Predicting Efficacy of a Purified Inactivated Zika Virus Vaccine in Flavivirus-Naïve Humans Using an Immunological Correlate of Protection in Non-Human Primates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source Data Studies
2.2. Comparing NHP and Human ZIKV NAb GMTs
2.3. Reference Definitions
2.4. Modeling the Relationship between NHP ZIKV NAb GMTs and Zika Viral Load
2.5. The Prentice Criteria
2.6. Estimating the Human Probability of Protection for Each Dose Group
2.7. Bayesian LR
3. Results
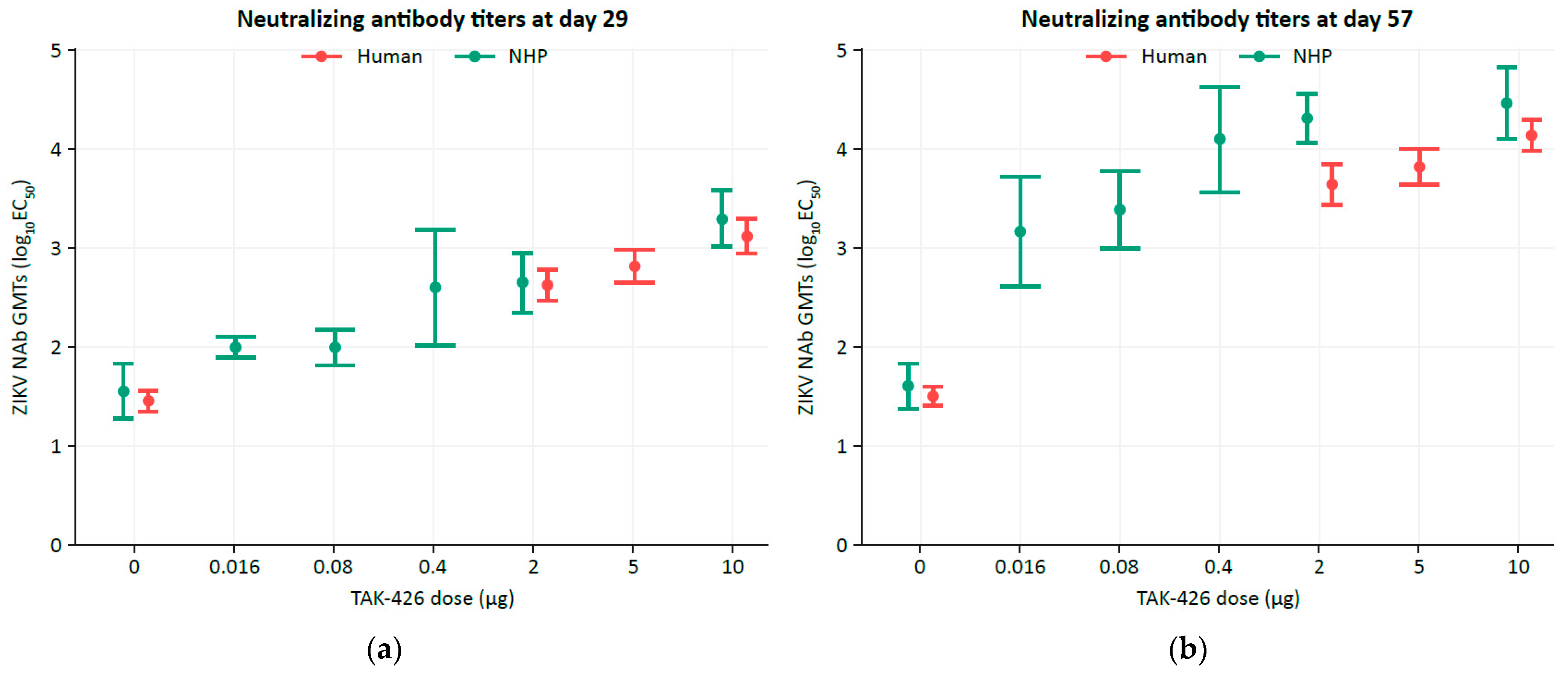
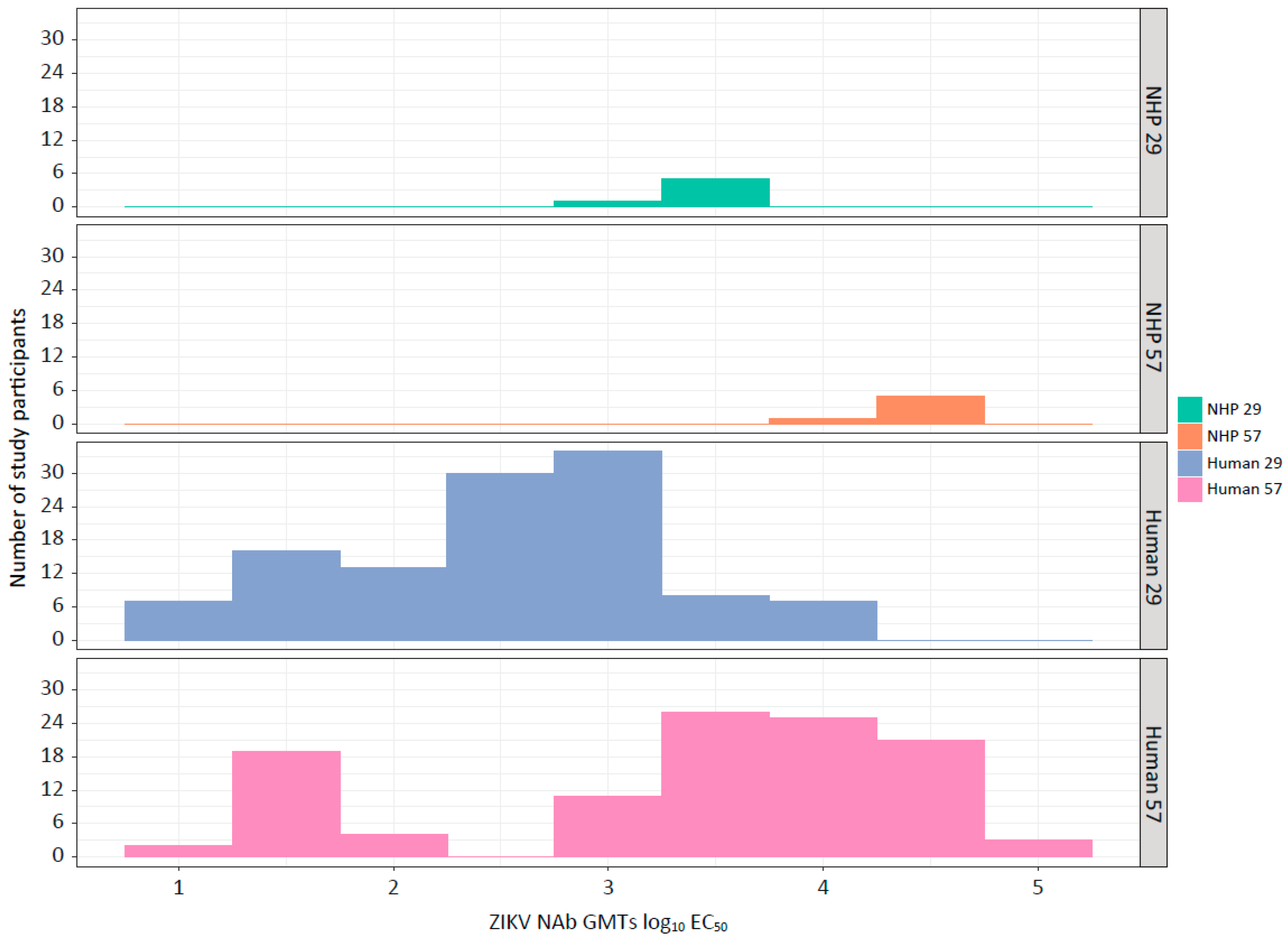
3.1. TAK-426 Induced Comparable Dose-Dependent ZIKV NAb Titer GMTs in Humans (FV-Naïve) and NHPs (FVs-Naïve)
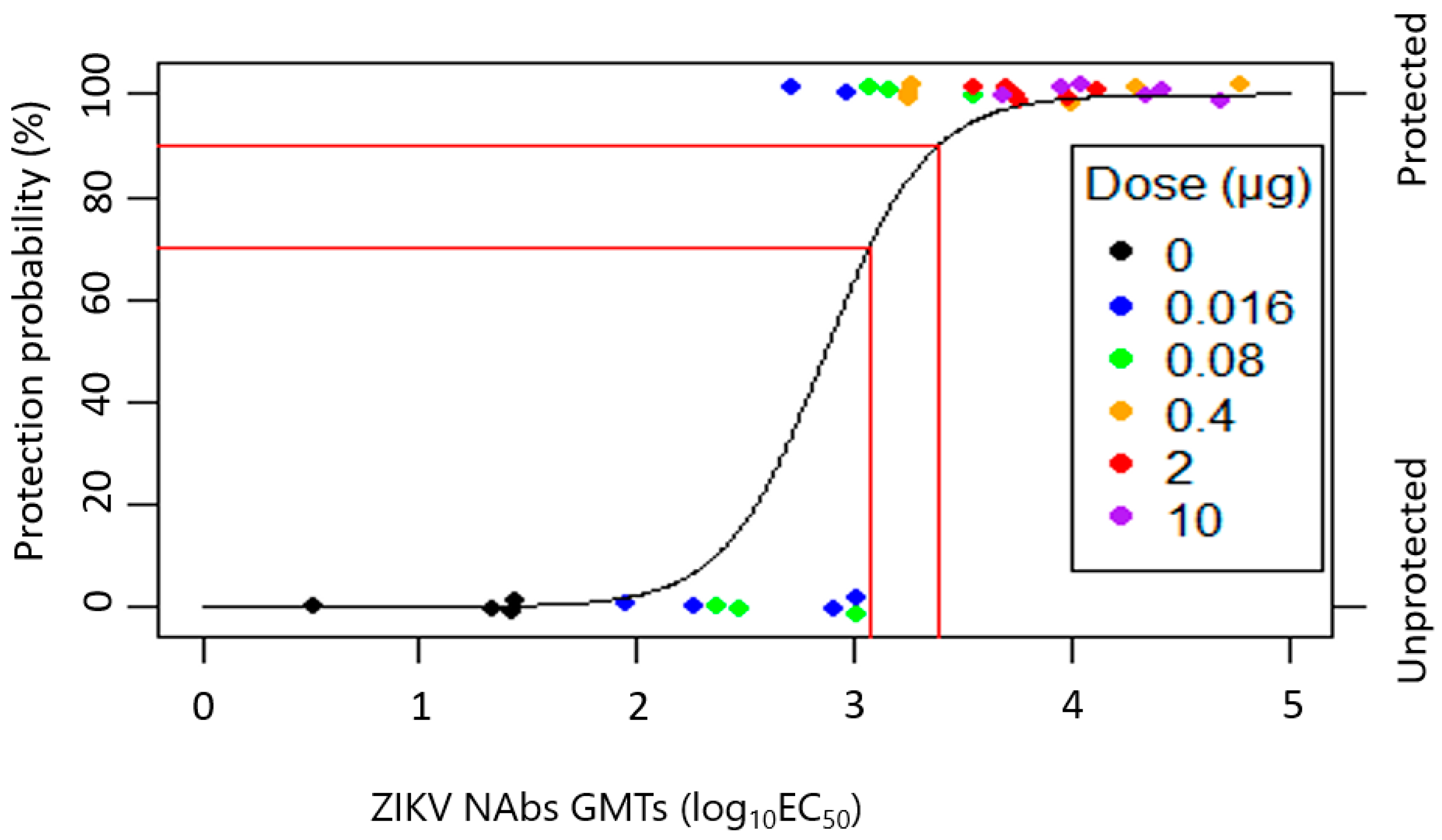
3.2. Estimated Probability of TAK-426–Induced Protection in NHPs (LR Fit Using Bias Reduction)
3.3. TAK-426 Elicited ZIKV NAbs Fulfilling the Prentice Criteria
3.4. Estimated Probability of TAK-426–Induced Protection in Phase 1 Trial Participants, LR with Bias Reduction
