Anti-HIV Activity and Immunomodulatory Properties of Fractionated Crude Extracts of Alternaria alternata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Secondary Metabolite Production
2.3. Fractionation of A. alternata PO4PR2 Using Mixed-Mode Solid-Phase Extraction
2.4. Screening of the Cytotoxic Effects and Cell Viability of A. alternata PO4PR2 Using MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5 Diphenyl Tetrazolium Bromide) Assay
2.5. Generation of Viruses (pNL4.3) by Transfection
2.6. Luciferase-Based Antiviral Assay
2.7. Determining the Mode of Action of the Fractionated A. alternata Crude Extracts and Their Inhibitory Mechanism in the HIV-1 Life Cycle Using Time of Addition Assay
2.7.1. HIV-1 p24 Time-Based ELISA to Measure the p24 Titre
2.7.2. Luciferase-Based Time of Addition Assay to Determine the Percentage Inhibition
2.8. Effect of the Fractionated Crude Extracts from A. alternata P04PR2 on CD4+ and CD8+ T Cell Activation and Exhaustion
2.8.1. Flow Cytometry Staining
2.8.2. Statistical Analysis
3. Results
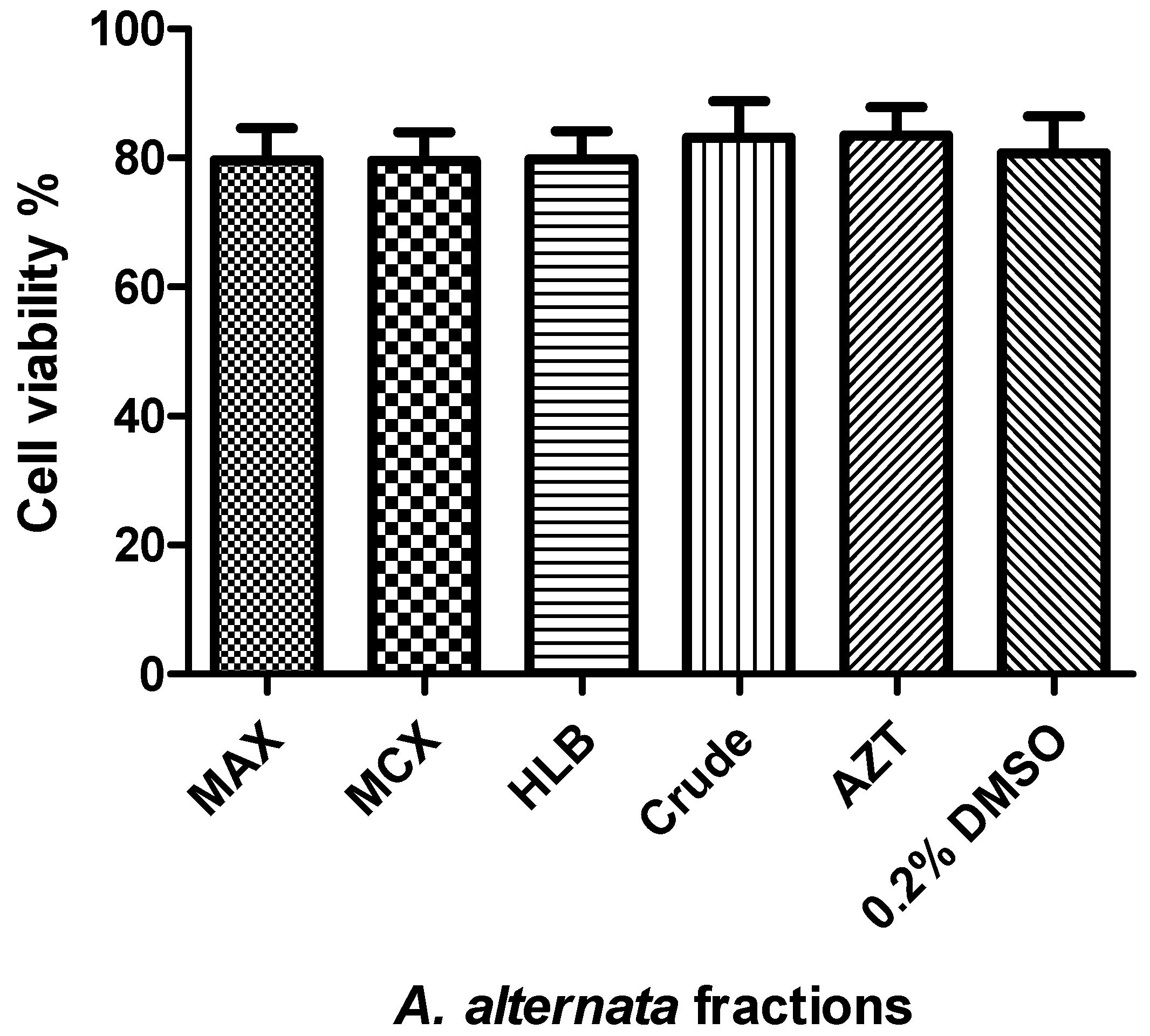
3.1. Cytotoxicity and Cell Viability of the Fractionated A. alternata Using MTT Assay
3.2. The Anti-HIV-1 Effects of Fractionated A. alternata PO4PR2 Crude Extract and Fractionated Fractions (MAX, MCX, HLB) Using Luciferase-Based Antiviral Assay
3.3. The Mode of Action of the Fractionated Crude Extracts and Their Tentative Inhibitory Mechanism in the HIV-1 Life Cycle Using Time of Addition Assay
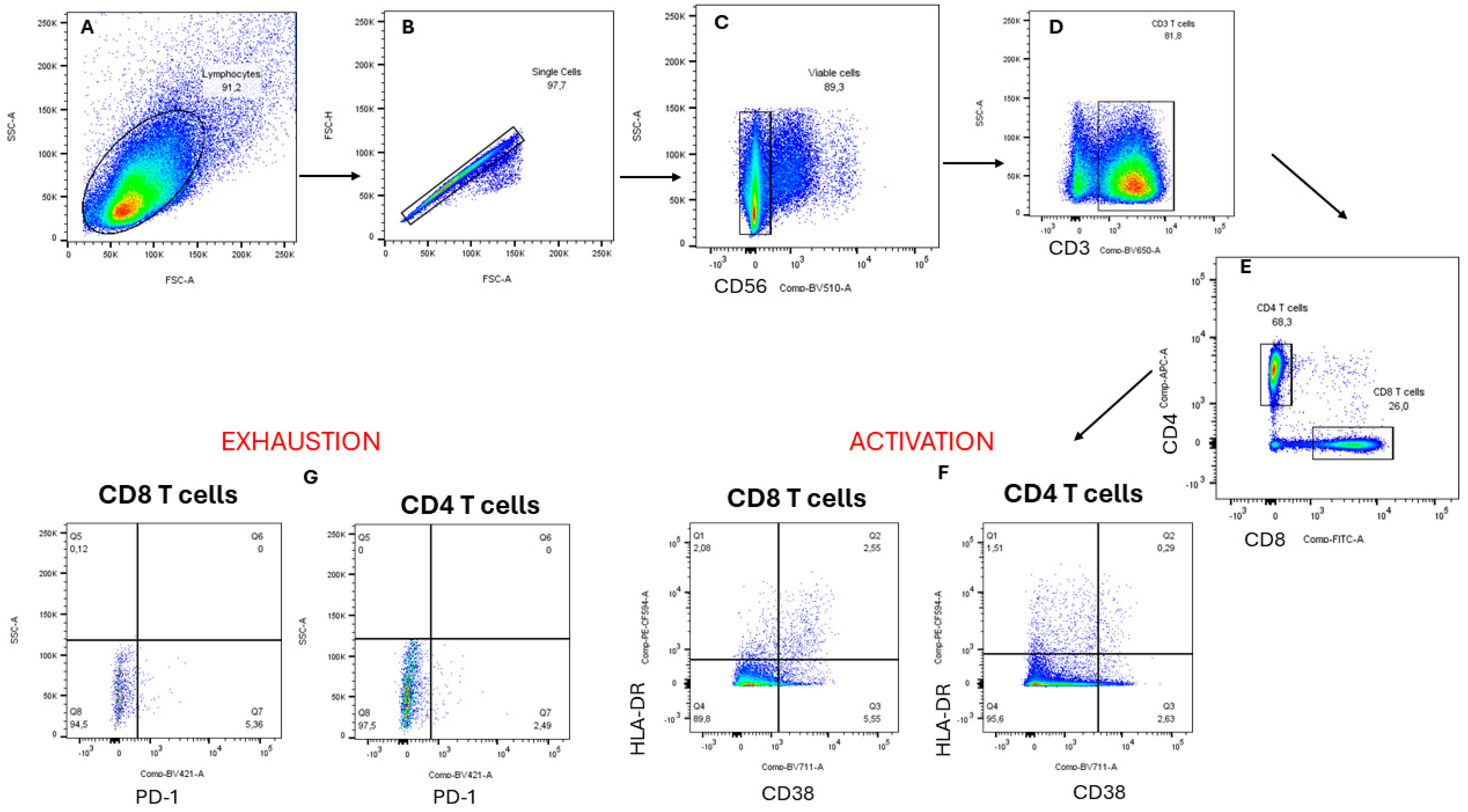
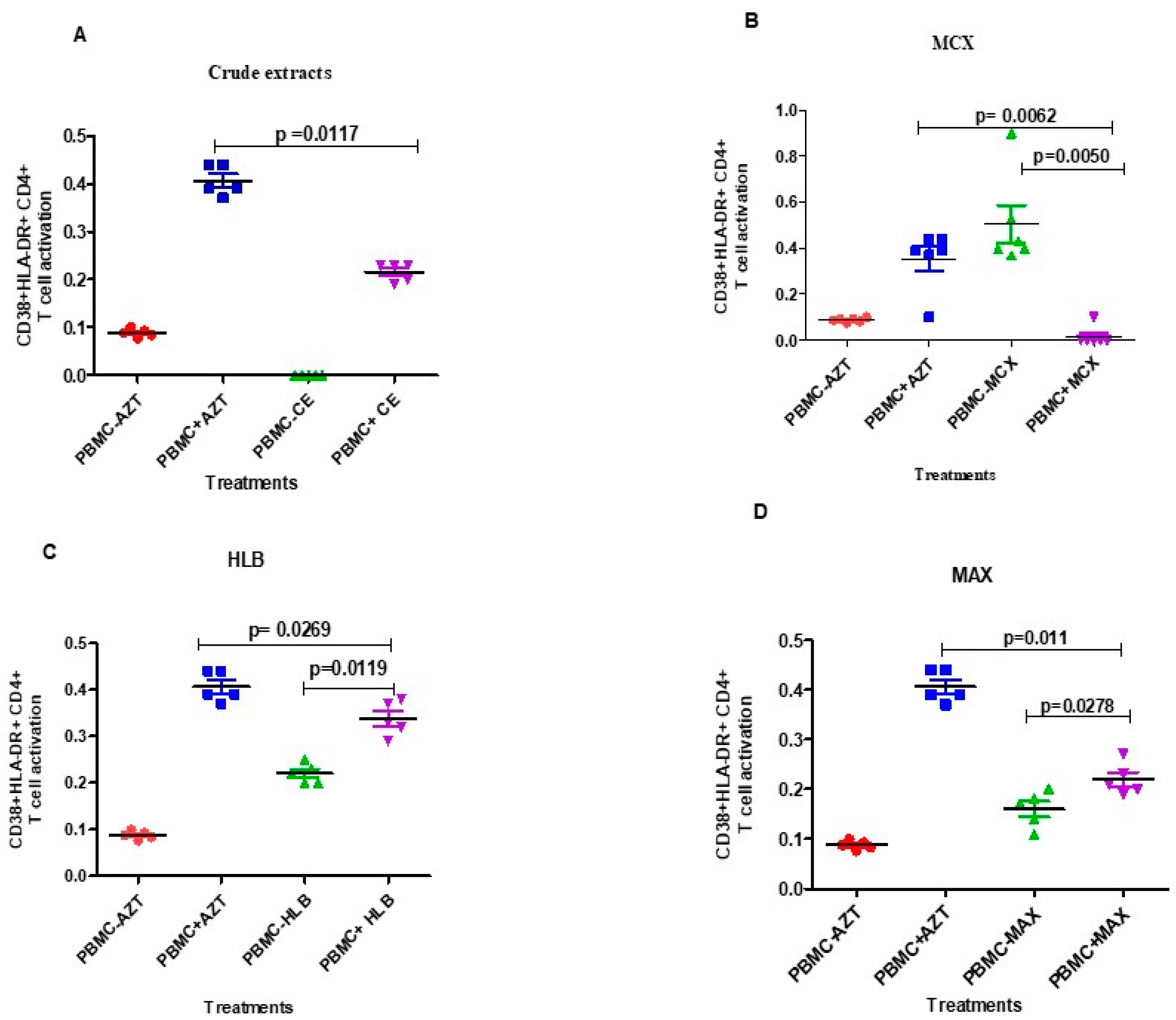
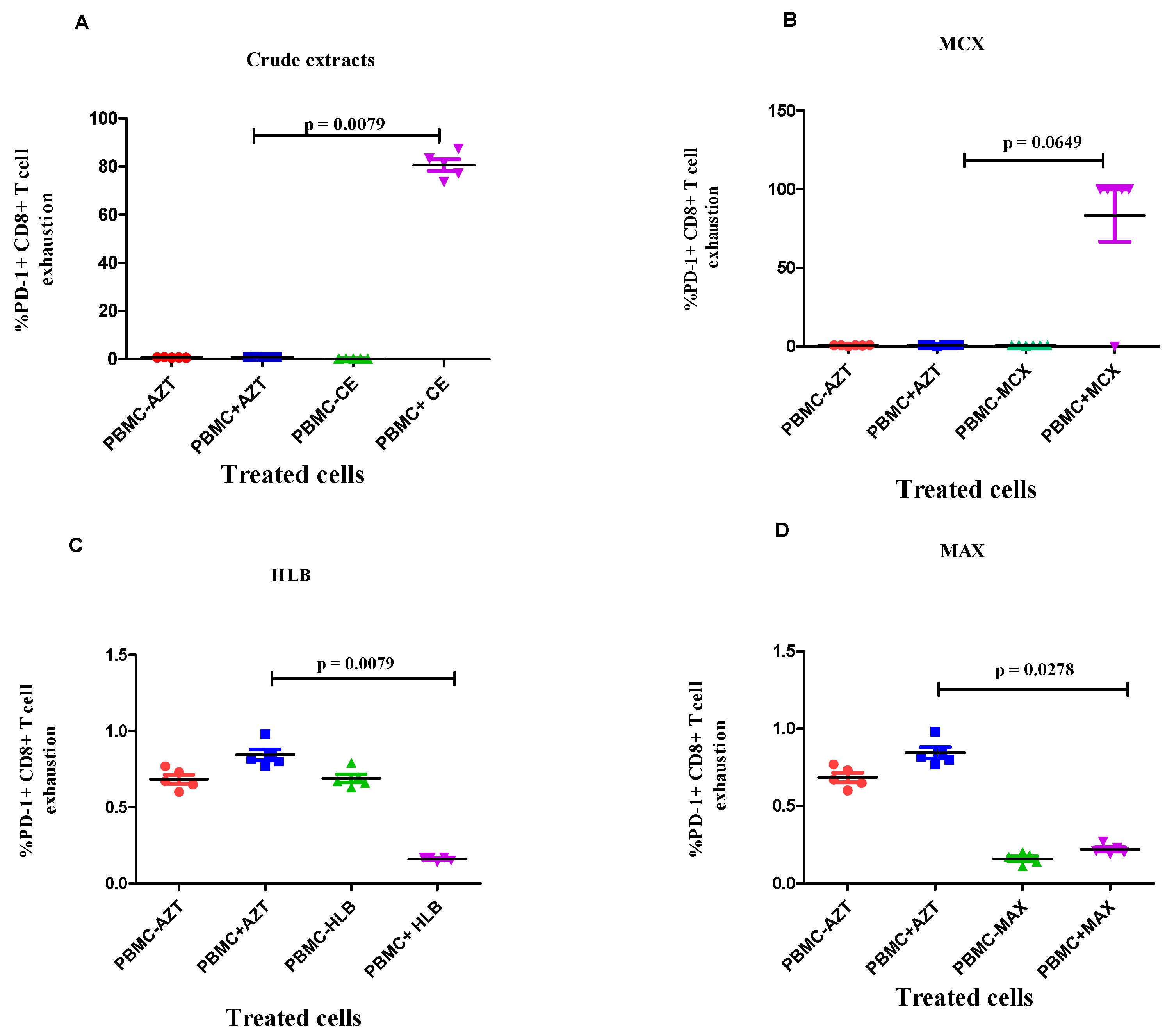
3.4. Evaluation of the Effects of Fractionated Crude Extracts from A. alternata on CD4+ and CD8+ T Cell Activation and Exhaustion
Flow Cytometry Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carr, A. Toxicity of antiretroviral therapy and implications for drug development. Nat. Rev. Drug Discov. 2003, 2, 624–634. [Google Scholar] [CrossRef]
