Highly Efficient Production of Cellulosic Ethanol from Poplar Using an Optimal C6/C5 Co-Fermentation Strain of Saccharomyces cerevisiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
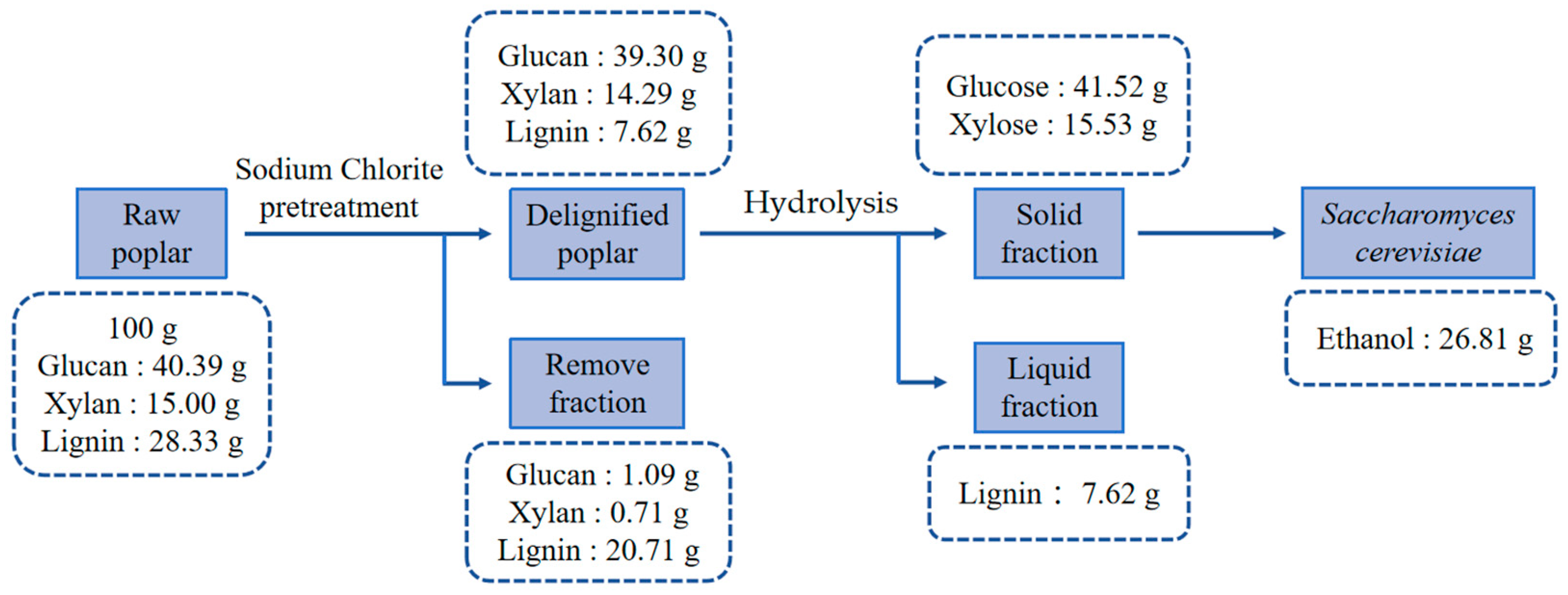
2.2. Delignification Process
2.3. Dilute Sulfuric acid Pretreatment and Enzymatic Hydrolysis
2.4. Fermentation and Strains
2.5. Analytical Methods
3. Results and Discussion
3.1. Chemical Compositions of Poplar
3.2. Delignification Pretreatment of Poplar with Sodium Chlorite
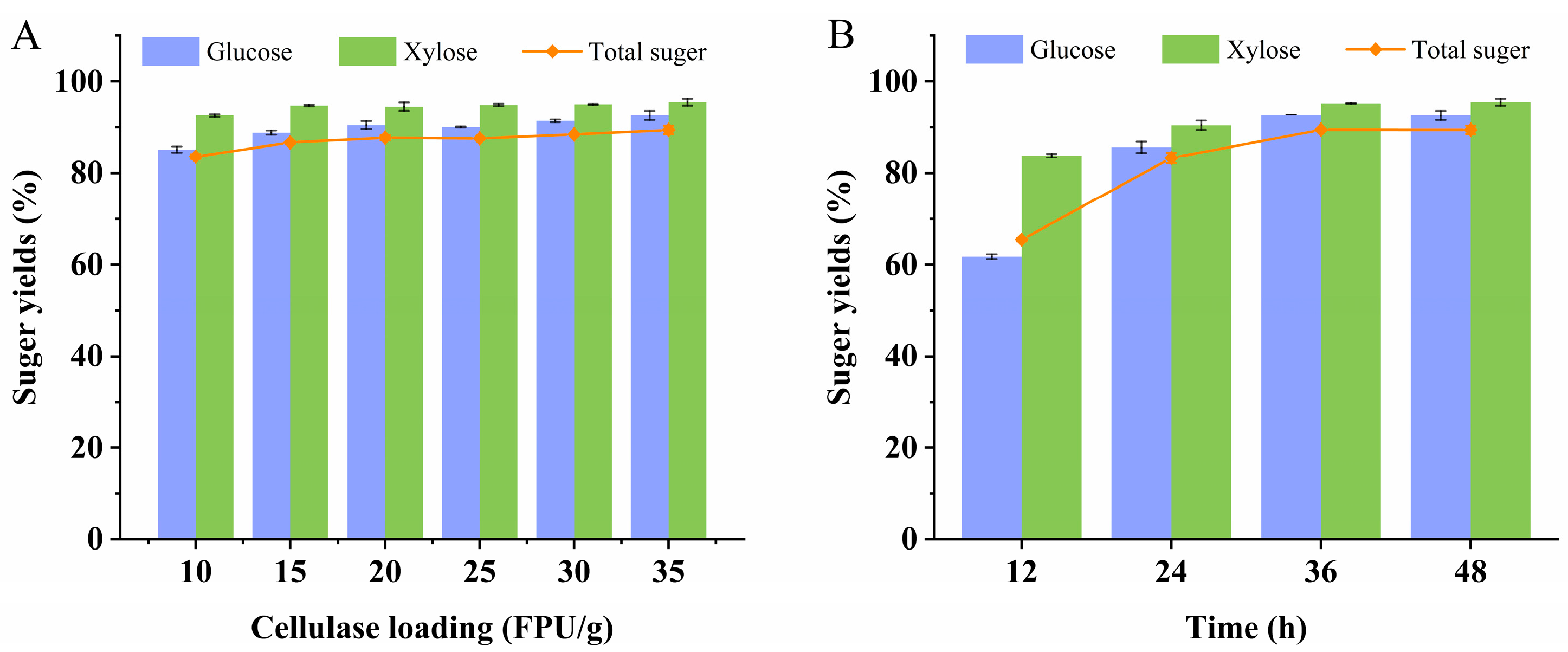
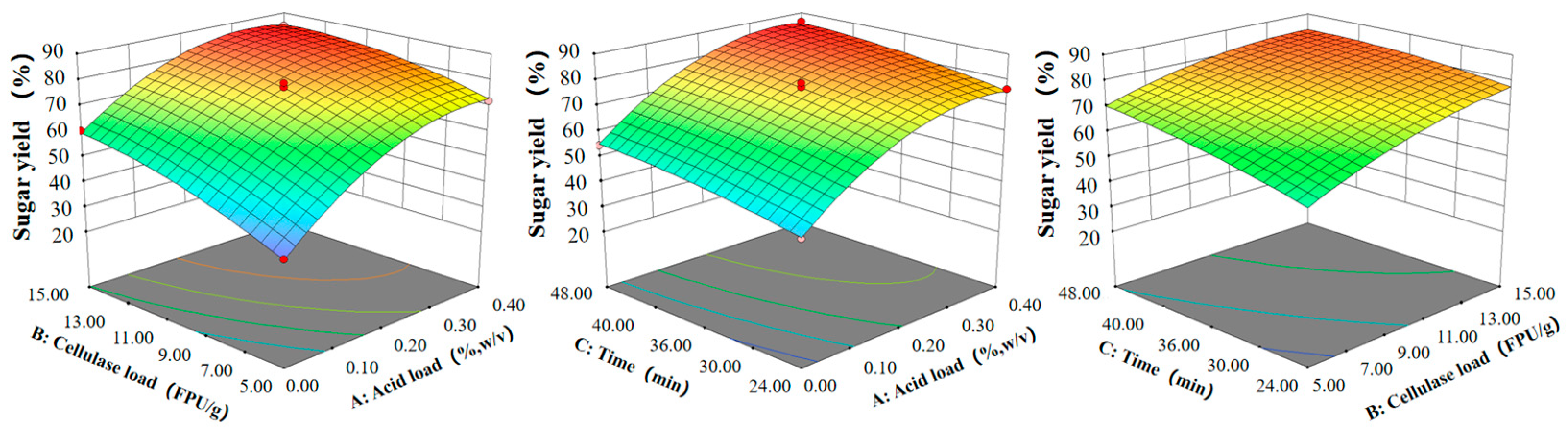
3.3. Depolymerization of Delignified Poplar by Sulfuric Acid and Cellulase
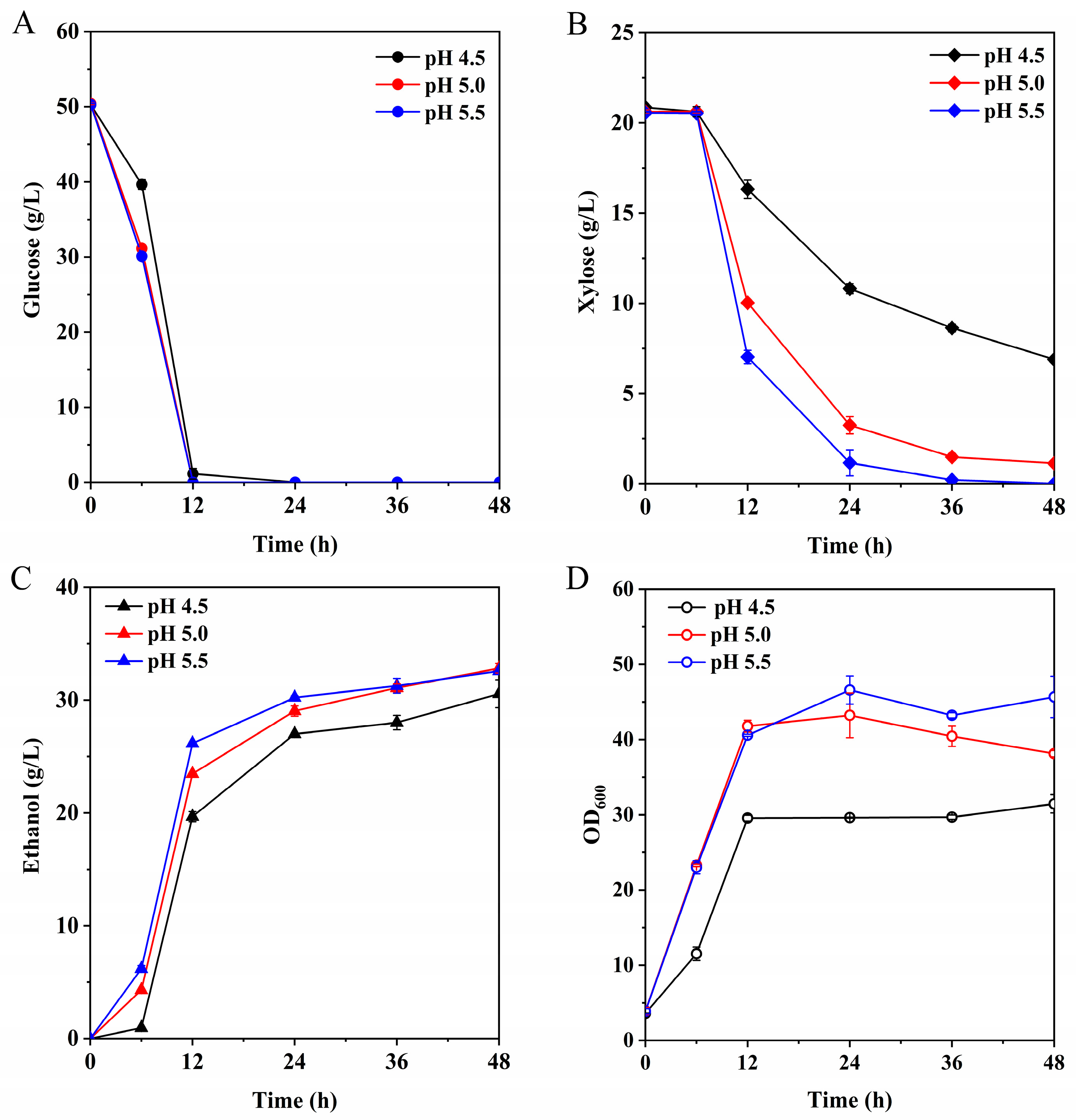
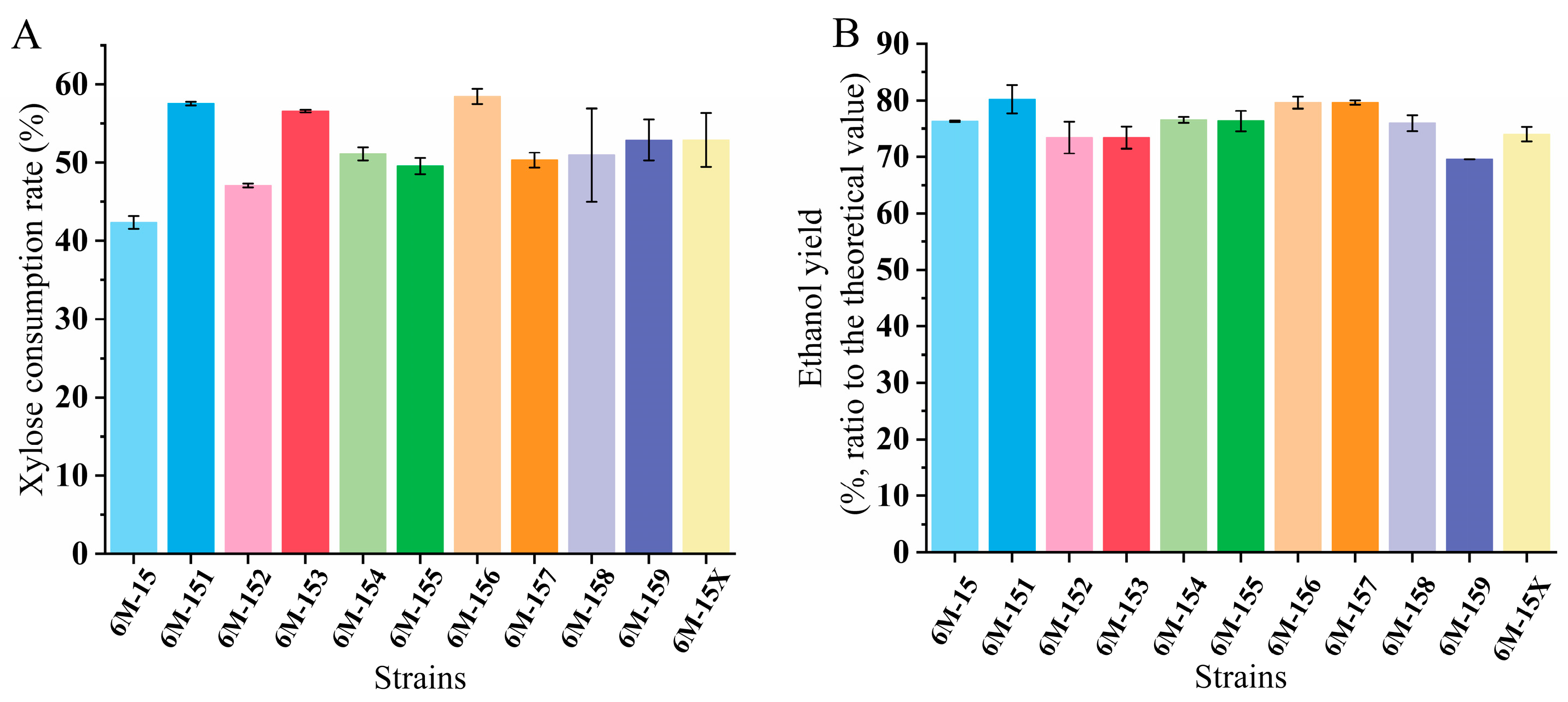
3.4. Evaluation of Yeast Fermentation of Poplar Biomass Hydrolysates
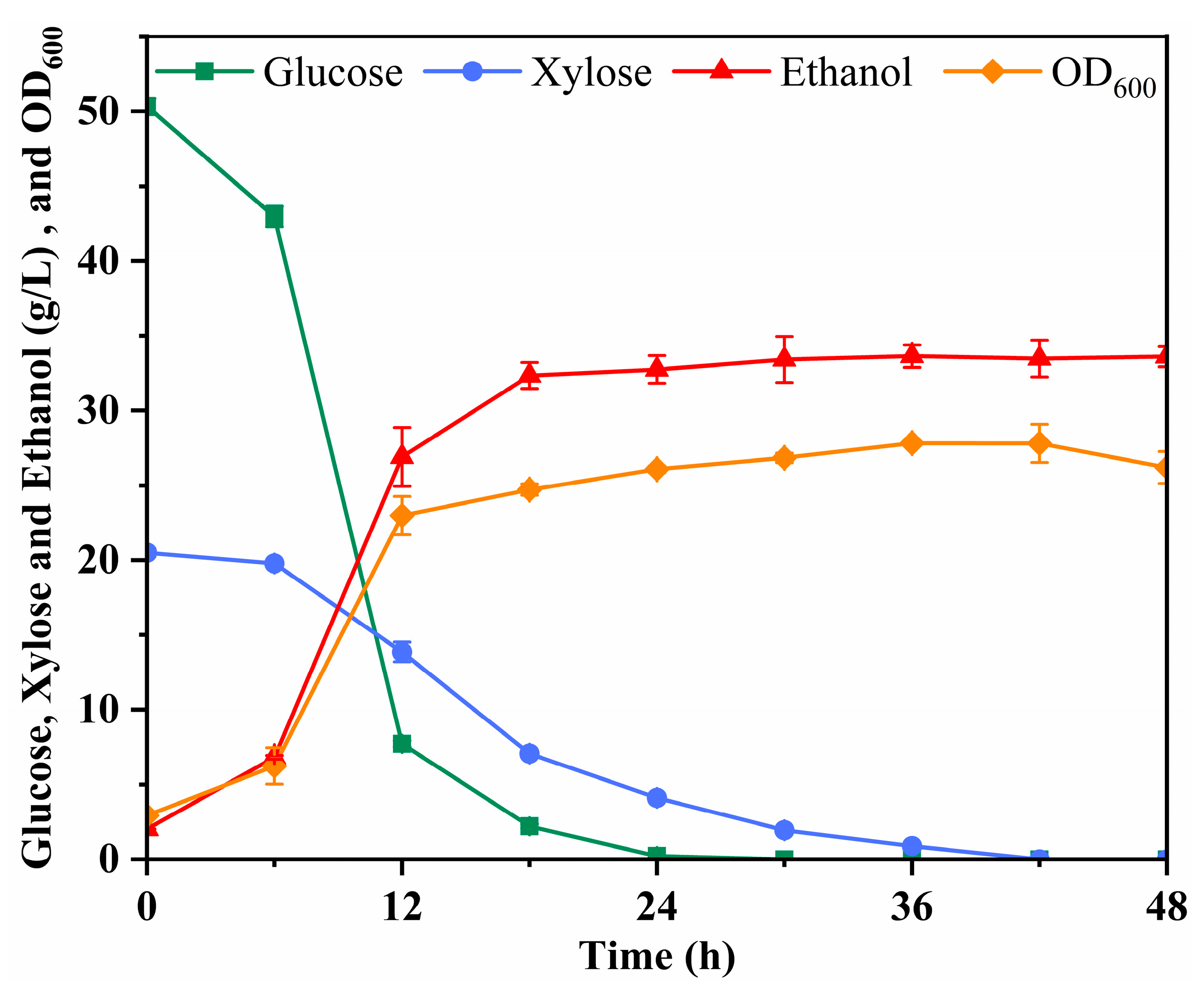
3.5. Effect of 20% Solid Loading Delignified Poplar Hydrolysis Products Ethanol Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Broda, M.; Yelle, D.J.; Serwańska, K. Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.T.L.; Fasahati, P.; Huang, K.; Maravelias, C.T. Utilizing Stillage in the Biorefinery: Economic, Technological and Energetic Analysis. Appl. Energy 2019, 241, 491–503. [Google Scholar] [CrossRef]
- Duwe, A.; Tippkötter, N.; Ulber, R. Lignocellulose-Biorefinery: Ethanol-Focused. In Biorefineries; Wagemann, K., Tippkötter, N., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Switzerland, 2017; Volume 166, pp. 177–215. ISBN 978-3-319-97117-9. [Google Scholar]
- Raj, T.; Chandrasekhar, K.; Naresh Kumar, A.; Rajesh Banu, J.; Yoon, J.-J.; Kant Bhatia, S.; Yang, Y.-H.; Varjani, S.; Kim, S.-H. Recent Advances in Commercial Biorefineries for Lignocellulosic Ethanol Production: Current Status, Challenges and Future Perspectives. Bioresour. Technol. 2022, 344, 126292. [Google Scholar] [CrossRef] [PubMed]
- Furtado, A.T.; Hekkert, M.P.; Negro, S.O. Of Actors, Functions, and Fuels: Exploring a Second Generation Ethanol Transition from a Technological Innovation Systems Perspective in Brazil. Energy Res. Soc. Sci. 2020, 70, 101706. [Google Scholar] [CrossRef]
- Reshmy, R.; Thomas, D.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Sirohi, R.; Varjani, S.; Pugazhendhi, A.; Pandey, A.; et al. Bioplastic Production from Renewable Lignocellulosic Feedstocks: A Review. Rev. Environ. Sci. Biotechnol. 2021, 20, 167–187. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Harfouche, A.; Casler, M.D.; Dylan Jones, H.; Macalpine, W.J.; Murphy-Bokern, D.; Smart, L.B.; Adler, A.; Ashman, C.; Awty-Carroll, D.; et al. Breeding Progress and Preparedness for Mass-scale Deployment of Perennial Lignocellulosic Biomass Crops Switchgrass, Miscanthus, Willow and Poplar. GCB Bioenergy 2019, 11, 118–151. [Google Scholar] [CrossRef] [PubMed]