3.5. Statistical Methodologies Comparison
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Countries and Territories with Current or Previous Zika Virus Transmission. Data as of February 2022. Available online: https://cdn.who.int/media/docs/default-source/documents/emergencies/zika/countries-with-zika-and-vectors-table_february-2022.pdf?sfvrsn=4dc1f8ab_9&download=true (accessed on 7 March 2024).
- World Health Organization. WHO Statement on the First Meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika Virus and Observed Increase in Neurological Disorders and Neonatal Malformations. Available online: https://www.who.int/news/item/01-02-2016-who-statement-on-the-first-meeting-of-the-international-health-regulations-(2005)-(ihr-2005)-emergency-committee-on-zika-virus-and-observed-increase-in-neurological-disorders-and-neonatal-malformations (accessed on 7 March 2024).
- Pan American Health Organization. Zika—Weekly Report. Available online: https://www3.paho.org/data/index.php/en/mnu-topics/zika-weekly-en/ (accessed on 7 March 2024).
- Ruchusatsawat, K.; Wongjaroen, P.; Posanacharoen, A.; Rodriguez-Barraquer, I.; Sangkitporn, S.; Cummings, D.A.T.; Salje, H. Long-term circulation of Zika virus in Thailand: An observational study. Lancet Infect. Dis. 2019, 19, 439–446. [Google Scholar] [CrossRef]
- Duong, V.; Ong, S.; Leang, R.; Huy, R.; Ly, S.; Mounier, U.; Ou, T.; In, S.; Peng, B.; Ken, S.; et al. Low circulation of Zika virus, Cambodia, 2007–2016. Emerg. Infect. Dis. 2017, 23, 296–299. [Google Scholar] [CrossRef]
- Kuadkitkan, A.; Wikan, N.; Sornjai, W.; Smith, D.R. Zika virus and microcephaly in Southeast Asia: A cause for concern? J. Infect. Public Health 2020, 13, 11–15. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Zika Cases in the United States. Available online: https://www.cdc.gov/zika/reporting/index.html (accessed on 7 March 2024).