- Chawla, A.; Wang, C.; Patton, C.; Murray, M.; Punekar, Y.; de Ruiter, A.; Steinhart, C. A Review of Long-Term Toxicity of Antiretroviral Treatment Regimens and Implications for an Aging Population. Infect. Dis. Ther. 2018, 7, 183–195. [Google Scholar] [CrossRef]
- Desai, M.; Iyer, G.; Dikshit, R.K. Antiretroviral drugs: Critical issues and recent advances. Indian J. Pharmacol. 2012, 44, 288–298. [Google Scholar] [CrossRef]
- Strobel, G.A. Endophytes as sources of bioactive products. Microbes Infect. 2003, 5, 535–544. [Google Scholar] [CrossRef]
- Bacon, C.W.; White Jr, J.F. Physiological adaptations in the evolution of endophytism in the Clavicipitaceae. In Microbial Endophytes; CRC Press: Boca Raton, FL, USA, 2000; pp. 251–276. [Google Scholar]
- Selim, K.A.; El-Beih, A.A.; Abdel-Rahman, T.M.; El-Diwany, A.I. Biological Evaluation of Endophytic Fungus, Chaetomium globosum JN711454, as Potential Candidate for Improving Drug Discovery. Cell Biochem. Biophys. 2014, 68, 67–82. [Google Scholar] [CrossRef]
- Singh, S.B.; Jayasuriya, H.; Dewey, R.; Polishook, J.D.; Dombrowski, A.W.; Zink, D.L.; Guan, Z.; Collado, J.; Platas, G.; Pelaez, F.; et al. Isolation, structure, and HIV-1-integrase inhibitory activity of structurally diverse fungal metabolites. J. Ind. Microbiol. Biotechnol. 2003, 30, 721–731. [Google Scholar] [CrossRef]
- Gunatilaka, A.A. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef]
- Ravindran, C.; Naveenan, T.; Varatharajan, G.R.; Rajasabapathy, R.; Meena, R.M. Antioxidants in mangrove plants and endophytic fungal associations. Bot. Mar. 2012, 55, 269–279. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Z.; Wang, W.; Zhou, Q.; Shi, G.; Wei, F.; Jiang, G. Developmental toxicity of synthetic phenolic antioxidants to the early life stage of zebrafish. Sci. Total Environ. 2018, 643, 559–568. [Google Scholar] [CrossRef]