- Selvi Gökkaya, D.; Çokkuvvetli, T.; Sağlam, M.; Yüksel, M.; Ballice, L. Hydrothermal Gasification of Poplar Wood Chips with Alkali, Mineral, and Metal Impregnated Activated Carbon Catalysts. J. Supercrit. Fluids 2019, 152, 104542. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Geiselman, G.M.; Liu, D.; Magurudeniya, H.D.; Rodriguez, A.; Chen, Y.-C.; Pidatala, V.; Unda, F.; Amer, B.; Baidoo, E.E.K.; et al. Evaluation of Engineered Low-Lignin Poplar for Conversion into Advanced Bioproducts. Biotechnol. Biofuels 2022, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J.; Zhu, C. Effect of Ultrasound Pretreatment on Enzymolysis and Physicochemical Properties of Corn Starch. Int. J. Biol. Macromol. 2018, 111, 848–856. [Google Scholar] [CrossRef]
- Saini, J.K.; Himanshu; Hemansi; Kaur, A.; Mathur, A. Strategies to Enhance Enzymatic Hydrolysis of Lignocellulosic Biomass for Biorefinery Applications: A Review. Bioresour. Technol. 2022, 360, 127517. [Google Scholar] [CrossRef]
- Van Der Cruijsen, K.; Al Hassan, M.; Van Erven, G.; Dolstra, O.; Trindade, L.M. Breeding Targets to Improve Biomass Quality in Miscanthus. Molecules 2021, 26, 254. [Google Scholar] [CrossRef]
- Tang, W.; Tang, Z.; Qian, H.; Huang, C.; He, Y.-C. Implementing Dilute Acid Pretreatment Coupled with Solid Acid Catalysis and Enzymatic Hydrolysis to Improve Bioconversion of Bamboo Shoot Shells. Bioresour. Technol. 2023, 381, 129167. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Shastri, Y. Economic Optimization of Acid Pretreatment: Structural Changes and Impact on Enzymatic Hydrolysis. Ind. Crops Prod. 2020, 147, 112236. [Google Scholar] [CrossRef]
- Halder, P.; Kundu, S.; Patel, S.; Setiawan, A.; Atkin, R.; Parthasarthy, R.; Paz-Ferreiro, J.; Surapaneni, A.; Shah, K. Progress on the Pre-Treatment of Lignocellulosic Biomass Employing Ionic Liquids. Renew. Sustain. Energy Rev. 2019, 105, 268–292. [Google Scholar] [CrossRef]
- Alayoubi, R.; Mehmood, N.; Husson, E.; Kouzayha, A.; Tabcheh, M.; Chaveriat, L.; Sarazin, C.; Gosselin, I. Low Temperature Ionic Liquid Pretreatment of Lignocellulosic Biomass to Enhance Bioethanol Yield. Renew. Energy 2020, 145, 1808–1816. [Google Scholar] [CrossRef]
- Ono, Y.; Takeuchi, M.; Isogai, A. Changes in Neutral Sugar Composition, Molar Mass and Molar Mass Distribution, and Solid-State Structures of Birch and Douglas Fir by Repeated Sodium Chlorite Delignification. Cellulose 2022, 29, 2119–2129. [Google Scholar] [CrossRef]