- European Centre for Disease Prevention and Control. Zika Virus Disease in Var Department, France. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/RRA-Zika-France-16-Oct-2019-corrected.pdf (accessed on 7 March 2024).
- Biswas, A.; Kodan, P.; Gupta, N.; Soneja, M.; Baruah, K.; Sharma, K.K.; Meena, S. Zika outbreak in India in 2018. J. Travel Med. 2020, 27, taaa001. [Google Scholar] [CrossRef]
- Khan, E.; Jindal, H.; Mishra, P.; Suvvari, T.K.; Jonna, S. The 2021 Zika outbreak in Uttar Pradesh state of India: Tackling the emerging public health threat. Trop. Dr. 2022, 52, 474–478. [Google Scholar] [CrossRef]
- Seers, T.; Rothe, C.; Hamer, D.H.; Denny, S.; Spindler, R.; Schwartz, E.; Johnston, V. Zika virus infection in European travellers returning from Thailand in 2022: A GeoSentinel case series. Trop. Med. Int. Health 2023, 28, 576–579. [Google Scholar] [CrossRef]
- Vannice, K.S.; Cassetti, M.C.; Eisinger, R.W.; Hombach, J.; Knezevic, I.; Marston, H.D.; Wilder-Smith, A.; Cavaleri, M.; Krause, P.R. Demonstrating vaccine effectiveness during a waning epidemic: A WHO/NIH meeting report on approaches to development and licensure of Zika vaccine candidates. Vaccine 2019, 37, 863–868. [Google Scholar] [CrossRef]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika virus and birth defects—Reviewing the evidence for causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef]
- Coelho, A.V.C.; Crovella, S. Microcephaly prevalence in infants born to Zika virus-infected women: A systematic review and meta-analysis. Int. J. Mol. Sci. 2017, 18, 1714. [Google Scholar] [CrossRef]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastere, S.; Valour, F.; Baudouin, L.; Mallet, H.; Musso, D.; Ghawche, F. Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Eurosurveillance 2014, 19, 20720. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Pan American Health Organization. Zika Cases and Congenital Syndrome Associated with Zika Virus. Available online: https://www.paho.org/hq/dmdocuments/2016/2016-nov-17-phe-ZIKV-cases.pdf (accessed on 7 March 2024).
- Araujo, L.M.; Ferreira, M.L.; Nascimento, O.J. Guillain-Barré syndrome associated with the Zika virus outbreak in Brazil. Arq. Neuropsiquiatr. 2016, 74, 253–255. [Google Scholar] [CrossRef]
- Mécharles, S.; Herrmann, C.; Poullain, P.; Tran, T.H.; Deschamps, N.; Mathon, G.; Landais, A.; Breurec, S.; Lannuzel, A. Acute myelitis due to Zika virus infection. Lancet 2016, 387, 1481. [Google Scholar] [CrossRef]
- World Health Organization. Zika Virus Situation Report. Available online: https://iris.who.int/bitstream/handle/10665/251905/zikasitrep8Dec2016-eng.pdf?sequence=1 (accessed on 7 March 2024).
- Pan American Health Organization. Zika—Epidemiological Update. Available online: https://www.paho.org/en/documents/27-april-2017-zika-epidemiological-update-0 (accessed on 7 March 2024).