- Hoque, N.; Khan, Z.R.; Rashid, P.T.; Begum, M.N.; Sharmin, S.; Hossain, M.J.; Rana, M.S.; Sohrab, M.H. Antimicrobial, antioxidant, and cytotoxic properties of endophytic fungi isolated from Thysanolaena maxima Roxb., Dracaena spicata Roxb. and Aglaonema hookerianum Schott. BMC Complement. Med. Ther. 2023, 23, 347. [Google Scholar] [CrossRef]
- Ding, L.; Münch, J.; Goerls, H.; Maier, A.; Fiebig, H.-H.; Lin, W.-H.; Hertweck, C. Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorg. Med. Chem. Lett. 2010, 20, 6685–6687. [Google Scholar] [CrossRef]
- Selim, K.A.; Elkhateeb, W.A.; Tawila, A.M.; El-Beih, A.A.; Abdel-Rahman, T.M.; El-Diwany, A.I.; Ahmed, E.F. Antiviral and Antioxidant Potential of Fungal Endophytes of Egyptian Medicinal Plants. Fermentation 2018, 4, 49. [Google Scholar] [CrossRef]
- Vora, J.; Velhal, S.; Sinha, S.; Patel, V.; Shrivastava, N. Bioactive phytocompound mulberroside C and endophytes of Morus alba as potential inhibitors of HIV-1 replication: A mechanistic evaluation. HIV Med. 2021, 22, 690–704. [Google Scholar] [CrossRef]
- Kumari, N.; Kulkarni, A.A.; Lin, X.; McLean, C.; Ammosova, T.; Ivanov, A.; Hipolito, M.; Nekhai, S.; Nwulia, E. Inhibition of HIV-1 by curcumin A, a novel curcumin analog. Drug Des. Dev. Ther. 2015, 9, 5051–5060. [Google Scholar] [CrossRef]
- Mazumder, A.; Raghavan, K.; Weinstein, J.; Kohn, K.W.; Pommier, Y. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem. Pharmacol. 1995, 49, 1165–1170. [Google Scholar] [CrossRef]
- Vajragupta, O.; Boonchoong, P.; Morris, G.M.; Olson, A.J. Active site binding modes of curcumin in HIV-1 protease and integrase. Bioorg. Med. Chem. Lett. 2005, 15, 3364–3368. [Google Scholar] [CrossRef]
- Ali, A.; Banerjea, A.C. Curcumin inhibits HIV-1 by promoting Tat protein degradation. Sci. Rep. 2016, 6, 27539. [Google Scholar] [CrossRef]
- Bashyal, B.P.; Wellensiek, B.P.; Ramakrishnan, R.; Faeth, S.H.; Ahmad, N.; Gunatilaka, A.L. Altertoxins with potent anti-HIV activity from Alternaria tenuissima QUE1Se, a fungal endophyte of Quercus emoryi. Bioorg. Med. Chem. 2014, 22, 6112–6116. [Google Scholar] [CrossRef]