- Melro, E.; Alves, L.; Antunes, F.E.; Medronho, B. A Brief Overview on Lignin Dissolution. J. Mol. Liq. 2018, 265, 578–584. [Google Scholar] [CrossRef]
- Siqueira, G.; Várnai, A.; Ferraz, A.; Milagres, A.M.F. Enhancement of Cellulose Hydrolysis in Sugarcane Bagasse by the Selective Removal of Lignin with Sodium Chlorite. Appl. Energy 2013, 102, 399–402. [Google Scholar] [CrossRef]
- Ouyang, S.; Qiao, H.; Xu, Q.; Zheng, Z.; Ouyang, J. Development of Two-Step Pretreatment of Chinese Fir Sawdust Using Dilute Sulfuric Acid Followed by Sodium Chlorite for Bioethanol Production. Cellulose 2019, 26, 8513–8524. [Google Scholar] [CrossRef]
- Hoang Nguyen Tran, P.; Ko, J.K.; Gong, G.; Um, Y.; Lee, S.-M. Improved Simultaneous Co-Fermentation of Glucose and Xylose by Saccharomyces Cerevisiae for Efficient Lignocellulosic Biorefinery. Biotechnol. Biofuels 2020, 13, 12. [Google Scholar] [CrossRef]
- Wen, P.; Zhang, T.; Xu, Y.; Zhang, J. Co-Production of Xylooligosaccharides and Monosaccharides from Poplar by a Two-Step Acetic Acid and Sodium Chlorite Pretreatment. Ind. Crops Prod. 2020, 152, 112500. [Google Scholar] [CrossRef]
- Li, M.; Xu, F.; Zhao, Y.; Sun, D.; Liu, J.; Yin, X.; Li, Z.; Zhao, J.; Li, H.; Bao, X. High-Efficient Production of Cellulosic Ethanol from Corn Fiber Based on the Suitable C5/C6 Co-Fermentation Saccharomyces Cerevisiae Strain. Fermentation 2023, 9, 743. [Google Scholar] [CrossRef]
- Hwang, S.H.; Koo, M.; Jo, S.; Cho, Y.S. A Comparison Study of Crude Protein Contents Obtained Utilizing the Kjeldahl Method and Dumas Combustion Method in Foods. Anal. Sci. Technol. 2020, 33, 143–150. [Google Scholar] [CrossRef]
- Loow, Y.-L.; Wu, T.Y.; Md. Jahim, J.; Mohammad, A.W.; Teoh, W.H. Typical Conversion of Lignocellulosic Biomass into Reducing Sugars Using Dilute Acid Hydrolysis and Alkaline Pretreatment. Cellulose 2016, 23, 1491–1520. [Google Scholar] [CrossRef]
- Shen, Y.; Li, H.; Wang, X.; Zhang, X.; Hou, J.; Wang, L.; Gao, N.; Bao, X. High Vanillin Tolerance of an Evolved Saccharomyces Cerevisiae Strain Owing to Its Enhanced Vanillin Reduction and Antioxidative Capacity. J. Ind. Microbiol. Biotechnol. 2014, 41, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Li, M.; Wang, M.; Li, H.; Li, Z.; Qin, W.; Wei, T.; Zhao, J.; Bao, X. A C6/C5 Co-fermenting Saccharomyces Cerevisiae Strain with the Alleviation of Antagonism between Xylose Utilization and Robustness. GCB Bioenergy 2021, 13, 83–97. [Google Scholar] [CrossRef]