- Dirlikov, E.; Major, C.G.; Mayshack, M.; Medina, N.; Matos, D.; Ryff, K.R.; Torres-Aponte, J.; Alkis, R.; Munoz-Jordan, J.; Colon-Sanchez, C.; et al. Guillain-Barré syndrome during ongoing Zika virus transmission—Puerto Rico, January 1–July 31, 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 910–914. [Google Scholar] [CrossRef]
- Dos Santos, T.; Rodriguez, A.; Almiron, M.; Sanhueza, A.; Ramon, P.; de Oliveira, W.K.; Coelho, G.E.; Badaró, R.; Cortez, J.; Ospina, M.; et al. Zika virus and the Guillain-Barré syndrome—Case series from seven countries. N. Engl. J. Med. 2016, 375, 1598–1601. [Google Scholar] [CrossRef]
- Barbi, L.; Coelho, A.V.C.; Alencar, L.C.A.; Crovella, S. Prevalence of Guillain-Barré syndrome among Zika virus infected cases: A systematic review and meta-analysis. Braz. J. Infect. Dis. 2018, 22, 137–141. [Google Scholar] [CrossRef]
- Mehta, R.; Soares, C.N.; Medialdea-Carrera, R.; Ellul, M.; da Silva, M.T.T.; Rosala-Hallas, A.; Jardim, M.R.; Burnside, G.; Pamplona, L.; Bhojak, M.; et al. The spectrum of neurological disease associated with Zika and chikungunya viruses in adults in Rio de Janeiro, Brazil: A case series. PLoS Negl. Trop. Dis. 2018, 12, e0006212. [Google Scholar] [CrossRef]
- Azevedo, R.S.; Araujo, M.T.; Martins Filho, A.J.; Oliveira, C.S.; Nunes, B.T.; Cruz, A.C.; Nascimento, A.G.; Medeiros, R.C.; Caldas, C.A.; Araujo, F.C.; et al. Zika virus epidemic in Brazil. I. Fatal disease in adults: Clinical and laboratorial aspects. J. Clin. Virol. 2016, 85, 56–64. [Google Scholar] [CrossRef]
- Arzuza-Ortega, L.; Polo, A.; Pérez-Tatis, G.; López-García, H.; Parra, E.; Pardo-Herrera, L.C.; Rico-Turca, A.M.; Villamil-Gómez, W.; Rodríguez-Morales, A.J. Fatal sickle cell disease and zika virus infection in girl from Colombia. Emerg. Infect. Dis. 2016, 22, 925–927. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Infectious Disease Accelerated Approvals Vaccines. Available online: https://www.fda.gov/drugs/accelerated-approval-program/ongoing-infectious-disease-accelerated-approvals-vaccines (accessed on 7 March 2024).
- Bohning, K.; Sonnberg, S.; Chen, H.L.; Zahralban-Steele, M.; Powell, T.; Hather, G.; Patel, H.K.; Dean, H.J. A high throughput reporter virus particle microneutralization assay for quantitation of Zika virus neutralizing antibodies in multiple species. PLoS ONE 2021, 16, e0250516. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef]
- Sumathy, K.; Kulkarni, B.; Gondu, R.K.; Ponnuru, S.K.; Bonguram, N.; Eligeti, R.; Gadiyaram, S.; Praturi, U.; Chougule, B.; Karunakaran, L.; et al. Protective efficacy of Zika vaccine in AG129 mouse model. Sci. Rep. 2017, 7, 46375. [Google Scholar] [CrossRef]
- Abbink, P.; Larocca, R.A.; De La Barrera, R.A.; Bricault, C.A.; Moseley, E.T.; Boyd, M.; Kirilova, M.; Li, Z.; Ng’ang’a, D.; Nanayakkara, O.; et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 2016, 353, 1129–1132. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA vaccines protect against Zika virus infection. Cell 2017, 169, 176. [Google Scholar] [CrossRef]
- Muthumani, K.; Griffin, B.D.; Agarwal, S.; Kudchodkar, S.B.; Reuschel, E.L.; Choi, H.; Kraynyak, K.A.; Duperret, E.K.; Keaton, A.A.; Chung, C.; et al. In vivo protection against ZIKV infection and pathogenesis through passive antibody transfer and active immunisation with a prMEnv DNA vaccine. NPJ Vaccines 2016, 1, 16021. [Google Scholar] [CrossRef]
- Larocca, R.A.; Abbink, P.; Peron, J.P.; Zanotto, P.M.; Iampietro, M.J.; Badamchi-Zadeh, A.; Boyd, M.; Ng’ang’a, D.; Kirilova, M.; Nityanandam, R.; et al. Vaccine protection against Zika virus from Brazil. Nature 2016, 536, 474–478. [Google Scholar] [CrossRef]
- Dowd, K.A.; Ko, S.Y.; Morabito, K.M.; Yang, E.S.; Pelc, R.S.; DeMaso, C.R.; Castilho, L.R.; Abbink, P.; Boyd, M.; Nityanandam, R.; et al. Rapid development of a DNA vaccine for Zika virus. Science 2016, 354, 237–240. [Google Scholar] [CrossRef]