- Govindappa, M.; Hemmanur, K.C.; Nithin, S.; Poojari, C.C.; Bhat, G.; Channabasava, K. In vitro anti-HIV activity of partially purified coumarin (s) isolated from fungal endophyte, Alternaria species of Calophyllum inophyllum. Pharmacol. Pharm. 2015, 6, 321–328. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Q.; Zhang, Y.; Liang, C. Coumarin-based derivatives with potential anti-HIV activity. Fitoterapia 2021, 150, 104863. [Google Scholar] [CrossRef]
- Sharapov, A.D.; Fatykhov, R.F.; Khalymbadzha, I.A.; Zyryanov, G.V.; Chupakhin, O.N.; Tsurkan, M.V. Plant Coumarins with Anti-HIV Activity: Isolation and Mechanisms of Action. Int. J. Mol. Sci. 2023, 24, 2839. [Google Scholar] [CrossRef]
- Nahar, L.; Talukdar, A.D.; Nath, D.; Nath, S.; Mehan, A.; Ismail, F.M.D.; Sarker, S.D. Naturally Occurring Calanolides: Occurrence, Biosynthesis, and Pharmacological Properties Including Therapeutic Potential. Molecules 2020, 25, 4983. [Google Scholar] [CrossRef]
- Khalymbadzha, I.A.; Fatykhov, R.F.; Butorin, I.I.; Sharapov, A.D.; Potapova, A.P.; Muthipeedika, N.J.; Zyryanov, G.V.; Melekhin, V.V.; Tokhtueva, M.D.; Deev, S.L.; et al. Bioinspired Pyrano[2,3-f]chromen-8-ones: Ring C-Opened Analogues of Calanolide A: Synthesis and Anti-HIV-1 Evaluation. Biomimetics 2024, 9, 44. [Google Scholar] [CrossRef]
- Ali Reza, A.S.M.; Nasrin, M.S.; Hossen, M.A.; Rahman, M.A.; Jantan, I.; Haque, M.A.; Sobarzo-Sánchez, E. Mechanistic insight into immunomodulatory effects of food-functioned plant secondary metabolites. Crit. Rev. Food Sci. Nutr. 2021, 63, 5546–5576. [Google Scholar] [CrossRef]
- Kaur, H.P.; Singh, B.; Thakur, A.; Kaur, A.; Kaur, S. Studies on immunomodulatory effect of endophytic fungus Alternaria alternata on Spodoptera litura. J. Asia-Pac. Entomol. 2015, 18, 67–75. [Google Scholar] [CrossRef]