- Khosravi, F.; Khaleghi, M.; Naghavi, H. Screening and Identification of Cellulose-Degrading Bacteria from Soil and Leaves at Kerman Province, Iran. Arch. Microbiol. 2022, 204, 88. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Chapla, D.; Shah, A. Bioconversion of Pretreated Sugarcane Bagasse Using Enzymatic and Acid Followed by Enzymatic Hydrolysis Approaches for Bioethanol Production. Renew. Energy 2017, 109, 323–331. [Google Scholar] [CrossRef]
- Li, H.; Shen, Y.; Wu, M.; Hou, J.; Jiao, C.; Li, Z.; Liu, X.; Bao, X. Engineering a Wild-Type Diploid Saccharomyces Cerevisiae Strain for Second-Generation Bioethanol Production. Bioresour. Bioprocess. 2016, 3, 51. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Vo, D.-V.N. Recent Advances and Sustainable Development of Biofuels Production from Lignocellulosic Biomass. Bioresour. Technol. 2022, 344, 126203. [Google Scholar] [CrossRef]
- Rivas, S.; Rigual, V.; Domínguez, J.C.; Alonso, M.V.; Oliet, M.; Parajó, J.C.; Rodriguez, F. A Biorefinery Strategy for the Manufacture and Characterization of Oligosaccharides and Antioxidants from Poplar Hemicelluloses. Food Bioprod. Process. 2020, 123, 398–408. [Google Scholar] [CrossRef]
- Bay, M.S.; Karimi, K.; Nasr Esfahany, M.; Kumar, R. Structural Modification of Pine and Poplar Wood by Alkali Pretreatment to Improve Ethanol Production. Ind. Crops Prod. 2020, 152, 112506. [Google Scholar] [CrossRef]
- Hou, J.; Qiu, Z.; Han, H.; Zhang, Q. Toxicity Evaluation of Lignocellulose-Derived Phenolic Inhibitors on Saccharomyces Cerevisiae Growth by Using the QSTR Method. Chemosphere 2018, 201, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, T.-T.; Shiu, B.-C.; Lou, C.-W.; Lin, J.-H. Enhanced Fluorescent Performance of Modacrylic/Cotton Blended Fabric by Pretreatment with Sodium Chlorite Bleaching. Text. Res. J. 2022, 92, 4722–4735. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Du, X.; Qu, Y. Selective Removal of Lignin to Enhance the Process of Preparing Fermentable Sugars and Platform Chemicals from Lignocellulosic Biomass. Bioresour. Technol. 2020, 303, 122846. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Shi, J.; Qiao, H.; Zheng, Z.; Ouyang, J.; Lai, C. The Key Role of Delignification in Overcoming the Inherent Recalcitrance of Chinese Fir for Biorefining. Bioresour. Technol. 2021, 319, 124154. [Google Scholar] [CrossRef]
- Nan, Y.; Jia, L.; Yang, M.; Xin, D.; Qin, Y.; Zhang, J. Simplified Sodium Chlorite Pretreatment for Carbohydrates Retention and Efficient Enzymatic Saccharification of Silvergrass. Bioresour. Technol. 2018, 261, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Xu, Y.; Zhang, J. Alkaline Incubation Improves the Saccharification of Poplar after Sodium Chlorite Pretreatment with Ultra-Low Cellulase Loading. Renew. Energy 2021, 170, 517–524. [Google Scholar] [CrossRef]