- Abbink, P.; Larocca, R.A.; Visitsunthorn, K.; Boyd, M.; De La Barrera, R.A.; Gromowski, G.D.; Kirilova, M.; Peterson, R.; Li, Z.; Nanayakkara, O.; et al. Durability and correlates of vaccine protection against Zika virus in rhesus monkeys. Sci. Transl. Med. 2017, 9, eaao4163. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, D.; Mendy, J.; Manayani, D.; Vang, L.; Wang, C.; Richard, T.; Guenther, B.; Aruri, J.; Avanzini, J.; Garduno, F.; et al. Passive transfer of immune sera induced by a Zika virus-like particle vaccine protects AG129 mice against lethal Zika virus challenge. eBioMedicine 2018, 27, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Modjarrad, K.; Lin, L.; George, S.L.; Stephenson, K.E.; Eckels, K.H.; De La Barrera, R.A.; Jarman, R.G.; Sondergaard, E.; Tennant, J.; Ansel, J.L.; et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: Phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet 2018, 391, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, N.J.; Martin, J.E.; Graham, B.S.; Nabel, G.J. Correlates of protective immunity for Ebola vaccines: Implications for regulatory approval by the animal rule. Nat. Rev. Microbiol. 2009, 7, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, J.; Skiadopoulos, M.H.; Hopkins, R.J. Licensure strategy for pre- and post-exposure prophylaxis of biothrax vaccine: The first vaccine licensed using the FDA animal rule. Expert. Rev. Vaccines 2016, 15, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Roques, P.; Fritzer, A.; Dereuddre-Bosquet, N.; Wressnigg, N.; Hochreiter, R.; Bossevot, L.; Pascal, Q.; Guehenneux, F.; Bitzer, A.; Corbic Ramljak, I.; et al. Effectiveness of CHIKV vaccine VLA1553 demonstrated by passive transfer of human sera. JCI Insight 2022, 7, e160173. [Google Scholar] [CrossRef]
- Osuna, C.E.; Whitney, J.B. Nonhuman primate models of Zika virus infection, immunity, and therapeutic development. J. Infect. Dis. 2017, 216, S928–S934. [Google Scholar] [CrossRef]
- Rayner, J.O.; Kalkeri, R.; Goebel, S.; Cai, Z.; Green, B.; Lin, S.; Snyder, B.; Hagelin, K.; Walters, K.B.; Koide, F. Comparative pathogenesis of asian and african-lineage Zika virus in Indian rhesus macaque’s and development of a non-human primate model suitable for the evaluation of new drugs and vaccines. Viruses 2018, 10, 229. [Google Scholar] [CrossRef]
- Azar, S.R.; Rossi, S.L.; Haller, S.H.; Yun, R.; Huang, J.H.; Plante, J.A.; Zhou, J.; Olano, J.P.; Roundy, C.M.; Hanley, K.A.; et al. ZIKV demonstrates minimal pathologic effects and mosquito infectivity in viremic cynomolgus macaques. Viruses 2018, 10, 661. [Google Scholar] [CrossRef]
- Kim, I.J.; Lanthier, P.A.; Clark, M.J.; De La Barrera, R.A.; Tighe, M.P.; Szaba, F.M.; Travis, K.L.; Low-Beer, T.C.; Cookenham, T.S.; Lanzer, K.G.; et al. Efficacy of an inactivated Zika vaccine against virus infection during pregnancy in mice and marmosets. NPJ Vaccines 2022, 7, 9. [Google Scholar] [CrossRef]
- Dudley, D.M.; Aliota, M.T.; Mohr, E.L.; Weiler, A.M.; Lehrer-Brey, G.; Weisgrau, K.L.; Mohns, M.S.; Breitbach, M.E.; Rasheed, M.N.; Newman, C.M.; et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat. Commun. 2016, 7, 12204. [Google Scholar] [CrossRef] [PubMed]
- Dudley, D.M.; Van Rompay, K.K.; Coffey, L.L.; Ardeshir, A.; Keesler, R.I.; Bliss-Moreau, E.; Grigsby, P.L.; Steinbach, R.J.; Hirsch, A.J.; MacAllister, R.P.; et al. Miscarriage and stillbirth following maternal Zika virus infection in nonhuman primates. Nat. Med. 2018, 24, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.; Friedrich, T.C.; O’Connor, D.H. Macaque monkeys in Zika virus research: 1947-present. Curr. Opin. Virol. 2017, 25, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, W.R.; Livengood, J.A.; Giebler, H.A.; Stovall, J.L.; Boroughs, K.L.; Sonnberg, S.; Bohning, K.J.; Dietrich, E.A.; Ong, Y.T.; Danh, H.K.; et al. Purified inactivated Zika vaccine candidates afford protection against lethal challenge in mice. Sci. Rep. 2018, 8, 16509. [Google Scholar] [CrossRef] [PubMed]