- Abood, W.N.; Fahmi, I.; Abdulla, M.A.; Ismail, S. Immunomodulatory effect of an isolated fraction from Tinospora crispa on intracellular expression of INF-γ, IL-6 and IL-8. BMC Complement. Altern. Med. 2014, 14, 205. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Sujarit, K.; Pattananandecha, T.; Saenjum, C.; Lumyong, S. Natural Bioactive Compounds from Fungi as Potential Candidates for Protease Inhibitors and Immunomodulators to Apply for Coronaviruses. Molecules 2020, 25, 1800. [Google Scholar] [CrossRef]
- Ujam, N.T.; Eze, P.; Chukwunwejim, C.R.; Okoye, F.B.C.; Esimone, C.O. Antimicrobial and immunomodulatory activities of secondary metabolites of an endophytic fungus isolated from Ageratum conyzoides. Life Sci. 2019, 5, 19–27. [Google Scholar]
- Nzimande, B.; Kumalo, H.M.; Ndlovu, S.I.; Mkhwanazi, N.P. Secondary metabolites produced by endophytic fungi, Alternaria alternata, as potential inhibitors of the human immunodeficiency virus. Front. Genet. 2022, 13, 1077159. [Google Scholar] [CrossRef]
- Makawana, J.A.; Mungra, D.C.; Patel, M.P.; Patel, R.G. Microwave assisted synthesis and antimicrobial evaluation of new fused pyran derivatives bearing 2-morpholinoquinoline nucleus. Bioorg. Med. Chem. Lett. 2011, 21, 6166–6169. [Google Scholar] [CrossRef]
- van der Kolk, M.R.; Janssen, M.A.; Rutjes, F.P.; Blanco-Ania, D. Cyclobutanes in small-molecule drug candidates. ChemMedChem 2022, 17, e202200020. [Google Scholar] [CrossRef]
- Ismail, G.A.; Gheda, S.F.; Abo-Shady, A.M.; Abdel-Karim, O.H. In vitro potential activity of some seaweeds as antioxidants and inhibitors of diabetic enzymes. Food Sci. Technol. 2019, 40, 681–691. [Google Scholar] [CrossRef]
- Stoszko, M.; Al-Hatmi, A.M.; Skriba, A.; Roling, M.; Ne, E.; Crespo, R.; Mueller, Y.M.; Najafzadeh, M.J.; Kang, J.; Ptackova, R. Gliotoxin, identified from a screen of fungal metabolites, disrupts 7SK snRNP, releases P-TEFb, and reverses HIV-1 latency. Sci. Adv. 2020, 6, eaba6617. [Google Scholar] [CrossRef]