- Novakovic, J.; Kontogianni, N.; Barampouti, E.M.; Mai, S.; Moustakas, K.; Malamis, D.; Loizidou, M. Towards Upscaling the Valorization of Wheat Straw Residues: Alkaline Pretreatment Using Sodium Hydroxide, Enzymatic Hydrolysis and Biogas Production. Environ. Sci. Pollut. Res. 2021, 28, 24486–24498. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Xu, L.-H.; Sun, Q.; Sun, S.-N.; Cao, X.-F.; Wen, J.-L.; Yuan, T.-Q. Ultrafast Alkaline Deep Eutectic Solvent Pretreatment for Enhancing Enzymatic Saccharification and Lignin Fractionation from Industrial Xylose Residue. Bioresour. Technol. 2022, 352, 127065. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. Efficiency of Dilute Sulfuric Acid Pretreatment of Distillery Stillage in the Production of Cellulosic Ethanol. Bioresour. Technol. 2018, 268, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of Lignocellulosic Biomass: A Review on Recent Advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E. Integrated Enzyme Production Lowers the Cost of Cellulosic Ethanol. Biofuels Bioprod. Bioref. 2016, 10, 164–174. [Google Scholar] [CrossRef]
- Zhou, X.; Ding, D.; You, T.; Zhang, X.; Takabe, K.; Xu, F. Synergetic Dissolution of Branched Xylan and Lignin Opens the Way for Enzymatic Hydrolysis of Poplar Cell Wall. J. Agric. Food Chem. 2018, 66, 3449–3456. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhou, W.; Du, X.; Huang, H.; Gong, Z. Combined Dilute Sulfuric Acid and Tween 80 Pretreatment of Corn Stover Significantly Improves the Enzyme Digestibility: Synergistic Removal of Hemicellulose and Lignin. Bioresour. Technol. 2023, 382, 129218. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, J.; Xu, N.; Jia, H.; Li, X.; Zhao, J.; Qu, Y. A Detoxification-Free Process for Enhanced Ethanol Production From Corn Fiber Under Semi-Simultaneous Saccharification and Fermentation. Front. Microbiol. 2022, 13, 861918. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Park, D.-H.; Song, S.H.; Wee, Y.-J.; Jeong, G.-T. Effect of Fermentation Inhibitors in the Presence and Absence of Activated Charcoal on the Growth of Saccharomyces Cerevisiae. Bioprocess. Biosyst. Eng. 2013, 36, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Ask, M.; Bettiga, M.; Duraiswamy, V.R.; Olsson, L. Pulsed Addition of HMF and Furfural to Batch-Grown Xylose-Utilizing Saccharomyces Cerevisiaeresults in Different Physiological Responses in Glucose and Xylose Consumption Phase. Biotechnol. Biofuels 2013, 6, 181. [Google Scholar] [CrossRef] [PubMed]