- Young, G.; Bohning, K.J.; Zahralban-Steele, M.; Hather, G.; Tadepalli, S.; Mickey, K.; Godin, C.S.; Sanisetty, S.; Sonnberg, S.; Patel, H.K.; et al. Complete protection in macaques conferred by purified inactivated Zika vaccine: Defining a correlate of protection. Sci. Rep. 2020, 10, 3488. [Google Scholar] [CrossRef] [PubMed]
- Young, G.; Zahralban-Steele, M.; Dean, H.J. Impact of prior flavivirus vaccination on immunogenicity and efficacy of an inactivated Zika vaccine in Indian rhesus macaques. Vaccine 2023, 41, 3024–3027. [Google Scholar] [CrossRef] [PubMed]
- Han, H.H.; Diaz, C.; Acosta, C.J.; Liu, M.; Borkowski, A. Safety and immunogenicity of a purified inactivated Zika virus vaccine candidate in healthy adults: An observer-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Acosta, C.J.; Diaz, C.; Nordio, F.; Han, H.H.; Moss, K.J.; Bohning, K.; Kumar, P.; Liu, M.; Patel, H.; Pacciarini, F.; et al. Persistence of immunogenicity of a purified inactivated Zika virus vaccine candidate in healthy adults: 2 years of follow-up compared with natural infection. J. Infect. Dis. 2023, 227, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.R. Surrogate endpoints and FDA’s accelerated approval process. Health Aff. 2005, 24, 67–78. [Google Scholar] [CrossRef]
- Firth, D. Bias reduction of maximum likelihood estimates. Biometrika 1993, 80, 27–38. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Prentice, R.L. Surrogate endpoints in clinical trials: Definition and operational criteria. Stat. Med. 1989, 8, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.P.; Follmann, D.A.; Lynn, F.; Schiffer, J.M.; Stark, G.V.; Kohberger, R.; Quinn, C.P.; Nuzum, E.O. Anthrax vaccine-induced antibodies provide cross-species prediction of survival to aerosol challenge. Sci. Transl. Med. 2012, 4, 151ra126. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A New Look at the Statistical Model Identification. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1998; pp. 215–222. [Google Scholar]
- Golding, H.; Khurana, S.; Zaitseva, M. What is the predictive value of animal models for vaccine efficacy in humans? The importance of bridging studies and species-independent correlates of protection. Cold Spring Harb. Perspect. Biol. 2018, 10, a028902. [Google Scholar] [CrossRef] [PubMed]
- Gruber, M.F.; Farizo, K.M.; Pratt, R.D.; Fink, D.L.; Finn, T.M.; Krause, P.R.; Borio, L.L.; Marks, P.W. Clinical development strategies and considerations for Zika vaccine licensure. J. Infect. Dis. 2017, 216, S964–S970. [Google Scholar] [CrossRef] [PubMed]
- Yellowlees, A.; Perry, R.H. Estimating vaccine efficacy using animal efficacy data. Eur. J. Pharmacol. 2015, 759, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.K.; Alera, M.T.; Lago, C.B.; Tac-An, I.A.; Villa, D.; Fernandez, S.; Thaisomboonsuk, B.; Klungthong, C.; Levy, J.W.; Velasco, J.M.; et al. High rate of subclinical chikungunya virus infection and association of neutralizing antibody with protection in a prospective cohort in the Philippines. PLoS Negl. Trop. Dis. 2015, 9, e0003764. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. IXCHIQ Vaccine. Available online: https://www.fda.gov/vaccines-blood-biologics/ixchiq (accessed on 7 March 2024).
- Chen, W. Chikungunya Vaccines. ACIP Meeting 2024. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2024-02-28-29/01-Chikungunya-chen-508.pdf (accessed on 7 March 2024).
- Durbin, A.P.; Whitehead, S.S. Zika vaccines: Role for controlled human infection. J. Infect. Dis. 2017, 216, S971–S975. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, B.; Noriega, F.; Dayan, G.H.; Rivera, D.M.; Arredondo, J.L.; Reynales, H.; Luz, K.; Deseda, C.; Bonaparte, M.I.; Langevin, E.; et al. Zika and dengue interactions in the context of a large dengue vaccine clinical trial in Latin America. Am. J. Trop. Med. Hyg. 2021, 104, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Van Rompay, K.K.A.; Keesler, R.I.; Ardeshir, A.; Watanabe, J.; Usachenko, J.; Singapuri, A.; Cruzen, C.; Bliss-Moreau, E.; Murphy, A.M.; Yee, J.L.; et al. DNA vaccination before conception protects Zika virus-exposed pregnant macaques against prolonged viremia and improves fetal outcomes. Sci. Transl. Med. 2019, 11, eaay2736. [Google Scholar] [CrossRef] [PubMed]