- Makhwitine, J.P.; Kumalo, H.M.; Ndlovu, S.I.; Mkhwanazi, N.P. Epigenetic Induction of Secondary Metabolites Production in Endophytic Fungi Penicillium chrysogenum and GC-MS Analysis of Crude Metabolites with Anti-HIV-1 Activity. Microorganisms 2023, 11, 1404. [Google Scholar] [CrossRef]
- Fanele, A.; Ndlovu, S.I. Endophytic fungal species Nigrospora oryzae and Alternaria alternata exhibit antimicrobial activity against gram-positive and gram-negative multi-drug resistant clinical bacterial isolates. BMC Complement. Med. Ther. 2023, 23, 323. [Google Scholar] [CrossRef]
- Louder, M.K.; Sambor, A.; Chertova, E.; Hunte, T.; Barrett, S.; Ojong, F.; Sanders-Buell, E.; Zolla-Pazner, S.; McCutchan, F.E.; Roser, J.D.; et al. HIV-1 envelope pseudotyped viral vectors and infectious molecular clones expressing the same envelope glycoprotein have a similar neutralization phenotype, but culture in peripheral blood mononuclear cells is associated with decreased neutralization sensitivity. Virology 2005, 339, 226–238. [Google Scholar] [CrossRef]
- Sarzotti-Kelsoe, M.; Bailer, R.T.; Turk, E.; Lin, C.L.; Bilska, M.; Greene, K.M.; Gao, H.; Todd, C.A.; Ozaki, D.A.; Seaman, M.S.; et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J. Immunol. Methods 2014, 409, 131–146. [Google Scholar] [CrossRef]
- Cherne, M.D.; Hall, J.; Kellner, A.; Chong, C.F.; Cole, A.L.; Cole, A.M. Avirulins, a Novel Class of HIV-1 Reverse Transcriptase Inhibitors Effective in the Female Reproductive Tract Mucosa. Viruses 2019, 11, 408. [Google Scholar] [CrossRef]
- Daelemans, D.; Pauwels, R.; De Clercq, E.; Pannecouque, C. A time-of–drug addition approach to target identification of antiviral compounds. Nat. Protoc. 2011, 6, 925–933. [Google Scholar] [CrossRef]
- Lara, H.; Garza Trevino, E.; De Zamacona, M.; Mureyko, L.; Ixtepan-Turrent, L. Luciferase Time-based, High-throughput Screening Assay for the Discovery of HIV-1 Inhibitors. J. Hum. Virol. Retrovirol. 2014, 1, 00017. [Google Scholar]