- Casey, E.; Sedlak, M.; Ho, N.W.Y.; Mosier, N.S. Effect of Acetic Acid and pH on the Cofermentation of Glucose and Xylose to Ethanol by a Genetically Engineered Strain of Saccharomyces Cerevisiae: Effect of Acetic Acid/pH on Xylose Fermentation. FEMS Yeast Res. 2010, 10, 385–393. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, L.; Zhu, J.; Xu, Y.; Yong, Q.; Yu, S. Quantitative Lipidomic Insights in the Inhibitory Response of Pichia Stipitis to Vanillin, 5-Hydroxymethylfurfural, and Acetic Acid. Biochem. Biophys. Res. Commun. 2018, 497, 7–12. [Google Scholar] [CrossRef]
- Farrell, A.E.; Plevin, R.J.; Turner, B.T.; Jones, A.D.; O’Hare, M.; Kammen, D.M. Ethanol Can Contribute to Energy and Environmental Goals. Science 2006, 311, 506–508. [Google Scholar] [CrossRef]
- Hamelinck, C.N.; Hooijdonk, G.V.; Faaij, A.P. Ethanol from Lignocellulosic Biomass: Techno-Economic Performance in Short-, Middle- and Long-Term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar] [CrossRef]
- Sheehan, J.; Aden, A.; Paustian, K.; Killian, K.; Brenner, J.; Walsh, M.; Nelson, R. Energy and Environmental Aspects of Using Corn Stover for Fuel Ethanol. J. Ind. Ecol. 2003, 7, 117–146. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2011; p. NREL/TP-5100-47764, 1013269. [Google Scholar]
- Raj, K.; Krishnan, C. Improved High Solid Loading Enzymatic Hydrolysis of Low-Temperature Aqueous Ammonia Soaked Sugarcane Bagasse Using Laccase-Mediator System and High Concentration Ethanol Production. Ind. Crops Prod. 2019, 131, 32–40. [Google Scholar] [CrossRef]
- Rochón, E.; Cabrera, M.N.; Scutari, V.; Cagno, M.; Guibaud, A.; Martínez, S.; Böthig, S.; Guchin, N.; Ferrari, M.D.; Lareo, C. Co-Production of Bioethanol and Xylosaccharides from Steam-Exploded Eucalyptus Sawdust Using High Solid Loads in Enzymatic Hydrolysis: Effect of Alkaline Impregnation. Ind. Crops Prod. 2022, 175, 114253. [Google Scholar] [CrossRef]
- Ying, W.; Sun, F.; Li, X.; Zhang, J. Efficient High Solid Loading Enzymatic Hydrolysis of Hydrogen Peroxide/Acetic Acid-Pretreated Bamboo for Monosaccharides Production. Ind. Crops Prod. 2023, 197, 116588. [Google Scholar] [CrossRef]
- Percival Zhang, Y.-H.; Himmel, M.E.; Mielenz, J.R. Outlook for Cellulase Improvement: Screening and Selection Strategies. Biotechnol. Adv. 2006, 24, 452–481. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, M.; Hong, D.; Fang, Z.; Xiao, Y.; Fang, W.; Zhang, X. Improving the Cellobiose Hydrolysis Activity of Glucose-Stimulating β-Glucosidase Bgl2A. Enzym. Microb. Technol. 2023, 169, 110289. [Google Scholar] [CrossRef]
- Hong, J.-W.; Gam, D.-H.; Kim, J.-H.; Jeon, S.-J.; Kim, H.-S.; Kim, J.-W. Process Development for the Detoxification of Fermentation Inhibitors from Acid Pretreated Microalgae Hydrolysate. Molecules 2021, 26, 2435. [Google Scholar] [CrossRef]