- da Costa Castilho, M.; de Filippis, A.M.B.; Machado, L.C.; de Lima Calvanti, T.Y.V.; Lima, M.C.; Fonseca, V.; Giovanetti, M.; Docena, C.; Neto, A.M.; Bôtto-Menezes, C.H.A.; et al. Evidence of Zika virus reinfection by genome diversity and antibody response analysis, Brazil. Emerg. Infect. Dis. 2024, 30, 310–320. [Google Scholar] [CrossRef]
| Criterion | Application |
|---|---|
| 1: Protection is significantly related to vaccine dose in the animal study | Fit LR with protection status as defined by vRNA as the response and log10 dose as the independent variable |
| 2: ZIKV NAbs are significantly related to vaccine dose in the animal study | Fit linear regression with ZIKV NAb GMTs as the response and log10 dose as the independent variable |
| 3: ZIKV NAbs are significantly related to protection in the animal study | Fit LR with protection status as the response and ZIKV NAb GMTs as the independent variable |
| 4: The full effect of the vaccine on protection is explained by ZIKV NAbs | Fit a similar model as in step 3, with the addition of a term for vaccine log10 dose. Determine that the log10 dose term does not contribute significantly to the model fit |
| N | TAK-426 Dose, µg | GMT (95% CI) | |||
|---|---|---|---|---|---|
| Baseline | Study Day 29 | Study Day 57 | |||
| Humans | 28 | 0 | 34 (27–44) | 28 (22–36) | 31 (25–40) |
| 25 | 2 | 37 (29–47) | 408 (274–607) | 3701 (2330–5878) | |
| 29 | 5 | 34 (28–40) | 688 (468–1010) | 6977 (4663–10,438) | |
| 30 | 10 | 48 (41–56) | 1310 (875–1961) | 13,604 (9560–19,359) | |
| NHPs | 4 | 0 | 45 (25–79) | 35 (19–67) | 40 (24–67) |
| 6 | 2 | 47 (29–75) | 437 (218–876) | 20,145 (11,363–35,714) | |
| 0 | 5 | N/A | N/A | N/A | |
| 6 | 10 | 57 (39–84) | 1959 (1014–3785) | 28,892 (12,607–66,212) | |
| Parameter | Estimate | 95% LCL | 95% UCL |
|---|---|---|---|
| Intercept | −12.39 | −22.87 | −1.91 |
| Slope | 4.31 | 0.85 | 7.77 |
| Hosmer–Lemeshow goodness-of-fit test statistic | 1.71 | N/A | N/A |
| Hosmer–Lemeshow test p-value | 0.99 | N/A | N/A |
| Area under the curve | 0.98 | N/A | N/A |
| Estimated Probability of Protection, against ZIKV, and 95% CI | |||
|---|---|---|---|
| Baseline | Day 29 | Day 57 | |
| Placebo | 1 (0–40) | 0 (0–36) | 0 (0–33) |
| TAK-426 2 µg | 1 (0–36) | 30 (13–66) | 87 (63–96) |
| TAK-426 5 µg | 0 (0–39) | 46 (25–74) | 95 (71–99) |
| TAK-426 10 µg | 1 (0–43) | 65 (42–84) | 98 (80–100) |
| Study Day | TAK-426 Dose, µg | Probability (Protection) Post, % | Lower Post, % | Upper Post, % | Probability (Protection) Boot, % | Lower Boot, % | Upper Boot, % |
|---|---|---|---|---|---|---|---|
| 29 | 2 | 21 | 3 | 55 | 21 | 2 | 57 |
| 5 | 42 | 13 | 72 | 42 | 11 | 75 | |
| 10 | 74 | 47 | 91 | 74 | 41 | 94 | |
| 57 | 2 | 95 | 80 | 100 | 95 | 78 | 100 |
| 5 | 99 | 89 | 100 | 98 | 89 | 100 | |
| 10 | 100 | 95 | 100 | 100 | 95 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta, C.J.; Nordio, F.; Boltz, D.A.; Baldwin, W.R.; Hather, G.; Kpamegan, E. Predicting Efficacy of a Purified Inactivated Zika Virus Vaccine in Flavivirus-Naïve Humans Using an Immunological Correlate of Protection in Non-Human Primates. Microorganisms 2024, 12, 1177. https://doi.org/10.3390/microorganisms12061177
Acosta CJ, Nordio F, Boltz DA, Baldwin WR, Hather G, Kpamegan E. Predicting Efficacy of a Purified Inactivated Zika Virus Vaccine in Flavivirus-Naïve Humans Using an Immunological Correlate of Protection in Non-Human Primates. Microorganisms. 2024; 12(6):1177. https://doi.org/10.3390/microorganisms12061177
Chicago/Turabian StyleAcosta, Camilo J., Francesco Nordio, David A. Boltz, Whitney R. Baldwin, Greg Hather, and Eloi Kpamegan. 2024. "Predicting Efficacy of a Purified Inactivated Zika Virus Vaccine in Flavivirus-Naïve Humans Using an Immunological Correlate of Protection in Non-Human Primates" Microorganisms 12, no. 6: 1177. https://doi.org/10.3390/microorganisms12061177