- Chachage, M.; Podola, L.; Clowes, P.; Nsojo, A.; Bauer, A.; Mgaya, O.; Kowour, D.; Froeschl, G.; Maboko, L.; Hoelscher, M. Helminth-associated systemic immune activation and HIV co-receptor expression: Response to albendazole/praziquantel treatment. PLoS Neglected Trop. Dis. 2014, 8, e2755. [Google Scholar] [CrossRef]
- Francke, S.; Orosz, C.G.; Hsu, J.; Mathes, L.E. Immunomodulatory effect of zidovudine (ZDV) on cytotoxic T lymphocytes previously exposed to ZDV. Antimicrob. Agents Chemother. 2002, 46, 2865–2871. [Google Scholar] [CrossRef]
- Tamargo, J.; Le Heuzey, J.Y.; Mabo, P. Narrow therapeutic index drugs: A clinical pharmacological consideration to flecainide. Eur. J. Clin. Pharmacol. 2015, 71, 549–567. [Google Scholar] [CrossRef]
- Muller, P.Y.; Milton, M.N. The determination and interpretation of the therapeutic index in drug development. Nat. Rev. Drug Discov. 2012, 11, 751–761. [Google Scholar] [CrossRef]
- Li, J.Z.; Segal, F.P.; Bosch, R.J.; Lalama, C.M.; Roberts-Toler, C.; Delagreverie, H.; Getz, R.; Garcia-Broncano, P.; Kinslow, J.; Tressler, R.; et al. Antiretroviral Therapy Reduces T-cell Activation and Immune Exhaustion Markers in Human Immunodeficiency Virus Controllers. Clin. Infect. Dis. 2019, 70, 1636–1642. [Google Scholar] [CrossRef]
- Freeman, M.L.; Shive, C.L.; Nguyen, T.P.; Younes, S.A.; Panigrahi, S.; Lederman, M.M. Cytokines and T-Cell Homeostasis in HIV Infection. J. Infect. Dis. 2016, 214 (Suppl. S2), S51–S57. [Google Scholar] [CrossRef]
- Schultz, H.S.; Reedtz-Runge, S.L.; Bäckström, B.T.; Lamberth, K.; Pedersen, C.R.; Kvarnhammar, A.M. Quantitative analysis of the CD4+ T cell response to therapeutic antibodies in healthy donors using a novel T cell:PBMC assay. PLoS ONE 2017, 12, e0178544. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, J.; Zhu, L.; Cheng, X.; Xia, B.; Jin, Y.; Qin, R.; Zhang, L.; Hu, H.; Yan, J.; et al. Improving the ex vivo expansion of human tumor-reactive CD8 + T cells by targeting toll-like receptors. Front. Bioeng. Biotechnol. 2022, 10, 1027619. [Google Scholar] [CrossRef]