- Wang, F.; Dong, Y.; Cheng, X.; Xie, H.; Song, A.; Zhang, Z. Effect of Detoxification Methods on ABE Production from Corn Stover Hydrolysate by Clostridium Acetobutylicum CICC 8016. Biotech. Appl. Biochem. 2020, 67, 790–798. [Google Scholar] [CrossRef]
- Yu, Y.; Christopher, L.P. Detoxification of Hemicellulose-Rich Poplar Hydrolysate by Polymeric Resins for Improved Ethanol Fermentability. Fuel 2017, 203, 187–196. [Google Scholar] [CrossRef]


| Components | Contents (%) | |
|---|---|---|
| Biomass component | Cellulose | 40.39 ± 1.54 |
| Hemicellulose | 15.00 ± 0.54 | |
| Protein | 0.42 ± 0.01 | |
| Lignin (acid-soluble) | 1.64 ± 0.05 | |
| Lignin (acid-insoluble) | 26.69 ± 0.23 | |
| Ash | 1.05 ± 0.03 | |
| Extractives | 8.21 ± 0.01 | |
| Glucose | 44.88 ± 1.71 | |
| Major monosaccharide | Xylose | 17.05 ± 0.61 |
| Arabinose | 0.09 ± 0.01 |
| Levels (%) | Solid Yield (%) * | Removal Ratio (%) | ||
|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | ||
| 2 | 90.86 ± 0.49 | 0.91 ± 0.09 | 2.24 ± 0.14 | 31.33 ± 0.28 |
| 3 | 86.37 ± 1.29 | 1.38 ± 0.67 | 3.34 ± 0.03 | 45.08 ± 0.21 |
| 4 | 82.66 ± 0.78 | 2.03 ± 0.25 | 4.45 ± 0.32 | 57.61 ± 0.72 |
| 5 | 80.31 ± 0.49 | 2.69 ± 0.67 | 4.72 ± 0.29 | 73.09 ± 0.19 |
| 6 | 79.74 ± 1.83 | 2.82 ± 0.39 | 6.09 ± 0.42 | 76.44 ± 1.62 |
| 7 | 78.39 ± 1.02 | 3.01 ± 0.17 | 7.54 ± 0.29 | 78.69 ± 0.78 |
| 8 | 77.80 ± 1.16 | 3.47 ± 0.21 | 8.30 ± 0.54 | 80.52 ± 1.23 |
| Levels (h) | Solid Yield (%) * | Removal Ratio (%) | ||
|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | ||
| 0.5 | 93.88 ± 1.29 | 0.84 ± 0.14 | 2.37 ± 0.17 | 28.74 ± 1.19 |
| 1 | 90.23 ± 0.65 | 0.99 ± 0.03 | 2.84 ± 0.21 | 43.28 ± 1.07 |
| 2 | 83.47 ± 0.63 | 1.11 ± 0.46 | 2.96 ± 0.54 | 59.77 ± 0.36 |
| 3 | 80.31 ± 0.49 | 2.69 ± 0.67 | 4.72 ± 0.29 | 73.09 ± 0.19 |
| 4 | 79.66 ± 0.84 | 2.82 ± 0.61 | 5.77 ± 1.1 | 72.06 ± 2.10 |
| 5 | 79.82 ± 0.40 | 3.08 ± 0.25 | 6.86 ± 0.38 | 73.98 ± 0.15 |
| 6 | 78.41 ± 0.24 | 3.37 ± 0.00 | 8.09 ± 0.13 | 74.69 ± 0.74 |
| RUN | H2SO4 Loading (%, w/v) | Cellulase Loading (FPU/g) | Time (h) | Glucose (g/L) | Xylose (g/L) | Total Sugar Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 0.4 | 5 | 36 | 40.02 | 18.50 | 71.98 |
| 2 | 0.4 | 10 | 24 | 43.42 | 18.54 | 76.57 |
| 3 | 0.2 | 5 | 48 | 39.11 | 18.19 | 71.02 |
| 4 | 0.2 | 5 | 24 | 29.18 | 16.22 | 56.13 |
| 5 | 0.2 | 10 | 36 | 43.47 | 18.80 | 76.37 |
| 6 | 0.2 | 10 | 36 | 43.53 | 18.63 | 77.02 |
| 7 | 0.4 | 15 | 36 | 48.78 | 20.00 | 84.70 |
| 8 | 0.2 | 10 | 36 | 44.68 | 19.18 | 78.83 |
| 9 | 0.4 | 10 | 48 | 49.55 | 20.59 | 86.28 |
| 10 | 0.2 | 10 | 36 | 43.46 | 18.69 | 76.22 |
