +AMF + 50% NPK | 15.67 ± 0.58 hi | 72.67 ± 15.53 b | 16.11 ± 1.53 bc | 2.38 ± 0.33 a–c | |
| Mixture + 50% NPK | 23.67 ± 1.15 a | 83.67 ± 4.62 ab | 17.81 ± 0.44 a | 2.46 ± 0.41 a | |
| D1 | 100% NPK | 7.67 ± 1.15 op | 38.67 ± 0.58 gh | 10.92 ± 0.67 g | 1.12 ± 0.20 l–n |
| 50% NPK | 5.33 ± 0.58 p | 28.00 ± 2.65 i | 9.31 ± 0.20 h | 0.85 ± 0.17 n | |
| Bradyrhiz. + 50% NPK | 18.33 ± 1.53 e–g | 39.00 ± 1.00 f–h | 10.24 ± 0.50 gh | 1.22 ± 0.15 k–m | |
| B. subtilis + 50% NPK | 8.33 ± 1.15 no | 37.00 ± 4.36 h | 10.18 ± 0.67 gh | 1.17 ± 0.03 l–n | |
| AMF + 50% NPK | 10.00 ± 1.00 l–o | 35.67 ± 4.04 hi | 11.31 ± 0.19 fg | 1.13 ± 0.23 l–n | |
| Bradyrhiz.+ B. subtilis + 50% NPK | 17.33 ± 3.79 f–h | 40.00 ± 3.61 f–h | 11.26 ± 1.08 fg | 1.07 ± 0.04 mn | |
| Bradyrhiz. + AMF + 50% NPK | 11.33 ± 1.15 j–m | 38.33 ± 1.15 gh | 10.34 ± 0.95 gh | 1.71 ± 0.18 g–j | |
| B. subtilis +AMF + 50% NPK | 9.33 ± 0.58 m–o | 37.33 ± 3.21 h | 11.34 ± 1.57 fg | 1.41 ± 0.05 j–l | |
| Mixture + 50% NPK | 17.00 ± 1.00 gh | 44.00 ± 1.00 e–h | 11.20 ± 1.01 fg | 1.53 ± 0.01 i–k | |
| D2 | 100% NPK | 10.00 ± 1.00 l–o | 44.67 ± 4.51 d–h | 13.10 ± 0.70 e | 1.63 ± 0.11 h–j |
| 50% NPK | 7.67 ± 0.58 op | 38.33 ± 1.15 gh | 10.34 ± 0.82 gh | 1.02 ± 0.03 mn | |
| Bradyrhiz. + 50% NPK | 23.33 ± 1.53 ab | 45.67 ± 1.53 d–h | 14.07 ± 0.78 de | 1.93 ± 0.07 e–h | |
| B. subtilis + 50% NPK | 10.33 ± 0.58 k–n | 51.00 ± 5.29 de | 12.59 ± 1.22 ef | 1.63 ± 0.02 h–j | |
| AMF + 50% NPK | 10.67 ± 0.58 k–n | 45.33 ± 5.13 d–h | 13.54 ± 0.38 e | 1.65 ± 0.30 h–j | |
| Bradyrhiz.+ B. subtilis + 50% NPK | 21.00 ± 1.73 b–d | 48.67 ± 1.15 d–f | 12.96 ± 0.29 e | 1.42 ± 0.18 j–l | |
| Bradyrhiz. + AMF + 50% NPK | 19.33 ± 2.08 d–g | 48.33 ± 1.53 d–g | 13.68 ± 1.52 de | 1.81 ± 0.09 f–i | |
| B. subtilis +AMF + 50% NPK | 11.67 ± 1.53 j–m | 44.33 ± 7.51 d–h | 12.66 ± 0.92 ef | 1.71 ± 0.10 g–j | |
| Mixture + 50% NPK | 19.67 ± 0.58 d–f | 52.33 ± 1.15 de | 13.67 ± 1.01 de | 2.04 ± 0.06 d–g | |
| Fertilization | *** | *** | *** | *** | |
| Drought | *** | *** | *** | *** | |
| Fertilization × Drought | *** | *** | *** | *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nader, A.A.; Hauka, F.I.A.; Afify, A.H.; El-Sawah, A.M. Drought-Tolerant Bacteria and Arbuscular Mycorrhizal Fungi Mitigate the Detrimental Effects of Drought Stress Induced by Withholding Irrigation at Critical Growth Stages of Soybean (Glycine max, L.). Microorganisms 2024, 12, 1123. https://doi.org/10.3390/microorganisms12061123
Nader AA, Hauka FIA, Afify AH, El-Sawah AM. Drought-Tolerant Bacteria and Arbuscular Mycorrhizal Fungi Mitigate the Detrimental Effects of Drought Stress Induced by Withholding Irrigation at Critical Growth Stages of Soybean (Glycine max, L.). Microorganisms. 2024; 12(6):1123. https://doi.org/10.3390/microorganisms12061123
Chicago/Turabian StyleNader, Aya Ahmed, Fathi I. A. Hauka, Aida H. Afify, and Ahmed M. El-Sawah. 2024. "Drought-Tolerant Bacteria and Arbuscular Mycorrhizal Fungi Mitigate the Detrimental Effects of Drought Stress Induced by Withholding Irrigation at Critical Growth Stages of Soybean (Glycine max, L.)" Microorganisms 12, no. 6: 1123. https://doi.org/10.3390/microorganisms12061123
APA StyleNader, A. A., Hauka, F. I. A., Afify, A. H., & El-Sawah, A. M. (2024). Drought-Tolerant Bacteria and Arbuscular Mycorrhizal Fungi Mitigate the Detrimental Effects of Drought Stress Induced by Withholding Irrigation at Critical Growth Stages of Soybean (Glycine max, L.). Microorganisms, 12(6), 1123. https://doi.org/10.3390/microorganisms12061123