- Antoine, P.; Olislagers, V.; Huygens, A.; Lecomte, S.; Liesnard, C.; Donner, C.; Marchant, A. Functional exhaustion of CD4+ T lymphocytes during primary cytomegalovirus infection. J. Immunol. 2012, 189, 2665–2672. [Google Scholar] [CrossRef]
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-cell exhaustion in HIV infection. Immunol. Rev. 2019, 292, 149–163. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef]
- Wang, Y.; Swiecki, M.; Cella, M.; Alber, G.; Schreiber, R.D.; Gilfillan, S.; Colonna, M. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe 2012, 11, 631–642. [Google Scholar] [CrossRef]




| A. alternata Fractions | IC50 (µg/mL) | % HIV Inhibition at the Maximum Concentration) |
|---|---|---|
| 5% HLB | 0.7232 | 64 |
| 45% HLB | 0.1130 | 48 |
| 95% HLB | 0.0338 | 32 |
| 5% MAX | 5.240 | 67 |
| 45% MAX | 0.03843 | 63 |
| 95% MAX | 0.03313 | 40 |
| 5% MCX | 0.3619 | 77 |
| 45% MCX | 6.750 | 74 |
| 95% MCX | 0.0110 | 53 |
| Crude Extract | CC50 (µg/mL) | IC50 (µg/mL) | Selective Index (CC50/IC50) |
|---|---|---|---|
| 5% MAX | 35.31 | 5.240 | 6.74 |
| 5% MCX | 62.31 | 0.3619 | 172.17 |
| 5% HLB | 81.81 | 0.7232 | 113 |
| Crude extract | 43.5 | 0.017 | 2559 |
| AZT | 1041 | 0.1322 | 7874 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubheka, M.X.; Ndlovu, S.I.; Mkhwanazi, N.P. Anti-HIV Activity and Immunomodulatory Properties of Fractionated Crude Extracts of Alternaria alternata. Microorganisms 2024, 12, 1150. https://doi.org/10.3390/microorganisms12061150
Kubheka MX, Ndlovu SI, Mkhwanazi NP. Anti-HIV Activity and Immunomodulatory Properties of Fractionated Crude Extracts of Alternaria alternata. Microorganisms. 2024; 12(6):1150. https://doi.org/10.3390/microorganisms12061150
Chicago/Turabian StyleKubheka, Mbali X., Sizwe I. Ndlovu, and Nompumelelo P. Mkhwanazi. 2024. "Anti-HIV Activity and Immunomodulatory Properties of Fractionated Crude Extracts of Alternaria alternata" Microorganisms 12, no. 6: 1150. https://doi.org/10.3390/microorganisms12061150