| 11 | 0 | 5 | 36 | 18.64 | 11.63 | 37.27 |
| 12 | 0 | 15 | 36 | 34.01 | 14.92 | 60.47 |
| 13 | 0 | 10 | 48 | 30.03 | 14.21 | 54.68 |
| 14 | 0 | 10 | 24 | 23.38 | 12.52 | 44.53 |
| 15 | 0.2 | 15 | 24 | 43.76 | 18.74 | 77.39 |
| 16 | 0.2 | 15 | 48 | 47.57 | 19.98 | 83.22 |
| 17 | 0.2 | 10 | 36 | 43.62 | 18.61 | 77.12 |
| Source | Sum of Squares | df | Mean Square | F-Value | Prob > F | Remark |
|---|---|---|---|---|---|---|
| Model | 3245.80 | 9 | 360.64 | 318.90 | <0.0001 | S |
| A- H2SO4 loading | 1878.23 | 1 | 1878.23 | 1660.80 | <0.0001 | S |
| B- Cellulase loading | 601.70 | 1 | 601.70 | 532.04 | <0.0001 | S |
| C-Time | 205.84 | 1 | 205.84 | 182.01 | <0.0001 | S |
| AB | 27.46 | 1 | 27.46 | 24.28 | 0.0017 | S |
| AC | 0.048 | 1 | 0.048 | 0.043 | 0.8420 | nS |
| BC | 20.52 | 1 | 20.52 | 18.15 | 0.0037 | S |
| A2 | 418.19 | 1 | 418.19 | 369.78 | <0.0001 | S |
| B2 | 52.79 | 1 | 52.79 | 46.68 | 0.0002 | S |
| C2 | 11.20 | 1 | 11.20 | 9.90 | 0.0162 | S |
| Residual | 7.92 | 7 | 1.13 | |||
| Lack of Fit | 3.61 | 3 | 1.20 | 1.12 | 0.4408 | nS |
| Pure Error | 4.31 | 4 | 1.08 | |||
| Cor Total | 3253.72 | 16 | ||||
| R2 | 0.9976 |
| Source | Glucose Yield (%) | Xylose Yield (%) | Total Sugar Yield (%) |
|---|---|---|---|
| Enzymolysis | 62.80 ± 1.23 | 88.24 ± 0.87 | 69.66 ± 0.97 |
| Enzymolysis+β-Glucosidase | 89.45 ± 2.74 | 94.16 ± 1.54 | 91.36 ± 2.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Sun, D.; Wang, Z.; Li, M.; Yin, X.; Li, H.; Xu, L.; Zhao, J.; Bao, X. Highly Efficient Production of Cellulosic Ethanol from Poplar Using an Optimal C6/C5 Co-Fermentation Strain of Saccharomyces cerevisiae. Microorganisms 2024, 12, 1174. https://doi.org/10.3390/microorganisms12061174
Xu F, Sun D, Wang Z, Li M, Yin X, Li H, Xu L, Zhao J, Bao X. Highly Efficient Production of Cellulosic Ethanol from Poplar Using an Optimal C6/C5 Co-Fermentation Strain of Saccharomyces cerevisiae. Microorganisms. 2024; 12(6):1174. https://doi.org/10.3390/microorganisms12061174
Chicago/Turabian StyleXu, Fadi, Dongming Sun, Zhaojiang Wang, Menglei Li, Xiaolong Yin, Hongxing Li, Lili Xu, Jianzhi Zhao, and Xiaoming Bao. 2024. "Highly Efficient Production of Cellulosic Ethanol from Poplar Using an Optimal C6/C5 Co-Fermentation Strain of Saccharomyces cerevisiae" Microorganisms 12, no. 6: 1174. https://doi.org/10.3390/microorganisms12061174
APA StyleXu, F., Sun, D., Wang, Z., Li, M., Yin, X., Li, H., Xu, L., Zhao, J., & Bao, X. (2024). Highly Efficient Production of Cellulosic Ethanol from Poplar Using an Optimal C6/C5 Co-Fermentation Strain of Saccharomyces cerevisiae. Microorganisms, 12(6), 1174. https://doi.org/10.3390/microorganisms12061174






